Nanoparticle Emulsions Enhance the Inhibition of NLRP3
Abstract
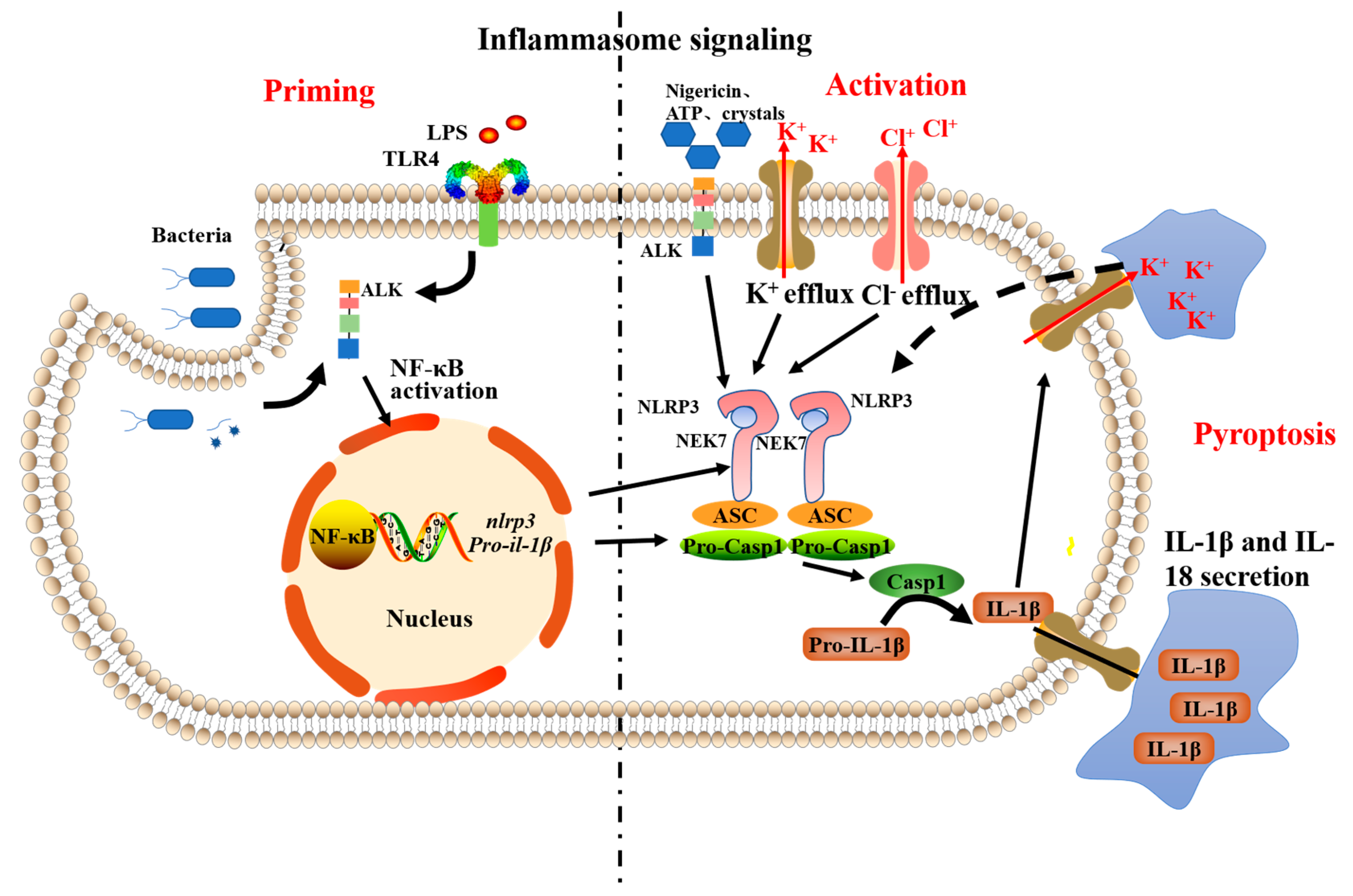
:1. Introduction
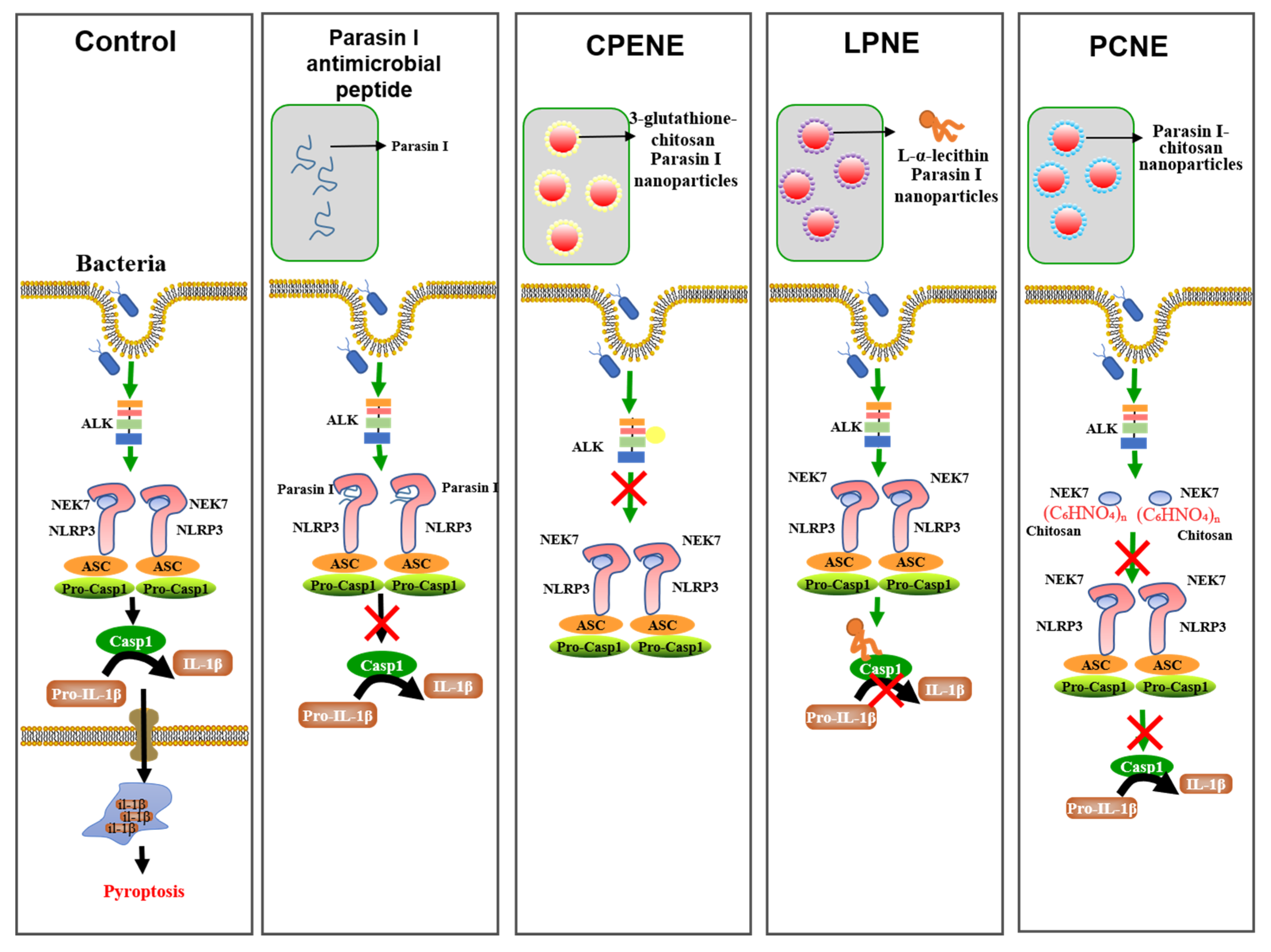
2. Results and Discussions
2.1. Molecular Docking
2.2. The NLRP3-Inflammasome Inhibition in Macrophages
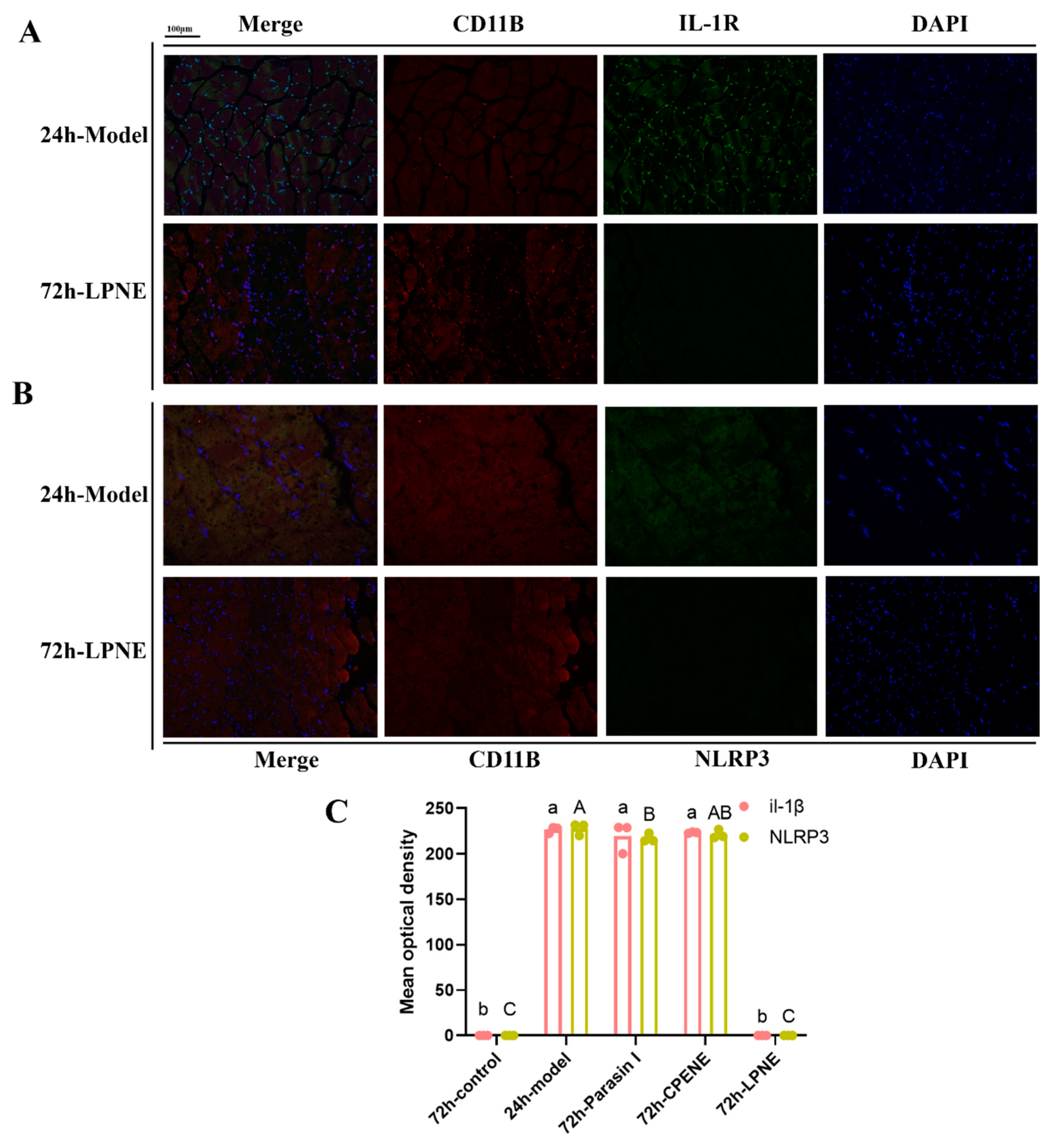
2.3. Immunofluorescence Analysis in Visceral Peritoneum
3. Material and Methods
3.1. Reagents
3.2. Pickering Emulsion Preparation and Quantification
3.3. Molecular Docking
3.4. Inflammasome Suppression in Macrophages
3.4.1. Inflammatory Cell Induction
3.4.2. Chloride Ion Concentration Detection
3.4.3. Potassium Ion Concentration Detection
3.4.4. Enzyme-Linked Immunosorbent Assay (Elisa)
3.4.5. Therapy of Mice Peritonitis Model
3.4.6. Tissue Staining and Immunohistochemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Pedron, C.N.; Higashikuni, Y.; Kramer, R.M.; Cardoso, M.H.; Oshiro, K.G.N.; Franco, O.L.; Silva Junior, P.I.; Silva, F.D.; Oliveira Junior, V.X.; et al. Structure-function-guided exploration of the antimicrobial peptide polybia-CP identifies activity determinants and generates synthetic therapeutic candidates. Commun. Biol. 2018, 1, 221. [Google Scholar] [CrossRef] [PubMed]
- Faya, M.; Kalhapure, R.S.; Kumalo, H.M.; Waddad, A.Y.; Omolo, C.; Govender, T. Conjugates and nano-delivery of antimicrobial peptides for enhancing therapeutic activity. J. Drug Deliv. Sci. Technol. 2017, 44, 153–171. [Google Scholar] [CrossRef]
- Huang, J.X.; Bishop-Hurley, S.L.; Cooper, M.A. Development of anti-infectives using phage display: Biological agents against bacteria, viruses, and parasites. Antimicrob. Agents Chemother. 2012, 56, 4569–4582. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Lee, H.E.; Lee, J.Y. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci. Rep. 2016, 6, 24399. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Okamoto, N.; Watanabe, Y.; Tsuneyama, K.; Hayashi, H.; Fujii, I.; Ikutani, M.; Hirai, Y. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-nduced adipose tissue inflammation. J. Leukoc. Biol. 2014, 96, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Robertson, A.A.B.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.B.; Cooper, M.A.; O’Neill, L.A.J.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav. Immunol. 2017, 61, 306–316. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Daniels, M.J.; Rivers-Auty, J.; Schilling, T.; Spencer, N.G.; Watremez, W.; Fasolino, V.; Booth, S.J.; White, C.S.; Baldwin, A.G.; Freeman, S.; et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 2016, 7, 12504. [Google Scholar] [CrossRef]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q.; et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017, 214, 3219–3238. [Google Scholar] [CrossRef]
- Pan, M.H.; Chiou, Y.S.; Tsai, M.L.; Ho, C.T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J. Tradit. Complement. Med. 2011, 1, 8–24. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, X.; Xu, L.; Wang, Y. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef]
- Hong, W.; Liu, L.; Zhao, Y.; Liu, Y.; Zhang, D.; Liu, M. Pluronic-based nano-self-assemblies of bacitracin A with a new mechanism of action for an efficient in vivo therapeutic effect against bacterial peritonitis. J. Nanobiotechnol. 2018, 16, 66. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Yao, H.; Li, W.; Mu, Q.; Li, H.; Hu, H.; Li, Y.; Huang, H. δ-Amyrone, a specific inhibitor of cyclooxygenase-2, exhibits anti-inflammatory effects in vitro and in vivo of mice. Int. Immunol. 2014, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Cao, M.; Regenstein, J. Slow-Release and Nontoxic Pickering Emulsion Platform for Antimicrobial Peptide. J. Agric. Food Chem. 2020, 68, 7453–7466. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 6, 3328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, W.; Qiu, J. ALK is required for NLRP3 inflammasome activation in macrophages. Biochem. Biophys. Res. Commun. 2018, 501, 246–252. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Wilson, C.H.; Kumar, S. Caspases in metabolic disease and their therapeutic potential. Cell Death Differ. 2018, 25, 1010–1024. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, X.; He, Q. Reconfigurable assembly of colloidal motors towards interactive soft materials and systems. J. Colloid Interface Sci. 2022, 612, 43–56. [Google Scholar] [CrossRef]
- Doman, T.N.; McGovern, S.L.; Witherbee, B.J.; Kasten, T.P.; Kurumbail, R.; Stallings, W.C.; Connolly, D.T.; Shoichet, B.K. Molecular docking and high-throughput screening for novel inhibitors of protein tyrosine phosphatase-1B. J. Med. Chem. 2002, 45, 2213–2221. [Google Scholar] [CrossRef]
- Yang, F.W.; Li, Y.X.; Ren, F.Z.; Luo, J.; Pang, G.F. Assessment of the endocrine-disrupting effects of organophosphorus pesticide triazophos and its metabolites on endocrine hormones biosynthesis, transport and receptor binding in silico. Food Chem. Toxicol. 2019, 133, 110759. [Google Scholar] [CrossRef]
- Yang, F.; Cui, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W.; Xie, Y. Identifying potential thyroid hormone disrupting effects among diphenyl ether structure pesticides and their metabolites in silico. Chemosphere 2022, 288 Pt 2, 132575. [Google Scholar] [CrossRef]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Lord, M.S.; Melrose, J.; Whitelock, J.M. The Role of Heparan Sulfate in Inflammation, and the Development of Biomimetics as Anti-Inflammatory Strategies. J. Histochem. Cytochem. 2018, 66, 321–336. [Google Scholar] [CrossRef]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Ranatunga, R.; Nguyen, C.T.; Wilson, B.A.; Shinoda, W.; Nielsen, S.O. Molecular dynamics study of nanoparticles and non-ionic surfactant at an oil–water interface. Soft Matter 2011, 7, 6942–6952. [Google Scholar] [CrossRef]
- Groß, C.J.; Mishra, R.; Schneider, K.S.; Médard, G.; Wettmarshausen, J.; Dittlein, D.C.; Shi, H.; Gorka, O.; Koenig, P.A.; Fromm, S.; et al. K(+) Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity 2016, 45, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Sanman, L.E.; Qian, Y.; Eisele, N.A.; Ng, T.M.; van der Linden, W.A.; Monack, D.M.; Weerapana, E.; Bogyo, M. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife 2016, 5, e13663. [Google Scholar] [CrossRef] [PubMed]
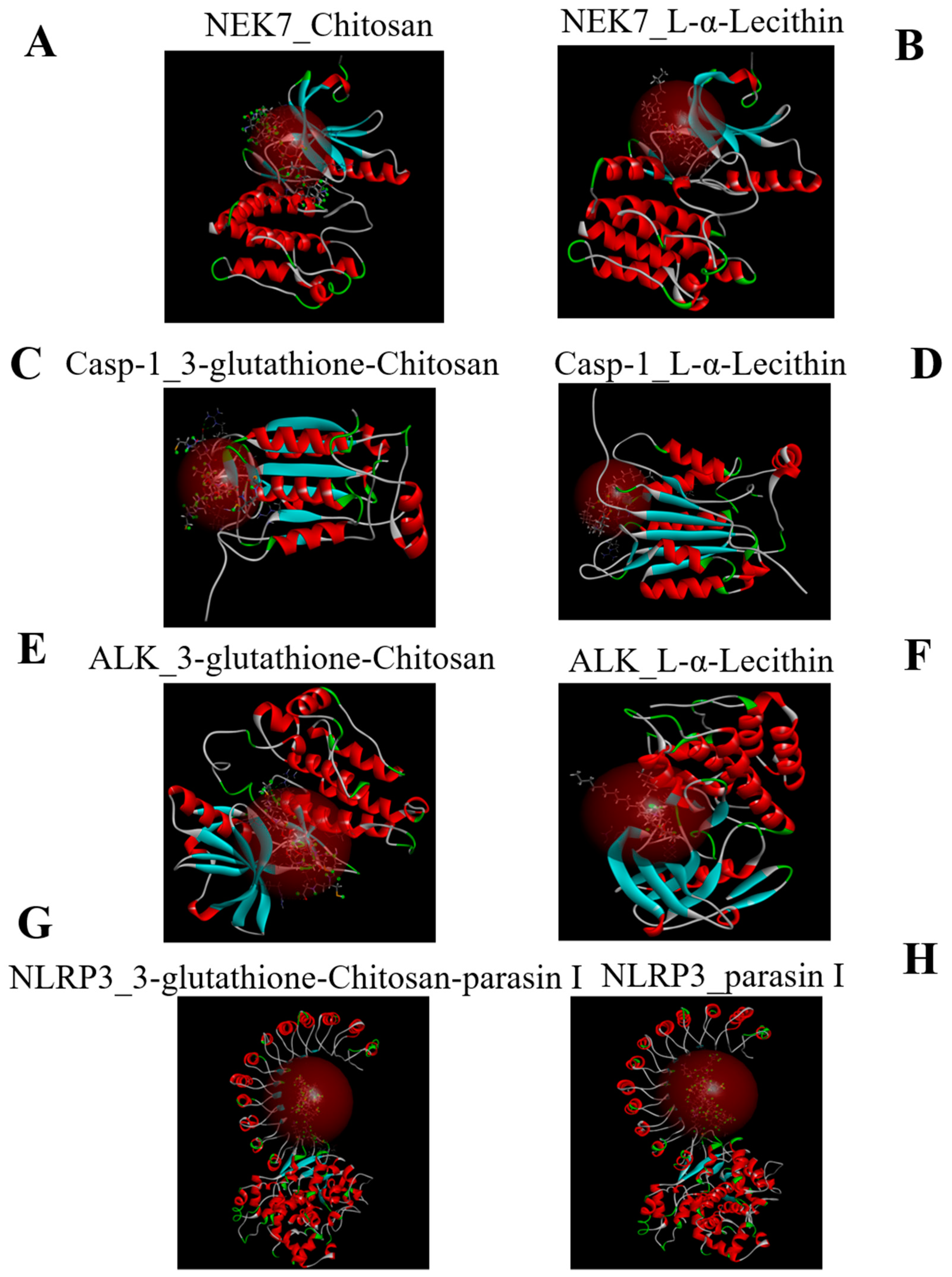




| Name | CDOCKER_INTERACTION_ENERGY (kcal/mol) |
|---|---|
| NEK7-L-α-Lecithin | −79.3303 |
| NEK7-Chitosan | −93.7484 |
| Casp-1-Chitosan-glutathione-3 | −97.0533 |
| Casp-1-L-α-Lecithin | −70.3612 |
| ALK-Chitosan-glutathione-3 | −106.754 |
| ALK-L-α-Lecithin | −79.8664 |
| NLRP3-3-glutathione-chitosan-parasin | −167.167 |
| NLRP3-parasin I | −138.395 |
| Time (h) | 0 | 12 | 24 | 72 |
|---|---|---|---|---|
| Control | 16 | 16 | 16 | 16 |
| Model | 16 | 13 | 11 | 7 |
| CPFX | 16 | 16 | 16 | 16 |
| Parasin I | 16 | 16 | 16 | 15 |
| CPENE | 16 | 16 | 15 | 15 |
| LPNE | 16 | 15 | 14 | 13 |
| PCNE | 16 | 14 | 14 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Cai, L. Nanoparticle Emulsions Enhance the Inhibition of NLRP3. Int. J. Mol. Sci. 2022, 23, 10168. https://doi.org/10.3390/ijms231710168
Cao M, Cai L. Nanoparticle Emulsions Enhance the Inhibition of NLRP3. International Journal of Molecular Sciences. 2022; 23(17):10168. https://doi.org/10.3390/ijms231710168
Chicago/Turabian StyleCao, Minjie, and Luyun Cai. 2022. "Nanoparticle Emulsions Enhance the Inhibition of NLRP3" International Journal of Molecular Sciences 23, no. 17: 10168. https://doi.org/10.3390/ijms231710168
APA StyleCao, M., & Cai, L. (2022). Nanoparticle Emulsions Enhance the Inhibition of NLRP3. International Journal of Molecular Sciences, 23(17), 10168. https://doi.org/10.3390/ijms231710168






