Laser-Processed PEN with Au Nanowires Array: A Biocompatibility Assessment
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Characterization
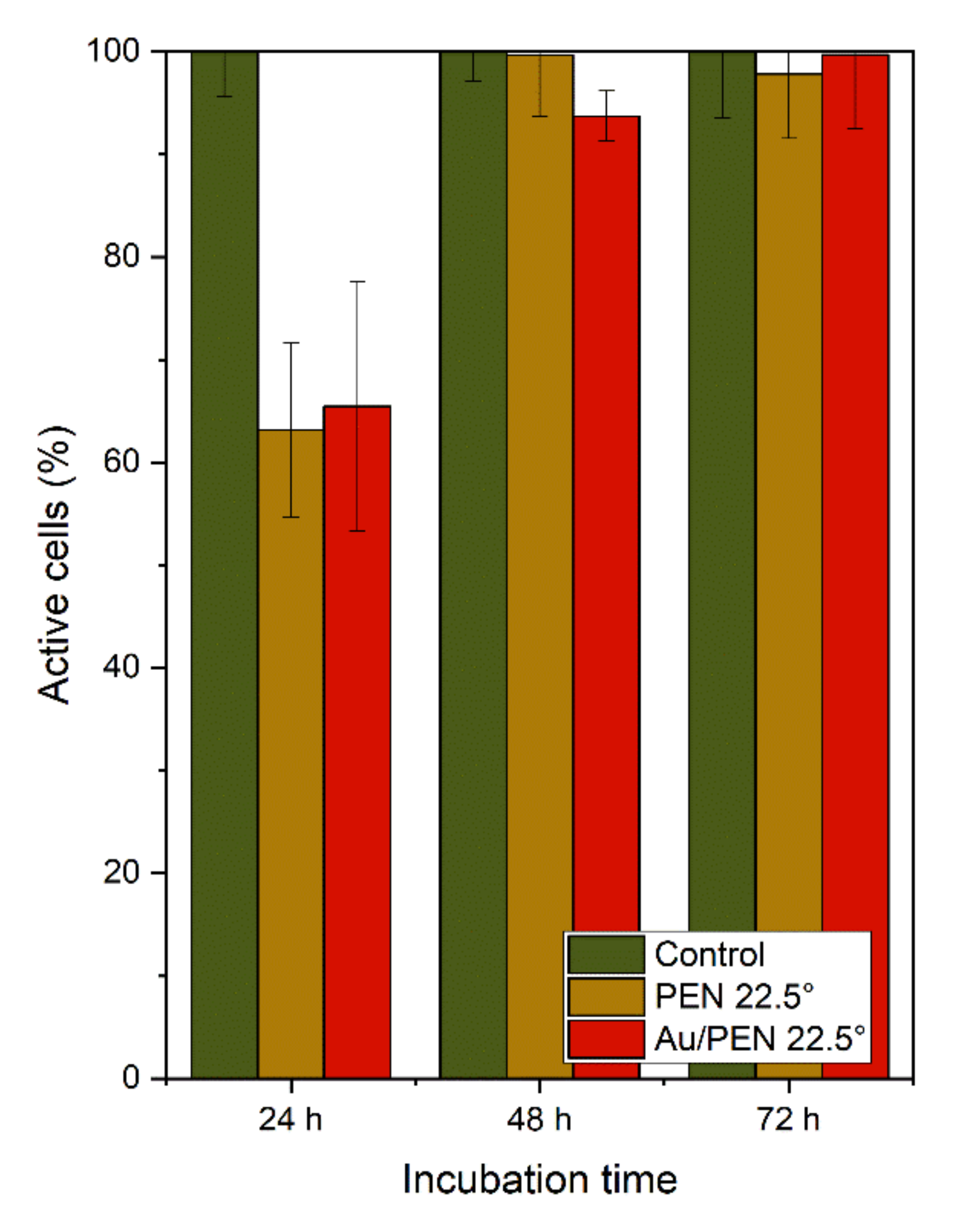
2.2. Cytotoxicity Tests
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Analytical Methods
3.3. Cytotoxicity Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Anderson, J.J.; Meenan, R.F. The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis. Results of two metaanalyses. Arthritis Rheumatol. 1990, 33, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Vollath, D. Optical properties. In Nanoparticles-Nanocomposites-Nanomaterials; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 181–228. [Google Scholar]
- Visaria, R.K.; Griffin, R.J.; Williams, B.W.; Ebbini, E.S.; Paciotti, G.F.; Song, C.W.; Bischof, J.C. Enhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-α delivery. Mol. Cancer Ther. 2006, 5, 1014–1020. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Park, J.-A.; Kim, H.-K.; Kim, J.-H.; Jeong, S.-W.; Jung, J.-C.; Lee, G.-H.; Lee, J.; Chang, Y.; Kim, T.-J. Gold nanoparticles functionalized by gadolinium–DTPA conjugate of cysteine as a multimodal bioimaging agent. Bioorg. Med. Chem. Lett. 2010, 20, 2287–2291. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Li, J.-C.; Chen, S.-H.; Liu, T.-Y.; Kuo, C.-H.; Huang, W.-T.; Lin, C.-S. An optical biosensing platform for proteinase activity using gold nanoparticles. Biomaterials 2010, 31, 6087–6095. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.Y.; Zhang, H.; Xia, Y.; Li, X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef]
- Kim, C.-k.; Ghosh, P.; Rotello, V.M. Multimodal drug delivery using gold nanoparticles. Nanoscale 2009, 1, 61–67. [Google Scholar] [CrossRef]
- Chung, E.; Nam, S.Y.; Ricles, L.M.; Emelianov, S.Y.; Suggs, L.J. Evaluation of gold nanotracers to track adipose-derived stem cells in a PEGylated fibrin gel for dermal tissue engineering applications. Int. J. Nanomed. 2013, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Pryjmaková, J.; Kaimlová, M.; Hubáček, T.; Švorčík, V.; Siegel, J. Nanostructured Materials for Artificial Tissue Replacements. Int. J. Mol. Sci. 2020, 21, 2521. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Colombo, M.; Prosperi, D.; Corsi, F.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Evaluation of gold nanoparticles biocompatibility: A multiparametric study on cultured endothelial cells and macrophages. J. Nanopart. Res. 2016, 18, 58. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Soritau, O.; Maniu, D.; Astilean, S. One-pot, green synthesis of gold nanoparticles by gelatin and investigation of their biological effects on Osteoblast cells. Colloids Surf. B Biointerfaces 2015, 132, 122–131. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.-J.; Kim, S.-Y.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.-Y.; Park, S.A. In situ gold nanoparticle growth on polydopamine-coated 3D-printed scaffolds improves osteogenic differentiation for bone tissue engineering applications: In vitro and in vivo studies. Nanoscale 2018, 10, 15447–15453. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Shevach, M.; Maoz, B.M.; Feiner, R.; Shapira, A.; Dvir, T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J. Mater. Chem. B 2013, 1, 5210–5217. [Google Scholar] [CrossRef]
- Shevach, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold Nanoparticle-Decellularized Matrix Hybrids for Cardiac Tissue Engineering. Nano Lett. 2014, 14, 5792–5796. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Polívková, M.; Štrublová, V.; Hubáček, T.; Rimpelová, S.; Švorčík, V.; Siegel, J. Surface characterization and antibacterial response of silver nanowire arrays supported on laser-treated polyethylene naphthalate. Mater. Sci. Eng. C 2017, 72, 512–518. [Google Scholar] [CrossRef]
- Asharani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, W.; Wang, H.; Zhuang, H.; Zhang, J. Shell thickness-dependent antibacterial activity and biocompatibility of gold@silver core–shell nanoparticles. RSC Adv. 2017, 7, 11355–11361. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Aparna, R.S.L.; Prasad, R.G.S.V. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 2014, 137, 75–78. [Google Scholar] [CrossRef]
- Navya, P.N.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 2019, 96, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Pryjmaková, J.; Kaimlová, M.; Vokatá, B.; Hubáček, T.; Slepička, P.; Švorčík, V.; Siegel, J. Bimetallic Nanowires on Laser-Patterned PEN as Promising Biomaterials. Nanomaterials 2021, 11, 2285. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.; Bor, Z. Laser-induced periodic surface structure formation on polyethylene-terephthalate. Appl. Surf. Sci. 1998, 133, 5–16. [Google Scholar] [CrossRef]
- Ehrhardt, M.; Lai, S.; Lorenz, P.; Zimmer, K. Guiding of LIPSS formation by excimer laser irradiation of pre-patterned polymer films for tailored hierarchical structures. Appl. Surf. Sci. 2020, 506, 144785. [Google Scholar] [CrossRef]
- Rebollar, E.; Pérez, S.; Hernández, J.J.; Martín-Fabiani, I.; Rueda, D.R.; Ezquerra, T.A.; Castillejo, M. Assessment and Formation Mechanism of Laser-Induced Periodic Surface Structures on Polymer Spin-Coated Films in Real and Reciprocal Space. Langmuir 2011, 27, 5596–5606. [Google Scholar] [CrossRef]
- Cui, J.; Nogales, A.; Ezquerra, T.A.; Rebollar, E. Influence of substrate and film thickness on polymer LIPSS formation. Appl. Surf. Sci. 2017, 394, 125–131. [Google Scholar] [CrossRef]
- Slepička, P.; Neděla, O.; Sajdl, P.; Kolská, Z.; Švorčík, V. Polyethylene naphthalate as an excellent candidate for ripple nanopatterning. Appl. Surf. Sci. 2013, 285, 885–892. [Google Scholar] [CrossRef]
- Slepicka, P.; Chaloupka, A.; Sajdl, P.; Heitz, J.; Hnatowicz, V.; Švorčík, V. Angle dependent laser nanopatterning of poly(ethylene terephthalate) surfaces. Appl. Surf. Sci. 2011, 257, 6021–6025. [Google Scholar] [CrossRef]
- Belardini, A.; Larciprete, M.C.; Centini, M.; Fazio, E.; Sibilia, C.; Bertolotti, M.; Toma, A.; Chiappe, D.; Mongeot, F.B.d. Tailored second harmonic generation from self-organized metal nano-wires arrays. Opt. Express 2009, 17, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Heitz, J.; Řezníčková, A.; Švorčík, V. Preparation and characterization of fully separated gold nanowire arrays. Appl. Surf. Sci. 2013, 264, 443–447. [Google Scholar] [CrossRef]
- Polivkova, M.; Valova, M.; Rimpelova, S.; Slepicka, P.; Svorcik, V.; Siegel, J. Pd nanowire coatings of laser-treated polyethylene naphthalate: Preparation, characterization and biological response. Express Polym. Lett. 2018, 12, 1039–1046. [Google Scholar] [CrossRef]
- Kaimlová, M.; Nemogová, I.; Kolářová, K.; Slepička, P.; Švorčík, V.; Siegel, J. Optimization of silver nanowire formation on laser processed PEN: Surface properties and antibacterial effects. Appl. Surf. Sci. 2019, 473, 516–526. [Google Scholar] [CrossRef]
- Bäuerle, D. Laser Processing and Chemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000. [Google Scholar]
- Pryjmaková, J.; Hryhoruk, M.; Veselý, M.; Slepička, P.; Švorčík, V.; Siegel, J. Engineered Cu-PEN Composites at the Nanoscale: Preparation and Characterisation. Nanomaterials 2022, 12, 1220. [Google Scholar] [CrossRef]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting Transition from the Cassie–Baxter State to the Wenzel State on Textured Polymer Surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef]
- Pessková, V.; Kubies, D.; Hulejová, H.; Himmlová, L. The influence of implant surface properties on cell adhesion and proliferation. J. Mater. Science. Mater. Med. 2007, 18, 465–473. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef]
- Ton-That, C.; Shard, A.G.; Bradley, R.H. Thickness of Spin-Cast Polymer Thin Films Determined by Angle-Resolved XPS and AFM Tip-Scratch Methods. Langmuir 2000, 16, 2281–2284. [Google Scholar] [CrossRef]
- Markossian, S.; Grossman, A.; Brimacombe, K. Assay Guidance Manual. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53196/ (accessed on 9 August 2022).
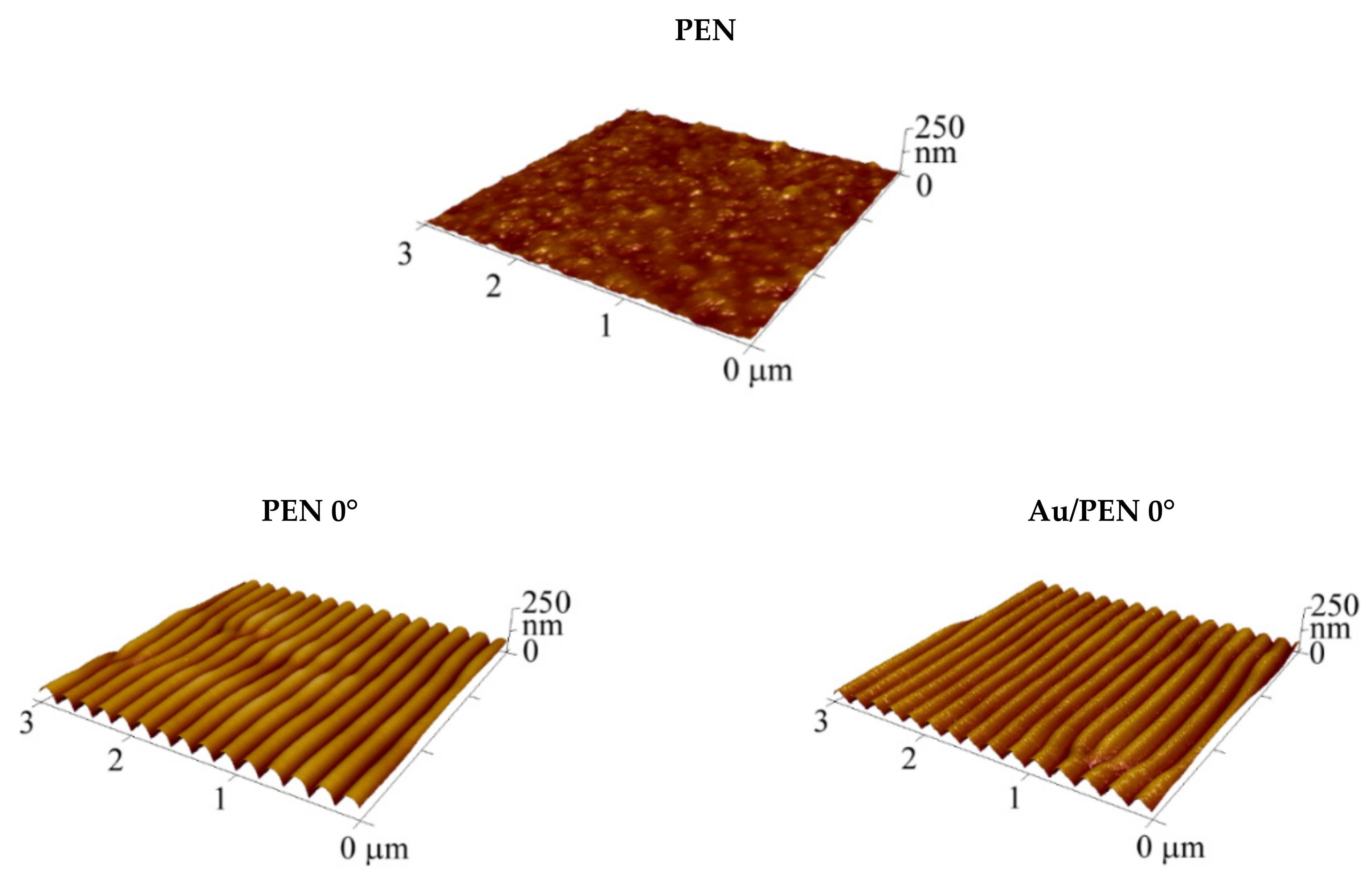
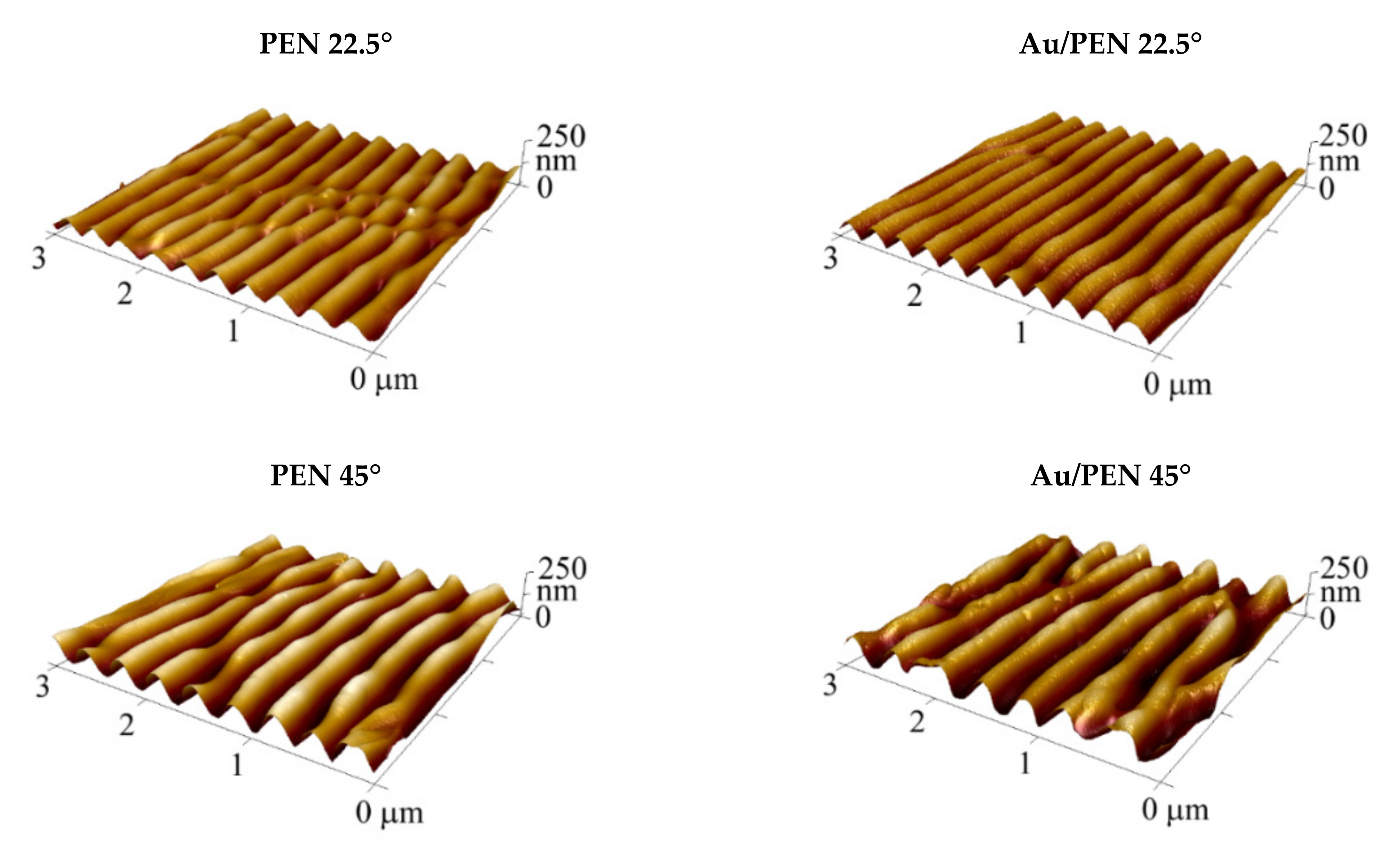
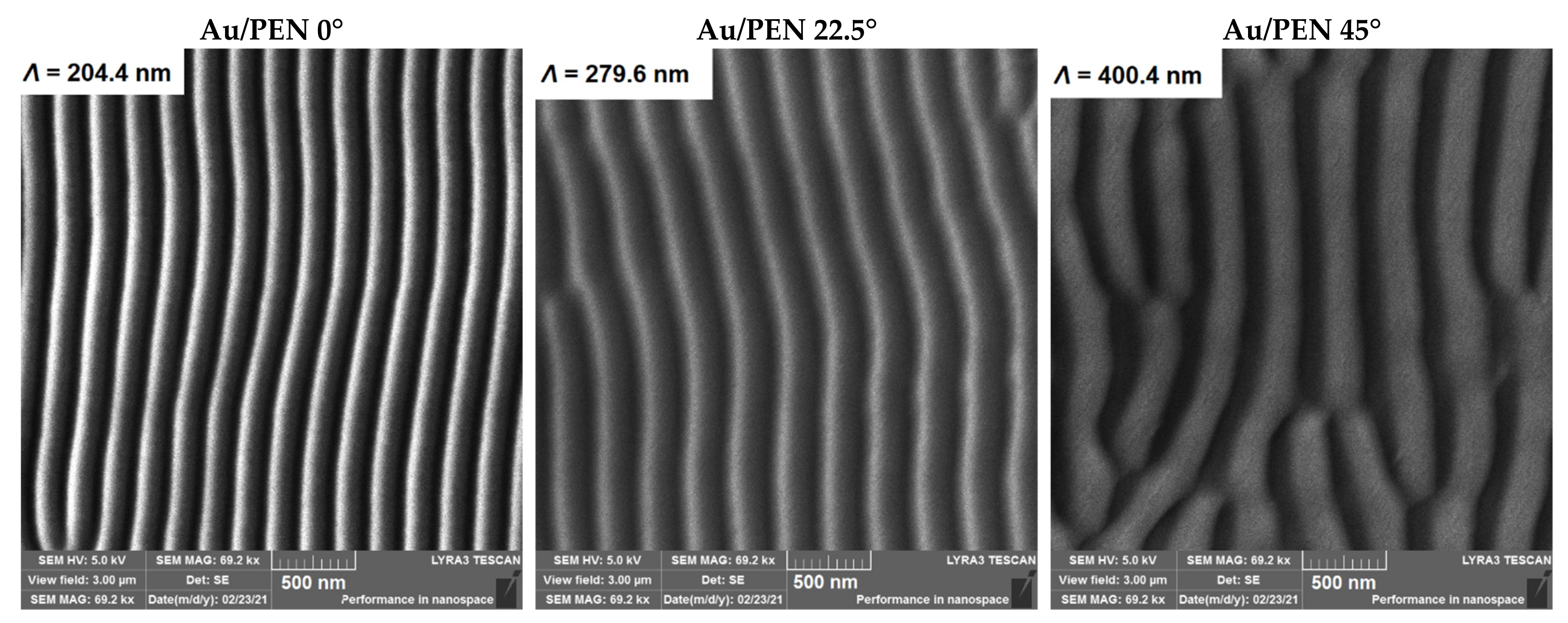
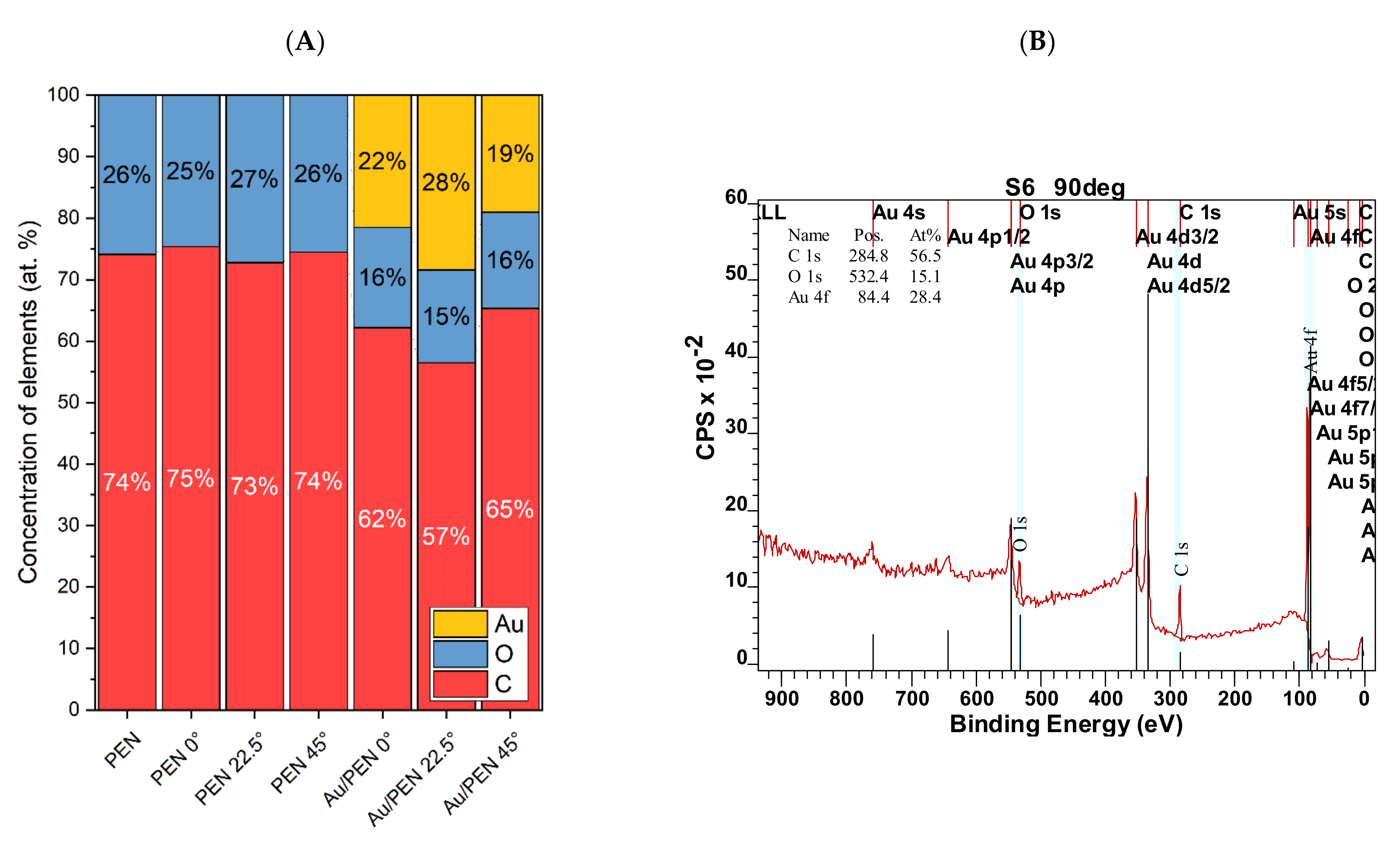
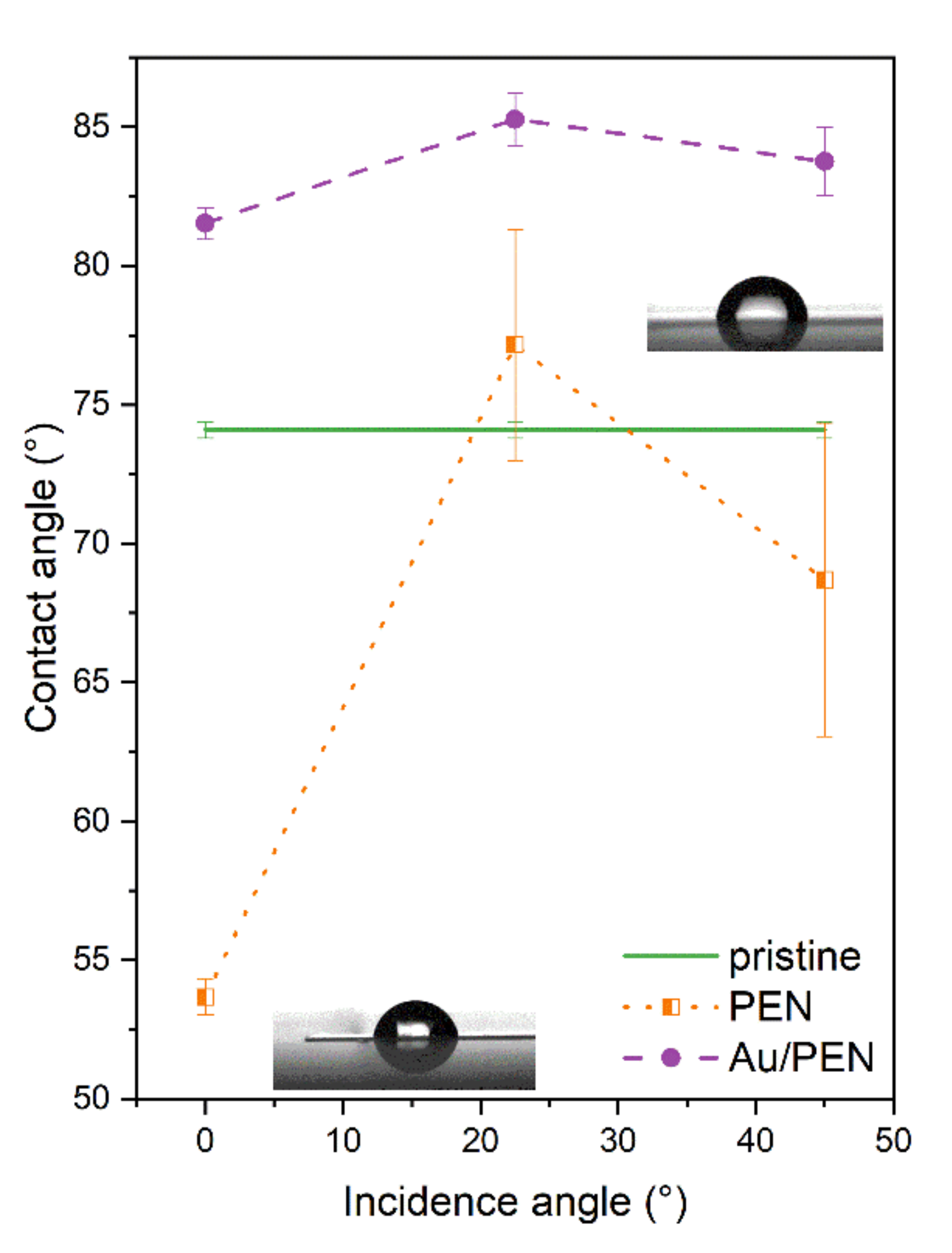
| Sample | Ra (nm) | Λ (nm) | h (nm) |
|---|---|---|---|
| PEN | 4.5 | - | - |
| PEN 0° | 16.0 | 207.6 ± 4.5 | 69.9 ± 2.2 |
| PEN 22.5° | 19.8 | 287.8 ± 3.5 | 88.1 ± 4.0 |
| PEN 45° | 34.3 | 339.6 ± 4.1 | 119.3 ± 2.7 |
| Au/PEN 0° | 14.9 | 204.4 ± 4.3 | 61.9 ± 3.2 |
| Au/PEN 22.5° | 20.6 | 279.6 ± 3.3 | 78.4 ± 2.9 |
| Au/PEN 45° | 40.6 | 400.4 ± 3.4 | 108.7 ± 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryjmaková, J.; Vokatá, B.; Slepička, P.; Siegel, J. Laser-Processed PEN with Au Nanowires Array: A Biocompatibility Assessment. Int. J. Mol. Sci. 2022, 23, 10953. https://doi.org/10.3390/ijms231810953
Pryjmaková J, Vokatá B, Slepička P, Siegel J. Laser-Processed PEN with Au Nanowires Array: A Biocompatibility Assessment. International Journal of Molecular Sciences. 2022; 23(18):10953. https://doi.org/10.3390/ijms231810953
Chicago/Turabian StylePryjmaková, Jana, Barbora Vokatá, Petr Slepička, and Jakub Siegel. 2022. "Laser-Processed PEN with Au Nanowires Array: A Biocompatibility Assessment" International Journal of Molecular Sciences 23, no. 18: 10953. https://doi.org/10.3390/ijms231810953
APA StylePryjmaková, J., Vokatá, B., Slepička, P., & Siegel, J. (2022). Laser-Processed PEN with Au Nanowires Array: A Biocompatibility Assessment. International Journal of Molecular Sciences, 23(18), 10953. https://doi.org/10.3390/ijms231810953








