SGLT2 Inhibitor Empagliflozin Modulates Ion Channels in Adult Zebrafish Heart
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
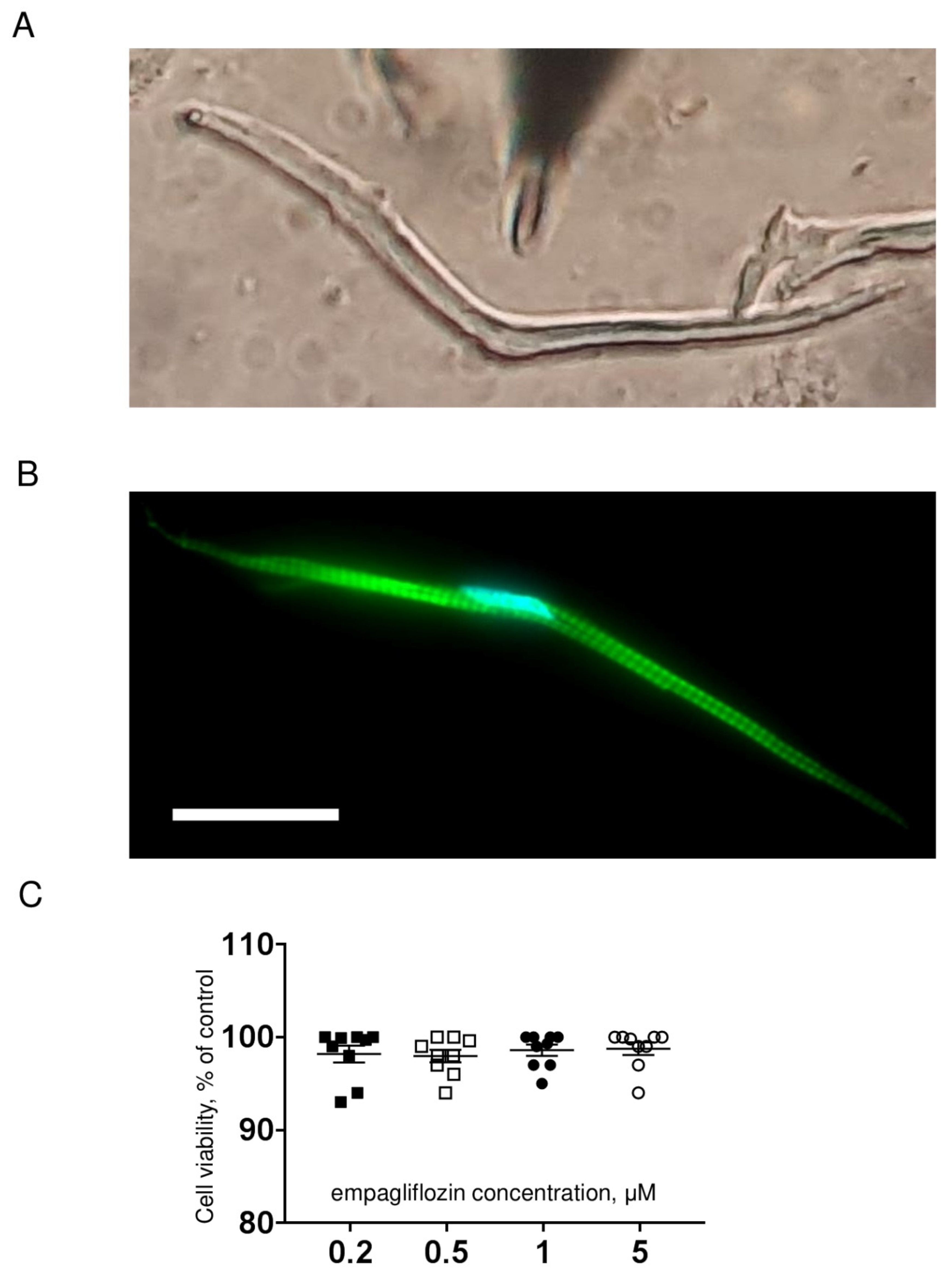
4.1. Isolation of Ventricular Cardiomyocytes
4.2. Cell Viability Assay
4.3. Actin Fluorescent Staining
4.4. Recording of Ionic Currents
4.5. Recording of Action Potentials
4.6. Empagliflozin Treatment
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | Action potential |
| APD20, APD50, and APD90 | Action potential duration at 20%, 50%, and 90% of repolarization, respectively |
| hERG | Human ether-a-go-go related gene |
| hiPSC | Human induced pluripotent stem cell |
| ICaL | L-type calcium current |
| ICaT | T-type calcium current |
| INa | Sodium current |
| IKr | Rapid component of delayed rectifier potassium current |
| IKs | Slow component of delayed rectifier potassium current |
| KV | Voltage-gated potassium channel |
| SGLT2 | Sodium-glucose co-transporter 2 |
| i(s)SGLT2 | Sodium-glucose cotransporter type 2 inhibitor(s) |
References
- Adabag, A.S.; Luepker, R.V.; Roger, V.L.; Gersh, B.J. Sudden cardiac death: Epidemiology and risk factors. Nat. Rev. Cardiol. 2010, 7, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Parajuli, N.; Levasseur, J.L.; Boisvenue, J.; Beker, D.L.; Masson, G.; Fedak, P.W.; Verma, S.; Dyck, J.R. Empagliflozin Prevents Worsening of Cardiac Function in an Experimental Model of Pressure Overload-Induced Heart Failure. JACC Basic Transl. Sci. 2017, 2, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.; Silljé, H.H.; Oberdorf Maass, S.U.; Schouten, E.; Giani, M.G.P.; Hillebrands, J.; Van Goor, H.; Van Veldhuisen, D.J.; De Boer, R.A.; Westenbrink, B.D. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur. J. Heart Fail. 2019, 21, 862–873. [Google Scholar] [CrossRef]
- Lim, V.G.; Bell, R.M.; Arjun, S.; Kolatsi-Joannou, M.; Long, D.A.; Yellon, D.M. SGLT2 Inhibitor, Canagliflozin, Attenuates Myocardial Infarction in the Diabetic and Nondiabetic Heart. JACC Basic Transl. Sci. 2019, 4, 15–26. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Ciuffreda, L.P.; Coppini, R.; Cozzolino, A.; Miccichè, A.; Dell'Aversana, C.; D’Amario, D.; Cianflone, E.; Scavone, C.; et al. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol. Res. 2020, 157, 104781. [Google Scholar] [CrossRef]
- Krasnova, M.; Kulikov, A.; Okovityi, S.; Ivkin, D.; Karpov, A.; Kaschina, E.; Smirnov, A. Comparative efficacy of empagliflozin and drugs of baseline therapy in post-infarct heart failure in normoglycemic rats. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2020, 393, 1649–1658. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Okunrintemi, V.; Mishriky, B.M.; Powell, J.R.; Cummings, D.M. Sodium glucose co transporter 2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes. Metab. 2020, 23, 276–280. [Google Scholar] [CrossRef]
- Li, H.-L.; Lip, G.Y.H.; Feng, Q.; Fei, Y.; Tse, Y.-K.; Wu, M.-Z.; Ren, Q.-W.; Tse, H.-F.; Cheung, B.-M.Y.; Yiu, K.-H. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Penalver, J.; Knijnik, L.; Mitrani, R.D.; Myerburg, R.J.; Goldberger, J.J. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Hearth Rhythm 2021, 18, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Garcia-Ropero, A.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Vargas-Delgado, A.P.; Flores-Umanzor, E.J.; Sanz, J.; et al. Empagliflozin Ameliorates Diastolic Dysfunction and Left Ventricular Fibrosis/Stiffness in Nondiabetic Heart Failure. JACC Cardiovasc. Imaging 2020, 14, 393–407. [Google Scholar] [CrossRef]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef]
- Wan, N.; Rahman, A.; Hitomi, H.; Nishiyama, A. The Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Sympathetic Nervous Activity. Front. Endocrinol. 2018, 9, 421. [Google Scholar] [CrossRef]
- Oshima, H.; Miki, T.; Kuno, A.; Mizuno, M.; Sato, T.; Tanno, M.; Yano, T.; Nakata, K.; Kimura, Y.; Abe, K.; et al. Empagliflozin, an SGLT2 Inhibitor, Reduced the Mortality Rate after Acute Myocardial Infarction with Modification of Cardiac Metabolomes and Antioxidants in Diabetic Rats. J. Pharmacol. Exp. Ther. 2018, 368, 524–534. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, J.M.; Lassen, T.R.; Hjortbak, M.V.; Jespersen, N.R.; Kvist, F.; Hansen, J.; Bøtker, H.E. Cardioprotective effects of empagliflozin after ischemia and reperfusion in rats. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated With Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Heart Fail. 2020, 13, e006277. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef] [PubMed]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Hernandez, J.M.; Shen, E.Y.; Habibi, N.R.; Bossuyt, J.; Bers, D.M. Empagliflozin Reverses Late Na + Current Enhancement and Cardiomyocyte Proarrhythmia in a Translational Murine Model of Heart Failure with Preserved Ejection Fraction. Circulation 2022, 145, 1029–1031. [Google Scholar] [CrossRef]
- Lee, T.-I.; Chen, Y.-C.; Lin, Y.-K.; Chung, C.-C.; Lu, Y.-Y.; Kao, Y.-H.; Chen, Y.-J. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef]
- Mustroph, J.; Wagemann, O.; Lücht, C.M.; Trum, M.; Hammer, K.; Sag, C.M.; Lebek, S.; Tarnowski, D.; Reinders, J.; Perbellini, F.; et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail. 2018, 5, 642–648. [Google Scholar] [CrossRef]
- Chung, Y.J.; Park, K.C.; Tokar, S.; Eykyn, T.R.; Fuller, W.; Pavlovic, D.; Swietach, P.; Shattock, M.J. Off-target effects of sodium-glucose co-transporter 2 blockers: Empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart. Cardiovasc. Res. 2020, 117, 2794–2806. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wust, R.C.; Fiolet, J.W.T.; Stienen, G.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 2016, 60, 568–573. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2017, 61, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Brette, F.; Luxan, G.; Cros, C.; Dixey, H.; Wilson, C.; Shiels, H.A. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2008, 374, 143–146. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Remme, C.A. Zebrafish: A novel research tool for cardiac (patho)electrophysiology and ion channel disorders. Front. Physiol. 2012, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Pharmacokinetic and Pharmacodynamic Profile of Empagliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor. Clin. Pharmacokinet. 2014, 53, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Schumacher, C.A.; Fiolet, J.W.T.; Kuschma, M.C.; Hollmann, M.W.; Coronel, R.; Weber, N.C.; Zuurbier, C.J. Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients. Front. Physiol. 2018, 9, 1575. [Google Scholar] [CrossRef]
- Sutanto, H.; Heijman, J. Integrative Computational Modeling of Cardiomyocyte Calcium Handling and Cardiac Arrhythmias: Current Status and Future Challenges. Cells 2022, 11, 1090. [Google Scholar] [CrossRef]
- Abramochkin, D.V.; Hassinen, M.; Vornanen, M. Transcripts of Kv7.1 and MinK channels and slow delayed rectifier K+ current (IKs) are expressed in zebrafish (Danio rerio) heart. Pflügers Arch. Eur. J. Physiol. 2018, 470, 1753–1764. [Google Scholar] [CrossRef]
- Gauvrit, S.; Bossaer, J.; Lee, J.; Collins, M.M. Modeling Human Cardiac Arrhythmias: Insights from Zebrafish. J. Cardiovasc. Dev. Dis. 2022, 9, 13. [Google Scholar] [CrossRef]
- Chen, L.; Sampson, K.J.; Kass, R.S. Cardiac Delayed Rectifier Potassium Channels in Health and Disease. Card. Electrophysiol. Clin. 2016, 8, 307–322. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. hERG Assay, QT Liability, and Sudden Cardiac Death. In Cardiac Safety of Noncardiac Drugs; Springer: Berlin/Heidelberg, Germany, 2007; pp. 67–81. [Google Scholar] [CrossRef]
- Bohnen, M.S.; Peng, G.; Robey, S.H.; Terrenoire, C.; Iyer, V.; Sampson, K.J.; Kass, R.S. Molecular Pathophysiology of Congenital Long QT Syndrome. Physiol. Rev. 2017, 97, 89–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-Q.; Yan, M.; Liu, L.-R.; Zhang, X.; Wang, X.; Geng, H.-Z.; Zhao, X.; Li, B.-X. High Glucose Represses hERG K+ Channel Expression through Trafficking Inhibition. Cell. Physiol. Biochem. 2015, 37, 284–296. [Google Scholar] [CrossRef]
- Ozturk, N.; Uslu, S.; Ozdemir, S. Diabetes-induced changes in cardiac voltage-gated ion channels. World J. Diabetes 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, J.; Maier, L.S.; Wagner, S. CaMKII regulation of cardiac K channels. Front. Pharmacol. 2014, 5, 20. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Varghese, A.; Lu, Y.; Bursill, J.A.; Mahaut-Smith, M.P.; Huang, C.L.-H. Temperature dependence of human ether-à-go-go-related gene K+ currents. Am. J. Physiol. Physiol. 2006, 291, C165–C175. [Google Scholar] [CrossRef]
- Walsh, K.B.; Begenisich, T.B.; Kass, R.S. Beta-adrenergic modulation of cardiac ion channels. Differential temperature sensitivity of potassium and calcium currents. J. Gen. Physiol. 1989, 93, 841–854. [Google Scholar] [CrossRef]
- Vornanen, M.; Hassinen, M. Zebrafish heart as a model for human cardiac electrophysiology. Channels 2016, 10, 101–110. [Google Scholar] [CrossRef]
- Vrhovac, I.; Eror, D.B.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radović, N.; Jadrijević, S.; Aleksic, I.; Walles, T.; et al. Localizations of Na+-d-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflügers Arch. Eur. J. Physiol. 2014, 467, 1881–1898. [Google Scholar] [CrossRef]
- Chen, J.; Williams, S.; Ho, S.; Loraine, H.; Hagan, D.; Whaley, J.M.; Feder, J.N. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010, 1, 57–92. [Google Scholar] [CrossRef]
- Zhou, L.; Cryan, E.V.; D'Andrea, M.R.; Belkowski, S.; Conway, B.R.; Demarest, K.T. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J. Cell. Biochem. 2003, 90, 339–346. [Google Scholar] [CrossRef]
- Ng, K.-M.; Lau, Y.-M.; Dhandhania, V.; Cai, Z.-J.; Lee, Y.-K.; Lai, W.-H.; Tse, H.-F.; Siu, C.-W. Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 14872. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Jung, H.S.; An, J.R.; Kang, M.; Heo, R.; Li, H.; Han, E.-T.; Yang, S.-R.; Cho, E.-H.; Bae, Y.M.; et al. Empagliflozin dilates the rabbit aorta by activating PKG and voltage-dependent K+ channels. Toxicol. Appl. Pharmacol. 2020, 403, 115153. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Engeszer, R.E.; Patterson, L.B.; Rao, A.A.; Parichy, D.M. Zebrafish in The Wild: A Review of Natural History And New Notes from The Field. Zebrafish 2007, 4, 21–40. [Google Scholar] [CrossRef] [PubMed]


| Control | n | Empagliflozin | n | |
|---|---|---|---|---|
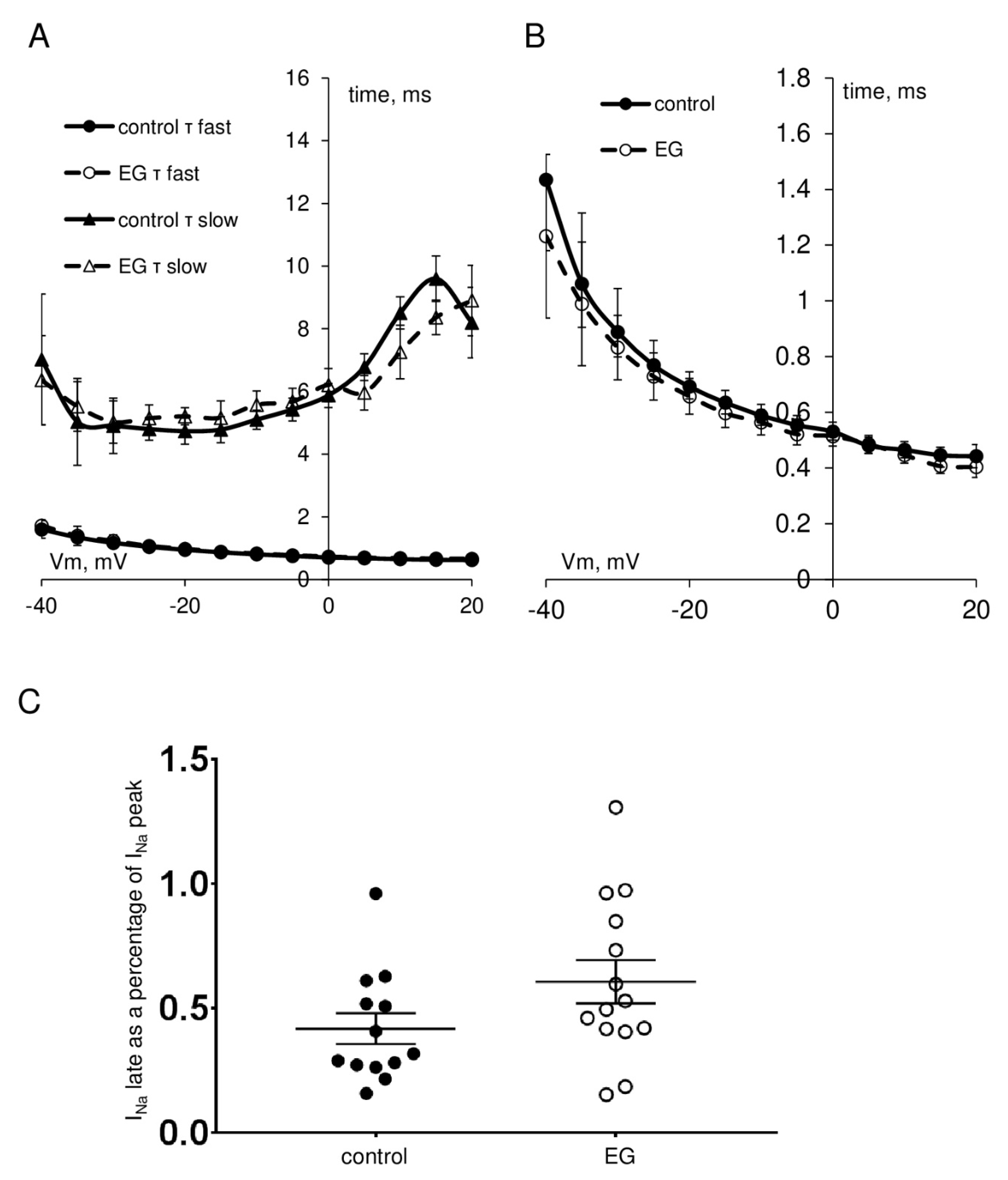
| INa peak current density at −35 mV, pA/pF | −317.9 ± 34.6 | 13 | −334.4 ± 37.4 | 14 |
| INa steady-state activation, V1/2, mV k, mV/e-fold | −46.5 ± 1.1 3.7 ± 0.2 | 13 | −48.3 ± 0.9 3.9 ± 0.3 | 14 |
| INa steady-state inactivation, V1/2, mV k, mV/e-fold | −77.3 ± 2.0 5.1 ± 0.2 | 13 | −76.1 ± 0.9 5.0 ± 0.1 | 14 |
| INa late current, % of INa peak current | 0.42 ± 0.09 | 13 | 0.61 ± 0.10 | 14 |
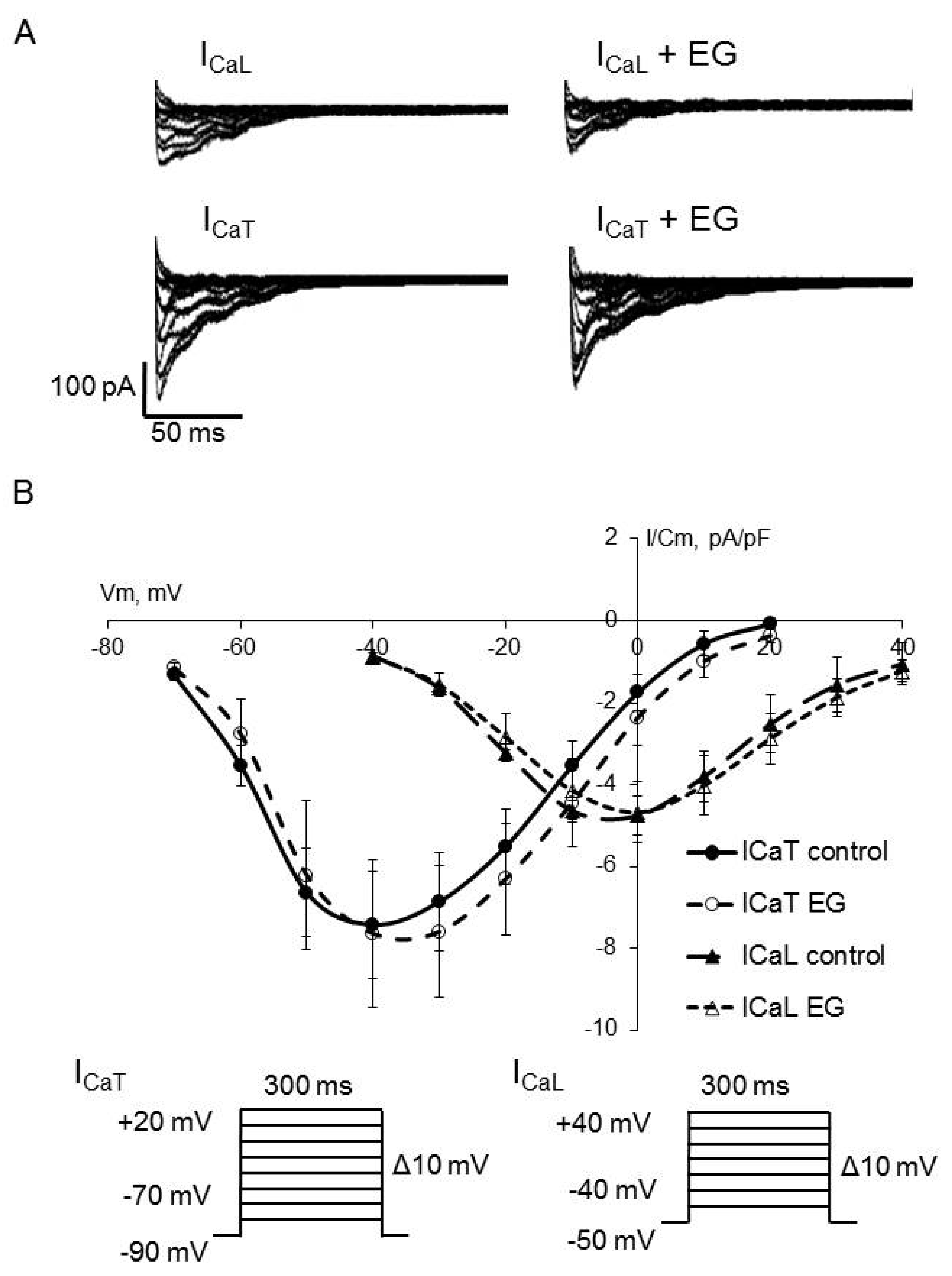
| ICaL peak current density at 0 mV, pA/pF | −4.8 ± 0.7 | 14 | −4.7 ± 0.7 | 14 |
| ICaT peak current density at −40 mV, pA/pF, ms | −7.4 ± 1.3 | 14 | −7.6 ± 1.8 | 14 |
| ICaL steady-state activation, V1/2, mV k, mV/e-fold | −19.3 ± 1.6 7.7 ± 0.3 | 14 | −16.0 ± 1.7 7.9 ± 0.5 | 14 |
| ICaT steady-state activation, V1/2, mV k, mV/e-fold | −50.2 ± 2.2 9.5 ± 1.3 | 14 | −46.6 ± 2.8 9.1 ± 1.2 | 14 |
| IKr peak step current density at 0 mV, pA/pF | 2.8 ± 0.3 | 18 | * 4.2 ± 0.7 | 19 |
| IKr peak tail current density at +10 mV, pA/pF | 5.1 ± 0.4 | 18 | * 7.6 ± 1.0 | 19 |
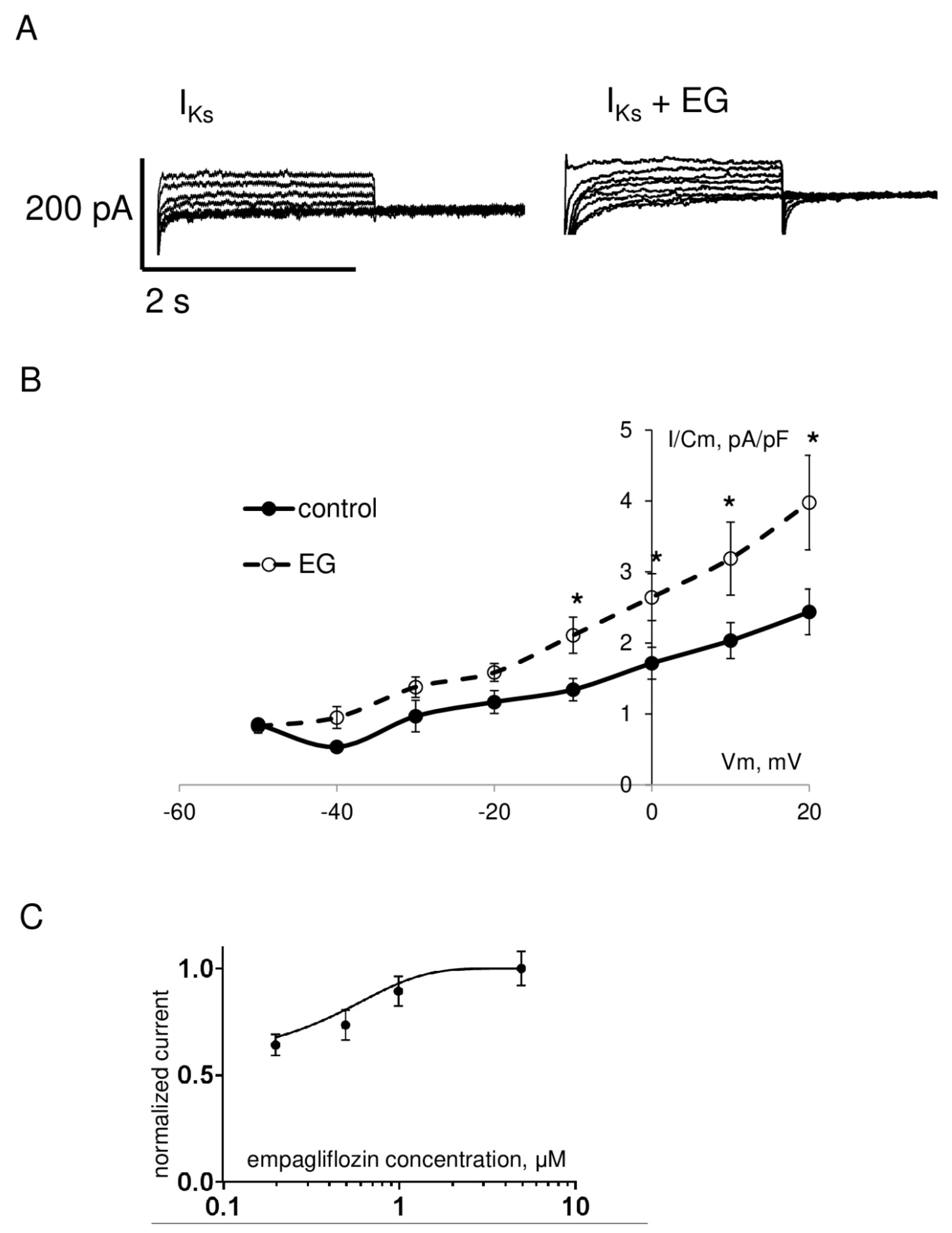
| IKs peak current density at +20 mV, pA/pF | 2.4 ± 0.3 | 18 | * 4.0 ± 0.7 | 19 |
| Control n = 5 | Empagliflozin n = 5 | |
|---|---|---|
| AP amplitude, mV | 82.8 ± 3.0 | 81.0 ± 2.7 |
| AP upstroke velocity, mV/ms | 11.2 ± 0.9 | 13.8 ± 2.0 |
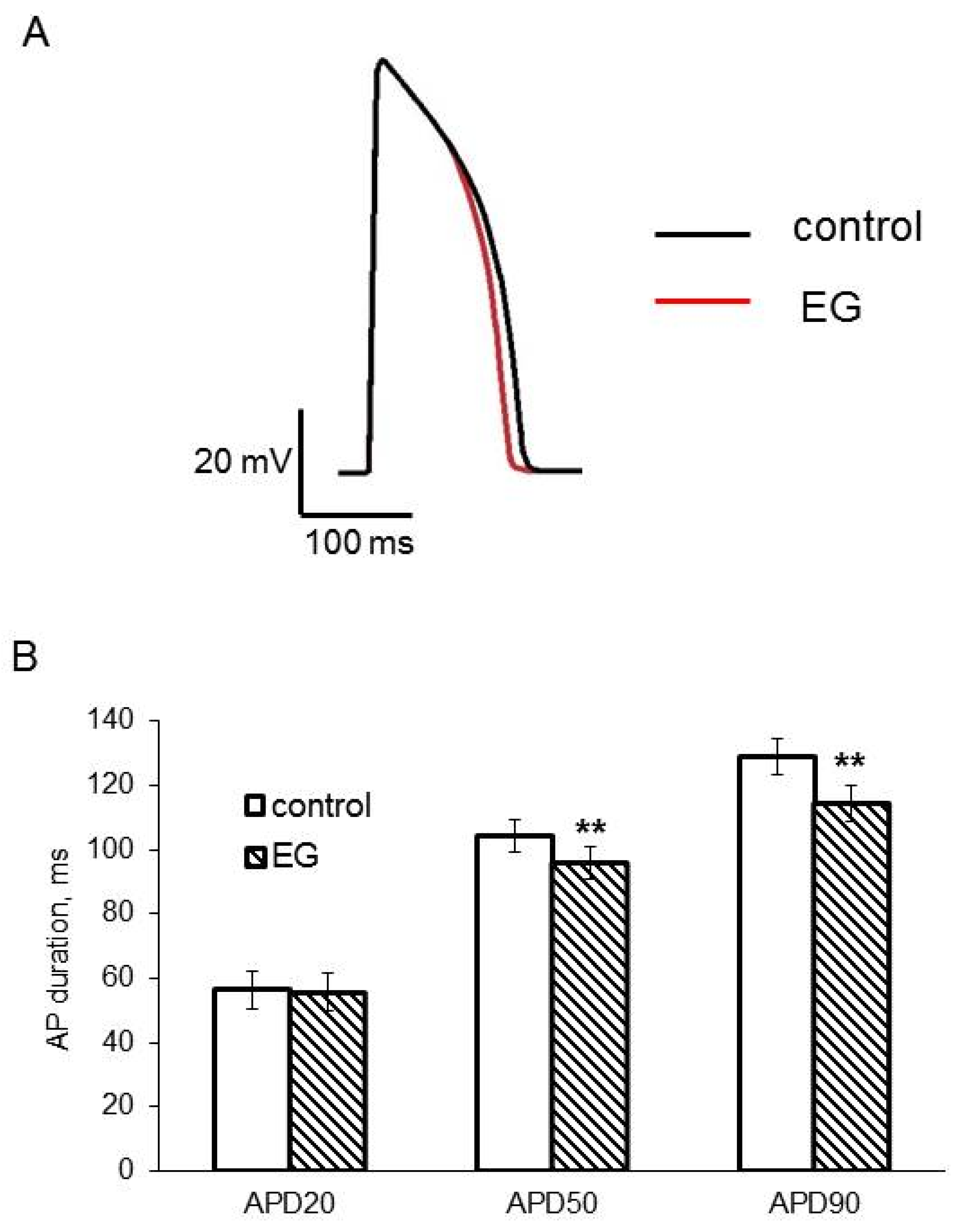
| APD20, ms | 56.5 ± 5.9 | 55.8 ± 5.9 |
| APD50, ms | 104.3 ± 5.0 | ** 95.7 ± 5.0 |
| APD90, ms | 128.8 ± 5.5 | ** 114.5 ± 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpushev, A.V.; Mikhailova, V.B.; Klimenko, E.S.; Kulikov, A.N.; Ivkin, D.Y.; Kaschina, E.; Okovityi, S.V. SGLT2 Inhibitor Empagliflozin Modulates Ion Channels in Adult Zebrafish Heart. Int. J. Mol. Sci. 2022, 23, 9559. https://doi.org/10.3390/ijms23179559
Karpushev AV, Mikhailova VB, Klimenko ES, Kulikov AN, Ivkin DY, Kaschina E, Okovityi SV. SGLT2 Inhibitor Empagliflozin Modulates Ion Channels in Adult Zebrafish Heart. International Journal of Molecular Sciences. 2022; 23(17):9559. https://doi.org/10.3390/ijms23179559
Chicago/Turabian StyleKarpushev, Alexey V., Valeria B. Mikhailova, Ekaterina S. Klimenko, Alexander N. Kulikov, Dmitry Yu. Ivkin, Elena Kaschina, and Sergey V. Okovityi. 2022. "SGLT2 Inhibitor Empagliflozin Modulates Ion Channels in Adult Zebrafish Heart" International Journal of Molecular Sciences 23, no. 17: 9559. https://doi.org/10.3390/ijms23179559
APA StyleKarpushev, A. V., Mikhailova, V. B., Klimenko, E. S., Kulikov, A. N., Ivkin, D. Y., Kaschina, E., & Okovityi, S. V. (2022). SGLT2 Inhibitor Empagliflozin Modulates Ion Channels in Adult Zebrafish Heart. International Journal of Molecular Sciences, 23(17), 9559. https://doi.org/10.3390/ijms23179559







