Lyophilization of Curcumin–Albumin Nanoplex with Sucrose as Cryoprotectant: Aqueous Reconstitution, Dissolution, Kinetic Solubility, and Physicochemical Stability
Abstract
1. Introduction
2. Results and Discussion
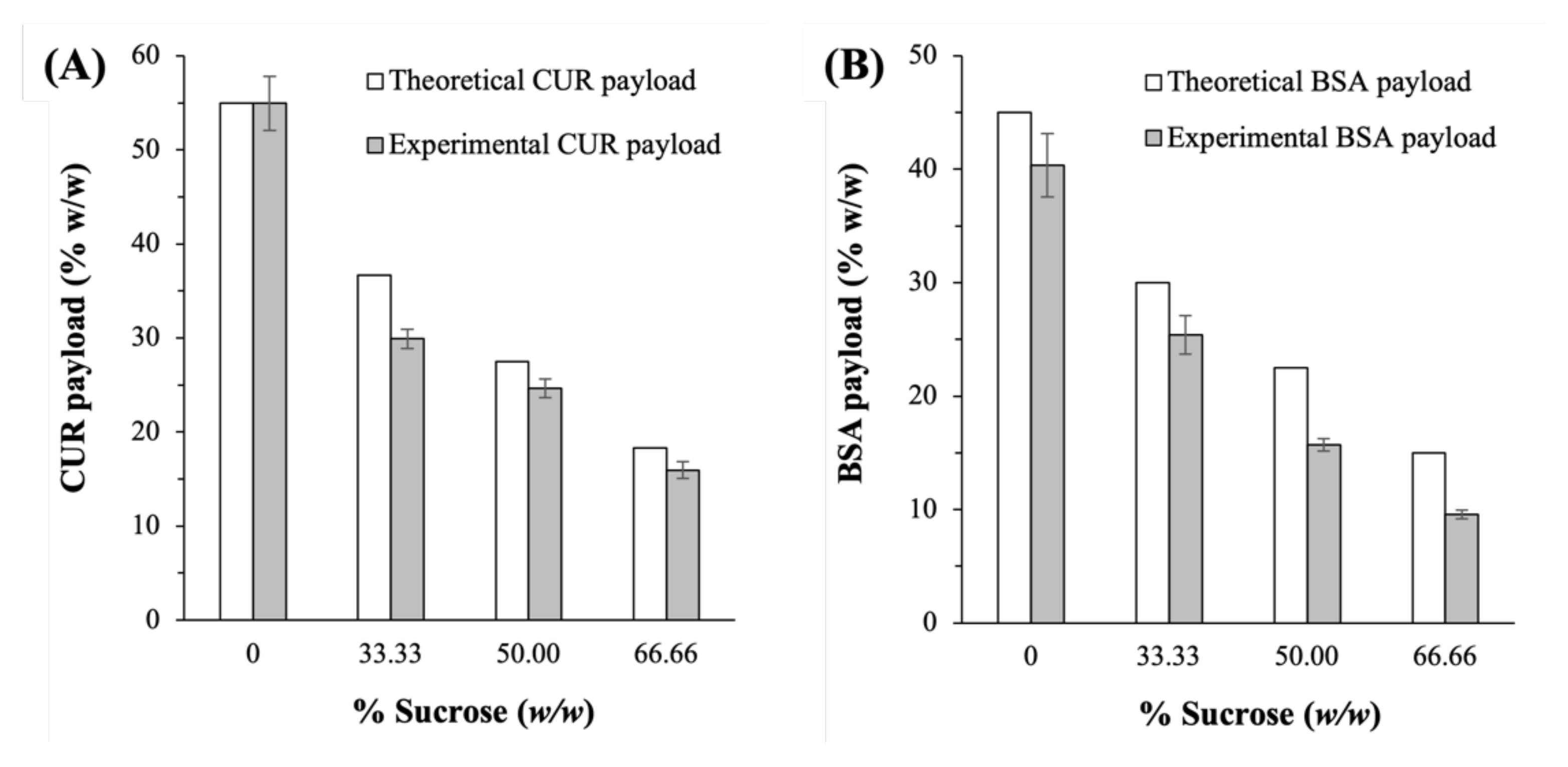
2.1. Physical Characteristics of the Lyophilized CUR-BSA Nanoplex
2.2. Feasibility of Sucrose as Cryoprotectant
2.2.1. Aqueous Reconstitution
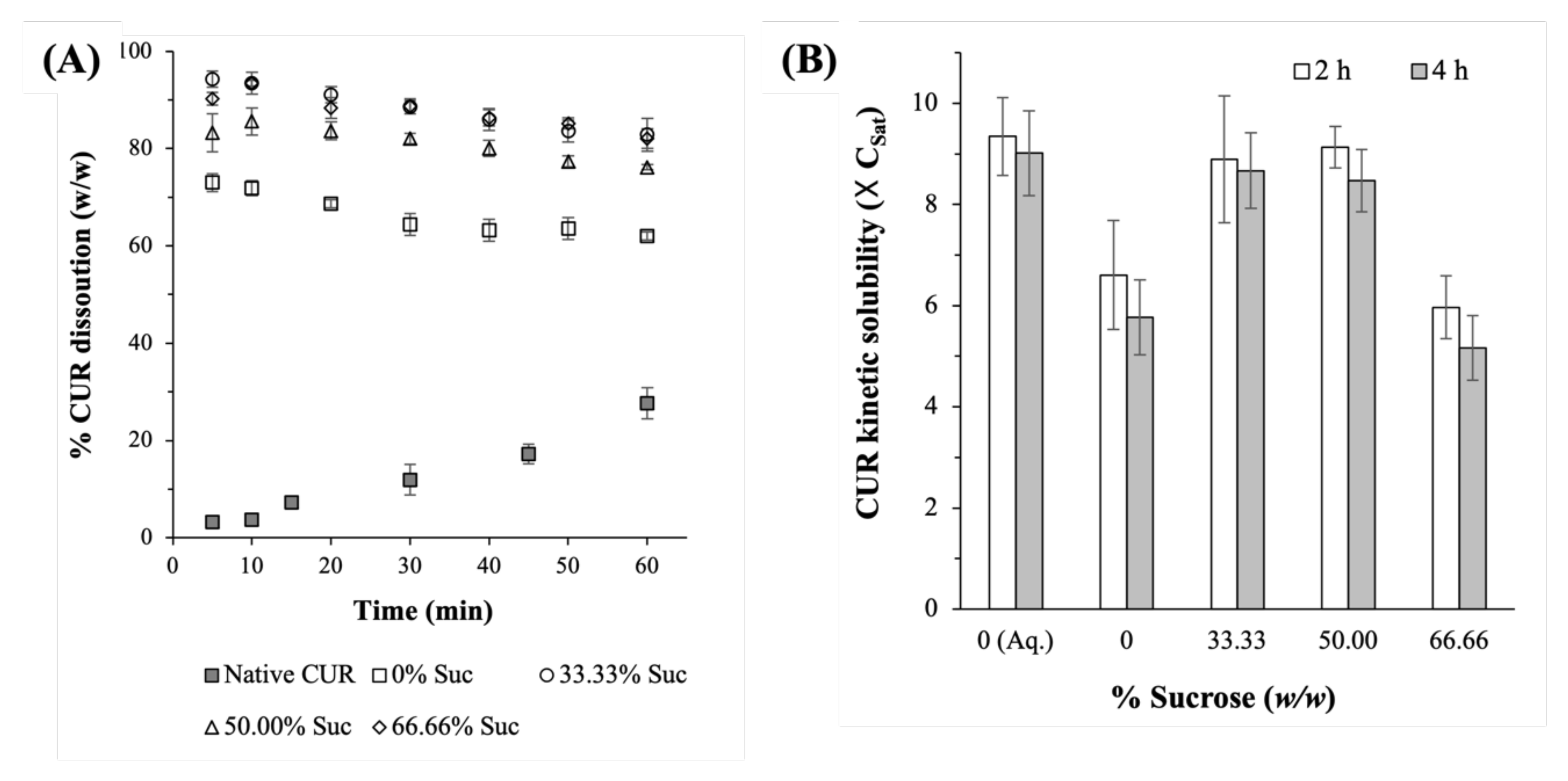
2.2.2. CUR Dissolution Profiles
2.2.3. Amorphous CUR Kinetic Solubility
2.3. Physicochemical Stability after 30-Day Exposures to 40 °C and 75% RH
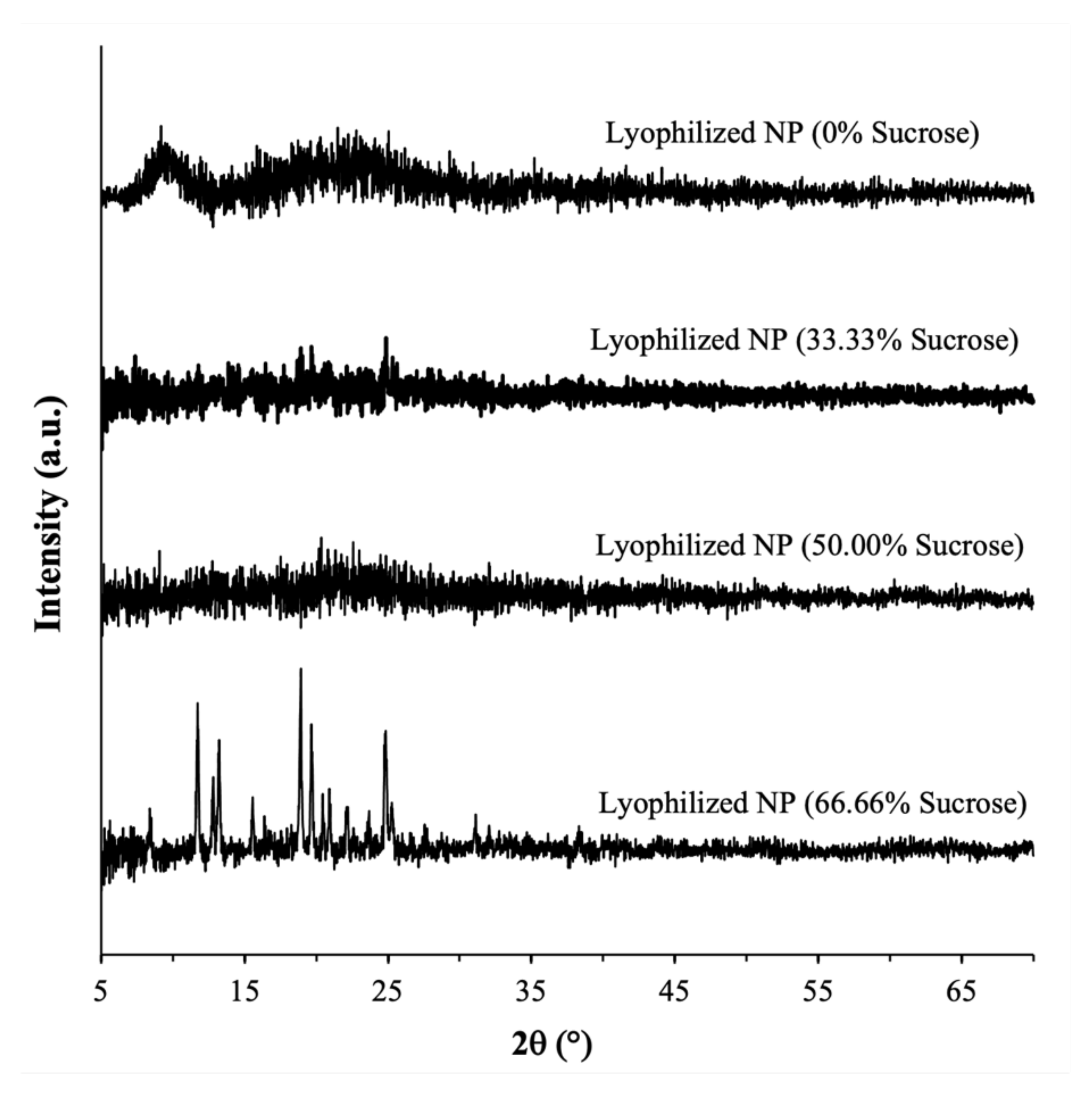
2.3.1. Amorphous Form
2.3.2. Aqueous Reconstitution
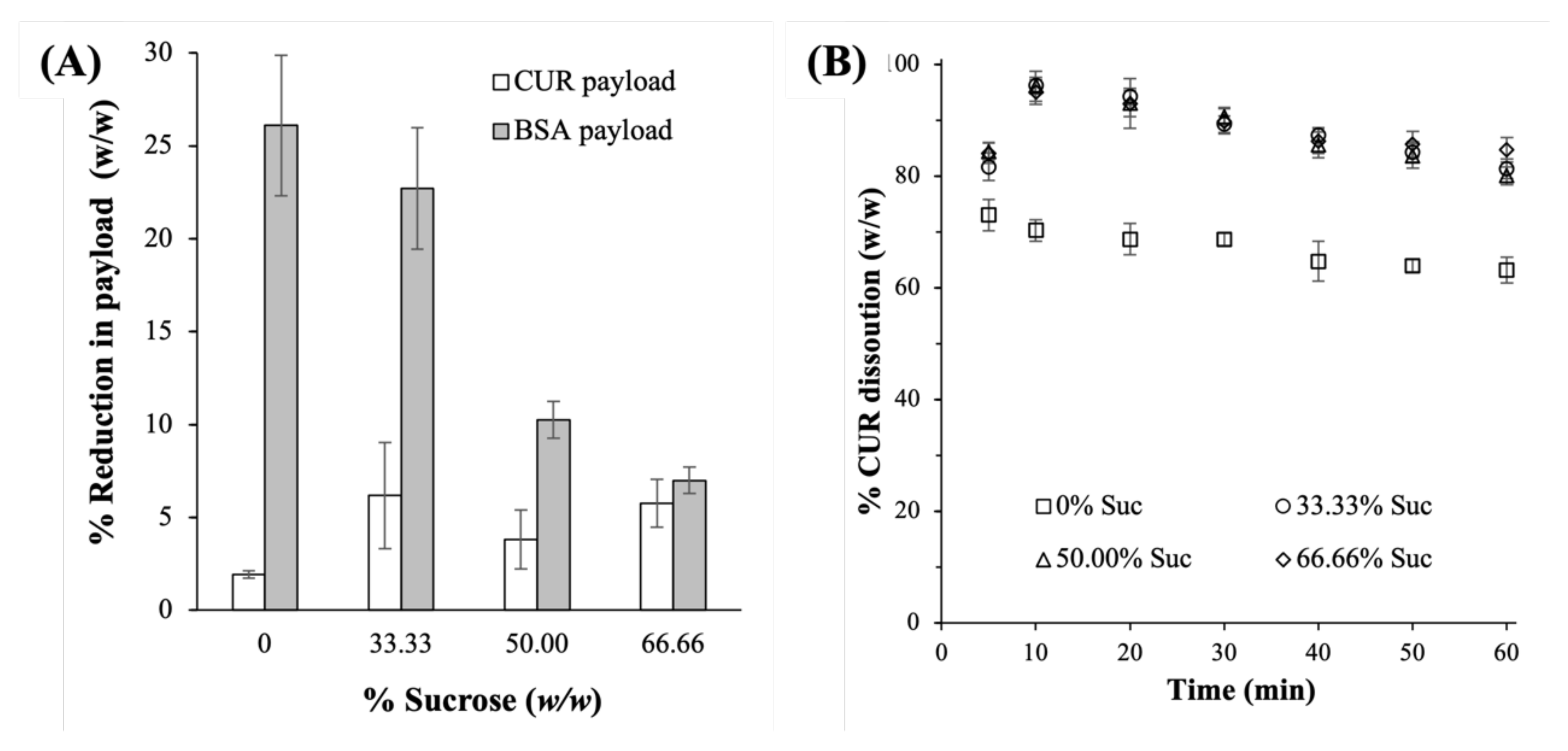
2.3.3. Payloads and Dissolution Profiles
2.3.4. Protein Structural Integrity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of CUR-BSA Nanoplex
3.2.2. Lyophilization of CUR-BSA Nanoplex
3.2.3. Aqueous Reconstitution
3.2.4. Experimental CUR and BSA Payloads
3.2.5. CUR Dissolution Profiles
3.2.6. Amorphous CUR Kinetic Solubility
3.2.7. Amorphous Form Stability
3.2.8. Protein Structural Integrity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| a.u. | Arbitrary unit |
| BSA | Bovine serum albumin |
| CSat | Thermodynamic saturation solubility |
| CUR | Curcumin |
| DLS | Dynamic light scattering |
| FESEM | Field emission scanning electron microscope |
| HPLC | High-performance liquid chromatography |
| MW | Molecular weight |
| NP | Nanoplex |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| pI | Isoelectric point |
| PXRD | Powder X-ray diffraction |
| RH | Relative humidity |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| Nanoplex size after reconstitution | |
| Original nanoplex size before lyophilization | |
| Suc | Sucrose |
| UV-Vis | Ultraviolet-visible |
References
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.; Trapani, D.; Hussain, S.; Wolf, D.; Willenbacher, W.; Spizzo, G.; Seeber, A. Curcumin: New Insights into an Ancient Ingredient against Cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Combining inkjet printing and amorphous nanonization to prepare personalized dosage forms of poorly-soluble drugs. Eur. J. Pharm. Biopharm. 2015, 96, 314–321. [Google Scholar] [CrossRef]
- Atiyah, N.A.; Albayati, T.M.; Atiya, M.A. Functionalization of mesoporous MCM-41 for the delivery of curcumin as an anti-inflammatory therapy. Adv. Powder Technol. 2022, 33, 103417. [Google Scholar] [CrossRef]
- Atiyah, N.A.; Albayati, T.M.; Atiya, M.A. Interaction behavior of curcumin encapsulated onto functionalized SBA-15 as an efficient carrier and release in drug delivery. J. Mol. Struct. 2022, 1260, 132879. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Lin, R.; Li, H.-J.; He, W.-l.; Du, J.-Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. WIREs Nanomed. Nanobiotechnology 2019, 11, e1519. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Aravind, S.R.; Krishnan, L.K. Curcumin-albumin conjugates as an effective anti-cancer agent with immunomodulatory properties. Int. Immunopharmacol. 2016, 34, 78–85. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef]
- Harshita; Barkat, M.A.; SarwarBeg; Pottoo, F.H.; JAhmad, F. Nanopaclitaxel therapy: An evidence based review on the battle for next-generation formulation challenges. Nanomedicine 2019, 14, 1323–1341. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Redox responsive curcumin-loaded human serum albumin nanoparticles: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 114, 759–766. [Google Scholar] [CrossRef]
- Kim, B.; Lee, C.; Lee, E.S.; Shin, B.S.; Youn, Y.S. Paclitaxel and curcumin co-bound albumin nanoparticles having antitumor potential to pancreatic cancer. Asian J. Pharm. Sci. 2016, 11, 708–714. [Google Scholar] [CrossRef]
- Hasanpoor, Z.; Mostafaie, A.; Nikokar, I.; Hassan, Z.M. Curcumin-human serum albumin nanoparticles decorated with PDL1 binding peptide for targeting PDL1-expressing breast cancer cells. Int. J. Biol. Macromol. 2020, 159, 137–153. [Google Scholar] [CrossRef]
- Salehiabar, M.; Nosrati, H.; Javani, E.; Aliakbarzadeh, F.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int. J. Biol. Macromol. 2018, 115, 83–89. [Google Scholar] [CrossRef]
- Yu, H.; Nguyen, M.H.; Cheow, W.S.; Hadinoto, K. A new bioavailability enhancement strategy of curcumin via self-assembly nano-complexation of curcumin and bovine serum albumin. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 75, 25–33. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Zhang, W.; Sui, X.; Yan, Z.; He, Z. Nanoparticle albumin-bound (NAB) technology is a promising method for anti-cancer drug delivery. Recent Pat. Anticancer. Drug Discov. 2009, 4, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Lee, S.E.; Tran, T.T.; Bui, C.B.; Nguyen, T.H.N.; Vu, N.B.D.; Tran, T.T.; Nguyen, T.H.P.; Nguyen, T.T.; Hadinoto, K. A simple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 98, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Zilker, M.; Sörgel, F.; Holzgrabe, U. A systematic review of the stability of finished pharmaceutical products and drug substances beyond their labeled expiry dates. J. Pharm. Biomed. Anal. 2019, 166, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mutukuri, T.T.; Wilson, N.E.; Zhou, Q. Pharmaceutical protein solids: Drying technology, solid-state characterization and stability. Adv. Drug Deliv. Rev. 2021, 172, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tran, T.T.; Teo, J.; Hadinoto, K. Dry powder aerosols of curcumin-chitosan nanoparticle complex prepared by spray freeze drying and their antimicrobial efficacy against common respiratory bacterial pathogens. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 504, 34–42. [Google Scholar] [CrossRef]
- Arsiccio, A.; Giorsello, P.; Marenco, L.; Pisano, R. Considerations on Protein Stability During Freezing and Its Impact on the Freeze-Drying Cycle: A Design Space Approach. J. Pharm. Sci. 2020, 109, 464–475. [Google Scholar] [CrossRef]
- Borzova, V.A.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of Thermal Denaturation and Aggregation of Bovine Serum Albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Luo, W.-C.; O’Reilly Beringhs, A.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef]
- Anhorn, M.G.; Mahler, H.-C.; Langer, K. Freeze drying of human serum albumin (HSA) nanoparticles with different excipients. Int. J. Pharm. 2008, 363, 162–169. [Google Scholar] [CrossRef]
- James, S.; McManus, J.J. Thermal and Solution Stability of Lysozyme in the Presence of Sucrose, Glucose, and Trehalose. J. Phys. Chem. B 2012, 116, 10182–10188. [Google Scholar] [CrossRef]
- Han, Y.; Jin, B.-S.; Lee, S.-B.; Sohn, Y.; Joung, J.-W.; Lee, J.-H. Effects of sugar additives on protein stability of recombinant human serum albumin during lyophilization and storage. Arch. Pharmacal Res. 2007, 30, 1124–1131. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Amorphous nanodrugs prepared by complexation with polysaccharides: Carrageenan versus dextran sulfate. Carbohydr. Polym. 2015, 117, 549–558. [Google Scholar] [CrossRef]
- Breen, E.D.; Curley, J.G.; Overcashier, D.E.; Hsu, C.C.; Shire, S.J. Effect of moisture on the stability of a lyophilized humanized monoclonal antibody formulation. Pharm. Res. 2001, 18, 1345–1353. [Google Scholar] [CrossRef]
- Sydykov, B.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Hydrogen Bonding Interactions and Enthalpy Relaxation in Sugar/Protein Glasses. J. Pharm. Sci. 2017, 106, 761–769. [Google Scholar] [CrossRef]
- Naksuriya, O.; van Steenbergen, M.J.; Torano, J.S.; Okonogi, S.; Hennink, W.E. A Kinetic Degradation Study of Curcumin in Its Free Form and Loaded in Polymeric Micelles. Aaps J 2016, 18, 777–787. [Google Scholar] [CrossRef]
- Pu, S.; Hadinoto, K. Continuous crystallization as a downstream processing step of pharmaceutical proteins: A review. Chem. Eng. Res. Des. 2020, 160, 89–104. [Google Scholar] [CrossRef]
- Edueng, K.; Mahlin, D.; Larsson, P.; Bergström, C.A.S. Mechanism-based selection of stabilization strategy for amorphous formulations: Insights into crystallization pathways. J. Control. Release 2017, 256, 193–202. [Google Scholar] [CrossRef]
- Morrow, E.A.; Terban, M.W.; Thomas, L.C.; Gray, D.L.; Bowman, M.J.; Billinge, S.J.L.; Schmidt, S.J. Effect of amorphization method on the physicochemical properties of amorphous sucrose. J. Food Eng. 2019, 243, 125–141. [Google Scholar] [CrossRef]
- Hellrup, J.; Mahlin, D. Pharmaceutical micro-particles give amorphous sucrose higher physical stability. Int. J. Pharm. 2011, 409, 96–103. [Google Scholar] [CrossRef]
- Hong, S.J.; Garcia, C.V.; Park, S.J.; Shin, G.H.; Kim, J.T. Retardation of curcumin degradation under various storage conditions via turmeric extract-loaded nanoemulsion system. LWT 2019, 100, 175–182. [Google Scholar] [CrossRef]
- Verdugo, M.; Ruiz Encinar, J.; Costa-Fernández, J.M.; Menendez-Miranda, M.; Bouzas-Ramos, D.; Bravo, M.; Quiroz, W. Study of conformational changes and protein aggregation of bovine serum albumin in presence of Sb(III) and Sb(V). PLoS ONE 2017, 12, e0170869. [Google Scholar] [CrossRef]
- Shil, S.; Das, N.; Sengupta, B.; Sen, P. Sucrose-Induced Stabilization of Domain-II and Overall Human Serum Albumin against Chemical and Thermal Denaturation. ACS Omega 2018, 3, 16633–16642. [Google Scholar] [CrossRef]
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of curcumin by complexation with divalent cations in glycerol/water system. Bioinorg Chem Appl 2010, 2010, 292760. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Bartelt-Hunt, S.; Rodenhausen, K.B.; Schubert, M.; Bartz, J.C. Investigation of Bovine Serum Albumin (BSA) Attachment onto Self-Assembled Monolayers (SAMs) Using Combinatorial Quartz Crystal Microbalance with Dissipation (QCM-D) and Spectroscopic Ellipsometry (SE). PLoS ONE 2015, 10, e0141282. [Google Scholar] [CrossRef] [PubMed]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul. Med. 2014, 87, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Teo, J.; Chew, J.W.; Hadinoto, K. Dry powder inhaler formulation of high-payload antibiotic nanoparticle complex intended for bronchiectasis therapy: Spray drying versus spray freeze drying preparation. Int. J. Pharm. 2016, 499, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Ingole, P.G. Development of polymer-based new high performance thin-film nanocomposite nanofiltration membranes by vapor phase interfacial polymerization for the removal of heavy metal ions. Chem. Eng. J. 2022, 446, 137303. [Google Scholar] [CrossRef]
- Borpatra Gohain, M.; Karki, S.; Yadav, D.; Yadav, A.; Thakare, N.R.; Hazarika, S.; Lee, H.K.; Ingole, P.G. Development of Antifouling Thin-Film Composite/Nanocomposite Membranes for Removal of Phosphate and Malachite Green Dye. Membranes 2022, 12, 768. [Google Scholar] [CrossRef]
- Alazzawi, H.F.; Salih, I.K.; Albayati, T.M. Drug delivery of amoxicillin molecule as a suggested treatment for covid-19 implementing functionalized mesoporous SBA-15 with aminopropyl groups. Drug Deliv. 2021, 28, 856–864. [Google Scholar] [CrossRef]
- Lim, L.M.; Tran, T.T.; Wong, J.J.L.; Wang, D.P.; Cheow, W.S.; Hadinoto, K. Amorphous ternary nanoparticle complex of curcumin-chitosan-hypromellose exhibiting built-in solubility enhancement and physical stability of curcumin. Colloids Surf. B-Biointerfaces 2018, 167, 483–491. [Google Scholar] [CrossRef]
- Boiero, C.; Allemandi, D.; Longhi, M.; Llabot, J.M. RP-HPLC method development for the simultaneous determination of timolol maleate and human serum albumin in albumin nanoparticles. J. Pharm. Biomed. Anal. 2015, 111, 186–189. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Cata Saady, N.M. Desulfurization of actual diesel fuel onto modified mesoporous material Co/MCM-41. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100635. [Google Scholar] [CrossRef]
- Ali, N.S.; Alismaeel, Z.T.; Majdi, H.S.; Salih, H.G.; Abdulrahman, M.A.; Cata Saady, N.M.; Albayati, T.M. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 2022, 8, e09737. [Google Scholar] [CrossRef]
- Kadhum, S.T.; Alkindi, G.Y.; Albayati, T.M. Remediation of phenolic wastewater implementing nano zerovalent iron as a granular third electrode in an electrochemical reactor. Int. J. Environ. Sci. Technol. 2022, 19, 1383–1392. [Google Scholar] [CrossRef]
- Liu, J.; Jing, H. Glycation of bovine serum albumin with monosaccharides inhibits heat-induced protein aggregation. RSC Adv. 2016, 6, 115183–115188. [Google Scholar] [CrossRef]




| % Sucrose (w/w) | Reconstituted NP size | PDI | Zeta potential (mV) | |
|---|---|---|---|---|
| 0 | 1135 ± 61 | 0.55 ± 0.08 | −27.9 ± 1.5 | 10.40 ± 0.61 |
| 33.33 | 100 ± 17 | 0.26 ± 0.03 | −24.8 ± 1.1 | 0.92 ± 0.11 |
| 50.00 | 103 ± 7 | 0.34 ± 0.02 | −24.6 ± 0.9 | 0.94 ± 0.06 |
| 66.67 | 99 ± 7 | 0.35 ± 0.02 | −25.1 ± 1.1 | 0.91 ± 0.06 |
| % Sucrose (w/w) | Reconstituted NP Size | PDI | Zeta Potential (mV) | |
|---|---|---|---|---|
| 0 | 1523 ± 99 | 0.70 ± 0.08 | −25.5 ± 0.9 | 12.52 ± 1.02 |
| 33.33 | 124 ± 34 | 0.70 ± 0.07 | −22.9 ± 1.8 | 1.13 ± 0.22 |
| 50.00 | 98 ± 37 | 0.61 ± 0.18 | −21.4 ± 0.9 | 0.90 ± 0.16 |
| 66.67 | 119 ± 36 | 0.68 ± 0.03 | 25.6 ± 1.2 | 1.09 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, A.; Tran, T.-T.; Pu, S.; Park, J.-W.; Hadinoto, K. Lyophilization of Curcumin–Albumin Nanoplex with Sucrose as Cryoprotectant: Aqueous Reconstitution, Dissolution, Kinetic Solubility, and Physicochemical Stability. Int. J. Mol. Sci. 2022, 23, 11731. https://doi.org/10.3390/ijms231911731
Chua A, Tran T-T, Pu S, Park J-W, Hadinoto K. Lyophilization of Curcumin–Albumin Nanoplex with Sucrose as Cryoprotectant: Aqueous Reconstitution, Dissolution, Kinetic Solubility, and Physicochemical Stability. International Journal of Molecular Sciences. 2022; 23(19):11731. https://doi.org/10.3390/ijms231911731
Chicago/Turabian StyleChua, Angeline, The-Thien Tran, Siyu Pu, Jin-Won Park, and Kunn Hadinoto. 2022. "Lyophilization of Curcumin–Albumin Nanoplex with Sucrose as Cryoprotectant: Aqueous Reconstitution, Dissolution, Kinetic Solubility, and Physicochemical Stability" International Journal of Molecular Sciences 23, no. 19: 11731. https://doi.org/10.3390/ijms231911731
APA StyleChua, A., Tran, T.-T., Pu, S., Park, J.-W., & Hadinoto, K. (2022). Lyophilization of Curcumin–Albumin Nanoplex with Sucrose as Cryoprotectant: Aqueous Reconstitution, Dissolution, Kinetic Solubility, and Physicochemical Stability. International Journal of Molecular Sciences, 23(19), 11731. https://doi.org/10.3390/ijms231911731







