Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma
Abstract
1. Introduction
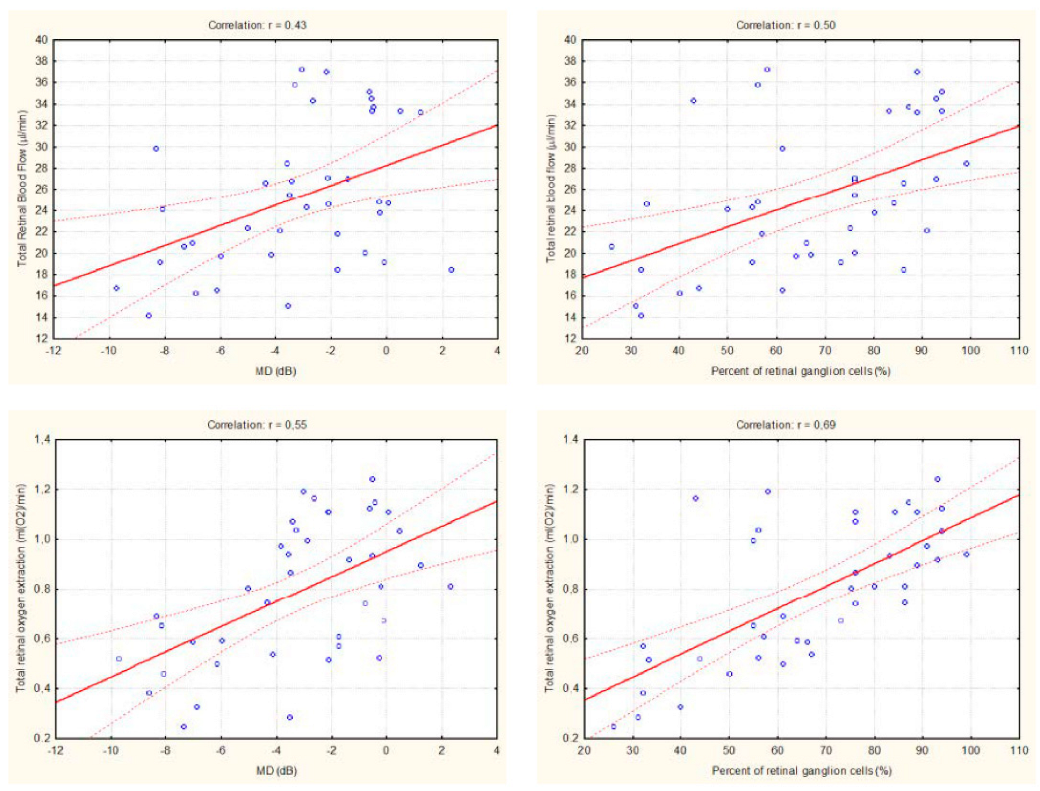
2. Results
3. Discussion
4. Methods and Materials
4.1. Subjects
4.2. Methods
4.2.1. Measurement of Total Retinal Blood Flow
4.2.2. Measurement of Retinal Oxygen Saturation
4.2.3. Calculation of Total Retinal Oxygen Extraction
4.2.4. Measurement of Retinal Nerve Fiber Layer Thickness
4.2.5. Estimation of Retinal Ganglion Cell Number
4.2.6. Measurement of Blood Pressure and Pulse Rate
4.2.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial, G. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Schmidl, D.; Schmetterer, L.; Garhöfer, G.; Popa-Cherecheanu, A. Pharmacotherapy of Glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 63–77. [Google Scholar] [CrossRef]
- Garway-Heath, D.F.; Crabb, D.P.; Bunce, C.; Lascaratos, G.; Amalfitano, F.; Anand, N.; Azuara-Blanco, A.; Bourne, R.R.; Broadway, D.C.; A Cunliffe, I.; et al. Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 385, 1295–1304. [Google Scholar] [CrossRef]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef]
- Cherecheanu, A.P.; Garhofer, G.; Schmidl, D.; Werkmeister, R.; Schmetterer, L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 36–42. [Google Scholar] [CrossRef]
- Schmetterer, L.; Garhofer, G. How can blood flow be measured? Surv. Ophthalmol. 2007, 52 (Suppl. S2), S134–S138. [Google Scholar] [CrossRef]
- Harris, A.; Kagemann, L.; Ehrlich, R.; Rospigliosi, C.; Moore, D.; Siesky, B. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can. J. Ophthalmol. 2008, 43, 328–336. [Google Scholar] [CrossRef]
- Sadda, S.R.; Maram, J.; Srinivas, S. Evaluating ocular blood flow. Indian J. Ophthalmol. 2017, 65, 337–346. [Google Scholar] [CrossRef]
- Wei, X.; Balne, P.K.; Meissner, K.E.; Barathi, V.A.; Schmetterer, L.; Agrawal, R. Assessment of flow dynamics in retinal and choroidal microcirculation. Surv. Ophthalmol. 2018, 63, 646–664. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, A.; Gil-Flamer, J.; Tan, O.; A Izatt, J.; Huang, D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br. J. Ophthalmol. 2009, 93, 634–637. [Google Scholar] [CrossRef]
- Baumann, B.; Potsaid, B.; Kraus, M.F.; Liu, J.J.; Huang, D.; Hornegger, J.; Cable, A.E.; Duker, J.S.; Fujimoto, J.G. Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT. Biomed. Opt. Express 2011, 2, 1539–1552. [Google Scholar] [CrossRef]
- Doblhoff-Dier, V.; Schmetterer, L.; Vilser, W.; Garhöfer, G.; Gröschl, M.; Leitgeb, R.A.; Werkmeister, R.M. Measurement of the total retinal blood flow using dual beam Fourier-domain Doppler optical coherence tomography with orthogonal detection planes. Biomed. Opt. Express 2014, 5, 630–642. [Google Scholar] [CrossRef]
- Haindl, R.; Trasischker, W.; Wartak, A.; Baumann, B.; Pircher, M.; Hitzenberger, C.K. Total retinal blood flow measurement by three beam Doppler optical coherence tomography. Biomed. Opt. Express 2016, 7, 287–301. [Google Scholar] [CrossRef]
- Tani, T.; Song, Y.-S.; Yoshioka, T.; Omae, T.; Ishibazawa, A.; Akiba, M.; Yoshida, A. Repeatability and Reproducibility of Retinal Blood Flow Measurement Using a Doppler Optical Coherence Tomography Flowmeter in Healthy Subjects. Investig. Opthalmol. Vis. Sci. 2017, 58, 2891–2898. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Dragostinoff, N.; Pircher, M.; Götzinger, E.; Hitzenberger, C.K.; Leitgeb, R.A.; Schmetterer, L. Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt. Lett. 2008, 33, 2967–2969. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Dragostinoff, N.; Palkovits, S.; Told, R.; Boltz, A.; Leitgeb, R.A.; Gröschl, M.; Garhöfer, G.; Schmetterer, L. Measurement of Absolute Blood Flow Velocity and Blood Flow in the Human Retina by Dual-Beam Bidirectional Doppler Fourier-Domain Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2012, 53, 6062–6071. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Palkovits, S.; Told, R.; Gröschl, M.; Leitgeb, R.A.; Garhöfer, G.; Schmetterer, L. Response of Retinal Blood Flow to Systemic Hyperoxia as Measured with Dual-Beam Bidirectional Doppler Fourier-Domain Optical Coherence Tomography. PLoS ONE 2012, 7, e45876. [Google Scholar] [CrossRef]
- Told, R.; Wang, L.; Cull, G.; Thompson, S.J.; Burgoyne, C.F.; Aschinger, G.C.; Schmetterer, L.; Werkmeister, R.M. Total Retinal Blood Flow in a Nonhuman Primate Optic Nerve Transection Model Using Dual-Beam Bidirectional Doppler FD-OCT and Microsphere Method. Investig. Opthalmol. Vis. Sci. 2016, 57, 1432. [Google Scholar] [CrossRef]
- Hammer, M.; Vilser, W.; Riemer, T.; Schweitzer, D. Retinal vessel oximetry-calibration, compensation for vessel diameter and fundus pigmentation, and reproducibility. J. Biomed. Opt. 2008, 13, 054015. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Schmidl, D.; Aschinger, G.; Doblhoff-Dier, V.; Palkovits, S.; Wirth, M.; Garhöfer, G.; Linsenmeier, R.A.; Leitgeb, R.; Schmetterer, L. Retinal oxygen extraction in humans. Sci. Rep. 2015, 5, 15763. [Google Scholar] [CrossRef]
- A Aref, A.; Maleki, S.; Tan, O.; Huang, D.; Varma, R.; Shahidi, M. Relating glaucomatous visual field loss to retinal oxygen delivery and metabolism. Acta Ophthalmol. 2019, 97, e968–e972. [Google Scholar] [CrossRef]
- Yoshioka, T.; Song, Y.; Kawai, M.; Tani, T.; Takahashi, K.; Ishiko, S.; Lavinsky, F.; Wollstein, G.; Ishikawa, H.; Schuman, J.S.; et al. Retinal blood flow reduction in normal-tension glaucoma with single-hemifield damage by Doppler optical coherence tomography. Br. J. Ophthalmol. 2020, 105, 124–130. [Google Scholar] [CrossRef]
- Sehi, M.; Goharian, I.; Konduru, R.; Tan, O.; Srinivas, S.; Sadda, S.R.; Francis, B.A.; Huang, D.; Greenfield, D.S. Retinal Blood Flow in Glaucomatous Eyes with Single-Hemifield Damage. Ophthalmology 2013, 121, 750–758. [Google Scholar] [CrossRef]
- Hwang, J.; Konduru, R.; Zhang, X.; Tan, O.; A Francis, B.; Varma, R.; Sehi, M.; Greenfield, D.S.; Sadda, S.R.; Huang, D. Relationship among Visual Field, Blood Flow, and Neural Structure Measurements in Glaucoma. Investig. Opthalmol. Vis. Sci. 2012, 53, 3020–3026. [Google Scholar] [CrossRef]
- Deokule, S.; Vizzeri, G.; Boehm, A.; Bowd, C.; Weinreb, R.N. Association of Visual Field Severity and Parapapillary Retinal Blood Flow in Open-Angle Glaucoma. J. Glaucoma 2010, 19, 293–298. [Google Scholar] [CrossRef]
- Yamada, Y.; Higashide, T.; Udagawa, S.; Takeshima, S.; Sakaguchi, K.; Nitta, K.; Sugiyama, K. The Relationship Between Interocular Asymmetry of Visual Field Defects and Optic Nerve Head Blood Flow in Patients with Glaucoma. J. Glaucoma 2019, 28, 231–237. [Google Scholar] [CrossRef]
- Resch, H.; Schmidl, D.; Hommer, A.; Rensch, F.; Jonas, J.B.; Fuchsjäger-Mayrl, G.; Garhöfer, G.; Vass, C.; Schmetterer, L. Correlation of optic disc morphology and ocular perfusion parameters in patients with primary open angle glaucoma. Acta Ophthalmol. 2011, 89, e544–e549. [Google Scholar] [CrossRef]
- Fan, X.; Ying, Y.; Zhai, R.; Sheng, Q.; Sun, Y.; Xu, H.; Kong, X. The characteristics of fundus microvascular alterations in the course of glaucoma: A narrative review. Ann. Transl. Med. 2022, 10, 527. [Google Scholar] [CrossRef]
- Lee, A.; Sung, K.R.; Shin, J.W. Progression detection capabilities of circumpapillary and macular vessel density in advanced glaucomatous eyes. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Calzetti, G.; Mursch-Edlmayr, A.S.; Bata, A.M.; Ungaro, N.; Mora, P.; Chua, J.; Schmidl, D.; Bolz, M.; Garhöfer, G.; Gandolfi, S.; et al. Measuring optic nerve head perfusion to monitor glaucoma: A study on structure–function relationships using laser speckle flowgraphy. Acta Ophthalmol. 2021, 100. [Google Scholar] [CrossRef]
- Kallab, M.; Hommer, N.; Schlatter, A.; Chua, J.; Tan, B.; Schmidl, D.; Hirn, C.; Findl, O.; Schmetterer, L.; Garhöfer, G.; et al. Combining vascular and nerve fiber layer thickness measurements to model glaucomatous focal visual field loss. Ann. New York Acad. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Chua, J.; Lin, E.; Tan, B.; Yao, X.; Chong, R.; Sng, C.; Lau, A.; Husain, R.; Aung, T.; et al. Focal Structure–Function Relationships in Primary Open-Angle Glaucoma Using OCT and OCT-A Measurements. Investig. Opthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Chua, J.; Tan, B.; Yao, X.; Chong, R.; Sng, C.C.A.; Husain, R.; Aung, T.; Garway-Heath, D.; Schmetterer, L. Combining OCT and OCTA for Focal Structure–Function Modeling in Early Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef]
- Yaoeda, K.; Shirakashi, M.; Fukushima, A.; Funaki, S.; Funaki, H.; Abe, H.; Tanabe, N. Relationship between optic nerve head microcirculation and visual field loss in glaucoma. Acta Ophthalmol. Scand. 2003, 81, 253–259. [Google Scholar] [CrossRef]
- Galassi, F.; Sodi, A.; Ucci, F.; Renieri, G.; Pieri, B.; Baccini, M. Ocular hemodynamics and glaucoma prognosis: A color Doppler imaging study. Arch. Ophthalmol. 2003, 121, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Satilmis, M.; Orgül, S.; Doubler, B.; Flammer, J. Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure. Am. J. Ophthalmol. 2003, 135, 664–669. [Google Scholar] [CrossRef]
- Siesky, B.; Harris, A.; Carr, J.; Vercellin, A.V.; Hussain, R.M.; Hembree, P.P.; Wentz, S.; Isaacs, M.; Eckert, G.; Moore, N.A. Reductions in Retrobulbar and Retinal Capillary Blood Flow Strongly Correlate with Changes in Optic Nerve Head and Retinal Morphology over 4 Years in Open-angle Glaucoma Patients of African Descent Compared with Patients of European Descent. J. Glaucoma 2016, 25, 750–757. [Google Scholar] [CrossRef]
- Zeitz, O.; Galambos, P.; Wagenfeld, L.; Wiermann, A.; Wlodarsch, P.; Praga, R.; Matthiessen, E.T.; Richard, G.; Klemm, M. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br. J. Ophthalmol. 2006, 90, 1245–1248. [Google Scholar] [CrossRef]
- Jeon, S.J.; Shin, D.-Y.; Park, H.-Y.L.; Park, C.K. Association of Retinal Blood Flow with Progression of Visual Field in Glaucoma. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.M.; Shen, R.; Lin, T.P.; Chan, P.P.; Wong, M.O.; Chan, N.C.; Tang, F.; Lam, A.K.; Leung, D.Y.; Tham, C.C.; et al. Optical coherence tomography angiography metrics predict normal tension glaucoma progression. Acta Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Kiyota, N.; Shiga, Y.; Omodaka, K.; Pak, K.; Nakazawa, T. Time-Course Changes in Optic Nerve Head Blood Flow and Retinal Nerve Fiber Layer Thickness in Eyes with Open-angle Glaucoma. Ophthalmology 2020, 128, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Berisha, F.; Feke, G.T.; Hirose, T.; McMeel, J.W.; Pasquale, L.R. Retinal Blood Flow and Nerve Fiber Layer Measurements in Early-Stage Open-Angle Glaucoma. Am. J. Ophthalmol. 2008, 146, 466–472.e2. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.K.; Cull, G.; Fortune, B.; Wang, L. Increased Optic Nerve Head Capillary Blood Flow in Early Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2019, 60, 3110–3118. [Google Scholar] [CrossRef] [PubMed]
- Fondi, K.; Wozniak, P.A.; Howorka, K.; Bata, A.M.; Aschinger, G.C.; Popa-Cherecheanu, A.; Witkowska, K.J.; Hommer, A.; Schmidl, D.; Werkmeister, R.M.; et al. Retinal oxygen extraction in individuals with type 1 diabetes with no or mild diabetic retinopathy. Diabetologia 2017, 60, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- E Riva, C.; E Grunwald, J.; Sinclair, S.H.; Petrig, B.L. Blood velocity and volumetric flow rate in human retinal vessels. Investig. Ophthalmol. Vis. Sci. 1985, 26. [Google Scholar]
- Polska, E.; Kircher, K.; Ehrlich, P.; Vecsei, P.V.; Schmetterer, L. RI in central retinal artery as assessed by CDI does not correspond to retinal vascular resistance. Am. J. Physiol. Circ. Physiol. 2001, 280, H1442–H1447. [Google Scholar] [CrossRef] [PubMed]
- Garhofer, G.; Werkmeister, R.; Dragostinoff, N.; Schmetterer, L. Retinal Blood Flow in Healthy Young Subjects. Investig. Opthalmol. Vis. Sci. 2012, 53, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Flanagan, J.G.; Patel, S.R.; Cheng, R.; Hudson, C. Retinal Blood Flow and Vascular Reactivity in Chronic Smokers. Investig. Opthalmol. Vis. Sci. 2014, 55, 4266–4276. [Google Scholar] [CrossRef][Green Version]
- Szegedi, S.; Hommer, N.; Kallab, M.; Puchner, S.; Schmidl, D.; Werkmeister, R.M.; Garhöfer, G.; Schmetterer, L. Repeatability and Reproducibility of Total Retinal Blood Flow Measurements Using Bi-Directional Doppler OCT. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Olafsdottir, O.B.; Hardarson, S.H.; Gottfredsdottir, M.S.; Harris, A.; Stefánsson, E. Retinal Oximetry in Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2011, 52, 6409–6413. [Google Scholar] [CrossRef]
- Shahidi, A.M.; Hudson, C.; Tayyari, F.; Flanagan, J.G. Retinal Oxygen Saturation in Patients with Primary Open-angle Glaucoma Using a Non-flash Hypespectral Camera. Curr. Eye Res. 2016, 42, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, E.; Pinto, L.A.; Olafsdottir, O.B.; De Clerck, E.; Stalmans, P.; Van Calster, J.; Zeyen, T.; Stefánsson, E.; Stalmans, I. Oximetry in glaucoma: Correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2013, 92, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Yap, Z.L.; Ong, C.; Lee, Y.F.; Tsai, A.; Cheng, C.; Nongpiur, M.E.; Perera, S. Retinal Oximetry in Subjects with Glaucomatous Hemifield Asymmetry. J. Glaucoma 2017, 26, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, S.; Lasta, M.; Told, R.; Schmidl, D.; Boltz, A.; Napora, K.J.; Werkmeister, R.M.; Popa-Cherecheanu, A.; Garhöfer, G.; Schmetterer, L. Retinal Oxygen Metabolism During Normoxia and Hyperoxia in Healthy Subjects. Investig. Opthalmol. Vis. Sci. 2014, 55, 4707–4713. [Google Scholar] [CrossRef]
- Palkovits, S.; Told, R.; Schmidl, D.; Boltz, A.; Napora, K.J.; Lasta, M.; Kaya, S.; Werkmeister, R.M.; Popa-Cherecheanu, A.; Garhöfer, G.; et al. Regulation of retinal oxygen metabolism in humans during graded hypoxia. Am. J. Physiol. Circ. Physiol. 2014, 307, H1412–H1418. [Google Scholar] [CrossRef][Green Version]
- Palkovits, S.; Lasta, M.; Told, R.; Schmidl, D.; Werkmeister, R.; Cherecheanu, A.P.; Garhöfer, G.; Schmetterer, L. Relation of retinal blood flow and retinal oxygen extraction during stimulation with diffuse luminance flicker. Sci. Rep. 2015, 5, 18291. [Google Scholar] [CrossRef]
- Kallab, M.; Hommer, N.; Schlatter, A.; Bsteh, G.; Altmann, P.; Popa-Cherecheanu, A.; Pfister, M.; Werkmeister, R.M.; Schmidl, D.; Schmetterer, L.; et al. Retinal Oxygen Metabolism and Haemodynamics in Patients with Multiple Sclerosis and History of Optic Neuritis. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Fondi, K.; Aschinger, G.C.; Bata, A.M.; Wozniak, P.A.; Liao, L.; Seidel, G.; Doblhoff-Dier, V.; Schmidl, D.; Garhöfer, G.; Werkmeister, R.M.; et al. Measurement of Retinal Vascular Caliber From Optical Coherence Tomography Phase Images. Investig. Opthalmol. Vis. Sci. 2016, 57. [Google Scholar] [CrossRef][Green Version]
- Harwerth, R.; Wheat, J.; Fredette, M.; Anderson, D. Linking structure and function in glaucoma. Prog. Retin. Eye Res. 2010, 29, 249–271. [Google Scholar] [CrossRef]
- Bata, A.M.; Fondi, K.; Szegedi, S.; Aschinger, G.C.; Hommer, A.; Schmidl, D.; Chua, J.; Werkmeister, R.M.; Garhöfer, G.; Schmetterer, L. Age-Related Decline of Retinal Oxygen Extraction in Healthy Subjects. Investig. Opthalmol. Vis. Sci. 2019, 60, 3162–3169. [Google Scholar] [CrossRef]

| POAG | Healthy Controls | p-Value * | |
|---|---|---|---|
| Age (years) | 58 ± 8 | 57 ± 7 | 0.687 |
| Sex (male/female) | 14/26 | 14/26 | - |
| Intraocular pressure (mmHg) | 16 ± 3 | 15 ± 3 | 0.826 |
| Systolic blood pressure (mmHg) | 132 ± 10 | 129 ± 9 | 0.211 |
| Diastolic blood pressure (mmHg) | 72 ± 8 | 70 ± 7 | 0.336 |
| Mean arterial pressure (mmHg) | 92 ± 8 | 90 ± 7 | 0.259 |
| Pulse rate (beats/min) | 67 ± 10 | 65 ± 9 | 0.417 |
| Intraocular Pressure (mmHg) | 17 ± 3 | 16 ± 3 | 0.677 |
| Retinal nerve fiber layer thickness (µm) | 73 ± 15 | 99 ± 8 | <0.001 |
| Mean deviation (MD) | −6.3 ± 3.3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garhöfer, G.; Bata, A.M.; Popa-Cherecheanu, A.; Hommer, A.; Vass, C.; Resch, H.; Schmidl, D.; Werkmeister, R.M.; Schmetterer, L. Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2022, 23, 10152. https://doi.org/10.3390/ijms231710152
Garhöfer G, Bata AM, Popa-Cherecheanu A, Hommer A, Vass C, Resch H, Schmidl D, Werkmeister RM, Schmetterer L. Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma. International Journal of Molecular Sciences. 2022; 23(17):10152. https://doi.org/10.3390/ijms231710152
Chicago/Turabian StyleGarhöfer, Gerhard, Ahmed M. Bata, Alina Popa-Cherecheanu, Anton Hommer, Clemens Vass, Hemma Resch, Doreen Schmidl, René M. Werkmeister, and Leopold Schmetterer. 2022. "Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma" International Journal of Molecular Sciences 23, no. 17: 10152. https://doi.org/10.3390/ijms231710152
APA StyleGarhöfer, G., Bata, A. M., Popa-Cherecheanu, A., Hommer, A., Vass, C., Resch, H., Schmidl, D., Werkmeister, R. M., & Schmetterer, L. (2022). Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma. International Journal of Molecular Sciences, 23(17), 10152. https://doi.org/10.3390/ijms231710152







