2.1. Experimental Solubility ()
Table 1 records the experimental solubility data of INH (substance 3) in (PEG 200 + W) cosolvent mixtures expressed in molar fraction (
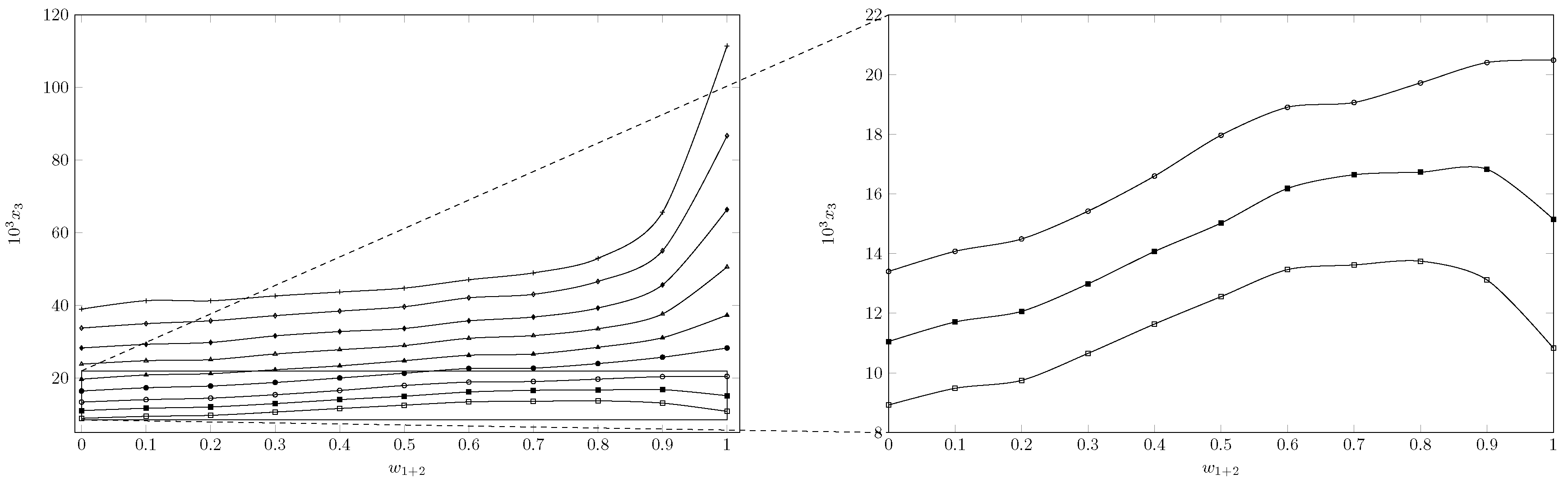
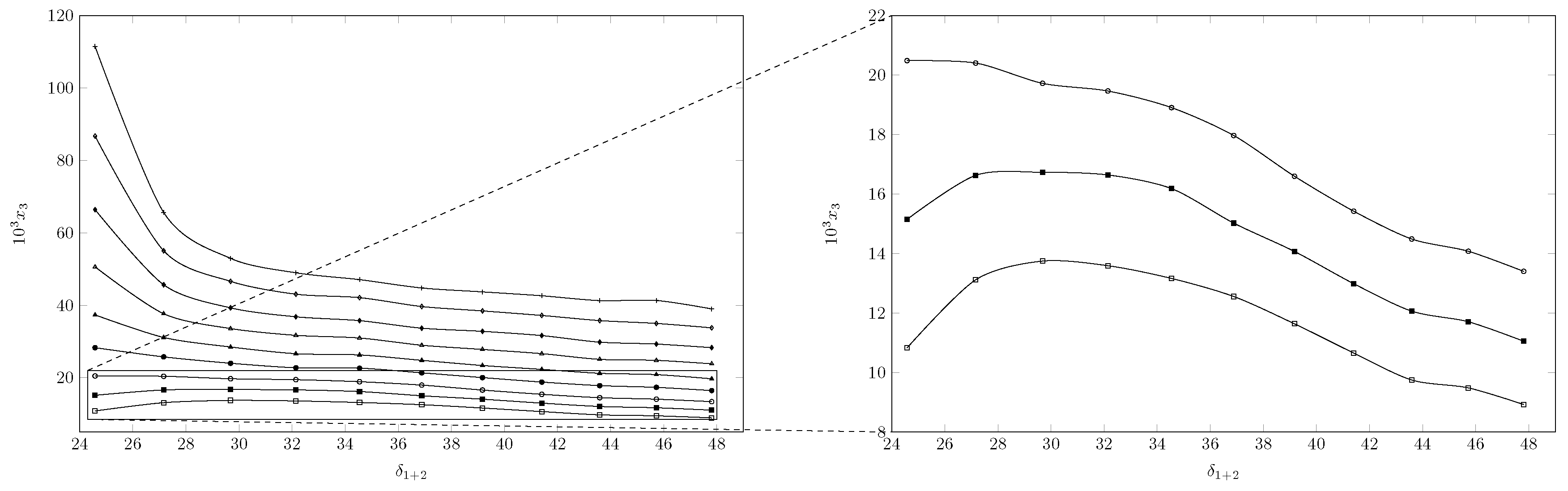
). The solubility of INH increases with increasing temperature in all cases, reaching the lowest solubility in pure water at 278.15 K and the highest solubility in pure PEG 200 at 318.15 K. As seen in
Figure 2 and
Figure 3, the effect of temperature is less in mixtures rich in water and intermediate or greater in mixtures rich in PEG 200.
When analyzing the cosolvent effect, it is observed that at 278.15 K and 283.15 K the maximum solubility is reached in the cosolvent mixture with
and
, respectively; as the temperature increases, the maximum solubility is reached in pure PEG 200 (
Figure 2 and
Figure 3). In general terms, between 278.15 K and 288.15 K the solubility of isoniazid increases approximately 1.5 times, changing the cosolvent composition where the maximum solubility is reached; from 288.15 K to 318.15 K, the cosolvent effect of PEG 200 is positive in all cases and increases linearly, achieving its greatest effect at 318.15 K where the solubility of isoniazid increases 2.9 times due to the addition of PEG 200.
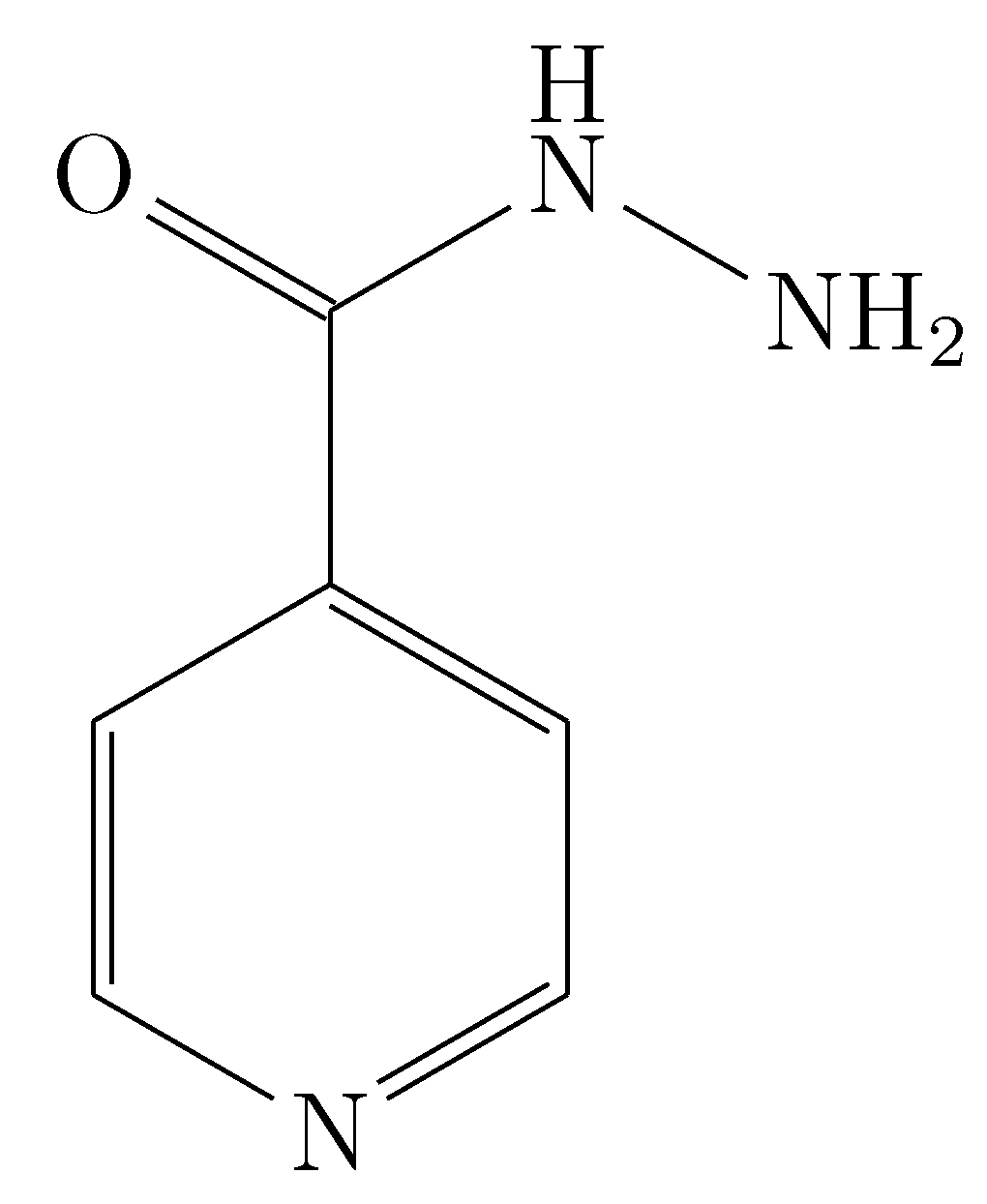
Bhat et al. reported the solubility of an INH analog, N-(4-chlorophenyl)-2-(pyridin-4-ylcarbonyl) hydrazinecarbothioamide (
Figure 4) [
16,
17]. When comparing the aqueous solubility of INH and the INH analog, it was observed that the INH analog had a solubility
times lower than INH; this may be due to solid state properties such as the enthalpy of fusion. Delgado et al. studied the solubility of three structurally related sulfonamides (sulfadiazine (SD), sulfamethazine (SMT), and sulfamerazine (SMT)) in three cosolvent systems (methanol + water; ethanol + water, and 1-propanol + water) [
22,
23,
24,
25,
26,
27]. When comparing the solubility of the three sulfonamides in a solvent, it was possible to establish that the solubility was related to the enthalpy of fusion, thus, SD (
kJ/mol [
23]) was less soluble than SMR (
kJ/mol [
25]) and it in turn was less soluble than SMT (
) kJ/mol [
26]). On the other hand, when comparing the solubility of INH in PEG 200 and that of the INH analog in PEG 400, the solubility of INH was three times lower than that of the INH analog; in this case there are two possible causes, the first one was described above (properties of the solid state) and the second one is related to the properties of the solvent. In the study of Delgado et al., it could be established that the solubility was related to the solubility parameter of the compounds; the greater the similarity between the solubility parameter of the solute and the solvent, the more favored the solubility of the drug; therefore, when comparing the solubility of the INH analog in PEG 400 and PG, it can be established that the INH analog, possibly presents a solubility parameter closer to PEG 400 (23.10 MPa
[
28]) than to PG (30.7 MPa
[
29]), since the solubility of the INH analog is higher in PEG 400.
Figure 3 shows the effect of the polarity of the cosolvent medium on the solubility of the drug; when calculating the solubility parameter of isoniazid using Fedor’s method [
29] (
Table 2), a solubility parameter of 30.54 MPa
was obtained, which was similar to the solubility parameter of the cosolvent mixture
= 0.8, whose solubility parameter was 29.7 MPa
and was where the maximum solubility was reached at 278.15 K. However, when increasing the temperature to 283.15 K, the point of maximum solubility moved to
= 0.9, whose calculated solubility parameter was 27.16 MPa
. From 283.15 K, the maximum solubility was reached in PEG 200 whose solubility parameter was 24.58 MPa
[
30,
31]. This could be due to the influence of temperature on Keesom interactions, which in turn would decrease the polarity of the system [
32,
33], especially in mixtures rich in PEG 200, which, due to its molecular size and molecular structure compared to water, would be more affected, which in turn would agree with the increase in the positive cosolvent effect of PEG 200 as the temperature increases.
In order to verify possible polymorphic changes that influence the solubility of isoniazid, three differential scanning calorimetry tests were performed on three samples of the drug in equilibrium with the solvent and contrasted with the DSC (differential scanning calorimetry) of the original sample.
Figure 5 shows the DSC spectra of the three INH samples in equilibrium with the solvents and the DSC of the original INH sample. It can be verified that the solid phase is the same in all cases, indicating that no polymorphic transition occurs due to the solvent.
Table 3 presents the results of the DSC analysis, that is, the melting, enthalpy data, and the melting temperature data of the four analyzed samples, which agree with the results reported by Forte et al. [
34], Duarte et al. [
35], and Gong et al. [
12].
Gong et al. [
12] reported the solubility data of INH in pure water at eight temperatures (283.15–323.15 K). When comparing their results with those obtained in this work (
Figure 6), a good correlation was observed with most of the data, presenting percentage differences of less than 1.0% at 288.15, 298.15, and 303.15 K; at 283.15, 308.15, and 313.15 K, the percentage difference was less than 5% and at 318.15 K, where the greatest difference between the data occurred, the difference was 8.1%.
2.2. Ideal Solubility and Activity Coefficients
The activity coefficient () allows the evaluation of the possible molecular interactions that may occur in the dissolution process of INH in the cosolvent system.
For the calculation of
, the ideal solubility must be calculated, which only depends on the physicochemical properties of INH, that is, it only involves the energy required to break the crystalline structure of the solute to melt and subsequently dissolve [
36]. Thus, the ideal solubility of INH was calculated according to the Equation (
1)
where
T and
are in K,
is the enthalpy of fusion (in kJ mol
) of the solute,
R is the gas constant (in kJ mol
K
), and
is the differential heat capacity of fusion (in kJ K
mol
) [
36]. Some researchers such as Hildebrand and Scott [
37], Neau and Flynn [
38], Neau et al. [
39], and Opperhuizen et al. [
40], assume
as the entropy of fusion (
), which is calculated as
.
Once
was calculated at the different study temperatures,
was calculated from the data of
(
Table 1) as:
Using Equation (
3), the results obtained with Equation (
2), can be analyzed, in terms of molecular solute–solute (
), solute–solvent (
), and solvent–solvent (
) interactions.
In this context,
Table 4 shows the activity coefficients of INH in (PEG 200 + W) cosolvent mixtures at different temperatures.
It is observed that as the temperature increases, the activity coefficient decreases, indicating a possible increase in molecular interactions , which in general terms favors the solution process. Regarding the cosolvent effect on , except for the results of at 278.15 K and 283.15 K where the values of closest to ideality are found in a cosolvent mixture, the addition of PEG 200 to the system also favors the interactions. In general terms, the solubility of INH in cosolvent mixtures (PEG 200 + W) presents a quasi-ideal behavior, since the values of are very close to 1.0.
2.3. Thermodynamic Functions of Solution
The thermodynamic solution functions (
Table 5) were calculated from experimental solubility data (
Table 1), following the Gibbs–van ’t Hoff approach, reformulated by Krug according to the following expressions [
41,
42]:
Here, , , and are the thermodynamic functions (in kJ mol) of the enthalpy, Gibbs energy, and entropy of the solution. is the harmonic temperature (in K), R is the gas constant (kJ molK), and y are the contributions of enthalpy and entropy to the Gibbs energy.
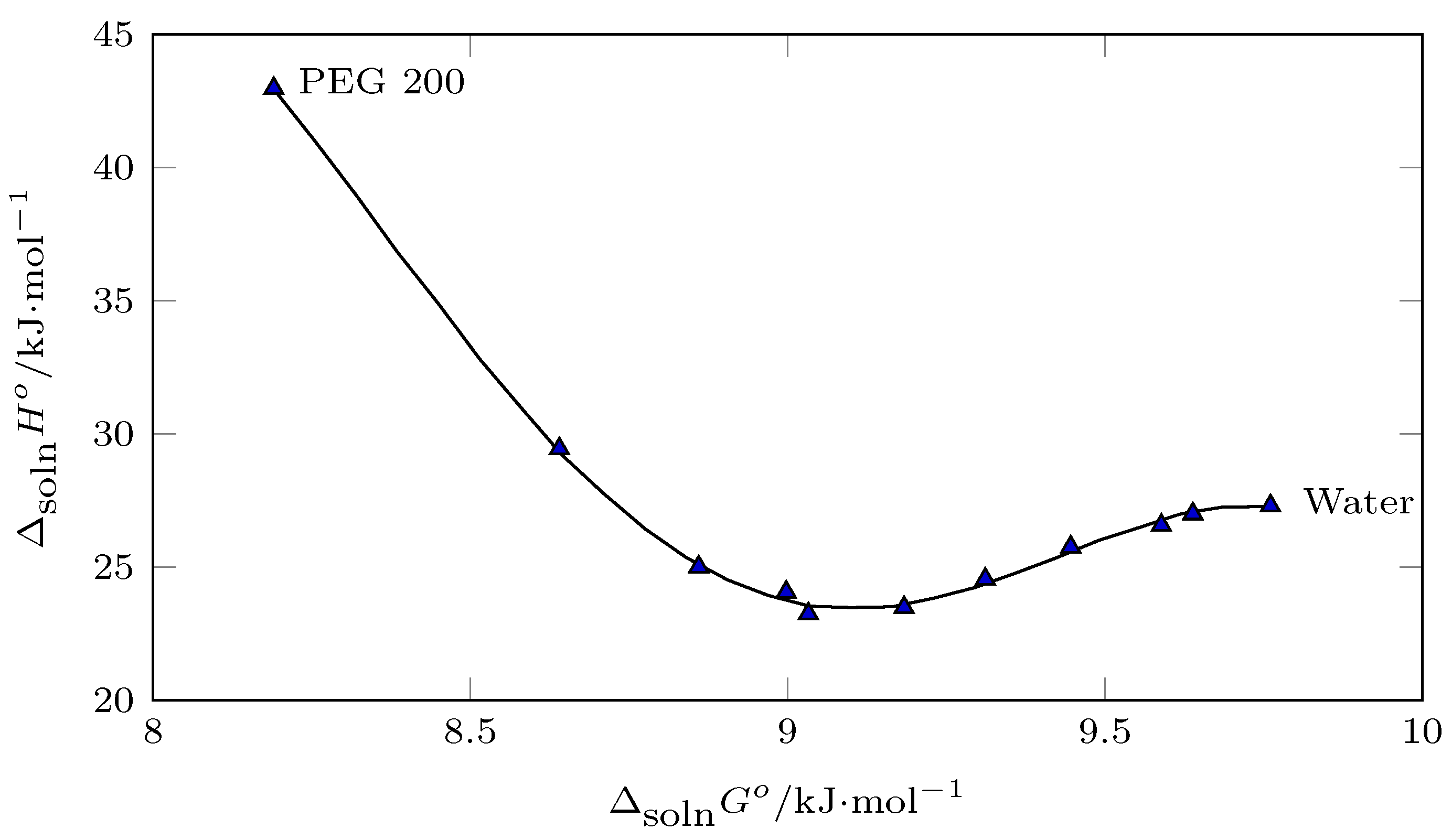
From
Figure 7, which shows a linear relationship between the
and
with
,
and
can be determined according to Equations (
4) and (
5).
In all cases,
is positive and decreases with the increase in the concentration of PEG 200 in the cosolvent mixture, as a consequence of the increase in the solubility of INH;
is also positive, which indicates that the solution process of INH in the (PEG 200 + W) cosolvent system is endothermic. The enthalpy values of solution decrease from pure water to the cosolvent mixture
= 0.6, which implies a flourishing of solute–solvent molecular interactions (
), increasing the solubility of INH. From
= 0.6 to
= 1.0, an increase in enthalpy occurs, possibly due to the formation of self-aggregations of PEG 200 in PEG 200-rich mixtures [
33]; as for
, it is positive, which favors the solution process. Like enthalpy, solution entropy decreases from pure water to
= 0.6 and increases from this PEG 200 concentration to pure PEG 200.
From the results of Equations (
7) and (
8), it is concluded that the energy component (
) predominates over the organizational component (
), contributing in all cases in more than 55%.
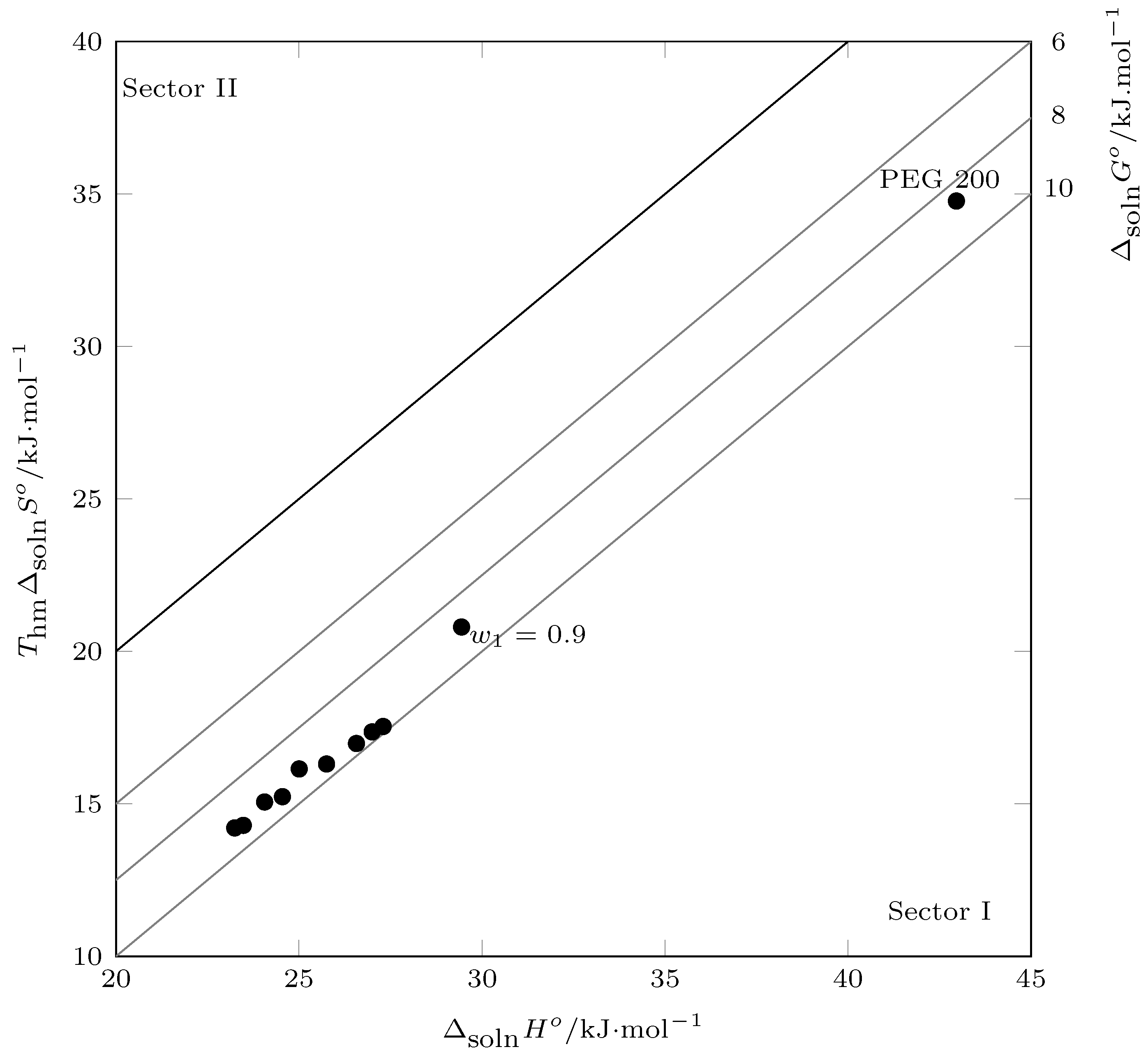
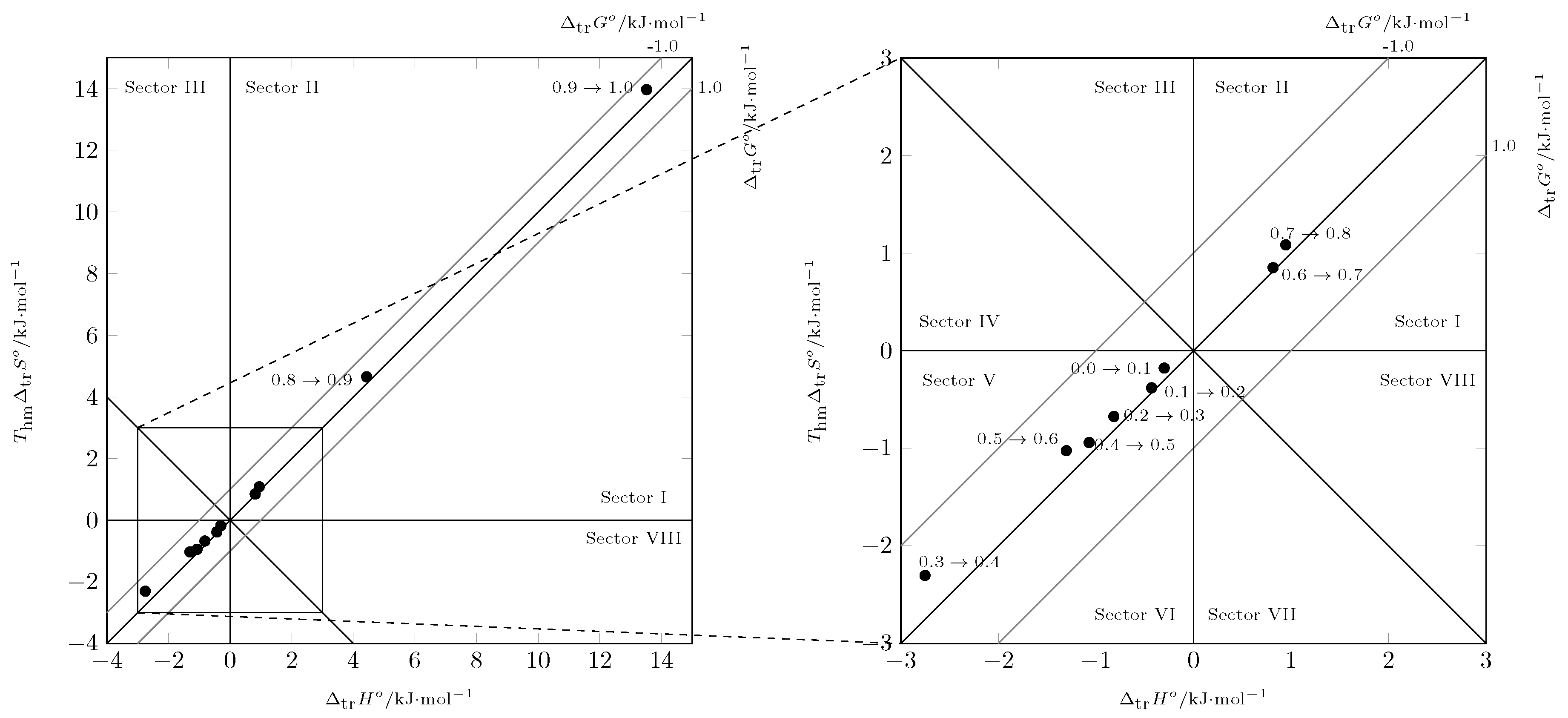
According to the Perlovich graph (
Figure 8), all data were recorded in sector I (
), indicating that the solution process was enthalpy-driven, as well as entropy-favored [
43,
44].
2.4. Thermodynamic Functions of Transfer
In
Table 6, the thermodynamic transfer functions calculated according to Equation (
9) are reported. Where
f represents the Gibbs energy, enthalpy or transfer entropy.
In this case, the transfer process is a hypothetical process between miscible solvents, which allows the evaluation of the influence of the cosolvent PEG 200 in the dissolution process of INH. Each thermodynamic function was calculated by subtracting the thermodynamic quantity of the medium of higher polarity from that of lower polarity.
Considering the addition of PEG 200 to water, which in principle decreases the polarity of the cosolvent system, the following occurred: from
(
MPa
[
29]) to
(
MPa
),
was negative, indicating that the hypothetical transfer process from pure water to any cosolvent mixture ≤
occurred, further indicating that PEG 200 favored the solution process, which was favored by
(negative) and disadvantaged by
(negative); from
to
(
MPa
),
was negative, so the transfer process was also energetically viable; however, in this composition range the process was favored by
(positive) and disadvantaged by
(positive).
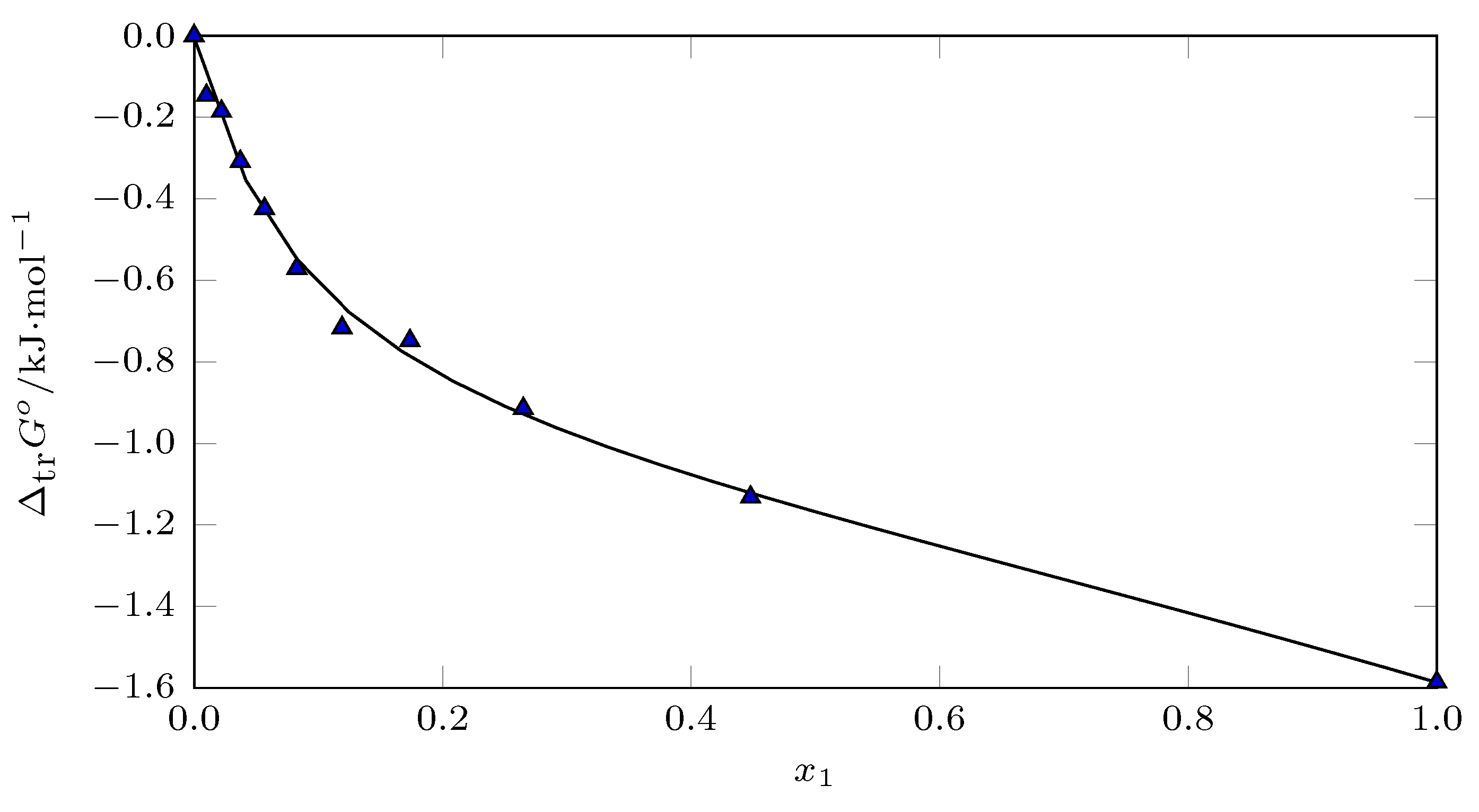
Analyzing the process using Perlovich’s graphic method [
43,
44] (
Figure 9) from
(
MPa
[
29]) to
(
MPa
), the data were recorded in sector V (
and
) and from
(
MPa
) to pure PEG 200, the data were recorded in sector I (
), indicating that in all cases the transfer process was driven by the transfer enthalpy.
2.5. Thermodynamic Functions of Mixing
The solution process involves the change of state of the solute (Solute
→ Solute
→ Solute
→ Solute
), the molecular rearrangement of solvent to form a cavity that allows the solute to lodge, and finally the process of mixture, which consists of the molecular interaction between the solute and the solvent to form the solution (Solute
→ Solute
) [
45].
The solution process can be described by Equation (
10)
Clearing
in (
10), we get:
Therefore, using Equation (
11), the thermodynamic mixing functions were calculated.
Table 7 shows the thermodynamic mixing functions of INH. The Gibbs energy of mixing is positive in all cases and decreases from pure water to pure PEG 200, although the mixing process is unfavorable to the solution process, and the addition of PEG 200 to the cosolvent system promotes solute–solvent interactions. As for the enthalpy of mixture, it is also positive in all cases, and it decreases from pure water to
and increases from this cosolvent mixture to PEG 200, where it reaches its highest value. The decrease in the enthalpy of the mixture in intermediate mixtures may be due to the fact that the PEG 200 – W interactions are less energetic than the W–W, and PEG 200–PEG 200 interactions; therefore, the formation of the cavity to house the solute is energetically more viable in intermediate mixtures. Finally, the mixing entropy is positive in all cases, indicating a favoring of the mixing process and, therefore, of the solution process.
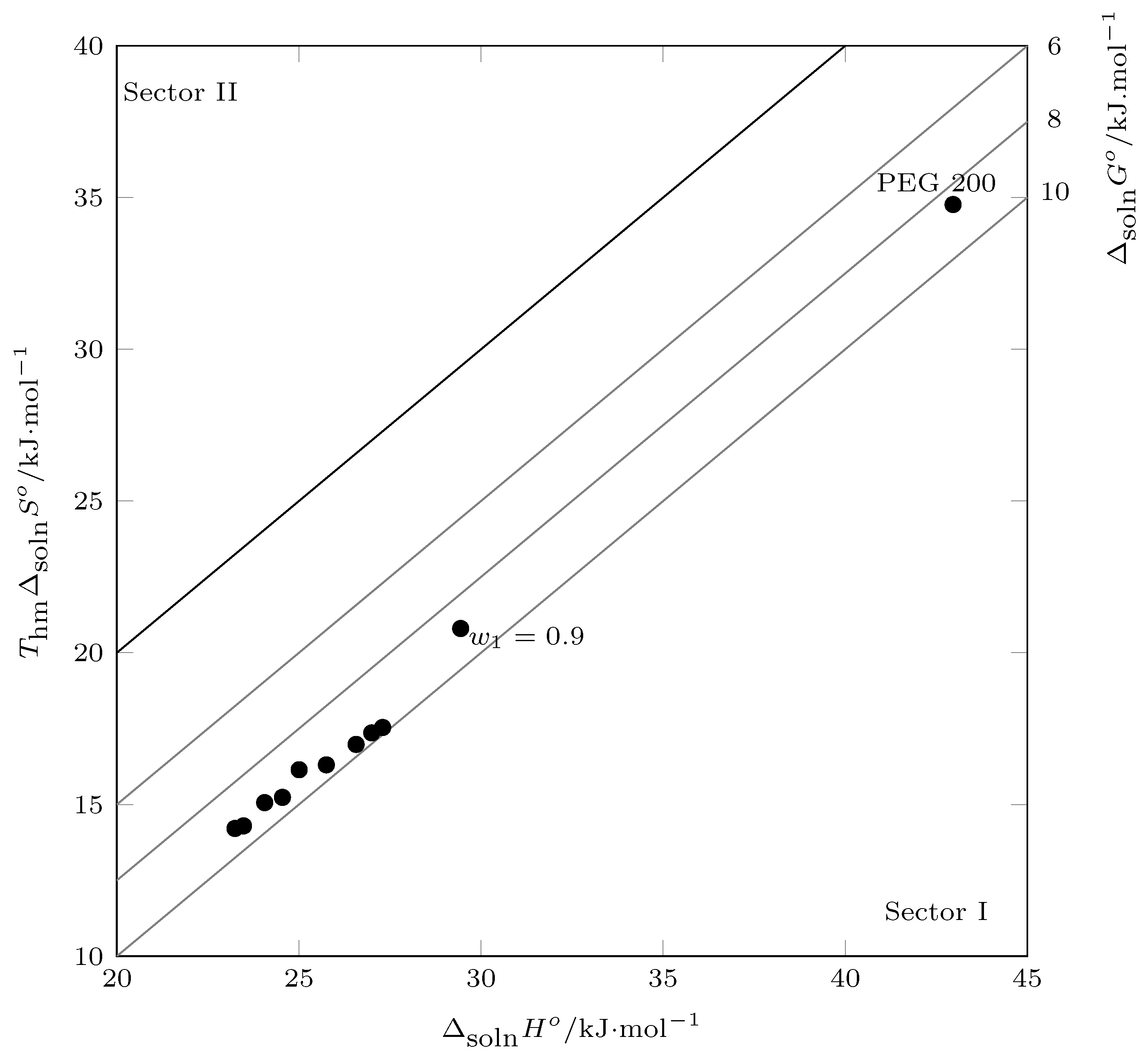
When performing Perlovich’s graphical analysis [
43,
44] (
Figure 10), the values of the thermodynamic mixing functions were recorded in sector I (
), indicating that the mixing process was driven by the enthalpy of mixing.
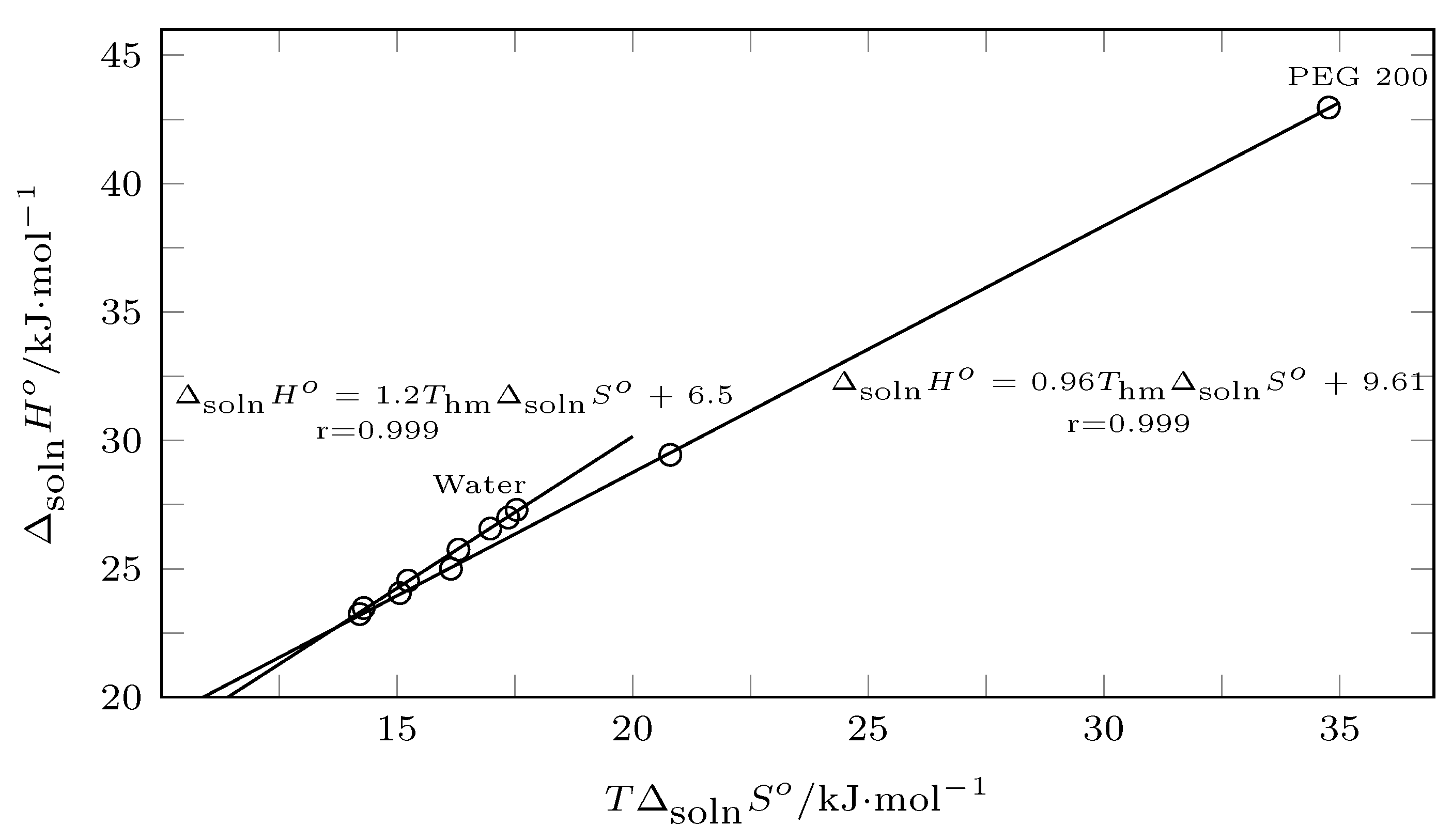
2.6. Enthalpy–Entropy Compensation Analysis
The enthalpy–entropy compensation analysis can be performed by plotting
vs.
, where positive slopes indicate enthalpy conduction and negative slopes indicate entropic conduction [
46,
47], or plotting
vs.
, where slopes greater than 1.0 indicate enthalpy conduction and slopes less than 1.0 indicate entropic conduction [
48].
In this context, from pure water to
, the process was driven by enthalpy according to
Figure 11 (positive slope), which was corroborated by
Figure 12 (slope > 1.0), and from
to PEG 200, the solution process was driven by entropy (
Figure 11, negative slope;
Figure 12 slope < 1.0).
Thus, in mixtures rich in water, the addition of PEG 200 promoted the formation of solute–solvent bonds, so the energy component () promoted an increase in the solubility of INH in terms of mixtures rich in PEG 200. Although there was an increase in , the organizational factor () was the one that governed the solution process, that is, by increasing the concentration of PEG 200, both the water and the PEG 200 tended to break down and interact with INH.
2.7. Preferential Solvation
A preferential solvation analysis allows the assessment of the possible interactions that occur at the molecular level between the solute molecules and the solvents that make up the cosolvent mixture. This analysis can be done by using the inverse Kirkwood–Buff integral (IKBI) method proposed by Ben-Naim, from which the preferential solvation parameters
and
are calculated, which indicate the tendency of the solute (3) to be solvated by solvents 1 or 2 [
49].
Ben-Naim developed the IKBI model, presenting the following equations [
50,
51]:
and
are the Kirkwood–Buff integrals (in cm
/mol),
is the correlation volume around solute (3) within which preferential solvation takes place (in cm
/mol),
is the isothermal compressibility of the mixtures (in
),
,
, and
are the partial molar volumes of the solute and solvents (in cm
/mol), and
D,
Q, and
(in kJ/mol) are expressed in Equations (
17) and (
18). The
of the cosolvent mixture was calculated as:
and the partial molar volumes were calculated from the density data reported by Yasmin et al. [
52]. The correlation volume was calculated by iteration due to dependency on the local mole fractions (
and
) given by Equations (
12) and (
16).
Table 8 shows the values of the different variables necessary for the calculation of the preferential solvation parameters. The transfer Gibbs energy of INH from the water to each PEG 200 + W cosolvent mixture was calculated from the INH solubility data according to:
From the
data (
Figure 13), the
D values were calculated. On the other hand, the
Q values were calculated from the
data reported by Ninni et al. [
53].
For the calculation of the Kirkwood–Buff integrals, the values of
PEG 200 0.42
[
52] and water 0.457
[
54] were taken,
was taken as 108.69 cm
/mol [
55], and
and
were calculated from the density data reported by Muñoz et al. [
56] according to Equations (
20) and (
22).
The value of
(0.351 nm), necessary for the calculation of
, was calculated from
(Equation (
22)).
Here, is the Avogadro number.
According to the results of the Kirkwood–Buff integrals, where negative values were obtained in all cases, it can be inferred that INH had affinity for the two solvents (PEG and W) [
57].
In relation to the values of
(
Table 8,
Figure 14), from pure water up to
, the INH tended to be solvated by water; from
up to pure PEG 200, the INH tended to be solvated by PEG 200; however, in all cases, the values of
were less than 0.01, so the results could be a consequence of the propagation of uncertainties in the calculations of the inverse integrals of Kirkwood–Buff rather than a real process of solvation by one of the solvents [
49,
58].
This may have occurred because the increase in solubility as a consequence of increasing the concentration of PEG 200 in the cosolvent mixture was relatively low, which could also be verified by reviewing the values of the thermodynamic functions of transfer, which showed that the addition of PEG 200 did not lead to a significant increase in the solubility of INH.







_Putnam.png)







