An Exploratory Study Provides Insights into MMP9 and Aβ Levels in the Vitreous and Blood across Different Ages and in a Subset of AMD Patients
Abstract
:1. Introduction
2. Results
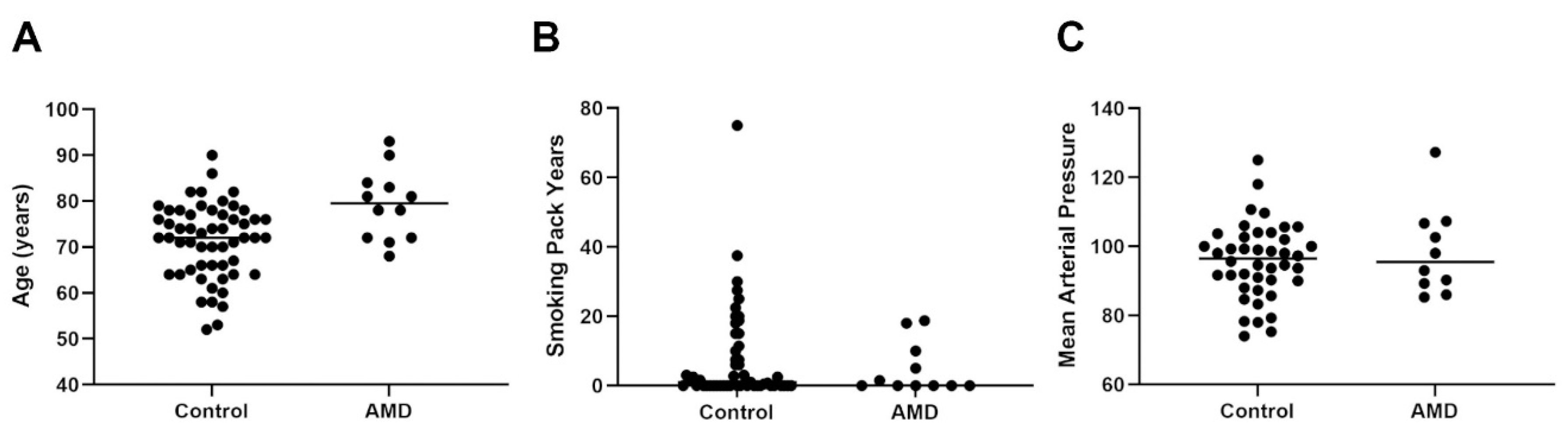
2.1. Scrutiny of the Control and AMD Cohorts Revealed a Mixed Picture of Age, Gender, Ocular History and Medication as Well as Lifestyle Demographics
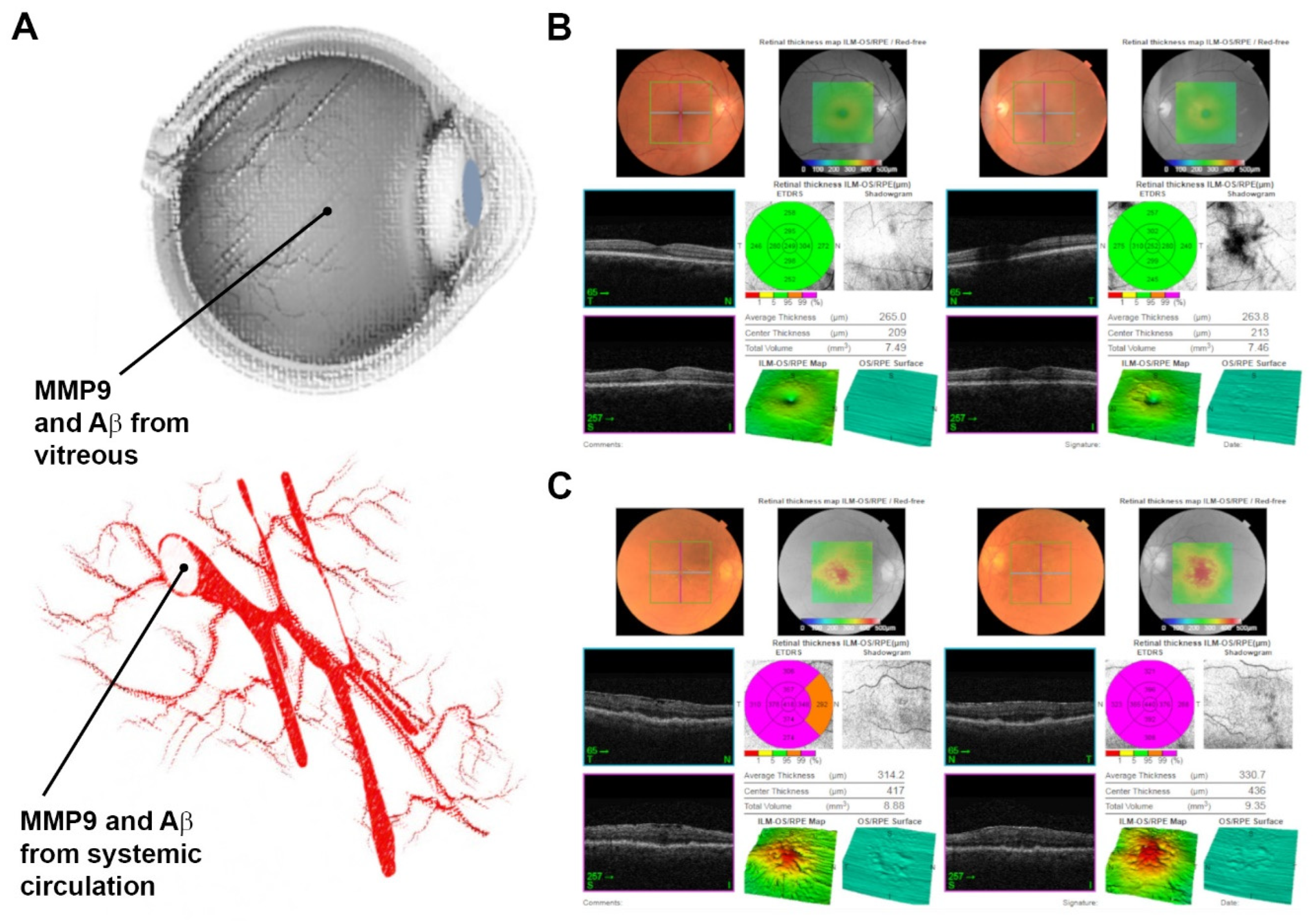
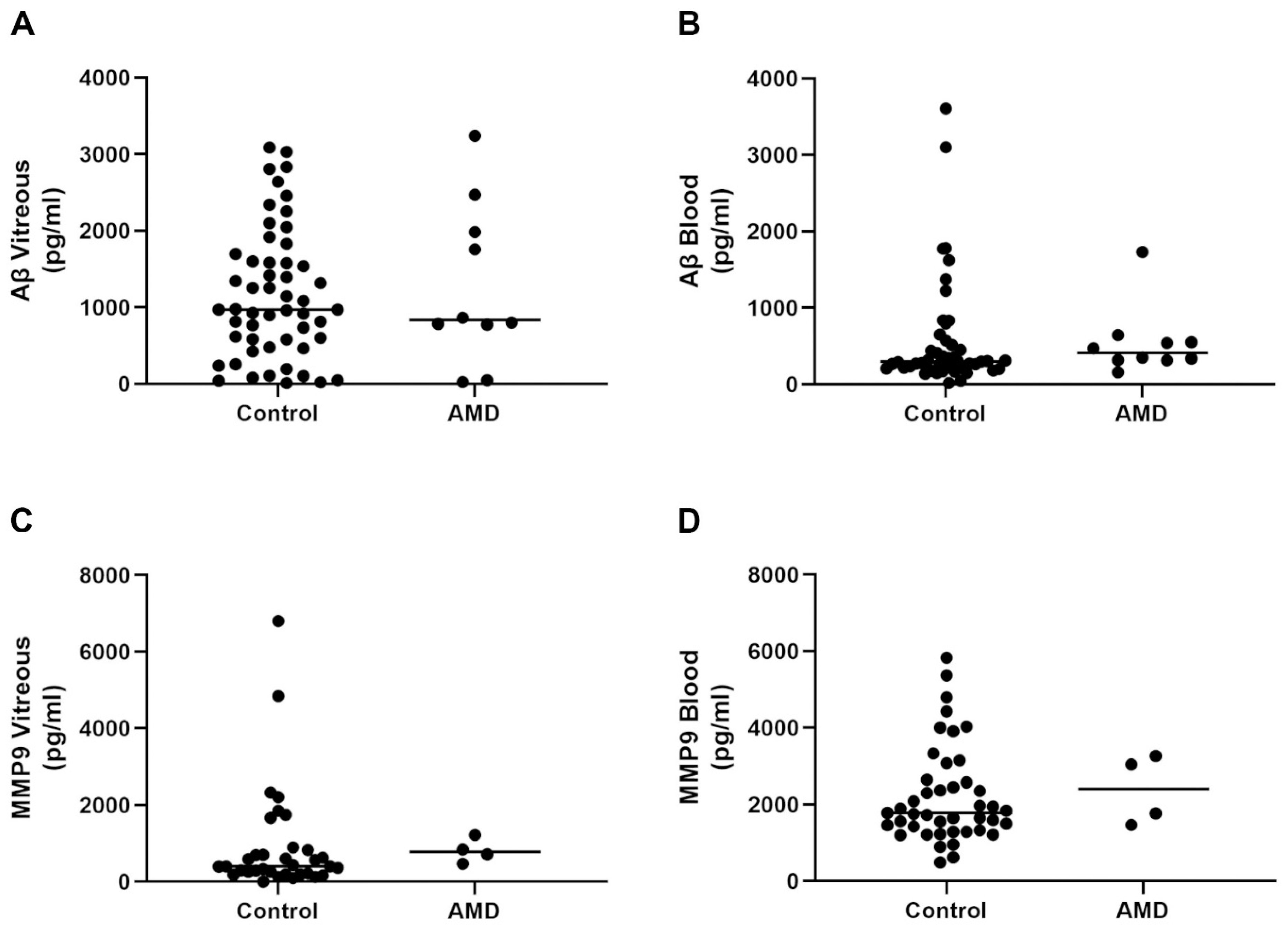
2.2. Aβ and MMP9 Levels in the Vitreous and Blood of Control Subjects and AMD Patients Showed Variable Levels across the Lifespan, Which Was Unrelated with Advanced Age or Retinopathy
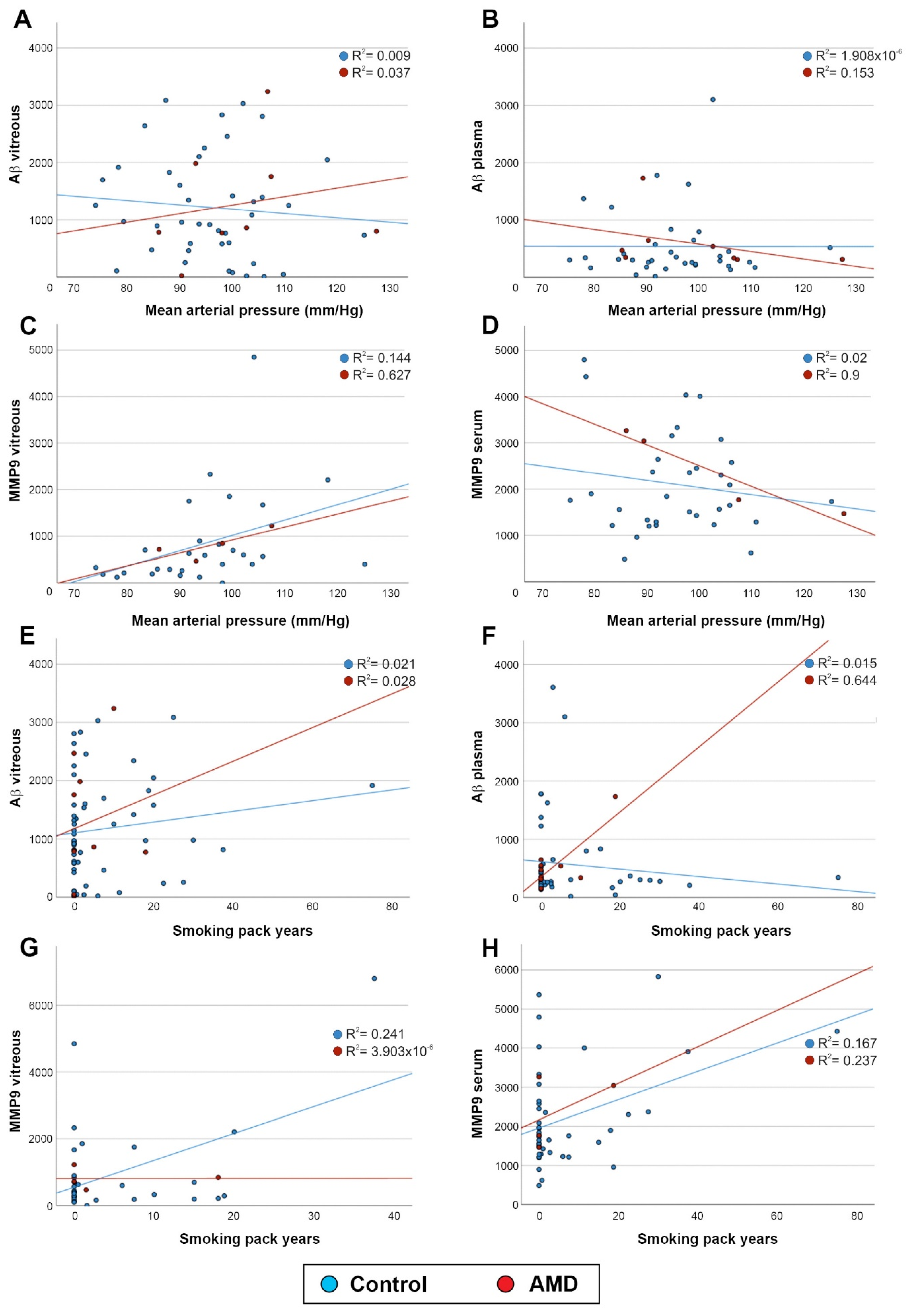
2.3. Aβ and MMP9 Levels in the Vitreous and Blood Were Unrelated to Mean Arterial Pressure and Smoking
2.4. A Bioinformatics Analysis Revealed Promising MMP9 and APP/Aβ Partners for Further Scrutiny, Many of Which Are Already Linked with Retinopathy
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Considerations for Recruiting Participants
5.2. Study Cohort Eligibility and Clinical Examination
5.3. Sample Processing
5.4. Enzyme-Linked Immunosorbent Assay for Quantifying MMP9
5.5. Enzyme-Linked Immunosorbent Assay for Quantifying Aβ
5.6. Bioinformatics Analysis
5.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration: Recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology 2017, 124, 464–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Pfau, M.; Blodi, B.A.; Holz, F.G.; Jaffe, G.J.; Liakopoulos, S.; Sadda, S.R.; Staurenghi, G.; Bjelopera, E.; Brown, T.; et al. OCT Signs of Early Atrophy in Age-Related Macular Degeneration: Interreader Agreement: Classification of Atrophy Meetings Report 6. Ophthalmol. Retina 2021, 6, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Shoda, C.; Kitagawa, Y.; Shimada, H.; Yuzawa, M.; Tateno, A.; Okubo, Y. Relationship of Area of Soft Drusen in Retina with Cerebral Amyloid-β Accumulation and Blood Amyloid-β Level in the Elderly. J. Alzheimer’s Dis. 2018, 62, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lynn, S.A.; Keeling, E.; Munday, R.; Gabha, G.; Griffiths, H.; Lotery, A.J.; Ratnayaka, J.A. The complexities underlying age-related macular degeneration: Could amyloid beta play an important role? Neural. Regen. Res. 2017, 12, 538–548. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020, 139, 813–836. [Google Scholar] [CrossRef] [Green Version]
- Ratnayaka, J.A.; Lynn, S. Alzheimer’s-Related Amyloid Beta Peptide Aggregates in the Ageing Retina: Implications for Sight Loss and Dementia, 1st ed.; Davide, M., Ed.; Intech Publishing: Rijeka, Croatia, 2016; Volume 1, p. 546. [Google Scholar]
- Le, J.T.; Agrón, E.; Keenan, T.D.L.; Clemons, T.E.; Brenowitz, W.D.; Yaffe, K.; Chew, E.Y. Assessing bidirectional associations between cognitive impairment and late age-related macular degeneration in the Age-Related Eye Disease Study 2. Alzheimer’s Dement. 2021, 18, 1296–1305. [Google Scholar] [CrossRef]
- Wen, L.Y.; Wan, L.; Lai, J.N.; Chen, C.S.; Chen, J.J.; Wu, M.Y.; Hu, K.C.; Chiu, L.T.; Tien, P.T.; Lin, H.J. Increased risk of Alzheimer’s disease among patients with age-related macular degeneration: A nationwide population-based study. PLoS ONE 2021, 16, e0250440. [Google Scholar] [CrossRef]
- Anisetti, B.; Youssef, H.; Elkhair, A.; Lin, M. Age-Related Macular Degeneration is Associated with Cerebral Amyloid Angiopathy (S35.003). Neurology 2022, 98, 2860. [Google Scholar]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Silvello, D.; Narvaes, L.B.; Albuquerque, L.C.; Forgiarini, L.F.; Meurer, L.; Martinelli, N.C.; Andrades, M.E.; Clausell, N.; dos Santos, K.G.; Rohde, L.E. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: Higher MMP-9 levels are associated with plaque vulnerability. Biomarkers 2014, 19, 49–55. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Albers, D.S.; Relkin, N.; Ngyuen, T.; Hilgenberg, S.L.; Chirichigno, J.; Cudkowicz, M.E.; Beal, M.F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003, 43, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2015, 48, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.A.; Lee, Y.; Zhang, J.J.; Marshall, J. Disturbed matrix metalloproteinase activity of Bruch’s membrane in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4459–4466. [Google Scholar] [CrossRef]
- Hollborn, M.; Stathopoulos, C.; Steffen, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4360–4367. [Google Scholar] [CrossRef] [Green Version]
- Krogh Nielsen, M.; Subhi, Y.; Rue Molbech, C.; Nilsson, L.L.; Nissen, M.H.; Sorensen, T.L. Imbalances in tissue inhibitors of metalloproteinases differentiate choroidal neovascularization from geographic atrophy. Acta Ophthalmol. 2018, 97, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, A.; Hjalmarsson, C.; Falk, P.; Ivarsson, M.L. Levels of matrix metalloproteinases differ in plasma and serum—Aspects regarding analysis of biological markers in cancer. Br. J. Cancer 2016, 115, 703–706. [Google Scholar] [CrossRef] [Green Version]
- Chau, K.Y.; Sivaprasad, S.; Patel, N.; Donaldson, T.A.; Luthert, P.J.; Chong, N.V. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye 2007, 21, 1511–1515. [Google Scholar] [CrossRef]
- Lauwen, S.; Lefeber, D.J.; Fauser, S.; Hoyng, C.B.; den Hollander, A.I. Increased pro-MMP9 plasma levels are associated with neovascular age-related macular degeneration and with the risk allele of rs142450006 near MMP9. Mol. Vis. 2021, 27, 142–150. [Google Scholar]
- Zeng, R.; Wen, F.; Zhang, X.; Su, Y. Serum levels of matrix metalloproteinase 2 and matrix metalloproteinase 9 elevated in polypoidal choroidal vasculopathy but not in age-related macular degeneration. Mol. Vis. 2013, 19, 729–736. [Google Scholar]
- Acera, A.; Vecino, E.; Duran, J.A. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8285–8291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannello, F. Serum or plasma samples? The “Cinderella” role of blood collection procedures: Preanalytical methodological issues influence the release and activity of circulating matrix metalloproteinases and their tissue inhibitors, hampering diagnostic trueness and leading to misinterpretation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 611–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakasam, A.; Muthuswamy, A.; Ablonczy, Z.; Greig, N.H.; Fauq, A.; Rao, K.J.; Pappolla, M.A.; Sambamurti, K. Differential accumulation of secreted AbetaPP metabolites in ocular fluids. J. Alzheimer’s Dis. 2010, 20, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Benilova, I.; Karran, E.; De, S.B. The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Talaga, K.C.; Rivest, A.J.; Barron, E.; Hageman, G.S.; Johnson, L.V. Characterization of beta amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Luibl, V.; Isas, J.M.; Kayed, R.; Glabe, C.G.; Langen, R.; Chen, J. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J. Clin. Investig. 2006, 116, 378–385. [Google Scholar] [CrossRef]
- Isas, J.M.; Luibl, V.; Johnson, L.V.; Kayed, R.; Wetzel, R.; Glabe, C.G.; Langen, R.; Chen, J. Soluble and mature amyloid fibrils in drusen deposits. Investig. Ophthalmol. Vis. Sci 2010, 51, 1304–1310. [Google Scholar] [CrossRef] [Green Version]
- Dentchev, T.; Milam, A.H.; Lee, V.M.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 9, 184–190. [Google Scholar]
- Zhu, J.; Su, T.; Wang, M.; Li, M.; Liu, L.; Wang, F. Highly Expressed Amyloid Beta-42 of Aqueous Humor in Patients with Neovascular Macular Degeneration. Semin. Ophthalmol. 2021, 36, 9–13. [Google Scholar] [CrossRef]
- Yoneda, S.; Hara, H.; Hirata, A.; Fukushima, M.; Inomata, Y.; Tanihara, H. Vitreous fluid levels of beta-amyloid((1–42)) and tau in patients with retinal diseases. Jpn. J. Ophthalmol. 2005, 49, 106–108. [Google Scholar] [CrossRef]
- Wright, L.M.; Stein, T.D.; Jun, G.; Chung, J.; McConnell, K.; Fiorello, M.; Siegel, N.; Ness, S.; Xia, W.; Turner, K.L.; et al. Association of Cognitive Function with Amyloid-β and Tau Proteins in the Vitreous Humor. J. Alzheimer’s Dis. 2019, 68, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.L.; Vig, V.; Chung, J.; Fiorello, M.G.; Xia, W.; Zetterberg, H.; Blennow, K.; Zetterberg, M.; Shareef, F.; Siegel, N.H.; et al. Neurofilament light chain in the vitreous humor of the eye. Alzheimer’s Res. Ther. 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, K.; Williams, T.L.; Morris, K.L.; Jonckheere, W.; Vandersteen, A.; Kelly, G.; Schymkowitz, J.; Rousseau, F.; Pastore, A.; Serpell, L.C.; et al. Structural basis for increased toxicity of pathological abeta42:abeta40 ratios in Alzheimer disease. J. Biol. Chem. 2012, 287, 5650–5660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashkari, K.; Teague, G.C.; Beattie, U.; Betts, J.; Kumar, S.; McLaughlin, M.M.; López, F.J. Plasma biomarkers of the amyloid pathway are associated with geographic atrophy secondary to age-related macular degeneration. PLoS ONE 2020, 15, e0236283. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.; Cipriani, T.; Rittenhouse, K.D.; Lim, L.; Robman, L.D.; Li, W.; Wang, W.; Deng, S.; Banerjee, P. Plasma levels of amyloid beta and other proinflammatory mediators in patients with age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Bibl, M.; Welge, V.; Esselmann, H.; Wiltfang, J. Stability of amyloid-β peptides in plasma and serum. Electrophoresis 2012, 33, 445–450. [Google Scholar] [CrossRef]
- Fan, F.; Montemari, A.L.; Rossi, S.; Parisi, G.; Lamoke, F.; Facchiano, F.; Ripandelli, G.; Bartoli, M. Up-regulation Of Soluble Amyloid Beta and Down-regulation Of Soluble RAGE in the Vitreous Of Age-related Macular Degeneration Patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6423. [Google Scholar]
- Parthasarathy, R.; Chow, K.M.; Derafshi, Z.; Fautsch, M.P.; Hetling, J.R.; Rodgers, D.W.; Hersh, L.B.; Pepperberg, D.R. Reduction of amyloid-beta levels in mouse eye tissues by intra-vitreally delivered neprilysin. Exp. Eye Res. 2015, 138, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.W.; Wang, S.J.; Wu, S.J.; Yang, C.C.; Huang, M.W.; Lin, C.H.; Cheng, I.H. Potential blood biomarker for disease severity in the Taiwanese population with Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Demen. 2013, 28, 75–83. [Google Scholar] [CrossRef]
- Dodel, R.C.; Du, Y.; Depboylu, C.; Hampel, H.; Frölich, L.; Haag, A.; Hemmeter, U.; Paulsen, S.; Teipel, S.J.; Brettschneider, S.; et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1472–1474. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.J.; Chang, M.L.; Zhang, F.F.; Li, T.; Gensler, G.; Schleicher, M.; Taylor, A. The relationship of major American dietary patterns to age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, J.M.; Cote, J.; Rosner, B. Progression of age-related macular degeneration: Association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 2003, 121, 1728–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogg, R.E.; Woodside, J.V.; McGrath, A.; Young, I.S.; Vioque, J.L.; Chakravarthy, U.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; et al. Mediterranean Diet Score and Its Association with Age-Related Macular Degeneration: The European Eye Study. Ophthalmology 2017, 124, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Gregoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Feart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, V.; Warwick, A.; Hingorani, A.; Tufail, A.; Cipriani, V.; Burgess, S.; Sofat, R. Association of Smoking, Alcohol Consumption, Blood Pressure, Body Mass Index, and Glycemic Risk Factors With Age-Related Macular Degeneration: A Mendelian Randomization Study. JAMA Ophthalmol 2021, 139, 1299–1306. [Google Scholar] [CrossRef]
- Keeling, E.; Lynn, S.A.; Koh, Y.M.; Scott, J.A.; Kendall, A.; Gatherer, M.; Page, A.; Cagampang, F.R.; Lotery, A.J.; Ratnayaka, J.A. A High Fat “Western-style” Diet Induces AMD-Like Features in Wildtype Mice. Mol. Nutr. Food Res. 2022, 66, e2100823. [Google Scholar] [CrossRef]
- Katsi, V.K.; Marketou, M.E.; Vrachatis, D.A.; Manolis, A.J.; Nihoyannopoulos, P.; Tousoulis, D.; Vardas, P.E.; Kallikazaros, I. Essential hypertension in the pathogenesis of age-related macular degeneration: A review of the current evidence. J. Hypertens. 2015, 33, 2382–2388. [Google Scholar] [CrossRef]
- Cougnard-Grégoire, A.; Delyfer, M.N.; Korobelnik, J.F.; Rougier, M.B.; Malet, F.; Le Goff, M.; Dartigues, J.F.; Colin, J.; Barberger-Gateau, P.; Delcourt, C. Long-term blood pressure and age-related macular degeneration: The ALIENOR study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1905–1912. [Google Scholar] [CrossRef]
- Velilla, S.; García-Medina, J.J.; García-Layana, A.; Dolz-Marco, R.; Pons-Vázquez, S.; Pinazo-Durán, M.D.; Gómez-Ulla, F.; Arévalo, J.F.; Díaz-Llopis, M.; Gallego-Pinazo, R. Smoking and age-related macular degeneration: Review and update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef]
- Klein, R.; Cruickshanks, K.J.; Nash, S.D.; Krantz, E.M.; Nieto, F.J.; Huang, G.H.; Pankow, J.S.; Klein, B.E. The prevalence of age-related macular degeneration and associated risk factors. Arch. Ophthalmol. 2010, 128, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Vingerling, J.R.; Hofman, A.; Grobbee, D.E.; de Jong, P.T. Age-related macular degeneration and smoking. The Rotterdam Study. Arch. Ophthalmol. 1996, 114, 1193–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delcourt, C.; Diaz, J.L.; Ponton-Sanchez, A.; Papoz, L. Smoking and age-related macular degeneration. The POLA Study. Pathologies Oculaires Liées à l’Age. Arch. Ophthalmol. 1998, 116, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Gliem, M.; Yusuf, I.H.; Birtel, J.; Müller, P.L.; Mangold, E.; Downes, S.M.; MacLaren, R.E.; Betz, C.; Bolz, H.J. A Specific Macula-Predominant Retinal Phenotype Is Associated With the CDHR1 Variant c.783G > A, a Silent Mutation Leading to In-Frame Exon Skipping. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3388–3397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ba-Abbad, R.; Robson, A.G.; Mahroo, O.A.; Wright, G.; Schiff, E.; Duignan, E.S.; Michaelides, M.; Arno, G.; Webster, A.R. A clinical study of patients with novel CDHR1 genotypes associated with late-onset macular dystrophy. Eye 2021, 35, 1482–1489. [Google Scholar] [CrossRef]
- Warwick, A.; Gibson, J.; Sood, R.; Lotery, A. A rare penetrant TIMP3 mutation confers relatively late onset choroidal neovascularisation which can mimic age-related macular degeneration. Eye 2016, 30, 488–491. [Google Scholar] [CrossRef]
- Dewing, J.M.; Carare, R.O.; Lotery, A.J.; Ratnayaka, J.A. The Diverse Roles of TIMP-3: Insights into Degenerative Diseases of the Senescent Retina and Brain. Cells 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Hongisto, H.; Dewing, J.M.; Christensen, D.R.G.; Scott, J.; Cree, A.J.; Nättinen, J.; Määttä, J.; Jylhä, A.; Aapola, U.; Uusitalo, H.; et al. In vitro stem cell modelling demonstrates a proof-of-concept for excess functional mutant TIMP3 as the cause of Sorsby Fundus Dystrophy. J. Pathol. 2020, 252, 138–150. [Google Scholar] [CrossRef]
- Sharma, K.; Tyagi, R.; Singh, R.; Sharma, S.K.; Anand, A. Serum Levels of TIMP-3, LIPC, IER3, and SLC16A8 in CFH-Negative AMD Cases. J. Cell Biochem. 2017, 118, 2087–2095. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, S.-J.; Jo, C.; Park, M.H.; Han, C.; Kim, E.-J.; Huh, G.Y.; Koh, Y.H. Altered TIMP-3 Levels in the Cerebrospinal Fluid and Plasma of Patients with Alzheimer’s Disease. J. Pers. Med. 2022, 12, 827. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Chen, W.; Schu, M.; Yaspan, B.L.; Yu, Y.; Thorleifsson, G.; Zack, D.J.; Arakawa, S.; Cipriani, V.; Ripke, S.; et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013, 45, 433. [Google Scholar] [CrossRef] [Green Version]
- Nahavandipour, A.; Krogh Nielsen, M.; Sørensen, T.L.; Subhi, Y. Systemic levels of interleukin-6 in patients with age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2020, 98, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef] [PubMed]
- Örnek, N.; Örnek, K.; Aydin, S.; Yilmaz, M.; Ölmez, Y. Serum vascular endothelial growth factor receptor-2 and adropin levels in age-related macular degeneration. Int. J. Ophthalmol. 2016, 9, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Z.; Hinz, B.J.; Greve, M.D.; Potter, M.J.; Hornan, D.; Samad, A.; To, E.; Matsubara, J.A. Expression of neuropilin-1 in choroidal neovascular membranes. Can. J. Ophthalmol. 2003, 38, 41–45. [Google Scholar] [CrossRef]
- Fernández-Robredo, P.; Selvam, S.; Powner, M.B.; Sim, D.A.; Fruttiger, M. Neuropilin 1 Involvement in Choroidal and Retinal Neovascularisation. PLoS ONE 2017, 12, e0169865. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, S.J.; Suh, E.J.; Ahn, J.; Park, J.H.; Hong, H.K.; Lee, J.E.; Ahn, S.J.; Hwang, D.J.; Kim, K.W.; et al. Identification of vinculin as a potential plasma marker for age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7166–7176. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Qi, X.; Kociok, N.; Skosyrski, S.; Emilio, A.; Ruan, Q.; Han, S.; Liu, L.; Chen, Z.; Bowes Rickman, C.; et al. beta-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 2012, 4, 980–991. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Zhao, Y.; Dua, P.; Rogaev, E.I.; Lukiw, W.J. microRNA-34a-Mediated Down-Regulation of the Microglial-Enriched Triggering Receptor and Phagocytosis-Sensor TREM2 in Age-Related Macular Degeneration. PLoS ONE 2016, 11, e0150211. [Google Scholar] [CrossRef] [Green Version]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Keeling, E.; Chatelet, D.S.; Johnston, D.A.; Page, A.; Tumbarello, D.A.; Lotery, A.J.; Ratnayaka, J.A. Oxidative Stress and Dysfunctional Intracellular Traffic Linked to an Unhealthy Diet Results in Impaired Cargo Transport in the Retinal Pigment Epithelium (RPE). Mol. Nutr. Food Res. 2019, 63, e1800951. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.B.; Zhang, Z.; Luo, P.; Wang, S.S.; Peng, Y.; Chu, S.F.; Chen, N.H. Lipid metabolism in Alzheimer’s disease. Brain Res. Bull. 2019, 144, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Nelson, A.; Sagare, A.; Zlokovic, B. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: The role, regulation and restoration of LRP1. Front. Aging Neurosci. 2015, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.; Zhu, M.; Liu, X.; Jiang, L.; Xu, J.; Chen, L.; Liang, J.; Li, L.; Zhou, T.; Wang, Y.; et al. Inhibition of TRAF6 alleviates choroidal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2018, 503, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Skeie, J.M.; Mahajan, V.B. Dissection of human vitreous body elements for proteomic analysis. J. Vis. Exp. 2011, 47, e2455. [Google Scholar] [CrossRef] [Green Version]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic analyses of the vitreous humour. Mediat. Inflamm. 2012, 2012, 148039. [Google Scholar] [CrossRef]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 50 Ethnicity: caucasian origin Control group Diagnosis confirmed by a consultant ophthalmologist for at least one of the following pathologies: • Cataract; • Epiretinal membrane; • Macular hole; • Vitreous floaters; • Vitreomacular traction syndrome. Experimental group Diagnosis confirmed by a consultant ophthalmologist for AMD: • Dry AMD; • Wet AMD. • Previous Wet AMD/Scar • AMD classification according to AREDS categories 2–4: Category 2: multiple small drusen/single intermediate drusen (63–124 µm) or RPE abnormalities. Category 3: extensive intermediate drusen, at least one large druse (≥125 µm) or GA not involving centre of fovea. Category 4: GA involving fovea and/or CNV. | Any ocular condition in which the vitreous proteome may be subject to systemic influence. Diagnosis confirmed by a consultant ophthalmologist for any of the following pathologies: • Diabetic retinopathy; • Retinal haemorrhage; • Retinal detachment; • Vitreous haemorrhage; • Retinal vein occlusion; • Wet AMD with vitreous haemorrhage. Age < 50 Participants lacking capacity to consent. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynn, S.A.; Soubigou, F.; Dewing, J.M.; Smith, A.; Ballingall, J.; Sass, T.; Nica, I.; Watkins, C.; Gupta, B.; Almuhtaseb, H.; et al. An Exploratory Study Provides Insights into MMP9 and Aβ Levels in the Vitreous and Blood across Different Ages and in a Subset of AMD Patients. Int. J. Mol. Sci. 2022, 23, 14603. https://doi.org/10.3390/ijms232314603
Lynn SA, Soubigou F, Dewing JM, Smith A, Ballingall J, Sass T, Nica I, Watkins C, Gupta B, Almuhtaseb H, et al. An Exploratory Study Provides Insights into MMP9 and Aβ Levels in the Vitreous and Blood across Different Ages and in a Subset of AMD Patients. International Journal of Molecular Sciences. 2022; 23(23):14603. https://doi.org/10.3390/ijms232314603
Chicago/Turabian StyleLynn, Savannah A., Flavie Soubigou, Jennifer M. Dewing, Amanda Smith, Joanna Ballingall, Thea Sass, Isabela Nica, Catrin Watkins, Bhaskar Gupta, Hussein Almuhtaseb, and et al. 2022. "An Exploratory Study Provides Insights into MMP9 and Aβ Levels in the Vitreous and Blood across Different Ages and in a Subset of AMD Patients" International Journal of Molecular Sciences 23, no. 23: 14603. https://doi.org/10.3390/ijms232314603
APA StyleLynn, S. A., Soubigou, F., Dewing, J. M., Smith, A., Ballingall, J., Sass, T., Nica, I., Watkins, C., Gupta, B., Almuhtaseb, H., Lash, S. C., Yuen, H. M., Cree, A., Newman, T. A., Lotery, A. J., & Ratnayaka, J. A. (2022). An Exploratory Study Provides Insights into MMP9 and Aβ Levels in the Vitreous and Blood across Different Ages and in a Subset of AMD Patients. International Journal of Molecular Sciences, 23(23), 14603. https://doi.org/10.3390/ijms232314603






