Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents
Abstract
:1. Introduction
2. Hys(V) Behavior Residing in Hyperpolarization-Activated Cation Current (Ih)
2.1. Pirfenidone (Esbriet®, 5-Methyl-1-Phenylpyridin-2[H-1]-One)
2.2. Dexmedetomidine
2.3. Oxaliplatin
2.4. Honokiol
2.5. Lutein (Xanthophyll, β,ε-Carotene-3,3′-Diol or 3,3′-Di-Hydroxy-β,α-Carotene)
3. Hys(V) Behavior Residing in Erg-Mediated K+ Current (IK(erg))
3.1. UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine))
3.2. SM-102 (1-Octylnonyl 8-[(2-Hydroxyethyl)[6-oxo-6(Undecyloxy)hexyl]amino]-Octanoate)
3.3. Isoplumbagin (5-Hydroxy-3-Methyl-1,4-Naphthoquinone) and Plumbagin (5-Hydroxy-2-Methyl-1,4-Naphthoquinone)
4. Hys(V) Behavior Residing in M-Type K+ Current (IK(M))
4.1. Remdesivir (Development Code: GS-5734)
4.2. QO-58 (5-(2,6-Dichloro-5-Fluoropyridin-3-yl)-3-Phenyl-2-(Trifluoromethyl)-1H-Pyrazolol[1,5-a]pyrimidin-7-One)
5. Hys(V) Behavior Residing in L-Type Ca2+ Current (ICa,L)
Zingerone (Ginerone, Vanillylacetone)
6. Hys(V) Behavior Residing in Persistent Na+ Current (INa(P))
6.1. Esaxerenone (Minnebro®)
6.2. Mirogabalin
6.3. Dapagliflozin (Foxiga®)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| erg | ether-à-go-go-related gene |
| Hys(V) | voltage-dependent hysteresis |
| HCN channel | hyperpolarization-activated cyclic nucleotide-gated channel |
| ICa,L | L-type Ca2+ current |
| Ih | hyperpolarization-activated cation current |
| IK(erg) | erg-mediated K+ current |
| IK(M) | M-type K+ current |
| INa(L) | late Na+ current |
| INa(P) | persistent Na+ current |
| INa(T) | transient (peak) Na+ current |
| Kerg channel | erg-mediated K+ channel |
| KM channel | M-type K+ channel |
| NaV channel | voltage-gated Na+ channel |
| SGLT | Na+-dependent glucose co-transporter |
| Vramp | ramp voltage |
References
- Krylov, D.; Velkos, G.; Chen, C.H.; Büchner, B.; Kostanyan, A.; Greber, T.; Avdoshenko, S.M.; Popov, A.A. Magnetic hysteresis and strong ferromagnetic coupling of sulfur-bridged Dy ions in clusterfullerene Dy(2)S@C(82). Inorg. Chem. Front. 2020, 7, 3521–3532. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Galea, C.A.; Chiem, A.T. Hysteretic Behavior in Voltage-Gated Channels. Front. Pharmacol. 2020, 11, 579596. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Benzoni, P.; Bertoli, G.; Giannetti, F.; Piantoni, C.; Milanesi, R.; Pecchiari, M.; Barbuti, A.; Baruscotti, M.; Bucchi, A. The funny current: Even funnier than 40 years ago. Uncanonical expression and roles of HCN/f channels all over the body. Prog. Biophys. Mol. Biol. 2021, 166, 189–204. [Google Scholar] [CrossRef]
- Combe, C.L.; Gasparini, S. I(h) from synapses to networks: HCN channel functions and modulation in neurons. Prog. Biophys. Mol. Biol. 2021, 166, 119–132. [Google Scholar] [CrossRef]
- Peters, C.H.; Liu, P.W.; Morotti, S.; Gantz, S.C.; Grandi, E.; Bean, B.P.; Proenza, C. Bidirectional flow of the funny current (I(f)) during the pacemaking cycle in murine sinoatrial node myocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2104668118. [Google Scholar] [CrossRef]
- Saponaro, A.; Bauer, D.; Giese, M.H.; Swuec, P.; Porro, A.; Gasparri, F.; Sharifzadeh, A.S.; Chaves-Sanjuan, A.; Alberio, L.; Parisi, G.; et al. Gating movements and ion permeation in HCN4 pacemaker channels. Mol. Cell 2021, 81, 2929–2943.e2926. [Google Scholar] [CrossRef]
- Depuydt, A.S.; Peigneur, S.; Tytgat, J. HCN channels in the heart. Curr. Cardiol. Rev. 2022, 18, e040222200836. [Google Scholar] [CrossRef]
- Yavuz, M.; Aydın, B.; Çarçak, N.; Onat, F. Decreased Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 2 Activity in a Rat Model of Absence Epilepsy and the Effect of ZD7288, an Ih Inhibitor, on the Spike-and-Wave Discharges. Pharmacology 2022, 107, 227–234. [Google Scholar] [CrossRef]
- Saito, S.; Alkhatib, A.; Kolls, J.K.; Kondoh, Y.; Lasky, J.A. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J. Thorac. Dis. 2019, 11, S1740–S1754. [Google Scholar] [CrossRef]
- Chang, W.T.; Gao, Z.H.; Li, S.W.; Liu, P.Y.; Lo, Y.C.; Wu, S.N. Characterization in Dual Activation by Oxaliplatin, a Platinum-Based Chemotherapeutic Agent of Hyperpolarization-Activated Cation and Electroporation-Induced Currents. Int. J. Mol. Sci. 2020, 21, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.T.; Ragazzi, E.; Liu, P.Y.; Wu, S.N. Effective block by pirfenidone, an antifibrotic pyridone compound (5-methyl-1-phenylpyridin-2[H-1]-one), on hyperpolarization-activated cation current: An additional but distinctive target. Eur. J. Pharmacol. 2020, 882, 173237. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Ramsay, G.; Mantz, J.; Sum-Ping, S.T. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J. Intensive Care Med. 2003, 18, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H.; Zeller, S.; Citrome, L.; Finman, J.; Goldberg, J.F.; Fava, M.; Kakar, R.; De Vivo, M.; Yocca, F.D.; Risinger, R. Effect of Sublingual Dexmedetomidine vs Placebo on Acute Agitation Associated With Bipolar Disorder: A Randomized Clinical Trial. JAMA 2022, 327, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Lu, T.J.; Wu, S.N. Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α(2)-Adrenergic Receptors. Int. J. Mol. Sci. 2020, 21, 9110. [Google Scholar] [CrossRef] [PubMed]
- Colthorpe, K.L.; Nalliah, J.; Anderson, S.T.; Curlewis, J.D. Adrenoceptor subtype involvement in suppression of prolactin secretion by noradrenaline. J. Neuroendocrinol. 2000, 12, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar] [CrossRef]
- Chen, B.S.; Peng, H.; Wu, S.N. Dexmedetomidine, an alpha2-adrenergic agonist, inhibits neuronal delayed-rectifier potassium current and sodium current. Br. J. Anaesth. 2009, 103, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.; Burnsed, J.; Heinan, K.; Letzkus, L.; Andris, R.; Fairchild, K.; Zanelli, S. Effect of dexmedetomidine on heart rate in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J. Neonatal. Perinatal. Med. 2022, 15, 47–54. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Lipp, H.P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Graham, J.; Mushin, M.; Kirkpatrick, P. Oxaliplatin. Nat. Rev. Drug Discov. 2004, 3, 11–12. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Jin, L.; Tan, Y.; Li, W.; Tang, J. HCN2 contributes to oxaliplatin-induced neuropathic pain through activation of the CaMKII/CREB cascade in spinal neurons. Mol. Pain 2018, 14, 1744806918778490. [Google Scholar] [CrossRef]
- Resta, F.; Micheli, L.; Laurino, A.; Spinelli, V.; Mello, T.; Sartiani, L.; Di Cesare Mannelli, L.; Cerbai, E.; Ghelardini, C.; Romanelli, M.N.; et al. Selective HCN1 block as a strategy to control oxaliplatin-induced neuropathy. Neuropharmacology 2018, 131, 403–413. [Google Scholar] [CrossRef]
- Yongning, Z.; Xianguang, L.; Hengling, C.; Su, C.; Fang, L.; Chenhong, L. The hyperpolarization-activated cyclic nucleotide-gated channel currents contribute to oxaliplatin-induced hyperexcitability of DRG neurons. Somatosens. Mot. Res. 2021, 38, 11–19. [Google Scholar] [CrossRef]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef] [Green Version]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Wang, Y.J.; Wu, P.Y.; Wu, S.N. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 127–135. [Google Scholar] [CrossRef]
- Hsiao, H.T.; Liu, Y.C.; Liu, P.Y.; Wu, S.N. Concerted suppression of I(h) and activation of I(K(M)) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef]
- Wu, S.N.; Huang, C.W. Editorial to the Special Issue “Electrophysiology”. Int. J. Mol. Sci. 2021, 22, 2956. [Google Scholar] [CrossRef]
- Bi, L.; Yu, Z.; Wu, J.; Yu, K.; Hong, G.; Lu, Z.; Gao, S. Honokiol Inhibits Constitutive and Inducible STAT3 Signaling via PU.1-Induced SHP1 Expression in Acute Myeloid Leukemia Cells. Tohoku J. Exp. Med. 2015, 237, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.H.; Chen, H.H.; Lo, Y.C.; Wu, S.N. Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K(+) Current. Int. J. Mol. Sci. 2020, 21, 4260. [Google Scholar] [CrossRef]
- Robinson, R.B.; Siegelbaum, S.A. Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev. Physiol. 2003, 65, 453–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodbury, A.; Yu, S.P.; Wei, L.; García, P. Neuro-modulating effects of honokiol: A review. Front. Neurol. 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodbury, A.; Yu, S.P.; Chen, D.; Gu, X.; Lee, J.H.; Zhang, J.; Espinera, A.; García, P.S.; Wei, L. Honokiol for the Treatment of Neonatal Pain and Prevention of Consequent Neurobehavioral Disorders. J. Nat. Prod. 2015, 78, 2531–2536. [Google Scholar] [CrossRef]
- Elinder, F.; Männikkö, R.; Pandey, S.; Larsson, H.P. Mode shifts in the voltage gating of the mouse and human HCN2 and HCN4 channels. J. Physiol. 2006, 575, 417–431. [Google Scholar] [CrossRef]
- Chuang, C.W.; Chang, K.P.; Cho, H.Y.; Chuang, T.H.; Yu, M.C.; Wu, C.L.; Wu, S.N. Characterization of Inhibitory Capability on Hyperpolarization-Activated Cation Current Caused by Lutein (β,ε-Carotene-3,3′-Diol), a Dietary Xanthophyll Carotenoid. Int. J. Mol. Sci. 2022, 23, 7186. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Jiang, Z.; Yue, W.W.S.; Chen, L.; Sheng, Y.; Yau, K.W. Cyclic-Nucleotide- and HCN-Channel-Mediated Phototransduction in Intrinsically Photosensitive Retinal Ganglion Cells. Cell 2018, 175, 652–664.e612. [Google Scholar] [CrossRef] [Green Version]
- Popova, E.; Kupenova, P. Effects of HCN channel blockade on the intensity-response function of electroretinographic ON and OFF responses in dark adapted frogs. Acta Neurobiol. Exp. 2020, 80, 192–204. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K(+) channels: Structure, function, and clinical significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinson, A.S.; van Rossum, D.B.; Diatta, F.H.; Layden, M.J.; Rhodes, S.A.; Martindale, M.Q.; Jegla, T. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc. Natl. Acad. Sci. USA 2014, 111, 5712–5717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.N.; Jan, C.R.; Li, H.F.; Chiang, H.T. Characterization of inhibition by risperidone of the inwardly rectifying K(+) current in pituitary GH(3) cells. Neuropsychopharmacology 2000, 23, 676–689. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yamasaki, M.; Abe, M.; Matsuda, Y.; Morino, H.; Kawakami, H.; Sakimura, K.; Watanabe, M.; Hashimoto, K. Kv11 (ether-à-go-go-related gene) voltage-dependent K(+) channels promote resonance and oscillation of subthreshold membrane potentials. J. Physiol. 2021, 599, 547–569. [Google Scholar] [CrossRef]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH(3) cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.P.; Thouta, S.; Claydon, T.W. Modulation of hERG K(+) Channel Deactivation by Voltage Sensor Relaxation. Front. Pharmacol. 2020, 11, 139. [Google Scholar] [CrossRef]
- Hsu, H.T.; Lo, Y.C.; Wu, S.N. Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels. Int. J. Mol. Sci. 2020, 21, 1441. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kolaj, M.; Renaud, L.P. Ca2+-dependent and Na+-dependent K+ conductances contribute to a slow AHP in thalamic paraventricular nucleus neurons: A novel target for orexin receptors. J. Neurophysiol. 2010, 104, 2052–2062. [Google Scholar] [CrossRef] [Green Version]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic. Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ferraresso, F.; Strilchuk, A.W.; Juang, L.J.; Poole, L.G.; Luyendyk, J.P.; Kastrup, C.J. Comparison of DLin-MC3-DMA and ALC-0315 for siRNA Delivery to Hepatocytes and Hepatic Stellate Cells. Mol. Pharm. 2022, 19, 2175–2182. [Google Scholar] [CrossRef]
- Ly, H.H.; Daniel, S.; Soriano, S.K.V.; Kis, Z.; Blakney, A.K. Optimization of Lipid Nanoparticles for saRNA Expression and Cellular Activation Using a Design-of-Experiment Approach. Mol. Pharm. 2022, 19, 1892–1905. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid. Biomedicines 2021, 9, 1367. [Google Scholar] [CrossRef]
- Zhou, J.; Augelli-Szafran, C.E.; Bradley, J.A.; Chen, X.; Koci, B.J.; Volberg, W.A.; Sun, Z.; Cordes, J.S. Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol. Pharmacol. 2005, 68, 876–884. [Google Scholar] [CrossRef]
- Srinivas, P.; Gopinath, G.; Banerji, A.; Dinakar, A.; Srinivas, G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol. Carcinog. 2004, 40, 201–211. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; He, C.; Chen, M.; Li, H. Anticancer Properties and Pharmaceutical Applications of Plumbagin: A Review. Am. J. Chin. Med. 2017, 45, 423–441. [Google Scholar] [CrossRef]
- Chen, L.; Cho, H.Y.; Chuang, T.H.; Ke, T.L.; Wu, S.N. The Effectiveness of Isoplumbagin and Plumbagin in Regulating Amplitude, Gating Kinetics, and Voltage-Dependent Hysteresis of erg-mediated K(+) Currents. Biomedicines 2022, 10, 780. [Google Scholar] [CrossRef]
- Schulze, M.M.; Löwe, R.; Pollex, R.; Mazik, M. Structure–extractability relationships for substituted 8-hydroxyquinolines: Solvent extraction of indium ions from acidic aqueous media. Mon. Für Chem. Chem. Mon. 2019, 150, 983–990. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, S.N.; Chen, C.P.; Shen, A.Y. Inhibition of Ca2+-activated and voltage-dependent K+ currents by 2-mercaptophenyl-1,4-naphthoquinone in pituitary GH3 cells: Contribution to its antiproliferative effect. Life Sci. 2002, 70, 1185–1203. [Google Scholar] [CrossRef]
- Tsao, Y.C.; Chang, Y.J.; Wang, C.H.; Chen, L. Discovery of Isoplumbagin as a Novel NQO1 Substrate and Anti-Cancer Quinone. Int. J. Mol. Sci. 2020, 21, 4378. [Google Scholar] [CrossRef]
- Gribkoff, V.K. The therapeutic potential of neuronal KCNQ channel modulators. Expert Opin. Ther. Targets 2003, 7, 737–748. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Effectiveness in Activating M-Type K(+) Current Produced by Solifenacin ([(3R)-1-azabicyclo [2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action. Int. J. Mol. Sci. 2021, 22, 2399. [Google Scholar] [CrossRef]
- Lo, Y.C.; Lin, C.L.; Fang, W.Y.; Lőrinczi, B.; Szatmári, I.; Chang, W.H.; Fülöp, F.; Wu, S.N. Effective Activation by Kynurenic Acid and Its Aminoalkylated Derivatives on M-Type K(+) Current. Int. J. Mol. Sci. 2021, 22, 1300. [Google Scholar] [CrossRef]
- Wulfsen, I.; Hauber, H.P.; Schiemann, D.; Bauer, C.K.; Schwarz, J.R. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J. Neuroendocrinol. 2000, 12, 263–272. [Google Scholar] [CrossRef]
- Wu, C.L.; Chuang, C.W.; Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Evidence for Effective Inhibition of I(Na) Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid), a Known Blocker of Ca(V) Channels. Int. J. Mol. Sci. 2022, 23, 3845. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Chang, W.T.; Liu, P.Y.; Gao, Z.H.; Lee, S.W.; Lee, W.K.; Wu, S.N. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of I (K(M)) or I (K(DR)) and in the Stimulation of I (MEP). Front. Pharmacol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; et al. Design, synthesis and biological activity of pyrazolo [1,5-a]pyrimidin-7(4H)-ones as novel Kv7/KCNQ potassium channel activators. Eur. J. Med. Chem. 2011, 46, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mi, Y.; Qi, J.L.; Li, J.W.; Si, M.; Guan, B.C.; Du, X.N.; An, H.L.; Zhang, H.L. Modulation of K(v)7 potassium channels by a novel opener pyrazolo [1,5-a]pyrimidin-7(4H)-one compound QO-58. Br. J. Pharmacol. 2013, 168, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.C.; Wu, S.N.; Huang, C.W. Zingerone Modulates Neuronal Voltage-Gated Na(+) and L-Type Ca(2+) Currents. Int. J. Mol. Sci. 2022, 23, 3123. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F.; Jan, C.R. Regulation of Ca2+-activated nonselective cationic currents in rat pituitary GH3 cells: Involvement in L-type Ca2+ current. Brain Res. 1998, 812, 133–141. [Google Scholar] [CrossRef]
- Lo, Y.K.; Wu, S.N.; Lee, C.T.; Li, H.F.; Chiang, H.T. Characterization of action potential waveform-evoked L-type calcium currents in pituitary GH3 cells. Pflugers Arch. 2001, 442, 547–557. [Google Scholar] [CrossRef]
- Simeone, K.A.; Sabesan, S.; Kim, D.Y.; Kerrigan, J.F.; Rho, J.M.; Simeone, T.A. L-Type calcium channel blockade reduces network activity in human epileptic hypothalamic hamartoma tissue. Epilepsia 2011, 52, 531–540. [Google Scholar] [CrossRef]
- Ortner, N.J.; Striessnig, J. L-type calcium channels as drug targets in CNS disorders. Channels 2016, 10, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Capelli, I.; Gasperoni, L.; Ruggeri, M.; Donati, G.; Baraldi, O.; Sorrenti, G.; Caletti, M.T.; Aiello, V.; Cianciolo, G.; La Manna, G. New mineralocorticoid receptor antagonists: Update on their use in chronic kidney disease and heart failure. J. Nephrol. 2020, 33, 37–48. [Google Scholar] [CrossRef]
- Morimoto, S.; Ichihara, A. Efficacy of esaxerenone-a nonsteroidal mineralocorticoid receptor blocker-on nocturnal hypertension. Hypertens. Res. 2022, 45, 376–377. [Google Scholar] [CrossRef]
- Munkhjargal, U.; Fukuda, D.; Ganbaatar, B.; Suto, K.; Matsuura, T.; Ise, T.; Kusunose, K.; Yamaguchi, K.; Yagi, S.; Yamada, H.; et al. A Selective Mineralocorticoid Receptor Blocker, Esaxerenone, Attenuates Vascular Dysfunction in Diabetic C57BL/6 Mice. J. Atheroscler. Thromb. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef]
- Wu, G.; Li, Q.; Liu, X.; Li-Byarlay, H.; He, B. Differential state-dependent effects of deltamethrin and tefluthrin on sodium channels in central neurons of Helicoverpa armigera. Pestic. Biochem. Physiol. 2021, 175, 104836. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Chen, B.S.; Hsu, T.I.; Peng, H.; Wu, Y.H.; Lo, Y.C. Analytical studies of rapidly inactivating and noninactivating sodium currents in differentiated NG108-15 neuronal cells. J. Theor. Biol. 2009, 259, 828–836. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent I(Na) in a pituitary cell line (GH(3)). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Yeung, S.Y.; Prestwich, S.; Pucovsky, V.; Greenwood, I. Electrophysiological and molecular identification of voltage-gated sodium channels in murine vascular myocytes. J. Physiol. 2005, 568, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Virsolvy, A.; Fort, A.; Erceau, L.; Charrabi, A.; Hayot, M.; Aimond, F.; Richard, S. Hypoxic Conditions Promote Rhythmic Contractile Oscillations Mediated by Voltage-Gated Sodium Channels Activation in Human Arteries. Int. J. Mol. Sci. 2021, 22, 2570. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Mukund, S.; Deng, L.; Khakh, K.; Chang, E.; Ho, H.; Shriver, S.; Young, C.; Lin, S.; Johnson, J.P., Jr.; et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science 2015, 350, aac5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. Alpha(2)delta ligands, gabapentin, pregabalin and mirogabalin: A review of their clinical pharmacology and therapeutic use. Expert Rev. Neurother. 2016, 16, 1263–1277. [Google Scholar] [CrossRef]
- Wu, C.L.; Fu, P.; Cho, H.Y.; Chuang, T.H.; Wu, S.N. Evidence for Dual Activation of I(K(M)) and I(K(Ca)) Caused by QO-58 (5-(2,6-Dichloro-5-fluoropyridin-3-yl)-3-phenyl-2-(trifluoromethyl)-1H-pyrazolol [1,5-a]pyrimidin-7-one). Int. J. Mol. Sci. 2022, 23, 7042. [Google Scholar] [CrossRef]
- Simasko, S.M. A background sodium conductance is necessary for spontaneous depolarizations in rat pituitary cell line GH3. Am. J. Physiol. 1994, 266, C709–C719. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Simasko, S.M. A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. Am. J. Physiol. 1996, 271, C1927–C1934. [Google Scholar] [CrossRef]
- Navarro, M.A.; Salari, A.; Lin, J.L.; Cowan, L.M.; Penington, N.J.; Milescu, M.; Milescu, L.S. Sodium channels implement a molecular leaky integrator that detects action potentials and regulates neuronal firing. Elife 2020, 9, e54940. [Google Scholar] [CrossRef]
- Guérineau, N.C.; Monteil, A.; Lory, P. Sodium background currents in endocrine/neuroendocrine cells: Towards unraveling channel identity and contribution in hormone secretion. Front. Neuroendocrinol. 2021, 63, 100947. [Google Scholar] [CrossRef]
- Martiszus, B.J.; Tsintsadze, T.; Chang, W.; Smith, S.M. Enhanced excitability of cortical neurons in low-divalent solutions is primarily mediated by altered voltage-dependence of voltage-gated sodium channels. Elife 2021, 10, e67914. [Google Scholar] [CrossRef]
- Wengert, E.R.; Patel, M.K. The Role of the Persistent Sodium Current in Epilepsy. Epilepsy Curr. 2021, 21, 40–47. [Google Scholar] [CrossRef]
- Nakamura, M.; Jang, I.S. Contribution of tetrodotoxin-resistant persistent Na(+) currents to the excitability of C-type dural afferent neurons in rats. J. Headache. Pain 2022, 23, 73. [Google Scholar] [CrossRef]
- Wu, S.N.; So, E.C.; Liao, Y.K.; Huang, Y.M. Reversal by ranolazine of doxorubicin-induced prolongation in the inactivation of late sodium current in rat dorsal root ganglion neurons. Pain Med. 2015, 16, 1032–1034. [Google Scholar] [CrossRef] [Green Version]
- Gorman, K.M.; Peters, C.H.; Lynch, B.; Jones, L.; Bassett, D.S.; King, M.D.; Ruben, P.C.; Rosch, R.E. Persistent sodium currents in SCN1A developmental and degenerative epileptic dyskinetic encephalopathy. Brain Commun. 2021, 3, fcab235. [Google Scholar] [CrossRef]
- Poulin, H.; Chahine, M. R1617Q epilepsy mutation slows Na(V) 1.6 sodium channel inactivation and increases the persistent current and neuronal firing. J. Physiol. 2021, 599, 1651–1664. [Google Scholar] [CrossRef]
- Chao, E.C.; Henry, R.R. SGLT2 inhibition--a novel strategy for diabetes treatment. Nat. Rev. Drug. Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Chien, S.; Wexler, D.; Shalayda, K.; Demarest, K.; Rothenberg, P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2011, 13, 669–672. [Google Scholar] [CrossRef]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, P.A.; Sherman, A.; Stojilkovic, S.S. Common and diverse elements of ion channels and receptors underlying electrical activity in endocrine pituitary cells. Mol. Cell Endocrinol. 2018, 463, 23–36. [Google Scholar] [CrossRef]

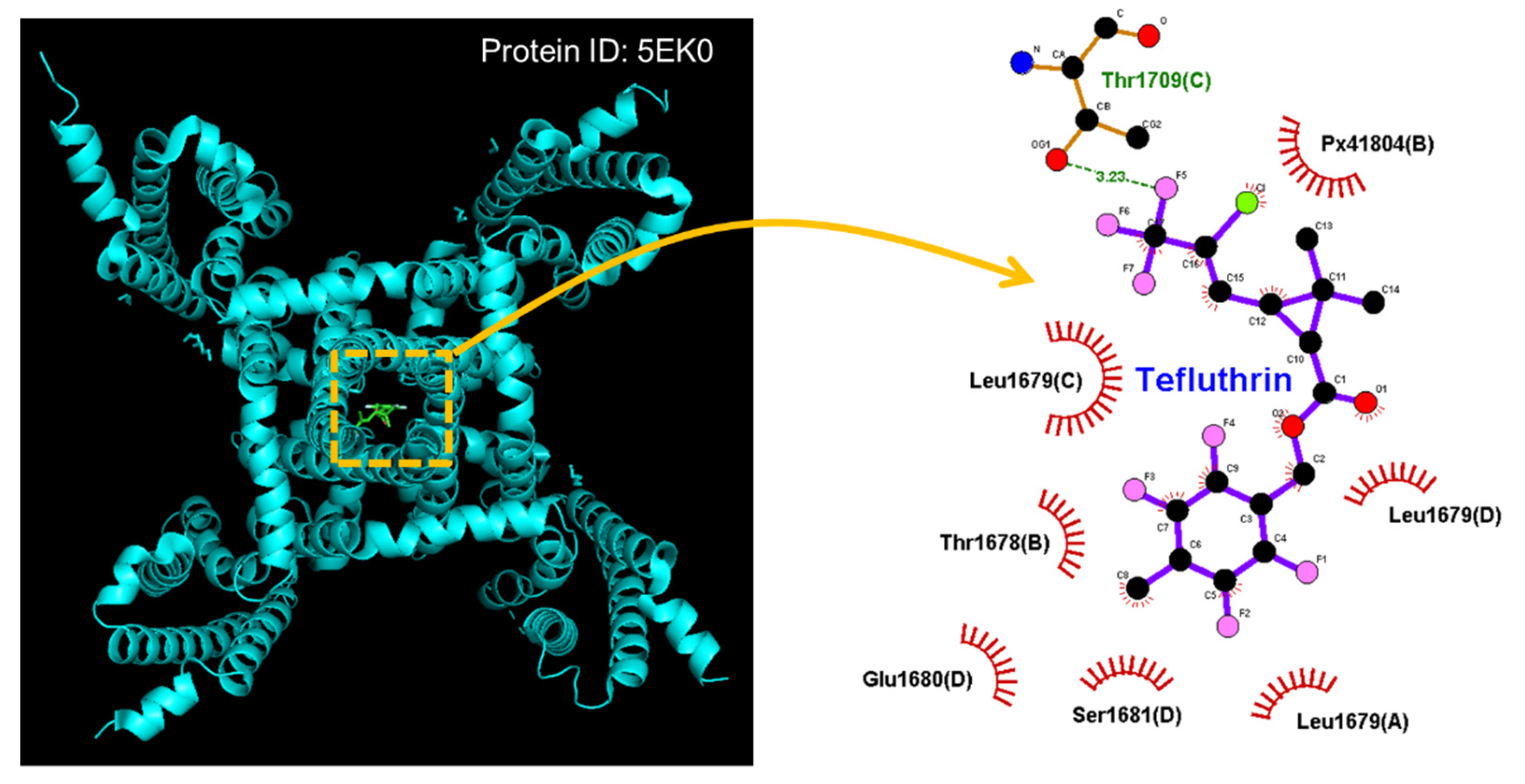

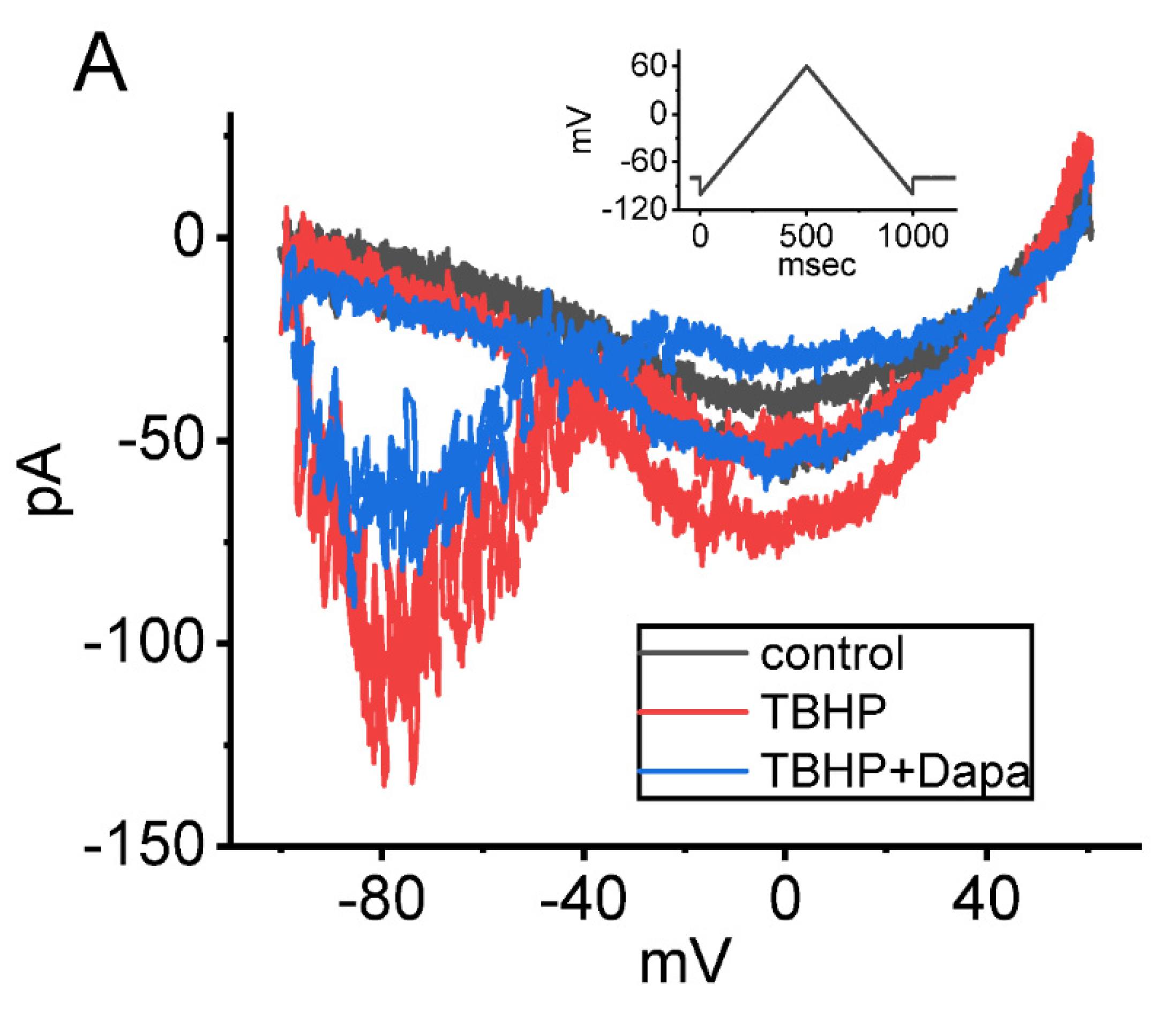
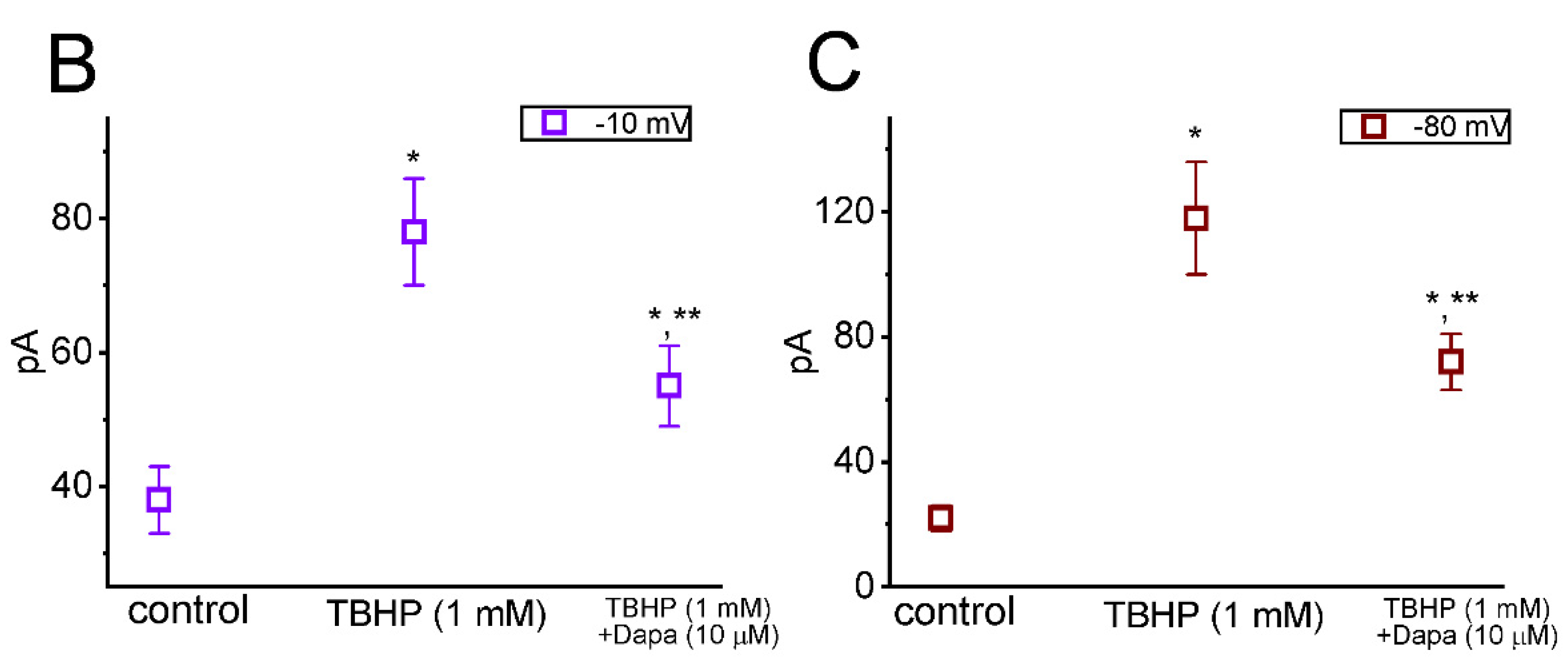
| Associated Ionic Currents | Small Molecules |
|---|---|
| hyperpolarization-activated cation current (Ih) | pirfenidone, oxaliplatin, lutein, dexmedetomidine, honokiol |
| erg-mediated K+ current (IK(erg)) | UCL-2077, SM-102, isoplumbagin, plumbagin |
| M-type K+ current (IK(M)) | remdesivir, QO-58 |
| L-type Ca2+ current (ICa,L) | zingerone |
| persistent Na+ current (INa(P)) | esaxerenone, tefluthrin, t-butyl hydroperoxide, mirogabalin, and dapagliflozin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-N.; Wu, C.-L.; Cho, H.-Y.; Chiang, C.-W. Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents. Int. J. Mol. Sci. 2022, 23, 9453. https://doi.org/10.3390/ijms23169453
Wu S-N, Wu C-L, Cho H-Y, Chiang C-W. Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents. International Journal of Molecular Sciences. 2022; 23(16):9453. https://doi.org/10.3390/ijms23169453
Chicago/Turabian StyleWu, Sheng-Nan, Chao-Liang Wu, Hsin-Yen Cho, and Chi-Wu Chiang. 2022. "Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents" International Journal of Molecular Sciences 23, no. 16: 9453. https://doi.org/10.3390/ijms23169453
APA StyleWu, S.-N., Wu, C.-L., Cho, H.-Y., & Chiang, C.-W. (2022). Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents. International Journal of Molecular Sciences, 23(16), 9453. https://doi.org/10.3390/ijms23169453






