Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes
Abstract
:1. Development of Testes
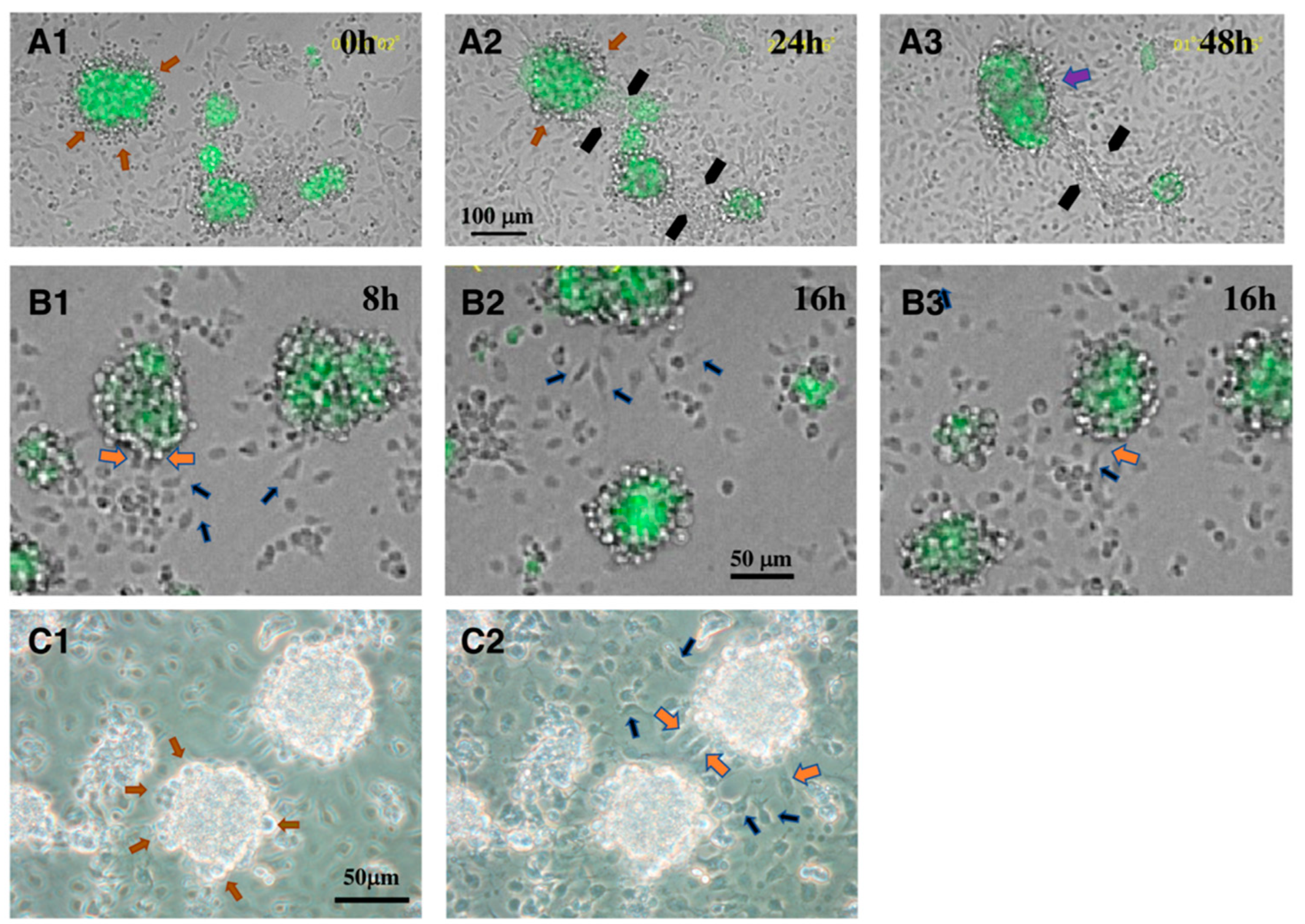
2. Roles of Interstitial Cells in the Reconstruction of Testicular Structures in 3D Re-Aggregate Culture
3. Interaction between CD34+ Telocytes and Other Testicular Cells In Vitro
4. Roles of CD34+ Mesenchymal Cells as a Constituent of Stem Cell Niche and/or Stem/Progenitor Cells
5. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMCs | peritubular myoid cells |
| ALCs | adult Leydig cells |
| FLCs | fetal Leydig cells |
| SLCs | stem cells of ALCs |
| HSD17β3 | 17β-hydroxysteroid dehydrogenase type 3 |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| PDGFRα | platelet-derived growth factor receptor α |
| KSR | KnockOut Serum Replacement |
| EDS | ethane dimethane sulfonate |
References
- Kim, Y.; Capel, B. Balancing the bipotential gonad between alternative organ fates: A new perspective on an old problem. Dev. Dyn. 2006, 235, 2292–2300. [Google Scholar] [CrossRef]
- Svingen, T.; Koopman, P. Building the mammalian testis: Origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013, 27, 2409–2426. [Google Scholar] [CrossRef] [Green Version]
- Ungewitter, E.K.; Yao, H.H. How to make a gonad: Cellular mechanisms governing formation of the testes and ovaries. Sex. Dev. 2013, 7, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Habert, R.; Lejeune, H.; Saez, J.M. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol. Cell. Endocrinol. 2001, 179, 47–74. [Google Scholar] [CrossRef]
- Mendis-Handagama, S.M.L.C.; Ariyaratne, H.B.S. Differentiation of the adult Leydig cell population in the postnatal testis. Biol. Reprod. 2001, 65, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Shima, Y.; Matsuzaki, S.; Miyabayashi, K.; Otake, H.; Baba, T.; Kato, S.; Huhtaniemi, I.; Morohashi, K.-I. Fetal Leydig cells persist as an androgen-independent subpopulation in the postnatal testis. Mol. Endocrinol. 2015, 29, 1581–1593. [Google Scholar] [CrossRef] [Green Version]
- Haider, S.G. Cell biology of Leydig cells in the testis. Int. Rev. Cytol. 2004, 233, 181–241. [Google Scholar] [CrossRef]
- Teerds, K.J.; Huhtaniemi, I.T. Morphological and functional maturation of Leydig cells: From rodent models to primates. Hum. Reprod. Update 2015, 21, 310–328. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Li, X.; Li, L.; Chen, H.; Ge, R.-S. Insights into the development of the adult Leydig cell lineage from stem Leydig cells. Front. Physiol. 2017, 8, 430. [Google Scholar] [CrossRef] [Green Version]
- Shima, Y. Development of fetal and adult Leydig cells. Reprod. Med. Biol. 2019, 18, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T.; et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol. Endocrinol. 2013, 27, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Cool, J.; DeFalco, T.; Capel, B. Testis formation in the fetal mouse: Dynamic and complex de novo tubulogenesis. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 847–859. [Google Scholar] [CrossRef]
- Heinrich, A.; DeFalco, T. Essential roles of interstitial cells in testicular development and function. Andrology 2020, 8, 903–914. [Google Scholar] [CrossRef] [Green Version]
- DeFalco, T.; Takahashi, S.; Capel, B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev. Biol. 2011, 352, 14–26. [Google Scholar] [CrossRef]
- Rotgers, E.; Jørgensen, A.; Yao, H.H.-C. At the crossroads of fate-somatic cell lineage specification in the fetal gonad. Endocr. Rev. 2018, 39, 739–759. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Ge, R.-S.; Zirkin, B.R. Leydig cell stem cells: Identification, proliferation and differentiation. Mol. Cell. Endocrinol. 2017, 445, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Shima, Y.; Miyabayashi, K.; Sato, T.; Suyama, M.; Ohkawa, Y.; Doi, M.; Okamura, H.; Suzuki, K. Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development 2018, 145, dev169136. [Google Scholar] [CrossRef] [Green Version]
- Gnessi, L.; Basciani, S.; Mariani, S.; Arizzi, M.; Spera, G.; Wang, C.; Bondjers, C.; Karlsson, L.; Betsholtz, C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A–deficient mice. J. Cell Biol. 2000, 149, 1019–1025. [Google Scholar] [CrossRef]
- Brennan, J.; Tilmann, C.; Capel, B. Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003, 17, 800–810. [Google Scholar] [CrossRef] [Green Version]
- Gnessi, L.; Emidi, A.; Jannini, E.A.; Carosa, E.; Maroder, M.; Arizzi, M.; Ulisse, S.; Spera, G. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J. Cell Biol. 1995, 131, 1105–1121. [Google Scholar] [CrossRef]
- Basciani, S.; Mariani, S.; Spera, G.; Gnessi, L. Role of platelet-derived growth factors in the testis. Endocr. Rev. 2010, 31, 916–939. [Google Scholar] [CrossRef] [Green Version]
- Abe, K.; Kameyama, H.; Abe, S.-I. CD34 is expressed in endothelial cells in embryonic testes and is additionally expressed in non-endothelial cells in postnatal mouse testes. Zool. Sci. 2022, 39, 1–9. [Google Scholar] [CrossRef]
- Kuroda, N.; Nakayama, H.; Miyazaki, E.; Hayashi, Y.; Toi, M.; Hiroi, M.; Enzan, H. Distribution and role of CD34-positive stromal cells and myofbroblasts in human normal testicular stroma. Histol. Histopathol. 2004, 19, 743–751. [Google Scholar] [CrossRef]
- Popescu, L.M.; Faussone-Pellegrini, M.-S. TELOCYTES—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Ahmad, N.; Hunag, Y.; Ullah, S.; Zhang, Q.; Waqas, Y.; Liu, Y.; Li, Q.; Hu, L.; Chen, Q. Telocytes: Novel interstitial cells present in the testis parenchyma of the Chinese soft-shelled turtle Pelodiscus sinensis. J. Cell. Mol. Med. 2015, 19, 2888–2899. [Google Scholar] [CrossRef]
- Marini, M.; Rosa, I.; Guasti, D.; Gacci, M.; Sgambati, E.; Ibba-Manneschi, L.; Manetti, M. Reappraising the microscopic anatomy of human testis: Identification of telocyte networks in the peritubular and intertubular stromal space. Sci. Rep. 2018, 8, 14780. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Wang, S.; Tarique, I.; Vistro, W.A.; Zhang, H.; Haseeb, A.; Gandahi, N.S.; Iqbal, A.; An, T.; et al. Identification and characterization of telocytes in rat testis. Aging 2019, 11, 5757–5768. [Google Scholar] [CrossRef]
- Pawlicki, P.; Hejmej, A.; Milon, A.; Lustofin, K.; Płachno, B.J.; Tworzydlo, W.; Gorowska-Wojtowicz, E.; Pawlicka, B.; Kotula-Balak, M.; Bilinska, B. Telocytes in the mouse testicular interstitium: Implications of G-protein-coupled estrogen receptor (GPER) and estrogen-related receptor (ERR) in the regulation of mouse testicular interstitial cells. Protoplasma 2019, 256, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Bei, Y.; Wang, F.; Yang, C.; Xiao, J. Telocytes in regenerative medicine. J. Cell. Mol. Med. 2015, 19, 1441–1454. [Google Scholar] [CrossRef]
- Manova, K.; Nocka, K.; Besmer, P.; Bachvarova, R.F. Gonadal expression of c-kit encoded at the W locus of the mouse. Development 1990, 110, 1057–1069. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Nishikawa, S.; Ogawa, M.; Hayashi, S.; Kunisada, T.; Fujimoto, T.; Nishikawa, S. Role of c-kit in mouse spermatogenesis: Identification of spermatogonia as a specific site of c-kit expression and function. Development 1991, 113, 689–699. [Google Scholar] [CrossRef]
- Aumuller, G.; Schulze, C.; Viebahn, C. Intermediate filaments in Sertoli cells. Microsc. Res. Tech. 1992, 20, 50–72. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Stukenborg, J.-B. Testicular organoids: A new model to study the testicular microenvironment in vitro? Hum. Reprod. Update 2018, 24, 176–191. [Google Scholar] [CrossRef] [Green Version]
- Richer, G.; Baert, Y.; Goossens, E. In-vitro spermatogenesis through testis modelling: Towards the generation of testicular organoids. Andrology 2020, 8, 879–891. [Google Scholar] [CrossRef] [Green Version]
- Sakib, S.; Goldsmith, T.; Voigt, A.; Dobrinski, I. Testicular organoids to study cell–cell interactions in the mammalian testis. Andrology 2020, 8, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Cham, T.-C.; Chen, X.; Honaramooz, A. Current progress, challenges, and future prospects of testis organoids. Biol. Reprod. 2021, 104, 942–961. [Google Scholar] [CrossRef]
- Zhang, J.; Hatakeyama, J.; Eto, K.; Abe, S.-I. Reconstruction of a seminiferous tubule-like structure in a 3 dimensional culture system of re-aggregated mouse neonatal testicular cells within a collagen matrix. Gen. Comp. Endocrinol. 2014, 205, 121–132. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhang, X.; Ren, L.; Shi, W.; Tian, Y.; Zhu, J.; Zhang, T. The use of KnockOut serum replacement (KSR) in three dimensional rat testicular cells co-culture model: An improved male reproductive toxicity testing system. Food Chem. Toxicol. 2017, 106, 487–495. [Google Scholar] [CrossRef]
- Abe, S.-I.; Abe, K.; Zhang, J.; Harada, T.; Mizumoto, G.; Oshikawa, H.; Akiyama, H.; Shimamura, K. Roles of CD34+ cells and ALK5 signaling in the reconstruction of seminiferous tubule-like structures in 3-D re- aggregate culture of dissociated cells from neonatal mouse testes. PLoS ONE 2017, 12, e0188705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, D.C.; Wakeling, S.I.; Stringer, J.M.; van den Bergen, J.A.; Wilhelm, D.; Sinclair, A.H.; Western, P.S. Signaling through the TGF beta-activin receptors ALK4/5/7 regulates testis formation and male germ cell development. PLoS ONE 2013, 8, e54606. [Google Scholar] [CrossRef] [Green Version]
- Abe, K.; Kon, S.; Kameyama, H.; Zhang, J.; Morohashi, K.-i.; Shimamura, K.; Abe, S.-I. VCAM1-α4β1 integrin interaction mediates interstitial tissue reconstruction in 3-D re-aggregate culture of dissociated prepubertal mouse testicular cells. Sci. Rep. 2021, 11, 18332. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.-H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [Green Version]
- Kitani, A.; Nakashima, N.; Izumihara, T.; Inagaki, M.; Baoui, X.; Yu, S.; Matsuda, T.; Matsuyama, T. Soluble VCAM-1 induces chemotaxis of Jurkat and synovial fluid T cells bearing high affinity very late antigen-4. J. Immunol. 1998, 161, 4931–4938. Available online: http://www.jimmunol.org/content/161/9/4931 (accessed on 30 July 2022).
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise Review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [Green Version]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Cretoiu, D.; Radu, B.M.; Banciu, A.; Banciu, D.D.; Cretoiu, S.M. Telocytes heterogeneity: From cellular morphology to functional evidence. Semin. Cell Dev. Biol. 2017, 64, 26–39. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Gayoso, S.; Gutiérrez, E.; Díaz-Flores, L., Jr.; Carrasco, J.L. Telocytes in the normal and pathological peripheral nervous system. Int. J. Mol. Sci. 2020, 21, 4320. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Sáez, F.J.; Díaz- Flores, L., Jr.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Díaz-Flores, L., Jr.; Goméz, M.G.; Sáez, F.J.; Madrid, J.F. Behaviour of telocytes during physiopathological activation. Semin. Cell Dev. Biol. 2016, 55, 50–61. [Google Scholar] [CrossRef]
- Rosa, I.; Marini, M.; Manetti, M. Telocytes: An emerging component of stem cell niche microenvironment. J. Histochem. Cytochem. 2021, 69, 795–818. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González, M.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L., Jr.; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.L.; DeFalco, T. A perivascular niche for multipotent progenitors in the fetal testis. Nat. Commun. 2018, 9, 4519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawamoto, K.; Nakao, N.; Kakishita, K.; Ogawa, Y.; Toyama, Y.; Yamamoto, A.; Yamaguchi, M.; Mori, K.; Goldman, S.A.; Itakura, T.; et al. Generation of dopaminergic neurons in the adult brain from mesencephalic precursor cells labeled with a nestin-GFP transgene. J. Neurosci. 2001, 21, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell. Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, K.; Yanazawa, M.; Sugiyama, N.; Miura, H.; Iizuka-Kogo, A.; Kusaka, M.; Omichi, K.; Suzuki, R.; Kato-Fukui, Y.; Kamiirisa, K.; et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 2002, 32, 359–369. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Katoh-Fukui, Y.; Ogawa, H.; Baba, T.; Shima, Y.; Sugiyama, N.; Kitamura, K.; Morohashi, K.-I. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS ONE 2013, 8, e68050. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.Y.; Tsai, M.J. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): Coming of age. Endocr. Rev. 1997, 18, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Ariyaratne, S.; Kim, I.; Mills, N.; Mason, I.; Mendis-Handagama, C. Effects of ethane dimethane sulfonate on the functional structure of the adult rat testis. Arch. Androl. 2003, 49, 313–326. [Google Scholar] [CrossRef]
- Davidoff, M.S.; Middendorff, R.; Enikolopov, G.; Riethmacher, D.; Holstein, A.F.; Muller, D. Progenitor cells of the testosterone-producing Leydig cells revealed. J. Cell Biol. 2004, 167, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.-S.; Dong, Q.; Sottas, C.M.; Papadopoulos, V.; Zirkin, B.R.; Hardy, M.P. In search of rat stem Leydig cells: Identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. USA 2006, 103, 2719–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, E.; Lin, C.-Y.; Jin, S.; Liu, J.; Sottas, C.M.; Ge, R.-S.; Zirkin, B.R.; Chen, H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology 2012, 153, 5002–5010. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Tsai, M.-J.; Tsai, S.Y. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE 2008, 3, e3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilcoyne, K.R.; Smith, L.B.; Atanassova, N.; Macpherson, S.; McKinnell, C.; van den Driesche, S.; Jobling, M.S.; Chambers, T.J.G.; De Gendt, K.; Verhoeven, G.; et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc. Natl. Acad. Sci. USA 2014, 111, E1924–E1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.H.; Cai, B.; Tuo, Y.; Wang, J.; Zang, Z.J.; Tu, X.; Gao, Y.; Su, Z.; Li, W.; Li, G.; et al. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014, 24, 1466–1485. [Google Scholar] [CrossRef] [Green Version]
- Zang, Z.J.; Wang, J.; Chen, Z.; Zhang, Y.; Gao, Y.; Su, Z.; Tuo, Y.; Liao, Y.; Zhang, M.; Yuan, Q.; et al. Transplantation of CD51+ stem Leydig cells: A new strategy for the treatment of testosterone deficiency. Stem Cells 2017, 35, 1222–1232. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Z.; Jiang, Z.; Guo, J.; Zhang, Y.; Li, C.; Chung, J.; Folmer, J.; Liu, J.; Lian, Q.; et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc. Natl. Acad. Sci. USA 2016, 113, 2666–2671. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, J.; Deng, C.; Jiang, M.H.; Feng, X.; Xia, K.; Li, W.; Lai, X.; Xiao, H.; Ge, R.-S.; et al. Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis. 2017, 8, e3123. [Google Scholar] [CrossRef]
- Stévant, I.; Neirijnck, Y.; Borel, C.; Escoffier, J.; Smith, L.B.; Antonarakis, S.E.; Dermitzakis, E.T.; Nef, S. Deciphering cell lineage specification during male sex determination with single-cell RNA sequencing. Cell Rep. 2018, 22, 1589–1599. [Google Scholar] [CrossRef] [Green Version]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-Seq. Dev. Cell 2018, 46, 651–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Shami, A.N.; Moritz, L.; Larose, H.; Manske, G.L.; Ma, Q.; Zheng, X.; Sukhwani, M.; Czerwinski, M.; Sultan, C.; et al. TCF21+ mesenchymal cells contribute to testis somatic cell development, homeostasis, and regeneration in mice. Nat. Commun. 2021, 12, 3876. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, P.; Ji, M.; Wen, X.; Chen, D.; Zhao, X.; Huang, F.; Wang, J.; Shao, J.; Xie, J.; et al. Identification of rat testicular Leydig precursor cells by single-cell-RNA-sequence analysis. Front. Cell Dev. Biol. 2022, 10, 805249. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Giustizieri, M.L.; Favale, A.; Fantini, M.C.; Campagnolo, L.; Konda, D.; Germano, F.; Farini, D.; Manna, C.; Siracusa, G. Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptors in mice suggest functional roles in testicular and epididymal morphogenesis. Biol. Reprod. 1999, 61, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, S.-i. Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes. Int. J. Mol. Sci. 2022, 23, 9585. https://doi.org/10.3390/ijms23179585
Abe S-i. Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes. International Journal of Molecular Sciences. 2022; 23(17):9585. https://doi.org/10.3390/ijms23179585
Chicago/Turabian StyleAbe, Shin-ichi. 2022. "Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes" International Journal of Molecular Sciences 23, no. 17: 9585. https://doi.org/10.3390/ijms23179585
APA StyleAbe, S.-i. (2022). Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes. International Journal of Molecular Sciences, 23(17), 9585. https://doi.org/10.3390/ijms23179585





