Abstract
Lead (Pb) is an important raw material for modern industrial production, they enter the aquatic environment in several ways and cause serious harm to aquatic ecosystems. Lead ions (Pb2+) are highly toxic and can accumulate continuously in organisms. In addition to causing biological deaths, it can also cause neurological damage in vertebrates. Our experiment found that Pb2+ caused decreased survival, delayed hatching, decreased frequency of voluntary movements at 24 hpf, increased heart rate at 48 hpf and increased malformation rate in zebrafish embryos. Among them, the morphology of spinal malformations varied, with 0.4 mg/L Pb2+ causing a dorsal bending of the spine of 72 hpf zebrafish and a ventral bending in 120 hpf zebrafish. It was detected that spinal malformations were mainly caused by Pb2+-induced endoplasmic reticulum stress and apoptosis. The genetic changes in somatic segment development which disrupted developmental polarity as well as osteogenesis, resulting in uneven myotomal development. In contrast, calcium ions can rescue the series of responses induced by lead exposure and reduce the occurrence of spinal curvature. This article proposes new findings of lead pollution toxicity in zebrafish.
1. Introduction
The heavy metal Pb is widely used in manufacturing as well as metallurgy, and with the variety of lead-containing wastes, it has become one of the most prevalent and toxic environmental pollutants. Although lead emissions have been reduced to a great extent globally, the impact of trace amounts of lead cannot be underestimated [1]. The hazard of trace amounts of lead to living organisms has received continuous attention. Studies have shown that Pb can cause damage to the human nervous system, hematopoietic system, digestive system, cardiovascular system, urinary system, reproductive system and immune system [2,3]. Heavy metals have been shown to compromise antioxidant activity. Lead forms a complex with glutathione, inhibits glutathione peroxidase, and interferes with oxidative phosphorylation. Saturation or compromise of these antioxidant processes results in accumulation of ROS and damage to macromolecules (proteins, lipids, and nucleotides), failure in cell function and death [4]. In some cases, very low doses of Pb can cause irreversible damage to the human body. Spinal malformations have a high environmental sensitivity [5] and are one of the common diseases caused by the long-term accumulation of heavy metals in living organisms. Witeska [6] concluded that heavy metals in living organisms first accumulate in bone tissue, leading to a decrease in Ca and P content causing changes in bone mechanical properties, including body and skeletal deformation.
Zebrafish have high reproductive rates, distinct developmental features, transparent embryos for easy observation, and a high degree of homology with advanced vertebrates in genetics and embryonic development [7]. Therefore, zebrafish has been widely used for toxicological assessment of heavy metals, drugs and organic pollutants [8]. Through a previous study, we found that lead-treated zebrafish produced severe spinal curvature, which is consistent with previous findings [9]. However, there is no convincing explanation for the mechanism by which Pb2+ cause spinal curvature. Here, we further explain the cause of spinal curvature in developing zebrafish due to Pb2+ by studying different time points, different lead ion concentrations, and the addition of rescue drugs. This study provides new ideas on the causes of spinal curvature in developing zebrafish due to lead exposure, and provides a scientific basis for evaluating the hazard of Pb2+ to aquatic organisms and guiding the monitoring of lead pollution in water bodies.
2. Results
2.1. Toxicity of Pb2+ to Zebrafish Embryos
Exercise frequency is an important index of zebrafish nervous system development. The frequency of exercise can reflect the stimulation or damage to the nervous system of zebrafish. As shown in Figure 1A, Pb2+ at concentrations higher than 0.2 mg/L significantly reduced the level of exercise frequency in zebrafish embryos, indicating some effects on the nervous system of zebrafish. The heart of zebrafish was basically developed by 48 hpf, and the blood circulation system was gradually established [10]. As shown in Figure 1B, Pb2+ (0.1–0.8 mg/L) caused heart rate acceleration. Low concentrations of Pb2+ (0.1 mg/L and 0.2 mg/L) significantly increased the heart rate and the stimulation to the heart was the most obvious, indicating some effect on the early development of the heart and the circulatory system in zebrafish. As shown in Figure 1C, the hatching rate decreased significantly with increasing Pb2+ concentration in a concentration-dependent manner. Delayed hatching occurred in the high concentration group. It indicates that Pb2+ delays the developmental period of zebrafish embryos.
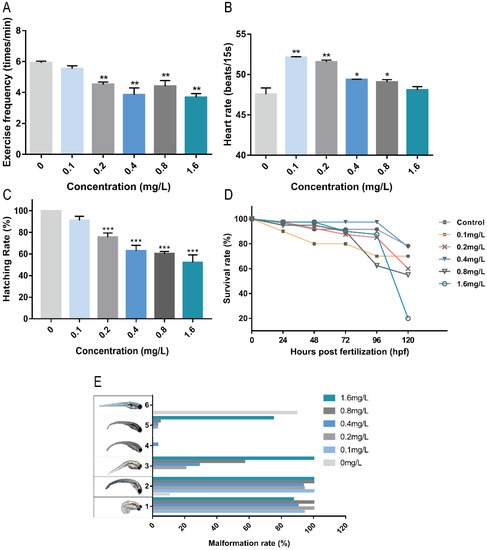
Figure 1.
The effects of different concentrations of Pb2+ on zebrafish embryos. (A) 24 hpf exercise frequency; (B) 48 hpf heart rate; (C) 57 hpf hatching rate; (D) 120 hpf survival rate; (E) 120 hpf Malformation rate (1, spinal curvature; 2, absence of swim bladder; 3, pericardial cavity edema; 4, pigment deficiency; 5, cerebral hemorrhage; 6, normal juvenile zebrafish). (* p < 0.05, ** p < 0.01, *** p < 0.001, n = 15, repeat three times).
The mortality rate reflects the acute toxicity of lead ions to zebrafish embryos. The cumulative survival rate of each group within 120 hpf was recorded. As shown in Figure 1D, the cumulative survival rates of zebrafish embryos were reduced under the exposure of Pb2+. The survival rate of low concentration of Pb2+ was higher. The survival rate of low concentration of Pb2+ was higher. The highest survival rate was 80% in the 0.4 mg/L group, while the highest concentration of 1.6 mg/L group survived only 20%, indicating that high concentrations of lead ions directly affected the survival of zebrafish. Subsequently, we observed the surviving zebrafish malformations and recorded five common malformations at 120 hpf, as shown in Figure 1E. The overall malformation rate was found to be 100% in each Pb2+ experimental group. The main malformations were curvature of the spine and absence of the swim bladder, and there was a co-occurrence of both. Some other malformations were observed in the Pb2+ group except for the 0.1 mg/L Pb2+ group. The incidence of pericardial cavity edema increased with increasing concentration in a concentration-dependent trend. In addition, a large number of brain hemorrhages were observed in the 1.6 mg/L group, and a unique pigment deficiency was observed in the 0.4 mg/L group. The above results indicate that both low and high concentrations of lead ions are toxic to zebrafish embryos, affecting cardiovascular and neurological development, leading to malformations and even death.
Based on the results of mortality and malformation rate, we found that the survival rate of 0.4 mg/L Pb2+ solution was high, and the malformation rate of spinal curvature was high at 120 hpf. Therefore, we chose 0.4 mg/L Pb2+ to study the reason of causing spinal curvature. The results showed that the spinal curvature caused by Pb2+ started at 72 hpf, and the type of malformation was upward curvature, with 52% of juvenile zebrafish showing upward curvature (Figure 2A). At 96 hpf, the main malformation changed to S-shaped curvature, with 85% of juvenile zebrafish showing S-shaped curvature (Figure 2B). At 120 hpf, the malformation changed to mainly downward curvature, with 100% of zebrafish showing downward curvature (Figure 2C). The specific statistical results are shown in Table 1.
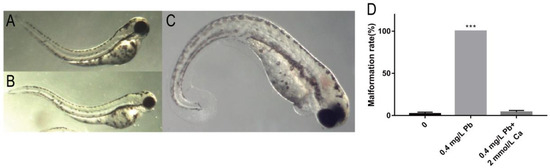
Figure 2.
Spinal malformation in zebrafish embryos at different periods of 0.4 mg/L Pb2+ exposure. (A) Bend upwards at 72 hpf; (B) S-shaped curved at 96 hpf; (C) Bend downward at 120 hpf; (D) Malformation rate at 120 hpf. (*** p < 0.001, n = 50, Repeat three times).

Table 1.
Statistics of Pb2+(0.4 mg/L) on spinal curvature of zebrafish in different periods.
It has been reported that calcium phosphate salts can be used as rescue drugs for Pb exposure [11]. Zebrafish embryos co-treated with 2.0 mmol/L calcium ions (Ca2+) and 0.4 mg/L Pb2+ showed almost the same malformation rate as the control group, indicating the elimination of the effect of Pb2+ at 120 hpf (Figure 2D).
In this study, the zebrafish spinal curvature model is established using Pb2+. Based on the statistics of malformation and lethality, it is clear that Pb2+ caused deformities such as curvature of the spine, and high concentrations have lethal effects, which is consistent with previous studies. From the statistics of malformation rate, it is found that the deformation of swim bladder and spinal curvature are more likely and concurrent. It is found that Pb2+ slows down the bone development of zebrafish embryos and causes irregularities in the musculature. Pb2+ can cause the loss of calcium ions in the body. The toxicity caused by lead ions can be mitigated by calcium ion rescue experiments.
2.2. The Mechanism of Spinal Curvature
Calcein binds Ca2+ in tissues and will appear yellow-green under fluorescence. It does not have a large effect on zebrafish and can be used as a good dye for in vivo staining of skeletal development to detect the degree of skeletal calcification in juvenile zebrafish. After staining, hard bones will appear yellow-green, and tissues containing calcium such as muscles can be seen clearly under the fluorescence microscope. This is a very convenient use for observing bones as well as muscle morphology.
Calcein staining was performed on zebrafish at 120 hpf to observe the skeletal ossification and somites development (Figure 3A). It was found that the ossification area of the Pb2+ group (0.4 mg/L) was smaller than that of the control group and the calcium rescue group (Figure 3B), and the number of somites was also smaller than that of the other two groups (Figure 3C). In addition, the Pb2+ group (0.4 mg/L) showed an uneven distribution of sarcomeres at the site of spinal curvature, showing shortened sarcomeres at one end and longer sarcomeres at the other, which differed significantly from the regular herringbone sarcomeres of the control group (Figure 3A). This suggests that Pb2+ contributes to spinal curvature in zebrafish by affecting sarcomeres development and bone formation. Subsequently, we look for the reason at the gene level.
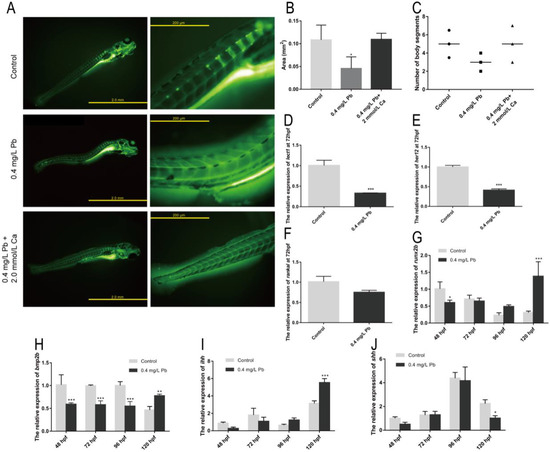
Figure 3.
Skeletal and somites development. (A) Calcein staining; (B) Area of ossification in juvenile zebrafish; (C) Body segment statistics. The expression of the somites development-related genes is shown for (D) lect1, (E) her12, (F) rankal (G) runx2b, (H) bmp2b. The expression of Hedgehog signaling pathway gene of zebrafish embryos is shown for (I) ihh and (J) shh, with ef1α as reference. (* p < 0.05, ** p < 0.01, *** p < 0.001, n = 50).
lect1 (chondromodulin) is expressed in cartilage and skull structures [12]. her12 (hairy-related 12) is involved in the Notch signaling pathway and regulates nervous system development [12]. rankal is expressed in head and scale, regulating osteoblast differentiation [12]. Bone morphogenetic protein 2b (bmp2b) [13] and RUNX family transcription factor 2b (runx2b) [14] together promote osteoblast maturation and differentiation. These genes play an important role in the development of the zebrafish skeletal system and represent the degree of development of the skeletal system. The expression of lect1 and her12 was significantly down-regulated after Pb2+ treatment, and rankal also showed a down-regulation trend (Figure 3D–F). This demonstrates that Pb2+ has serious effects on zebrafish somites and skeletal development. During osteoblast formation, bmp2b and runx2b were also significantly down-regulated (Figure 3G,H). The decreased expression levels of lect1, her12, bmp2b and runx2b together affect the skeletal and somatic segment development in zebrafish. This may be one of the important reasons for the spinal curvature.
In addition, we found that zebrafish with spinal curvature caused by Pb2+ were often accompanied by swim bladder deficiency. There is a linkage relationship between these two deformities. The Hedgehog signaling pathway is an important pathway that controls swim bladder development [15]. As shown in Figure 3I, the expression of Indian hedgehog (ihh) gene was not significantly different from the control until 96 hpf. However, this gene was significantly upregulated at 120 hpf. Meanwhile, the Sonic hedgehog (shh) gene was significantly decreased at 120 hpf (Figure 3J), indicating that Pb2+ affected swim bladder development through the Hedgehog pathway. Changes in the expression levels of ihh and shh may affect developmental polarity leading to the appearance of spinal curvature [16]. The above results suggest that Pb2+ regulates the development of zebrafish somites mainly through lept1 and her12, inhibits skeletal development in zebrafish through bmp2b and runx2b, and affects the process of skeletal and swim bladder development through ihh and shh.
2.3. Pb2+ Induce Apoptosis by Activating Endoplasmic Reticulum (ER) Stress
Current studies have shown that oxidative damage is one of the important causes of organismal damage caused by Pb2+. It is also reported that the ER stress pathway is also involved in the regulation of runx2b, a gene related to bone development. Among them, perk/chop are important genes involved in ER stress, and they can regulate runx2b with osteoblast differentiation and maturation. It was found that both perk and chop were significantly upregulated in the Pb2+-treated group compared with the control group at 48 hpf, as shown in Figure 4A,B. Therefore, it is demonstrated that Pb2+ induce ER stress in zebrafish embryos. Activation of ER stress chop signaling pathway triggers apoptosis.
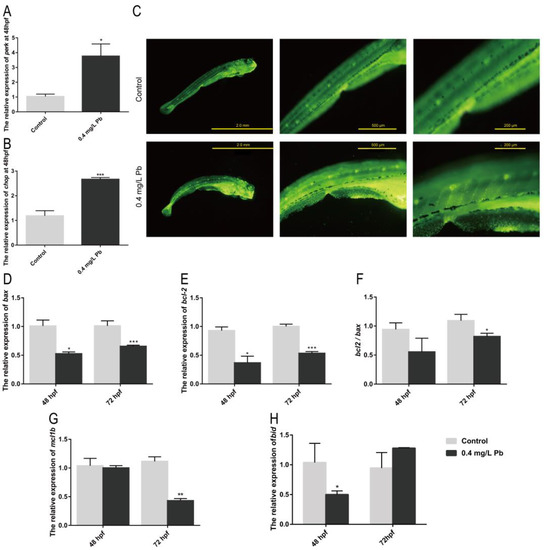
Figure 4.
mRNA expression of ER stress and apoptosis-related genes in zebrafish. mRNA expression of ER stress-related genes in zebrafish embryos at 48 hpf using ef1α as reference: (A) perk, (B) chop. (C) AO staining; mRNA expression of apoptosis-related genes in zebrafish embryos at 48 and 72 hpf; (D) bax; (E) bcl-2; (F) bcl-2/bax ratio; (G) mcl1b; (H) bid. (* p < 0.05, ** p < 0.01, *** p < 0.001, n = 50.)
In apoptotic cells, apoptotic bodies are formed due to chromatin consolidation or breakage into pieces of varying sizes. Acridine orange stained them with yellow-green fluorescence to detect apoptotic cells, as shown in Figure 4C. It was found that a large amount of green fluorescence appeared in the zebrafish muscular segment part in the Pb2+ experimental group. This indicates that Pb2+ promotes the occurrence of apoptosis in zebrafish muscle cells. Combined with the previous deformation and uneven distribution of muscle nodes, it suggests that abnormal muscle cell development and apoptosis may be closely associated with their spinal curvature.
One of the key indicators of apoptosis is whether the ratio of anti-apoptotic gene bcl-2 to the pro-apoptotic gene bax is down-regulated. The expression of bcl-2 and bax genes was detected by RT-PCR (Figure 4D,E). Both genes showed significant down-regulation at 48 hpf as well as 72 hpf relative to the control group. Additionally, at 72 hpf, the bcl-2 and bax ratios appeared significantly down-regulated (Figure 4F). This indicates that apoptosis occurred at 72 hpf. Meanwhile the anti-apoptotic gene mcl1b was significantly decreased at 72 hpf, and the pro-apoptotic gene bid was increased (Figure 4G,H). It further demonstrated that Pb2+ promoted apoptosis in myocytes.
3. Discussion
Studies have shown that Pb2+ increases oxidative stress levels, causing endoplasmic reticulum stress early in development [3]. Pb2+ trigger ER stress, which is involved in protein folding and calcium homeostasis. ER stress leads to abnormal endoplasmic reticulum function, triggering apoptosis and inflammation [17]. The molecular chaperone of PERK, BIP, is one of the most abundant proteins in the ER. PERK is considered a major sensor of the unfolded protein response, and BIP separates from PERK when the ER stress response is received, activating PERK to cause apoptosis [18]. CHOP is considered a marker protein to promote apoptosis [19]. The increase in PERK and CHOP in this study indicates that the ER stress response activates the onset of apoptosis.
On the one hand, the accumulation of Pb2+ in vivo triggers apoptosis, which deforms the musculature and leads to spinal curvature. On the other hand, Pb2+ stimulates the Hedgehog signaling pathway, causing the downward curvature of the spine. During endochondral ossification, secreted signal, Ihh has been shown to regulate the onset of hypertrophic differentiation of chondrocytes. BMPs, family of secreted factors regulating bone formation, have been implicated as potential interactors of the IHH [20]. Functional integration or Synergistic regulation between Hedgehog signaling pathway and BMPs signaling pathway promotes ALP expression and osteogenic differentiation [21]. In addition, Pb2+ affects the formation of bones and muscle nodes by suppressing the notochord-associated shh. It is because lead ions can lead to the loss of bone calcium [22]. We suppose that the cause of Pb2+-induced spinal curvature is the loss of calcium in vivo, which is caused by the competition between Pb2+ and Ca2+. The reduced activity of calcium-binding calmodulin protein causes downregulation of the shh, which affects bmp, causing changes in ihh, runx2b, and leading to spinal curvature.
This study provides two possible mechanisms of Pb2+-induced spinal curvature, namely, endoplasmic reticulum stress–apoptosis pathway and bone development-related gene regulation pathway. Both exhibit obvious changes, but which one is the former needs further research. It provides a scientific basis for evaluating the hazard of Pb2+ to aquatic organisms and guiding the monitoring of lead pollution in water bodies.
4. Materials and Methods
4.1. Materials and Reagents
Lead nitrate, calcium phosphate, Tricaine and paraformaldehyde used in the experiments were purchased from Biopped, Paterson, NJ, USA. Alizarin red, calcein and acridine orange were purchased from Solarbio Co., Ltd. (Beijing, China). Trizol was purchased from TaKaRa, Kyoto, Japan.
4.2. Breeding and Spawning of Zebrafish
The AB strain zebrafish used in this experiment was obtained from the Heilongjiang Fisheries Research Institute under the Chinese Academy of Fisheries Science, and was raised in our laboratory for more than two months. The water temperature was 28 °C, and the light/dark cycle was 14 h/10 h. The food was fed twice a day, at 9:00 and 15:00, with live Artemia cysts (Beijing, China).
4.3. Zebrafish Pb2+ Exposure Experiment
Deionized water (DW) was used to wash fertilized eggs and then transferred to 24-well cell culture plates for Pb2+ exposure experiments. The maximum allowed discharge concentration of Pb2+ is 1.0 mg/L according to the National Comprehensive Discharge Standard GB 8978-1996 of the People’s Republic of China. Therefore, this experiment used lead nitrate at concentrations of 0.1 mg/L, 0.2 mg/L, 0.4 mg/L, 0.8 mg/L and 1.6 mg/L according to the national standard concentration (1.0 mg/L), with deionized water as the control. Three parallel replicates were set up with 45 fertilized eggs per group using DW as control. The culture plates were placed in a biochemical constant-temperature incubator, at a constant 28 °C. The culture medium was changed every 24 h during the experiment, and dead individuals were removed.
The frequency of exercise was observed at 24 hpf, the heart rate was measured for 15 s at 48 hpf, and the hatching rate was counted at 54 hpf. The number of surviving individuals was recorded every 24 h during the entire cycle of the experiment. Hatched larvae were anesthetized with Tricaine and fixed in 4% paraformaldehyde. Photographs and statistics of deformities were taken with stereomicroscope.
4.4. Alizarin Red Staining
Zebrafish embryos were fixed with 4% PFA for 2 h, rinsed twice with PBS, bleached with 1.5% H2O2/1% KOH for 5 min, rinsed twice with 25% glycerol/0.1% KOH for 20 min, stained with 0.05% alizarin red for 30 min avoiding light, rinsed twice with 50% glycerol/0.1% KOH, and stored at 4 °C with 50% glycerol/0.1% KOH.
4.5. Calcein Staining
Zebrafish embryos were added with 0.2% calcein solution, kept in the dark for 1 h, washed with E3 solution and left for 2 h to release the excess dye in the embryos, and washed twice with PBS. After zebrafish embryos were anesthetized with 0.05% Tricaine for 5 min, the development of fish skeleton was observed and photographed by fluorescence microscope.
4.6. Acridine Orange Staining
The zebrafish embryos were stained with 2 mg/L AO dye, and stained at room temperature for 20 min in the dark, and then washed twice with PBS solution. Zebrafish embryos were anesthetized with 0.05% Tricaine for 5 min. Apoptosis was observed in fluorescence microscopy and photographed.
4.7. Semi-Quantitative Polymerase Chain Reaction (PCR)
In this experiment, RNA was extracted by Trizol method. The purity and concentration of RNA samples were detected by gel electrophoresis and UV spectrophotometer and stored at −80 °C. PrimeScriptTM RT reagent Kit with gDNA Eraser was purchased from TaKaRa, Inc. The Premix rTag kit (TaKaRa) was used, using reverse-transcribed cDNA as the template, and the housekeeping gene used was ef1α. The primer sequences are shown in Table S1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23179571/s1.
Author Contributions
All authors contributed to the study conception and design. Conceptualization: L.L., methodology: R.L. and C.M., formal analysis and investigation: L.Y., writing—original draft preparation: X.L., writing—review and editing: C.C. and M.H., resources: J.J., B.L. and Y.Z., supervision: L.L. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Technologies R&D Program of China under Grant Number: 2019YFA0705202, the National Natural Science Foundation of China (No. 31701296, 11474076 and 21974097), the Education Department of Guangdong Province (No. 2020KSYS004), the National Key Research and Development Program of China (No. 2021YFC3200804), and the Open Project of State Key Laboratory of Urban Water Resources and Water Environment, Harbin Institute of Technology (No. ES202116).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the zebrafish used in this experiment is under the 5 dpf (European Animal Research Association 2010/63/EU).
Data Availability Statement
Data are available on request due to restrictions, e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available as part of the data will be used for another unpublished article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Resongles, E.; Dietze, V.; Green, D.C.; Harrison, R.M.; Ochoa-Gonzalez, R.; Tremper, A.H.; Weiss, D.J. Strong evidence for the continued contribution of lead deposited during the 20th century to the atmospheric environment in London of today. Proc. Natl. Acad. Sci. USA 2021, 118, e2102791118. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E. Low level environmental lead exposure–a continuing challenge. Clin. Biochem. Rev. 2008, 29, 63. [Google Scholar] [PubMed]
- Roy, N.M.; De Wolf, S.; Carneiro, B. Evaluation of the developmental toxicity of lead in the Danio rerio body. Aquat. Toxicol. 2015, 158, 138–148. [Google Scholar] [CrossRef]
- Dunham, J. Encyclopedia of Environmental Health. Libr. J. 2011, 136, 104. [Google Scholar]
- Kolstad, K.; Thorland, I.; Refstie, T.; Gjerde, B. Genetic variation and genotype by location interaction in body weight, spinal deformity and sexual maturity in Atlantic cod (Gadus morhua) reared at different locations off Norway. Aquaculture 2006, 259, 66–73. [Google Scholar] [CrossRef]
- Witeska, M.; Jezierska, B. The effects of environmental factors on metal toxicity to fish (review). Fresen. Environ. Bull 2003, 12, 824–829. [Google Scholar]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a Model System to Study Toxicology. Env. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef]
- Dou, C.M.; Zhang, J. Effects of lead on neurogenesis during zebrafish embryonic brain development. J. Hazard Mater. 2011, 194, 277–282. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic-Development of the Zebrafish. Dev. Dynam. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.Q.; Chen, M.; Singh, S.P.; Harris, W.G. Impacts of phosphate amendments on lead biogeochemistry at a contaminated site. Environ. Sci. Technol. 2002, 36, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Mundlos, S.; Olsen, B.R. Heritable diseases of the skeleton. Part I: Molecular insights into skeletal development-transcription factors and signaling pathways. FASEB J. 1997, 11, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Huang, Z.; Liu, N.; Su, R.; Xie, G.; Zhong, B.; Zhang, K.; Wang, S.; Hu, X.; Zhang, J.; et al. MicroRNA-140-5p impairs zebrafish embryonic bone development via targeting BMP-2. FEBS Lett. 2016, 590, 1438–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meulen, T.; Kranenbarg, S.; Schipper, H.; Samallo, J.; van Leeuwen, J.L.; Franssen, H. Identification and characterisation of two runx2 homologues in zebrafish with different expression patterns. Biochim. Biophys. Acta 2005, 1729, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Winata, C.L.; Korzh, S.; Kondrychyn, I.; Zheng, W.L.; Korzh, V.; Gong, Z.Y. Development of zebrafish swimbladder: The requirement of Hedgehog signaling in specification and organization of the three tissue layers. Dev. Biol. 2009, 331, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, J.A.; Robbins, A.E.; Stewart, S.; Stankunas, K. Basal epidermis collective migration and local Sonic hedgehog signaling promote skeletal branching morphogenesis in zebrafish fins. Dev. Biol. 2021, 477, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Musatov, A.; Robinson, N.C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic. Res. 2012, 46, 1313–1326. [Google Scholar] [CrossRef]
- Huang, R.; Hui, Z.; Wei, S.; Li, D.; Li, W.; Daping, W.; Alahdal, M. IRE1 signaling regulates chondrocyte apoptosis and death fate in the osteoarthritis. J. Cell Physiol. 2022, 237, 118–127. [Google Scholar] [CrossRef]
- Schonthal, A.H. Endoplasmic reticulum stress: Its role in disease and novel prospects for therapy. Science 2012, 2012, 857516. [Google Scholar] [CrossRef] [Green Version]
- Minina, E.; Wenzel, H.; Kreschel, C.; Karp, S.; Gaffield, W.; McMahon, A.; Vortkamp, A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 2001, 128, 4523–4534. [Google Scholar] [CrossRef]
- Reichert, J.; Schmalzl, J.; Prager, P.; Gilbert, F.; Quent, V.; Steinert, A.; Rudert, M.; Nöth, U. Synergistic effect of Indian hedgehog and bone morphogenetic protein-2 gene transfer to increase the osteogenic potential of human mesenchymal stem cells. Stem Cell Res. Ther. 2013, 4, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak-Juszczak, L. Impact of strontium on skeletal deformities in Baltic cod (Gadus morhua callaris L.). Chemosphere 2011, 83, 486–491. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).