Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance
Abstract
:1. Introduction
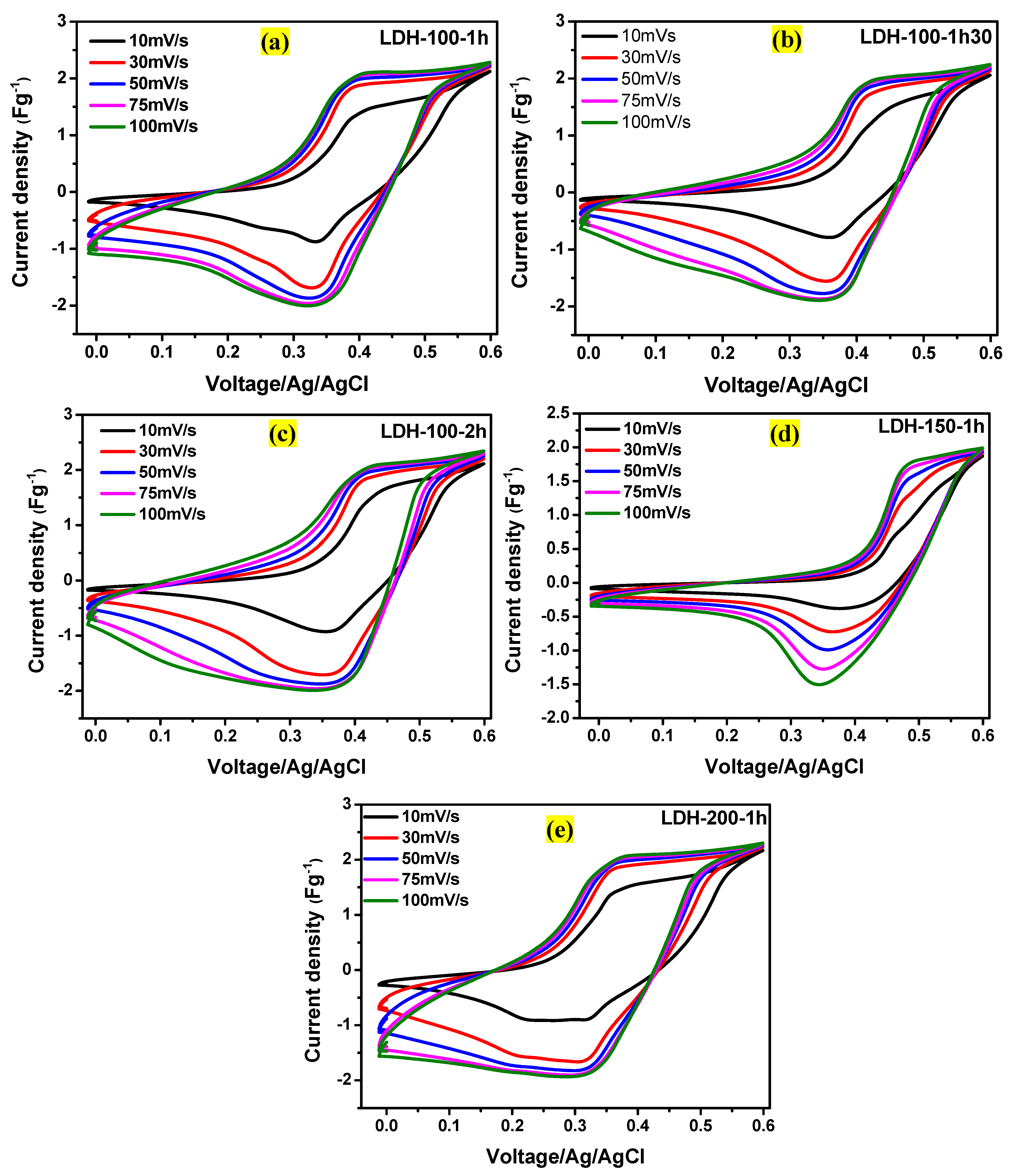
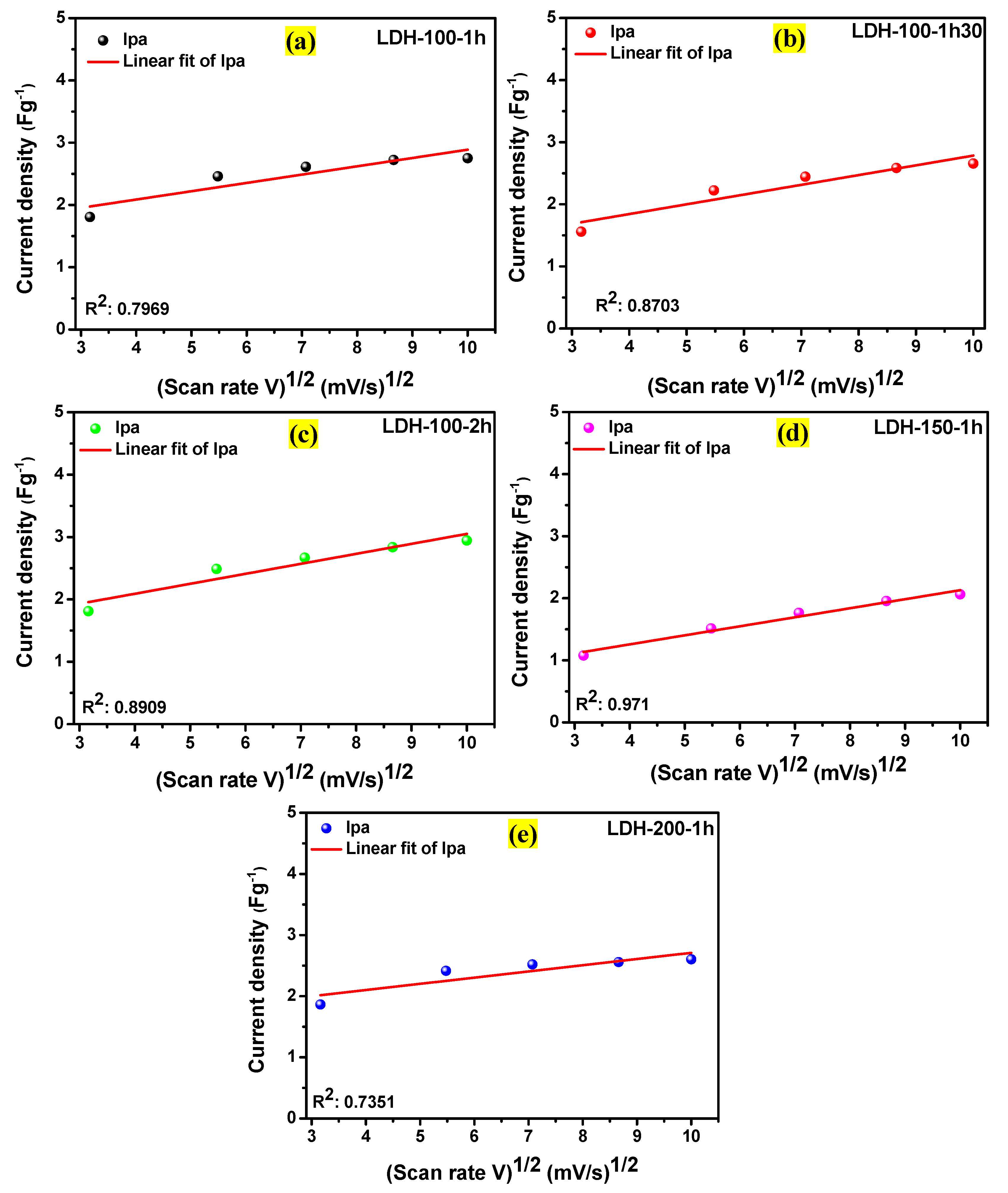
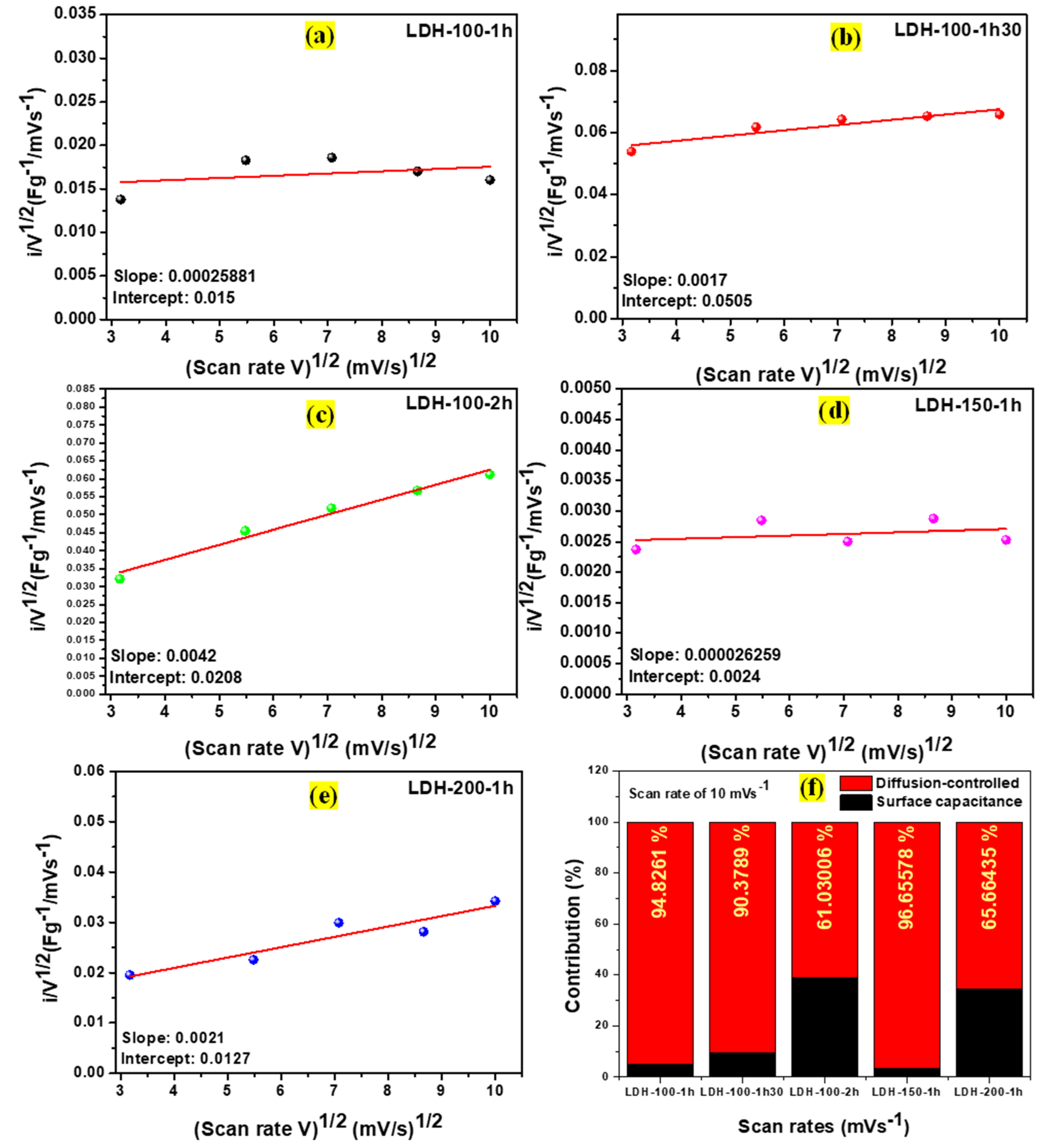
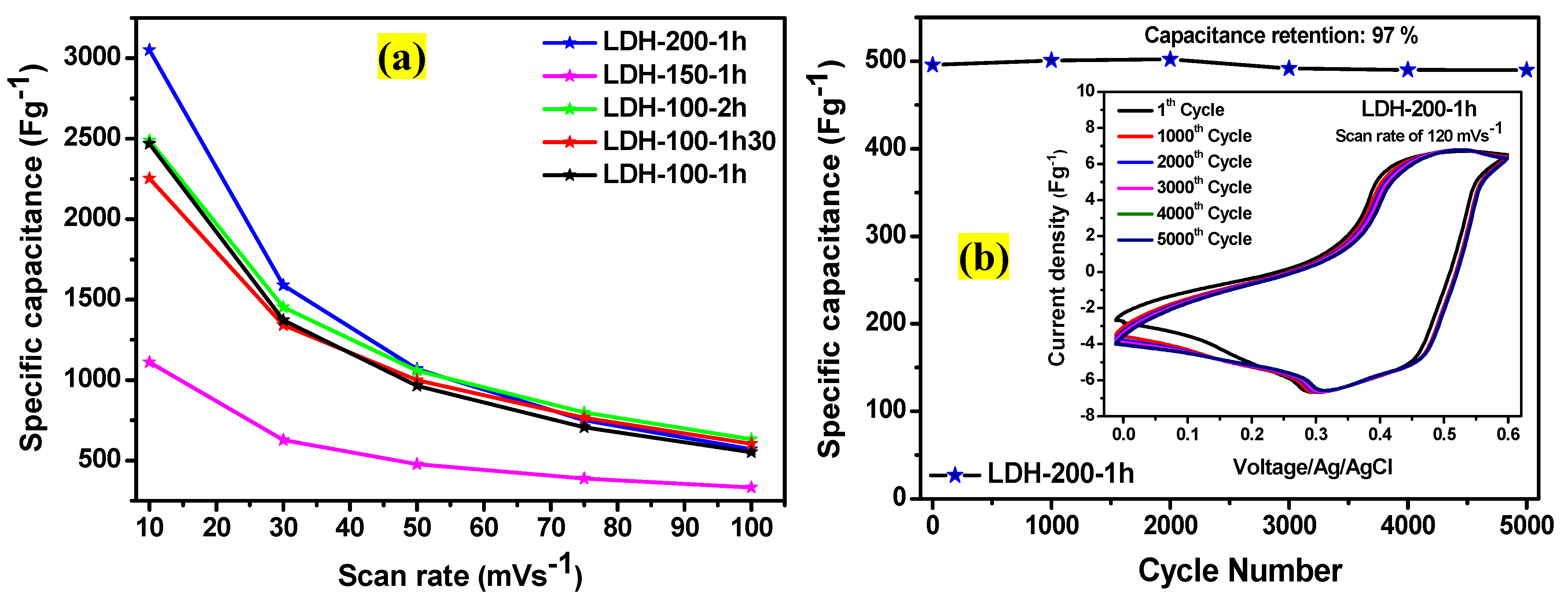
2. Results and Discussion
3. Materials and Methods
3.1. Binder-Free LDH Electrodes Preparation
3.2. Materials Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, A.; Ramos, M.K.; Pinto, C.S.; Pereira, A.F.; Souza, V.H.R.; Zarbin, A.J.G. Electrode fabrication at liquid interfaces: Towards transparency and flexibility. Electrochem. Commun. 2022, 134, 107183. [Google Scholar] [CrossRef]
- Wehner, L.A.; Mittal, N.; Liu, T.; Niederberger, M. Multifunctional batteries: Flexible, transient, and transparent. ACS Cent. Sci. 2021, 7, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Zhai, S.; Wang, S.; Ru, Q.; Hou, X.; San Hui, K.; Nam Hui, K.; Chen, F. Recent Progress in Binder-Free Electrodes Synthesis for Electrochemical Energy Storage Application. Batter. Supercaps 2021, 4, 860–880. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, R.; Huang, X.; Shi, Y.; Wang, C.; Gao, Y.; Han, Z. One-step growth of NiCoAl layered double hydroxides microspheres toward high energy density supercapacitors. J. Alloys Compd. 2021, 859, 157879. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, H.; Lin, S.; Bai, J.; Dai, J.; Liang, C.; Zhu, X.; Sun, Y.; Dou, S. Heterostructures of Ni-Co-Al layered double hydroxide assembled on V 4 C 3 MXene for high-energy hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 2291–2300. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yan, X.; Lyu, M.; Wang, L.; Bell, J.; Wang, H. 2-Methylimidazole-Derived Ni-Co Layered Double Hydroxide Nanosheets as High Rate Capability and High Energy Density Storage Material in Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Q.; Liu, J.; Li, R.; Zhang, H.; Chen, R.; Wang, J. Hierarchical NiCo2O4@NiCoAl-layered double hydroxide core/shell nanoforest arrays as advanced electrodes for high-performance asymmetric supercapacitors. J. Alloys Compd. 2017, 724, 130–138. [Google Scholar] [CrossRef]
- Li, P.; Jiao, Y.; Yao, S.; Wang, L.; Chen, G. Dual role of nickel foam in NiCoAl-LDH ensuring high-performance for asymmetric supercapacitors. New J. Chem. 2019, 43, 3139–3145. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Zhong, Y.; Cui, L.; Wei, D.; Zheng, R.; Liu, J. Construction of hierarchical Cu2+1O@NiCoAl-layered double hydroxide nanorod arrays electrode for high-performance supercapacitor. J. Alloys Compd. 2020, 835. [Google Scholar] [CrossRef]
- Wang, G.X.; Ahn, J.H.; Yao, J.; Lindsay, M.; Liu, H.K.; Dou, S.X. Preparation and characterization of carbon nanotubes for energy storage. J. Power Source 2003, 119–121, 16–23. [Google Scholar] [CrossRef]
- Karthick, R.; Chen, F. Free-standing graphene paper for energy application: Progress and future scenarios. Carbon 2019, 150, 292–310. [Google Scholar] [CrossRef]
- Zhai, S.; Wei, L.; Karahan, H.E.; Wang, Y.; Wang, C.; Montoya, A.; Shao, Q.; Wang, X.; Chen, Y. Ultrafast hydrothermal assembly of nanocarbon microfibers in near-critical water for 3D microsupercapacitors. Carbon 2018, 132, 698–708. [Google Scholar] [CrossRef]
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, 1804881. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, G.; Peng, S.; Wang, J.; Yan, W.; Ramakrishna, S. One-dimensional nanomaterials toward electrochemical sodium-ion storage applications via electrospinning. Energy Storage Mater. 2020, 25, 443–476. [Google Scholar] [CrossRef]
- Oakes, L.; Westover, A.; Mahjouri-Samani, M.; Chatterjee, S.; Puretzky, A.A.; Rouleau, C.; Geohegan, D.B.; Pint, C.L. Uniform, homogenous coatings of carbon nanohorns on arbitrary substrates from common solvents. ACS Appl. Mater. Interfaces 2013, 5, 13153–13160. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Liu, D.; Lu, Q.; Sun, X.; Asiri, A.M. Nickel promoted cobalt disulfide nanowire array supported on carbon cloth: An efficient and stable bifunctional electrocatalyst for full water splitting. Electrochem. Commun. 2016, 63, 60–64. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.X.; Wang, Z.L.; Meng, F.L.; Zhang, X.B. Integrated Three-Dimensional Carbon Paper/Carbon Tubes/Cobalt-Sulfide Sheets as an Efficient Electrode for Overall Water Splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Lee, K. Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef]
- Poserin, V.; Marcuson, S.; Shu, J.; Wilkinson, D.S. CVD technique for Inco nickel foam production. Adv. Eng. Mater. 2004, 6, 454–459. [Google Scholar] [CrossRef]
- Queheillalt, D.T.; Hass, D.D.; Sypeck, D.J.; Wadley, H.N.G. Synthesis of open-cell metal foams by templated directed vapor deposition. J. Mater. Res. 2001, 16, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Nyongombe, G.; Kabongo, G.; Noto, L.; Dhlamini, S. Layered double hydroxides (LDHs) as promising electrode materials for commercial supercapacitors. In Applications and Industrialisation of Nanotechnology; Maciel, D.M.A.M., Ed.; One Central Press: Manchester, UK, 2022; pp. 71–90. ISBN 9781910086230. [Google Scholar]
- Nyongombe, G.E.; Kabongo, G.L.; Noto, L.L.; Dhlamini, M.S. Up-scalable synthesis of highly crystalline electroactive Ni-Co LDH nanosheets for supercapacitor applications. Int. J. Electrochem. Sci. 2020, 15, 4494–4502. [Google Scholar] [CrossRef]
- Polat, S.; Atun, G. Enhanced cycling stability performance for supercapacitor application of NiCoAl-LDH nanofoam on modified graphite substrate. J. Ind. Eng. Chem. 2021, 99, 107–116. [Google Scholar] [CrossRef]
- Liu, L.; Guan, T.; Fang, L.; Wu, F.; Lu, Y.; Luo, H.; Song, X.; Zhou, M.; Hu, B.; Wei, D.; et al. Self-supported 3D NiCo-LDH/Gr composite nanosheets array electrode for high-performance supercapacitor. J. Alloys Compd. 2018, 763, 926–934. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Madito, M.J.; Momodu, D.Y.; Dangbegnon, J.K.; Guellati, O.; Harat, A.; Guerioune, M.; Barzegar, F.; Manyala, N. High performance asymmetric supercapacitor based on CoAl-LDH/GF and activated carbon from expanded graphite. RSC Adv. 2016, 6, 46723–46732. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: Recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Nyongombe, G.; Bello, I.T.; Otun, K.O.; Kabongo, G.L.; Mothudi, B.M.; Noto, L.; Dhlamini, M.S. Unveiling the benefits of Dimethyl Sulfoxide as a binder solvent on the electrochemical performance of Layered Double Hydroxides. Electrochim. Acta 2022, 419, 140386. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Beneficial rheological properties of lithium-ion battery cathode slurries from elevated mixing and coating temperatures. J. Energy Storage 2019, 26, 100994. [Google Scholar] [CrossRef]
- Nyongombe, G.; Kabongo, G.L.; Noto, L.L.; Dhlamini, M.S. Investigating the Impact of the Washing Steps of Layered Double Hydroxides (LDH) on the Electrochemical Performance. Nanomaterials 2022, 12, 578. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Richetta, M.; Medaglia, P.; Mattoccia, A.; Varone, A.; Pizzoferrato, R. Layered Double Hydroxides: Tailoring Interlamellar Nanospace for a Vast Field of Applications. J. Mater. Sci. Eng. 2017, 6, 2169-0022. [Google Scholar] [CrossRef]
- Gamil, S.; El Rouby, W.M.A.; Antuch, M.; Zedan, I.T. Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation. RSC Adv. 2019, 9, 13503–13514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Zhu, J.; Zhang, X.; San Hui, K.; Hui, K.N. 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials. J. Mater. Chem. A 2013, 1, 9046–9053. [Google Scholar] [CrossRef]
- Xiao, K.; Xia, L.; Liu, G.; Wang, S.; Ding, L.X.; Wang, H. Honeycomb-like NiMoO4 ultrathin nanosheet arrays for high-performance electrochemical energy storage. J. Mater. Chem. A 2015, 3, 6128–6135. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.W.; Wang, Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef]
- Sun, S.; Diao, P.; Feng, C.; Ungureanu, E.M.; Tang, Y.; Hu, B.; Hu, Q. Nickel-foam-supported β-Ni(OH)2 as a green anodic catalyst for energy efficient electrooxidative degradation of azo-dye wastewater. RSC Adv. 2018, 8, 19776–19785. [Google Scholar] [CrossRef]
- Tessier, C.; Haumesser, P.H.; Bernard, P.; Delmas, C. The Structure of Ni (OH)2: From the Ideal Material to the Electrochemically Active One. J. Electrochem. Soc. 1999, 146, 2059–2067. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Raman and Infrared Spectroscopy of α and β Phases of Thin Nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef]
- Nyongombe, G.; Kabongo, G.L.; Bello, I.T.; Noto, L.L.; Dhlamini, M.S. The impact of drying temperature on the crystalline domain and the electrochemical performance of NiCoAl-LDH. Energy Rep. 2022, 8, 1151–1158. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. A spectroscopic study of the effect of the trivalent cation on the thermal decomposition behaviour of Co-based hydrotalcites. J. Mater. Chem. 2001, 11, 2529–2536. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–23. [Google Scholar] [CrossRef]
- Chernysh, O.; Khomenko, V.; Makyeyeva, I.; Barsukov, V. Effect of binder’s solvent on the electrochemical performance of electrodes for lithium-ion batteries and supercapacitors. Mater. Today Proc. 2019, 6, 42–47. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Su, D.; Park, J.; Ahn, H.; Wang, G. Enhance electrochemical performance of lithium sulfur battery through a solution-based processing technique. J. Power Source 2012, 202, 389–393. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Review—The Importance of Chemical Interactions between Sulfur Host Materials and Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A2567–A2576. [Google Scholar] [CrossRef]
- Silva Neto, L.D.; Anchieta, C.G.; Duarte, J.L.S.; Meili, L.; Freire, J.T. Effect of Drying on the Fabrication of MgAl Layered Double Hydroxides. ACS Omega 2021, 6, 21819–21829. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Depège, C.; Ennaqadi, A.; De Roy, A.; Besse, J.P. Rehydration of Zn-Al layered double hydroxides. Clays Clay Miner. 1997, 45, 92–98. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Ling, Z.; Qiu, J.; Gogotsi, Y. Facile fabrication of MWCNT-doped NiCoAl-layered double hydroxide nanosheets with enhanced electrochemical performances. J. Mater. Chem. A 2013, 1, 1963–1968. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, C.; Chu, H.; Qiu, S.; McLeod, J.; She, Z.; Xu, F.; Sun, L.; Zou, Y. Binary Co–Ni oxide nanoparticle-loaded hierarchical graphitic porous carbon for high-performance supercapacitors. J. Mater. Sci. Technol. 2020, 37, 135–142. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Huang, C.; Hao, C.; Ge, C.; Guo, Y. Fabrication of NiCoAl-layered double hydroxide/N-GO for high energy all-solid-state asymmetric supercapacitors. Appl. Surf. Sci. 2020, 527, 146891. [Google Scholar] [CrossRef]
- Wang, S.; Zou, Y.; Xu, F.; Xiang, C.; Peng, H.; Zhang, J.; Sun, L. Morphological control and electrochemical performance of NiCo2O4@NiCo layered double hydroxide as an electrode for supercapacitors. J. Energy Storage 2021, 41, 102862. [Google Scholar] [CrossRef]
- Wang, X.L.; Jin, E.M.; Chen, J.; Bandyopadhyay, P.; Jin, B.; Jeong, S.M. Facile in situ synthesis of Co(OH)2–Ni3S2 nanowires on Ni foam for use in high-energy-density supercapacitors. Nanomaterials 2022, 12, 34. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Chhetri, K.; Kim, H.; Ji, S.; Chae, S.H.; Kim, T.; Kim, H.Y. Self-assembled polypyrrole hierarchical porous networks as the cathode and porous three dimensional carbonaceous networks as the anode materials for asymmetric supercapacitor. J. Energy Storage 2021, 33, 102080. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, C.; Fu, X.; Shen, M.; Li, K.; Liu, X.; Yao, H.C.; Zhang, Y.; Yao, K.X. Synthesis of porous NiCoS nanosheets with Al leaching on ordered mesoporous carbon for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123367. [Google Scholar] [CrossRef]
- Cao, X.J.; Zeng, H.Y.; Cao, X.; Xu, S.; Alain, G.B.F.C.; Zou, K.M.; Liu, L. 3D-hierarchical mesoporous CuCo2S4@NiCoAl hydrotalcite/Ni foam material for high-performance supercapacitors. Appl. Clay Sci. 2020, 199, 10586. [Google Scholar] [CrossRef]
- Liu, L.; Fang, L.; Wu, F.; Hu, J.; Zhang, S.; Luo, H.; Hu, B.; Zhou, M. Self-supported core-shell heterostructure MnO2/NiCo-LDH composite for flexible high-performance supercapacitor. J. Alloys Compd. 2020, 824, 153929. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Liu, Y.; Bao, J.; Han, M.; Dai, Z. Integrating ultrathin and modified NiCoAl-layered double-hydroxide nanosheets with N-doped reduced graphene oxide for high-performance all-solid-state supercapacitors. Nanoscale 2019, 11, 9896–9905. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Deng, J.; Duan, X.; Guo, J.; Liu, P. Improving cyclic stability of polyaniline by thermal crosslinking as electrode material for supercapacitors. RSC Adv. 2015, 5, 78545–78552. [Google Scholar] [CrossRef]
- Bhise, S.C.; Awale, D.V.; Vadiyar, M.M.; Patil, S.K.; Kokare, B.N.; Kolekar, S.S. Facile synthesis of CuO nanosheets as electrode for supercapacitor with long cyclic stability in novel methyl imidazole-based ionic liquid electrolyte. J. Solid State Electrochem. 2017, 21, 2585–2591. [Google Scholar] [CrossRef]
- Lu, X.; Liu, T.; Zhai, T.; Wang, G.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Improving the cycling stability of metal-nitride supercapacitor electrodes with a thin carbon shell. Adv. Energy Mater. 2014, 4, 1300994. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
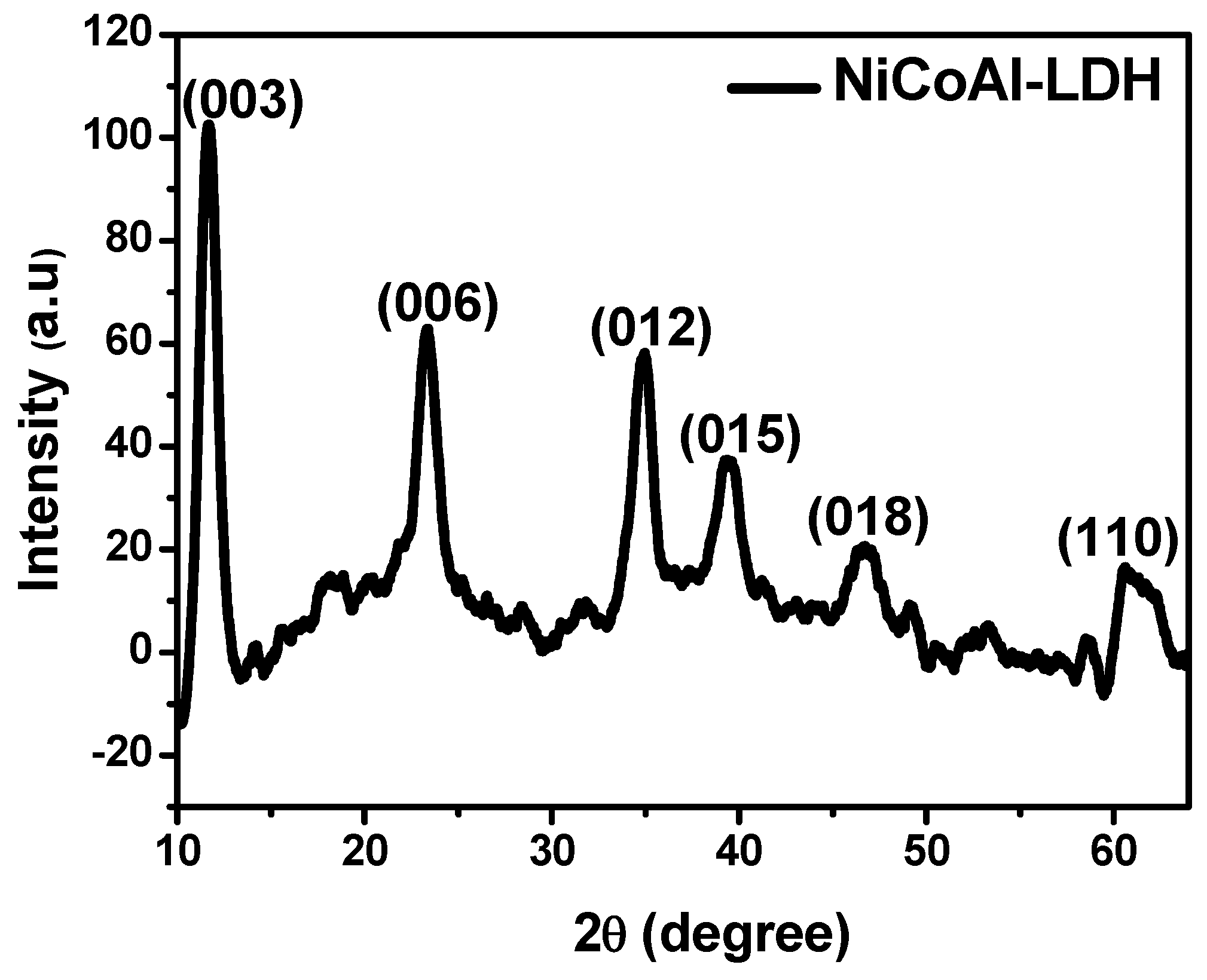
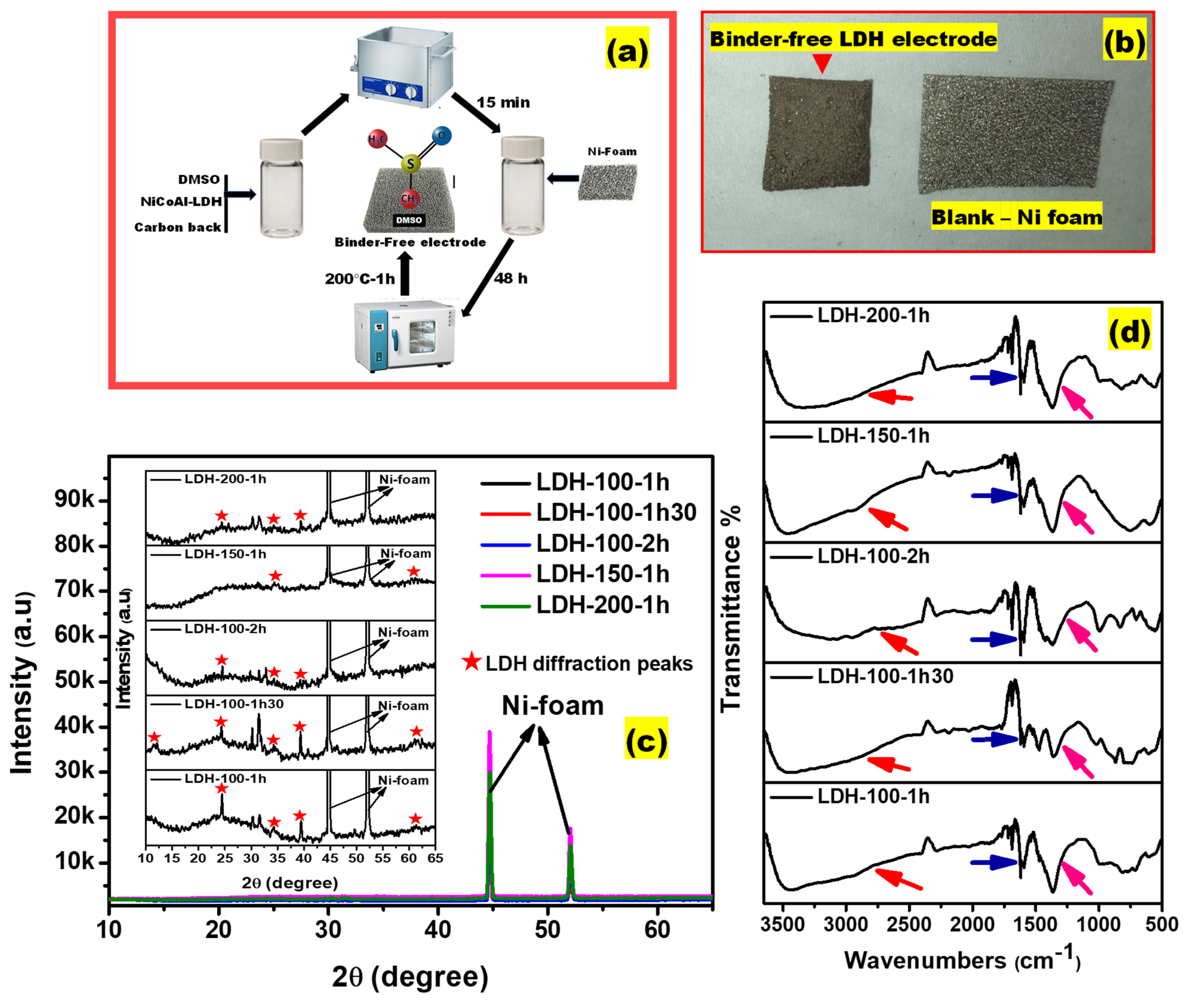

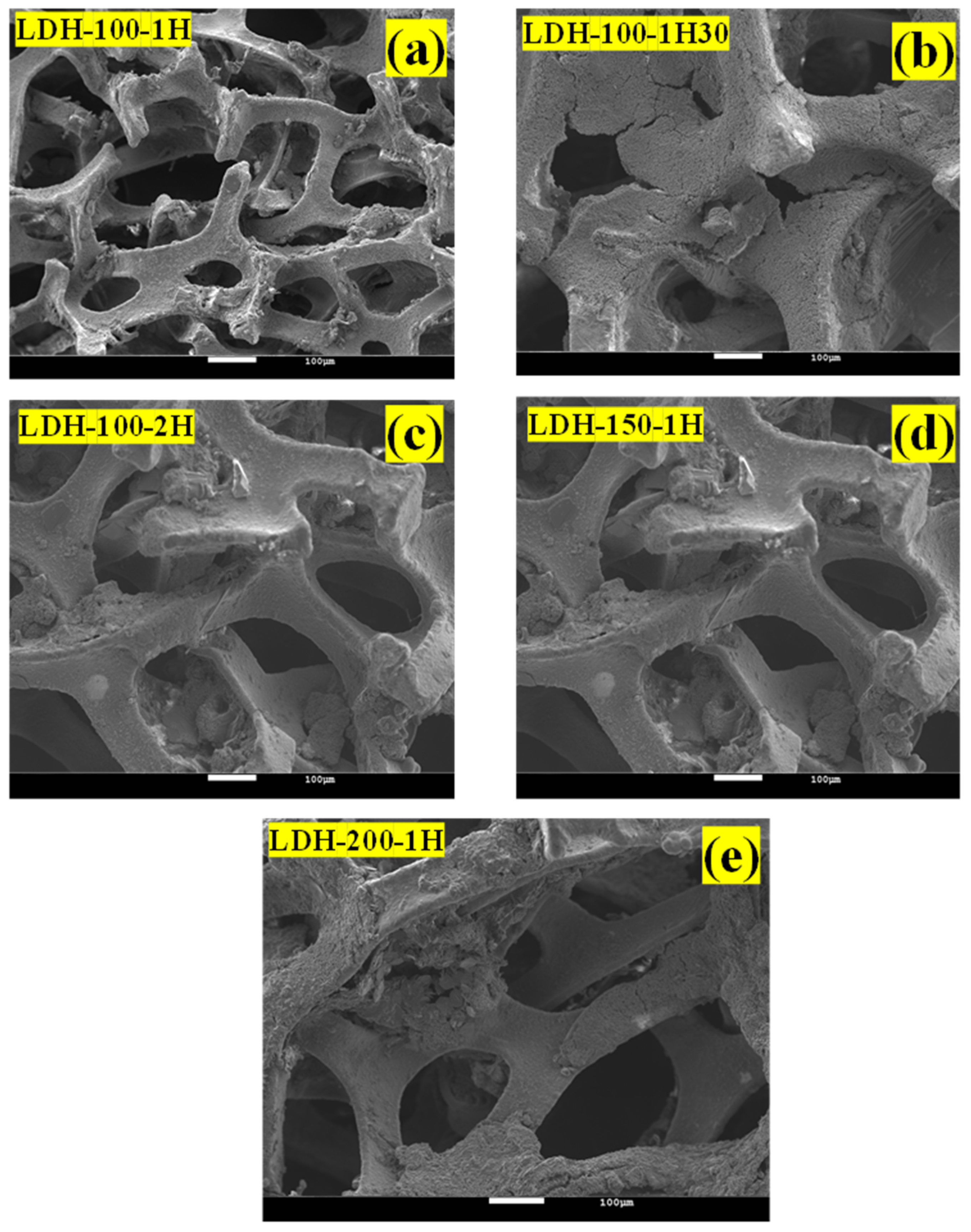
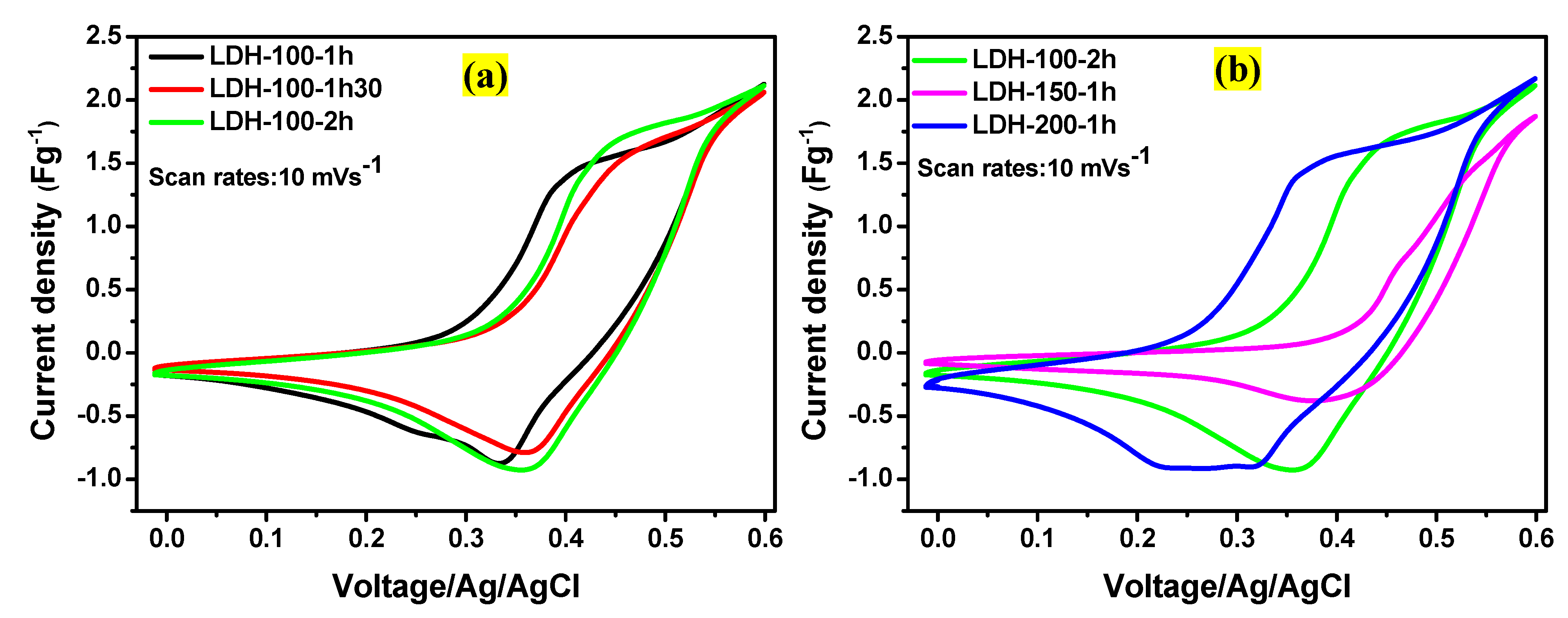

| Electrodes | Mass Loading (g) |
|---|---|
| LDH-100-1h | 0.028 |
| LDH-100-1h30 | 0.026 |
| LDH-100-2h | 0.028 |
| LDH-150-1h | 0.026 |
| LDH-200-1h | 0.029 |
| Electrodes | Specific Capacitances | Scan Rate |
|---|---|---|
| LDH-100-1h | 2467.70 Fg−1 | 10 mVs−1 |
| LDH-100-1h30 | 2252.43 Fg−1 | 10 mVs−1 |
| LDH-100-2h | 2489.34 Fg−1 | 10 mVs−1 |
| LDH-150-1h | 1110.16 Fg−1 | 10 mVs−1 |
| LDH-200-1h | 3050.95 Fg−1 | 10 mVs−1 |
| Electrodes | Specific Capacitance | Electrolyte | References |
|---|---|---|---|
| NiCoS@SBA-C | 1757 Fg−1–1 A g−1 | 6 M KOH | [55] |
| CuCo2S4@NiCoAl-LDH/NF | 1876 Fg−1–1 A g−1 | 6 M KOH | [56] |
| Cu2+1O@NiCoAl-LDH | 2932 Fg−1–0.75 A g−1 | 6 M KOH | [57] |
| m-LDH/NRG NHs | 1877.0 Fg−1–1 A g−1 | 6 M KOH | [58] |
| NiCo2Al-LDH/N-GO | 1136.67Fg−1–1 A g−1 | 2 M KOH | [51] |
| NiCoAl-LDH | 5691.25 mF cm−2–1 mA cm−2 | 3 M KOH | [8] |
| NiCo2O4@NiCoAl-LDH | 1814.24 Fg−1–1 A g−1 | 2 M KOH | [7] |
| LDH-200-1h | 3050.95 Fg−1–10 mVs−1 | 1 M KOH | This work |
| LDH-100-1h | LDH-100-1h30 | LDH-100-2h | LDH-150-1h | LDH-200-1h | |
|---|---|---|---|---|---|
| Rct (Ω) | 0.78 | 0.98 | 0.76 | 4.01 | 0.68 |
| Electrodes | Rct (Ω) |
|---|---|
| LDH-100-1h | 0.78 |
| LDH-100-1h30 | 0.98 |
| LDH-100-2h | 0.76 |
| LDH-150-1h | 4.01 |
| LDH-200-1h | 0.68 |
| NiCoAl-NMP | 1.5 |
| NiCoAl-DMSO | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyongombe, G.; Kabongo, G.L.; Noto, L.L.; Dhlamini, M.S. Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance. Int. J. Mol. Sci. 2022, 23, 10192. https://doi.org/10.3390/ijms231710192
Nyongombe G, Kabongo GL, Noto LL, Dhlamini MS. Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance. International Journal of Molecular Sciences. 2022; 23(17):10192. https://doi.org/10.3390/ijms231710192
Chicago/Turabian StyleNyongombe, Gayi, Guy L. Kabongo, Luyanda L. Noto, and Mokhotjwa S. Dhlamini. 2022. "Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance" International Journal of Molecular Sciences 23, no. 17: 10192. https://doi.org/10.3390/ijms231710192
APA StyleNyongombe, G., Kabongo, G. L., Noto, L. L., & Dhlamini, M. S. (2022). Advantage of Dimethyl Sulfoxide in the Fabrication of Binder-Free Layered Double Hydroxides Electrodes: Impacts of Physical Parameters on the Crystalline Domain and Electrochemical Performance. International Journal of Molecular Sciences, 23(17), 10192. https://doi.org/10.3390/ijms231710192









