Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences
Abstract
:1. Introduction
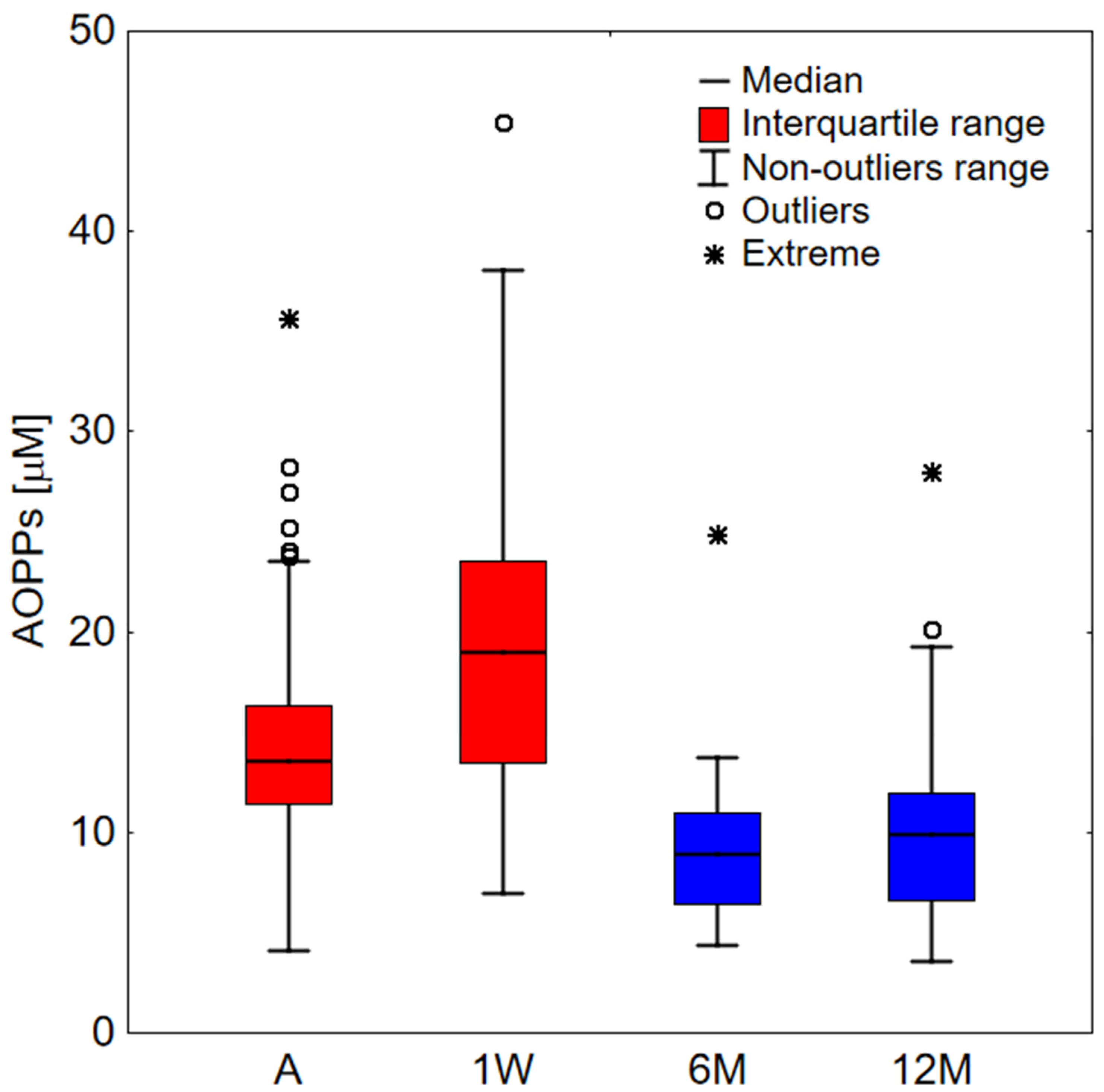
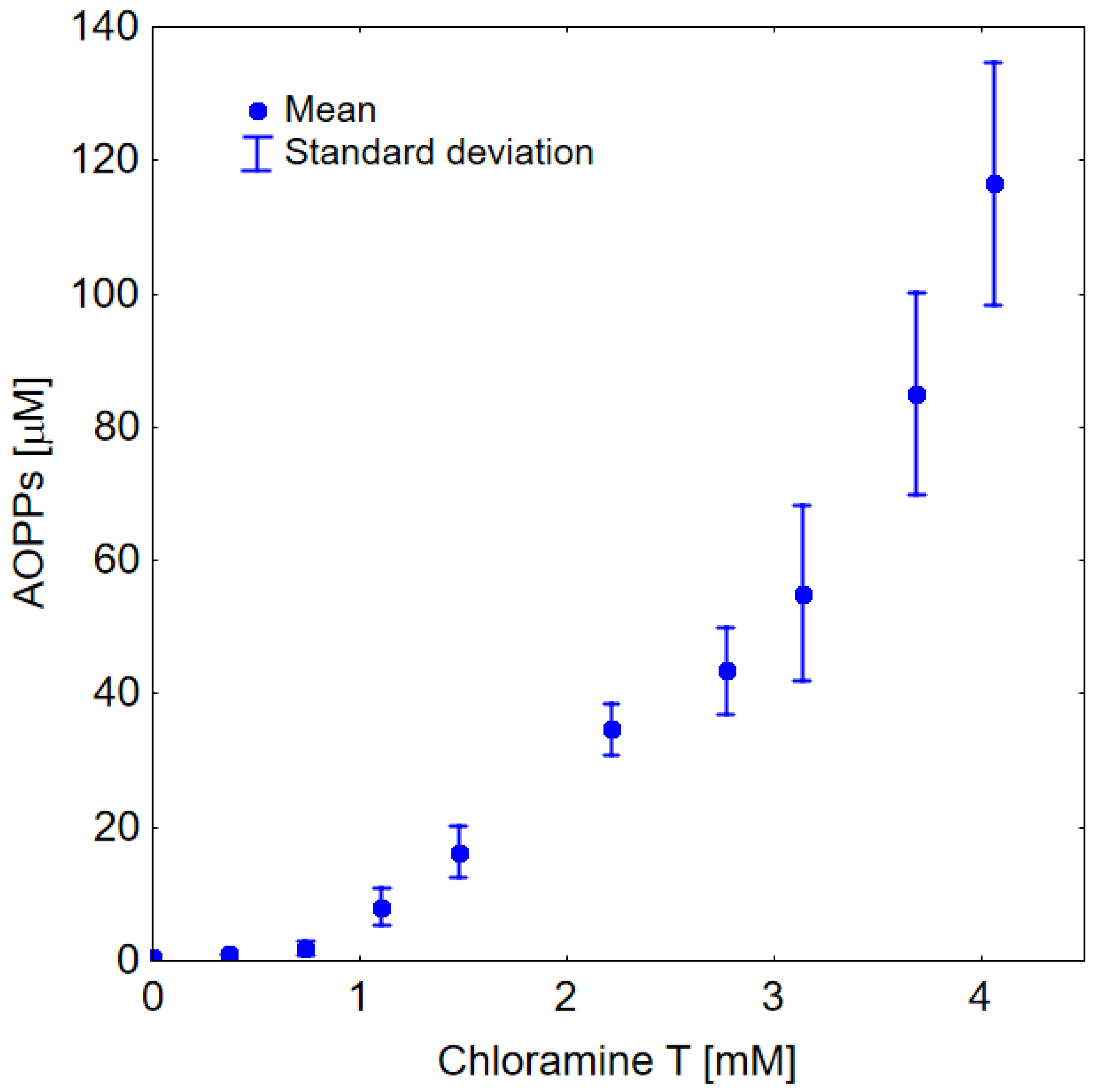
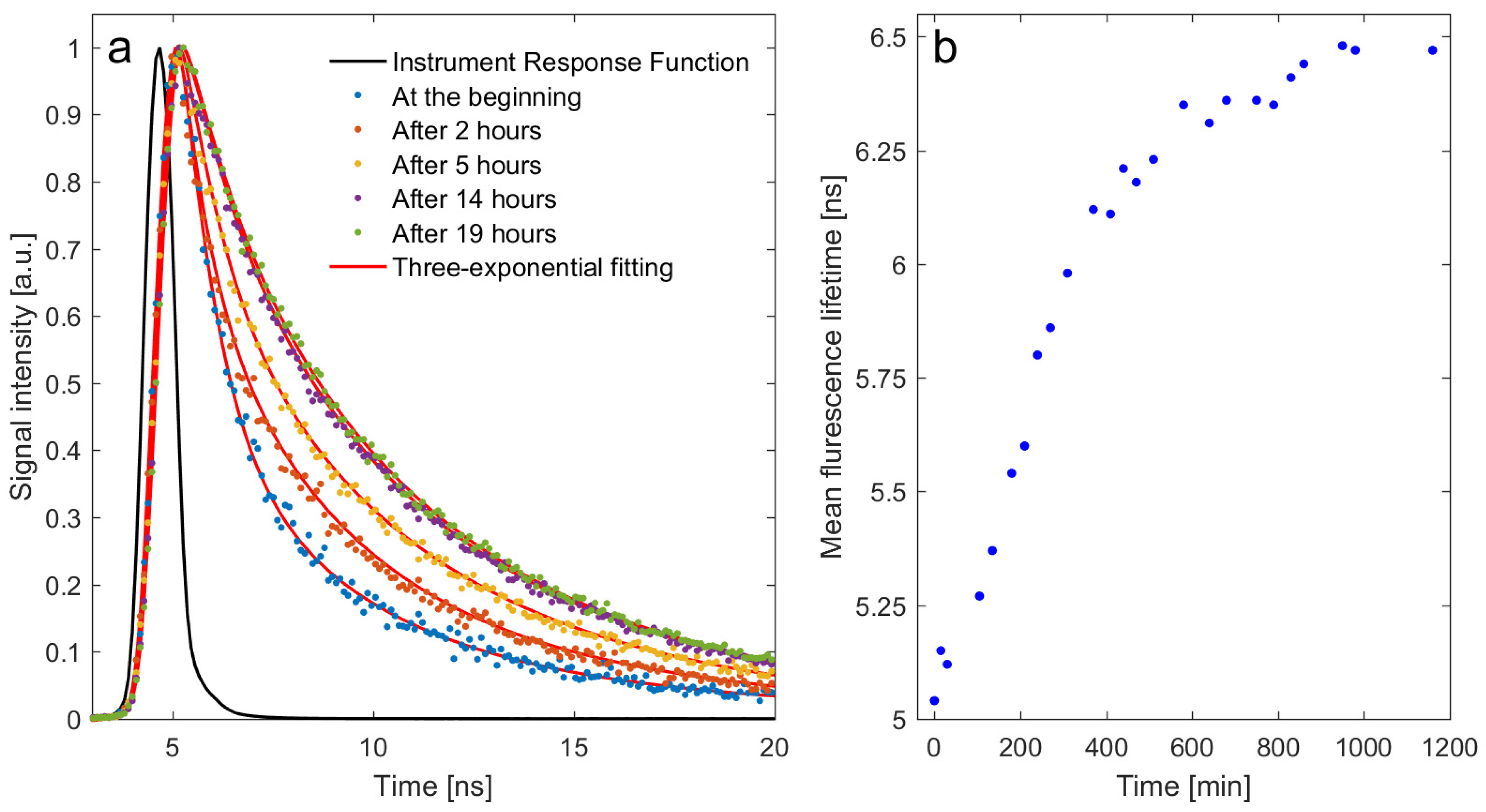
2. Results
2.1. Clinical Study
2.2. In Vitro Study
3. Discussion
4. Materials and Methods
4.1. Clinical Study
4.2. Sample Preparation
4.3. In Vitro Study
4.4. AOPPs Measurements
4.5. Time-Resolved Fluorescence Spectroscopy Measurements
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling Serum Cytokines in COVID-19 Patients Reveals IL-6 and IL-10 Are Disease Severity Predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, H.G.; Liu, W.; Liu, J.; Liu, K.; Shang, J.; Deng, Y.; Wei, S. Analysis of Clinical Features of 29 Patients with 2019 Novel Coronavirus Pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, e005. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Michot, J.M.; Albiges, L.; Chaput, N.; Saada, V.; Pommeret, F.; Griscelli, F.; Balleyguier, C.; Besse, B.; Marabelle, A.; Netzer, F.; et al. Tocilizumab, an Anti-IL-6 Receptor Antibody, to Treat COVID-19-Related Respiratory Failure: A Case Report. Ann. Oncol. 2020, 31, 961–964. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 Infection Pathogenesis Is Related to Oxidative Stress as a Response to Aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections. Oxid. Med. Cell. Longev. 2022, 2022, 5589089. [Google Scholar] [CrossRef]
- Yildiz, H.; Alp, H.H.; Ekin, S.; Arisoy, A.; Gunbatar, H.; Asker, S.; Cilingir, B.M.; Sunnetcioglu, A.; Celikel, M.; Esen, N.; et al. Analysis of Endogenous Oxidative Damage Markers and Association with Pulmonary Involvement Severity in Patients with SARS-CoV-2 Pneumonia. Infect. Dis. 2021, 51, 429–434. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the Development of Pulmonary Fibrosis Using Serial Thin-Section CT and Clinical Features in Patients Discharged after Treatment for COVID-19 Pneumonia. Korean J. Radiol. 2020, 21, 746–755. [Google Scholar] [CrossRef]
- Sun, X.; Xue, Z.; Yasin, A.; He, Y.; Chai, Y.; Li, J.; Zhang, K. Colorectal Cancer and Adjacent Normal Mucosa Differ in Apoptotic and Inflammatory Protein Expression. Eng. Regen. 2021, 2, 279–287. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-Covid Syndrome in Individuals Admitted to Hospital with COVID-19: Retrospective Cohort Study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef]
- Artigas, A.; Wernerman, J.; Arroyo, V.; Vincent, J.L.; Levy, M. Role of Albumin in Diseases Associated with Severe Systemic Inflammation: Pathophysiologic and Clinical Evidence in Sepsis and in Decompensated Cirrhosis. J. Crit. Care 2016, 33, 62–70. [Google Scholar] [CrossRef]
- Rozga, J.; Piatek, T.; Małkowski, P. Human Albumin: Old, New, and Emerging Applications. Ann. Transplant. 2013, 18, 205–217. [Google Scholar] [CrossRef]
- Sun, L.; Yin, H.; Liu, M.; Xu, G.; Zhou, X.; Ge, P.; Yang, H.; Mao, Y. Impaired Albumin Function: A Novel Potential Indicator for Liver Function Damage? Ann. Med. 2019, 51, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Bartimoccia, S.; Carnevale, R.; Stefanini, L.; Angelico, F.; del Ben, M. Oxidative Stress Mediated Platelet Activation in Patients with Congenital Analbuminemia: Effect of Albumin Infusion. J. Thromb. Haemost. 2021, 19, 3090–3094. [Google Scholar] [CrossRef]
- Iles, J.; Zmuidinaite, R.; Sadee, C.; Gardiner, A.; Lacey, J.; Harding, S.; Ule, J.; Roblett, D.; Heeney, J.; Baxendale, H.; et al. SARS-CoV-2 Spike Protein Binding of Glycated Serum Albumin-Its Potential Role in the Pathogenesis of the COVID-19 Clinical Syndromes and Bias towards Individuals with Pre-Diabetes/Type 2 Diabetes and Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 4126. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The Antioxidant Properties of Serum Albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Sitar, M.; Aydin, S.; Cakatay, U. Human Serum Albumin and Its Relation with Oxidative Stress. Clin. Lab. 2013, 59, 945–952. [Google Scholar] [CrossRef]
- Michelis, R.; Kristal, B.; Zeitun, T.; Shapiro, G.; Fridman, Y.; Geron, R.; Sela, S. Albumin Oxidation Leads to Neutrophil Activation in Vitro and Inaccurate Measurement of Serum Albumin in Patients with Diabetic Nephropathy. Free Radic. Biol. Med. 2013, 60, 49–55. [Google Scholar] [CrossRef]
- Magzal, F.; Sela, S.; Szuchman-Sapir, A.; Tamir, S.; Michelis, R.; Kristal, B. In-Vivo Oxidized Albumin- a pro-Inflammatory Agent in Hypoalbuminemia. PLoS ONE 2017, 12, e0177799. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Maras, J.S.; Hussain, M.S.; Sharma, S.; David, P.; Sukriti, S.; Shasthry, S.M.; Maiwall, R.; Trehanpati, N.; Singh, T.P.; et al. Hyperoxidized Albumin Modulates Neutrophils to Induce Oxidative Stress and Inflammation in Severe Alcoholic Hepatitis. Hepatology 2017, 65, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Ulfig, A.; Leichert, L.I. The Effects of Neutrophil-Generated Hypochlorous Acid and Other Hypohalous Acids on Host and Pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef]
- Tabata, F.; Wada, Y.; Kawakami, S.; Miyaji, K. Serum Albumin Redox States: More Than Oxidative Stress Biomarker. Antioxidants 2021, 10, 503. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Machado, M.V. New Insights about Albumin and Liver Disease. Ann. Hepatol. 2018, 17, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Das, S.; Yadav, G.; Chaudhary, S.; Vyas, A.; Islam, M.; Gupta, A.C.; Bajpai, M.; Maiwall, R.; Maras, J.S.; et al. Hyperoxidized Albumin Modulates Platelets and Promotes Inflammation Through CD36 Receptor in Severe Alcoholic Hepatitis. Hepatol. Commun. 2020, 4, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Pasterk, L.; Lemesch, S.; Leber, B.; Trieb, M.; Curcic, S.; Stadlbauer, V.; Schuligoi, R.; Schicho, R.; Heinemann, A.; Marsche, G. Oxidized Plasma Albumin Promotes Platelet-Endothelial Crosstalk and Endothelial Tissue Factor Expression. Sci. Rep. 2016, 6, 22104. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Kukia, N.; Abbasi, A.; Pakravan, N.; Hassan, Z.M. Measurement of Oxidized Albumin: An Opportunity for Diagnoses or Treatment of COVID-19. Bioorg. Chem. 2020, 105, 104429. [Google Scholar] [CrossRef]
- Badawy, M.A.; Yasseen, B.A.; El-Messiery, R.M.; Abdel-Rahman, E.A.; Elkhodiry, A.A.; Kamel, A.G.; El-Sayed, H.; Shedra, A.M.; Hamdy, R.; Zidan, M.; et al. Neutrophil-Mediated Oxidative Stress and Albumin Structural Damage Predict COVID-19-Associated Mortality. Elife 2021, 10, e69417. [Google Scholar] [CrossRef]
- Ducastel, M.; Chenevier-Gobeaux, C.; Ballaa, Y.; Meritet, J.F.; Brack, M.; Chapuis, N.; Pene, F.; Carlier, N.; Szwebel, T.A.; Roche, N.; et al. Oxidative Stress and Inflammatory Biomarkers for the Prediction of Severity and ICU Admission in Unselected Patients Hospitalized with COVID-19. Int. J. Mol. Sci. 2021, 22, 7462. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Zhao, X.; Tao, M.; Yan, W.; Fu, Y. Hypoalbuminemia—An Indicator of the Severity and Prognosis of COVID-19 Patients: A Multicentre Retrospective Analysis. Infect. Drug Resist. 2021, 14, 3699–3710. [Google Scholar] [CrossRef]
- Rabbani, G.; Ahn, S.N. Review: Roles of Human Serum Albumin in Prediction, Diagnoses and Treatment of COVID-19. Int. J. Biol. Macromol. 2021, 193, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Kaikita, K.; Tsujita, K. Hypoalbuminemia and Inflammation as Prognostic Markers in Patients Undergoing Percutaneous Coronary Intervention. Circ. J. 2017, 81, 1268–1269. [Google Scholar] [CrossRef]
- Acharya, R.; Poudel, D.; Bowers, R.; Patel, A.; Schultz, E.; Bourgeois, M.; Paswan, R.; Stockholm, S.; Batten, M.; Kafle, S.; et al. Low Serum Albumin Predicts Severe Outcomes in COVID-19 Infection: A Single-Center Retrospective Case-Control Study. J. Clin. Med. Res. 2021, 13, 258–267. [Google Scholar] [CrossRef]
- Park, J.E.; Jung, S.; Kim, A. MERS Transmission and Risk Factors: A Systematic Review. BMC Public Health 2018, 18, 574. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Omar, H.R.; Sweiss, N. Hypoalbuminemia Is Related to Inflammation Rather than Malnutrition in Sarcoidosis. Eur. J. Intern. Med. 2018, 53, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.C.; Sun, J.; Qureshi, A.R.; Dai, L.; Snaedal, S.; Bárány, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P. The Higher Mortality Associated with Low Serum Albumin Is Dependent on Systemic Inflammation in End-Stage Kidney Disease. PLoS ONE 2018, 13, e0190410. [Google Scholar] [CrossRef] [PubMed]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum Albumin Levels and Inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef]
- Hang, J.; Xue, P.; Yang, H.; Li, S.; Chen, D.; Zhu, L.; Huang, W.; Ren, S.; Zhu, Y.; Wang, L. Pretreatment C-Reactive Protein to Albumin Ratio for Predicting Overall Survival in Advanced Pancreatic Cancer Patients. Sci. Rep. 2017, 7, 2993. [Google Scholar] [CrossRef]
- Manani, S.M.; Virzì, G.M.; Clementi, A.; Brocca, A.; de Cal, M.; Tantillo, I.; Ferrando, L.; Crepaldi, C.; Ronco, C. Pro-Inflammatory Cytokines: A Possible Relationship with Dialytic Adequacy and Serum Albumin in Peritoneal Dialysis Patients. Clin. Kidney J. 2016, 9, 153–157. [Google Scholar] [CrossRef]
- Bologa, R.M.; Levine, D.M.; Parker, T.S.; Cheigh, J.S.; Serur, D.; Stenzel, K.H.; Rubin, A.L. Interleukin-6 Predicts Hypoalbuminemia, Hypocholesterolemia, and Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 1998, 32, 107–114. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Publishing, B.; Don, B.R.; Kaysen, G. POOR NUTRITIONAL STATUS AND INFLAMMATION: Serum Albumin: Relationship to Inflammation and Nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. The Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Iwao, Y.; Anraku, M.; Hiraike, M.; Kawai, K.; Nakajou, K.; Kai, T.; Suenaga, A.; Otagiri, M. The Structural and Pharmacokinetic Properties of Oxidized Human Serum Albumin, Advanced Oxidation Protein Products (AOPP). Drug Metab. Pharmacokinet. 2006, 21, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Ou, H.; Huang, Z.; Mo, Z.; Xiao, J. The Characteristics and Roles of Advanced Oxidation Protein Products in Atherosclerosis. Cardiovasc. Toxicol. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, A. Advanced Oxidation Protein Products. Part I. Mechanism of the Formation, Characteristics and Property. Pol. Merkur. Lekarski 2010, 28, 166–169. [Google Scholar] [PubMed]
- Garibaldi, S.; Barisione, C.; Marengo, B.; Ameri, P.; Brunelli, C.; Balbi, M.; Ghigliotti, G. Advanced Oxidation Protein Products-Modified Albumin Induces Differentiation of RAW264.7 Macrophages into Dendritic-like Cells Which Is Modulated by Cell Surface Thiols. Toxins 2017, 9, 27. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Altomare, A.; Rusconi, F.; Giustarini, D.; Portinaro, N.; Garavaglia, M.L.; Rossi, R.; Dalle-Donne, I.; Milzani, A. Thiol Oxidation and Di-Tyrosine Formation in Human Plasma Proteins Induced by Inflammatory Concentrations of Hypochlorous Acid. J. Proteomics 2017, 152, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.; Figarola, J.L.; Li, Y.; Swiderski, P.M.; Rahbar, S.; Natarajan, R. Proinflammatory Effects of Advanced Lipoxidation End Products in Monocytes. Diabetes 2008, 57, 879–888. [Google Scholar] [CrossRef]
- Mol, M.; Degani, G.; Coppa, C.; Baron, G.; Popolo, L.; Carini, M.; Aldini, G.; Vistoli, G.; Altomare, A. Advanced Lipoxidation End Products (ALEs) as RAGE Binders: Mass Spectrometric and Computational Studies to Explain the Reasons Why. Redox Biol. 2019, 23, 101083. [Google Scholar] [CrossRef]
- Vistoli, G.; de Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced Glycoxidation and Lipoxidation End Products (AGEs and ALEs): An Overview of Their Mechanisms of Formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef]
- Guarneri, F.; Custurone, P.; Papaianni, V.; Gangemi, S. Involvement of RAGE and Oxidative Stress in Inflammatory and Infectious Skin Diseases. Antioxidants 2021, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Nawroth, P.P. Multiple Levels of Regulation Determine the Role of the Receptor for AGE (RAGE) as Common Soil in Inflammation, Immune Responses and Diabetes Mellitus and Its Complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, F.; Moinuddin; Alam, K.; Ali, A. Physicochemical and Immunological Studies on 4-Hydroxynonenal Modified HSA: Implications of Protein Damage by Lipid Peroxidation Products in the Etiopathogenesis of SLE. Hum. Immunol. 2012, 73, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Moinuddin; Mir, A.R.; Islam, S.; Abidi, M.; Husain, M.A.; Khan, R.H. Unsaturated Aldehyde, 4-Hydroxynonenal (HNE) Alters the Structural Integrity of HSA with Consequences in the Immuno-Pathology of Rheumatoid Arthritis. Int. J. Biol. Macromol. 2018, 112, 306–314. [Google Scholar] [CrossRef]
- Prasad, A.; Rossi, C.; Manoharan, R.R.; Sedlářová, M.; Cangeloni, L.; Rathi, D.; Tamasi, G.; Pospíšil, P.; Consumi, M. Bioactive Compounds and Their Impact on Protein Modification in Human Cells. Int. J. Mol. Sci. 2022, 23, 7424. [Google Scholar] [CrossRef]
- Qiang, M.; Xu, Y.; Lu, Y.; He, Y.; Han, C.; Liu, Y.; He, R. Autofluorescence of MDA-Modified Proteins as an in Vitro and in Vivo Probe in Oxidative Stress Analysis. Protein Cell 2014, 5, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Piwowar, A. Biochemical and Clinical Aspects of Advanced Oxidation Protein Products in Kidney Diseases and Metabolic Disturbances. Postepy Hig. Med. Dosw. 2014, 68, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Winsz-Szczotka, K.; Kuznik-Trocha, K.; Klimek, K.; Olczyk, K. Age- and Gender-Related Alteration in Plasma Advanced Oxidation Protein Products (AOPP) and Glycosaminoglycan (GAG) Concentrations in Physiological Ageing. Clin. Chem. Lab. Med. 2012, 50, 557–563. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Ładny, J.R. Salivary Antioxidant Barrier, Redox Status, and Oxidative Damage to Proteins and Lipids in Healthy Children, Adults, and the Elderly. Oxid. Med. Cell. Longev. 2019, 2019, 4393460. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and Diabetes as High-Risk Factors for Severe Coronavirus Disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Motaib, I.; Zbiri, S.; Elamari, S.; Haoudar, A.; Chadli, A.; el Kettani, C. Cardiovascular Risk Factors and the Severity of COVID-19 Disease. Cureus 2021, 13, e15486. [Google Scholar] [CrossRef] [PubMed]
- Mozzini, C.; Girelli, D. The Role of Neutrophil Extracellular Traps in Covid-19: Only an Hypothesis or a Potential New Field of Research? Thromb. Res. 2020, 191, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Nakashima, R.; Enomoto, M.; Koike, Y.; Zhao, X.; Yip, K.; Huang, S.H.; Waldron, J.N.; Ikura, M.; Liu, F.F.; et al. Plasma Redox Imbalance Caused by Albumin Oxidation Promotes Lung-Predominant NETosis and Pulmonary Cancer Metastasis. Nat. Commun. 2018, 9, 5116. [Google Scholar] [CrossRef]
- Szturmowicz, M.; Demkow, U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021, 22, 8854. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef]
- Stig, B.; Hajdú, N. Endproducts and Receptors of Advanced Glycation and Lipoxidation (AGE, ALE, RAGE) and Chronic Diseases from the Perspective of Food and Nutrition. Orv. Hetil. 2008, 149, 771–778. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sánchez-de Prada, L.; Gorgojo, Ó.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid Peroxidation as a Hallmark of Severity in COVID-19 Patients. Redox Biol. 2021, 48, 102181. [Google Scholar] [CrossRef] [PubMed]
- Žarković, N.; Orehovec, B.; Milković, L.; Baršić, B.; Tatzber, F.; Wonisch, W.; Tarle, M.; Kmet, M.; Mataić, A.; Jakovčević, A.; et al. Preliminary Findings on the Association of the Lipid Peroxidation Product 4-Hydroxynonenal with the Lethal Outcome of Aggressive COVID-19. Antioxidants 2021, 10, 1341. [Google Scholar] [CrossRef]
- Domingues, R.M.; Domingues, P.; Melo, T.; Pérez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation Adducts with Peptides and Proteins: Deleterious Modifications or Signaling Mechanisms? J. Proteomics 2013, 92, 110–131. [Google Scholar] [CrossRef]
- Snell, J.A.; Jandova, J.; Wondrak, G.T. Hypochlorous Acid: From Innate Immune Factor and Environmental Toxicant to Chemopreventive Agent Targeting Solar UV-Induced Skin Cancer. Front. Oncol. 2022, 12, 887220. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; García-Vicente, R.; Morales, M.L.; Ortiz-Ruiz, A.; Martínez-López, J.; Linares, M. Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies. Antioxidants 2020, 9, 1212. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and Biochemistry of 4-Hydroxynonenal, Malonaldehyde and Related Aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Burcham, P.C.; Kuhan, Y.T. Introduction of Carbonyl Groups into Proteins by the Lipid Peroxidation Product, Malondialdehyde. Biochem. Biophys. Res. Commun. 1996, 220, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Schultz, M.B.; Sinclair, D.A. NAD+ in COVID-19 and Viral Infections. Trends Immunol. 2022, 43, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.M.; Almaliki, M.S. Influence of NAD+ as an Ageing-Related Immunomodulator on COVID 19 Infection: A Hypothesis. J. Infect. Public Health 2020, 13, 1196–1201. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Chellappa, K.; Baur, J.A. Age-Related NAD+ Decline. Exp. Gerontol. 2020, 134, 110888. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD + in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Nacarelli, T.; Zhang, R. NAD+ Metabolism Controls Inflammation during Senescence. Mol. Cell. Oncol. 2019, 6, 1605819. [Google Scholar] [CrossRef]
- Zapata-Pérez, R.; Wanders, R.J.A.; Karnebeek, C.D.M.; Houtkooper, R.H. NAD + Homeostasis in Human Health and Disease. EMBO Mol. Med. 2021, 13, e13943. [Google Scholar] [CrossRef]
- Miller, R.; Wentzel, A.R.; Richards, G.A. COVID-19: NAD+ Deficiency May Predispose the Aged, Obese and Type2 Diabetics to Mortality through Its Effect on SIRT1 Activity. Med. Hypotheses 2020, 144, 110044. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.; Pang, H.; Ma, T.; Ye, Q.; Chen, Q.; Chen, H.; Hu, Z.; Qin, C.F.; Xu, Z. Treatment of SARS-CoV-2-Induced Pneumonia with NAD+ and NMN in Two Mouse Models. Cell Discov. 2022, 8, 38. [Google Scholar] [CrossRef]
- Chatterjee, N.A.; Jensen, P.N.; Harris, A.W.; Nguyen, D.D.; Huang, H.D.; Cheng, R.K.; Savla, J.J.; Larsen, T.R.; Gomez, J.M.D.; Du-Fay-de-Lavallaz, J.M.; et al. Admission Respiratory Status Predicts Mortality in COVID-19. Influenza Other Respir. Viruses 2021, 15, 569–572. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Marzio, M.A.L.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic Factors for Severity and Mortality in Patients Infected with COVID-19: A Systematic Review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef] [PubMed]
- Akoumianaki, E.; Vaporidi, K.; Bolaki, M.; Georgopoulos, D. Happy or Silent Hypoxia in COVID-19–A Misnomer Born in the Pandemic Era. Front. Physiol. 2021, 12, 745634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, B.; Zhang, J.; He, D.; Zhang, Q.; Pan, C.; Yuan, Q.; Shi, Y.; Tang, H.; Xu, F.; et al. ALDH2 (Aldehyde Dehydrogenase 2) Protects against Hypoxia-Induced Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2303–2319. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Grune, T.; Müller, R.; Siems, W.G.; Wauer, R.R. Increased Levels of Lipid Peroxidation Products Malondialdehyde and 4-Hydroxynonenal after Perinatal Hypoxia. Pediatr. Res. 1996, 40, 15–20. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Longini, M.; Terzuoli, L.; Bracci, R. Total Hydroperoxide and Advanced Oxidation Protein Products in Preterm Hypoxic Babies. Pediatr. Res. 2000, 47, 221–224. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abdel-Baset, A.A.; Younis, M.A.; Mahmoud, M.G.; Shafik, N.S. Liver Function Test Abnormalities in COVID-19 Patients and Factors Affecting Them—A Retrospective Study. Clinic. Exp. Hepatol. 2021, 7, 297–304. [Google Scholar] [CrossRef]
- Ceci, F.M.; Fiore, M.; Gavaruzzi, F.; Angeloni, A.; Lucarelli, M.; Scagnolari, C.; Bonci, E.; Gabanella, F.; di Certo, M.G.; Barbato, C.; et al. Early Routine Biomarkers of SARS-CoV-2 Morbidity and Mortality: Outcomes from an Emergency Section. Diagnostics 2022, 12, 176. [Google Scholar] [CrossRef]
- Łykowska-Szuber, L.; Wołodźko, K.; Rychter, A.M.; Krela-Kaźmierczak, I.; Dobrowolska, A.; Szymczak-Tomczak, A. Liver Injury in Patients with Coronavirus Disease 2019 (COVID-19)—A Narrative Review. J. Clin. Med. 2021, 10, 5048. [Google Scholar] [CrossRef]
- Neu, B.; Wenby, R.; Meiselman, H.J. Effects of Dextran Molecular Weight on Red Blood Cell Aggregation. Biophys. J. 2008, 95, 3059–3065. [Google Scholar] [CrossRef] [Green Version]
- Levin, G.Y.; Egorihina, M.N. The Role of Oxidized Albumin in Blood Cell Aggregation Disturbance in Burn Disease. Int. J. Burn. Trauma 2013, 3, 115–121. [Google Scholar]
- Litvinov, R.I.; Weisel, J.W. Role of Red Blood Cells in Haemostasis and Thrombosis. ISBT Sci. Ser. 2017, 12, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, A.H.; Doctor, A. Red Blood Cell Contribution to Hemostasis. Front. Pediatrics 2021, 9, 629824. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Red Blood Cells in Thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef]
- Heinrich, F.; Roedl, K.; Jarczak, D.; Goebels, H.L.; Heinemann, A.; Schäfer, U.; Ludwig, F.; Bachmann, M.; Bein, B.; Weber, C.F.; et al. New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19-A Large Postmortem and Clinical Analysis. Viruses 2022, 14, 811. [Google Scholar] [CrossRef]
- Jenner, W.J.; Gorog, D.A. Incidence of Thrombotic Complications in COVID-19: On Behalf of ICODE: The International COVID-19 Thrombosis Biomarkers Colloquium. J. Thromb. Thrombolysis 2021, 52, 999–1006. [Google Scholar] [CrossRef]
- Basili, S.; Carnevale, R.; Nocella, C.; Bartimoccia, S.; Raparelli, V.; Talerico, G.; Stefanini, L.; Romiti, G.F.; Perticone, F.; Corazza, G.R.; et al. Serum Albumin Is Inversely Associated with Portal Vein Thrombosis in Cirrhosis. Hepatol. Commun. 2019, 3, 504–512. [Google Scholar] [CrossRef]
- Violi, F.; Ceccarelli, G.; Loffredo, L.; Alessandri, F.; Cipollone, F.; D’Ardes, D.; D’Ettorre, G.; Pignatelli, P.; Venditti, M.; Mastroianni, C.M.; et al. Albumin Supplementation Dampens Hypercoagulability in COVID-19: A Preliminary Report. Thromb. Haemost. 2021, 121, 102–105. [Google Scholar] [CrossRef]
- Zhai, M.; Cao, S.; Lu, J.; Xu, H.; Xia, M.; Li, Z. The Relationship between the Fibrinogen to Albumin Ratio and Early Outcomes in Patients with Acute Pontine Infarction. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296211067260. [Google Scholar] [CrossRef]
- Wybranowski, T.; Ziomkowska, B.; Cyrankiewicz, M.; Kruszewski, S. The Impact of Oxidative Stress on Binding of Drugs with Plasma Proteins Studied by Fluorescence Anisotropy Methods. Gen. Physiol. Biophys. 2018, 37, 647–655. [Google Scholar] [CrossRef]
- Oettl, K.; Birner-Gruenberger, R.; Spindelboeck, W.; Stueger, H.P.; Dorn, L.; Stadlbauer, V.; Putz-Bankuti, C.; Krisper, P.; Graziadei, I.; Vogel, W.; et al. Oxidative Albumin Damage in Chronic Liver Failure: Relation to Albumin Binding Capacity, Liver Dysfunction and Survival. J. Hepatol. 2013, 59, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Kragh-Hansen, U.; Kawai, K.; Maruyama, T.; Yamasaki, Y.; Takakura, Y.; Otagiri, M. Validation of the Chloramine-T Induced Oxidation of Human Serum Albumin as a Model for Oxidative Damage in Vivo. Pharm. Res. 2003, 20, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Mera, K.; Takeo, K.; Izumi, M.; Maruyama, T.; Nagai, R.; Otagiri, M. Effect of Reactive-Aldehydes on the Modification and Dysfunction of Human Serum Albumin. J. Pharm. Sci. 2010, 99, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G. Albumin Infusion in Critically Ill COVID-19 Patients: Hemodilution and Anticoagulation. Int. J. Mol. Sci. 2021, 22, 7126. [Google Scholar] [CrossRef]
- Boldt, J. The Good, the Bad, and the Ugly: Should We Completely Banish Human Albumin from Our Intensive Care Units? Anesth. Analg. 2000, 91, 887–895. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, T.; Xu, F.; Ding, J.; Yang, B.; Ma, Q.; Wu, Z.; Lyu, J.; Wang, Z. Infusion of Human Albumin on Acute Pancreatitis Therapy: New Tricks for Old Dog? Front. Pharmacol. 2022, 13, 842108. [Google Scholar] [CrossRef]
- Melia, D.; Post, B. Human Albumin Solutions in Intensive Care: A Review. J. Intensive Care Soc. 2021, 22, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.J.; Orellana-Jimenez, C.; Melot, C.; de Backer, D.; Berre, J.; Leeman, M.; Brimioulle, S.; Appoloni, O.; Creteur, J.; Vincent, J.L. Albumin Administration Improves Organ Function in Critically Ill Hypoalbuminemic Patients: A Prospective, Randomized, Controlled, Pilot Study. Crit. Care Med. 2006, 34, 2536–2540. [Google Scholar] [CrossRef]
- Uhlig, C.; Silva, P.L.; Deckert, S.; Schmitt, J.; de Abreu, M.G. Albumin versus Crystalloid Solutions in Patients with the Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Crit. Care 2014, 18, R10. [Google Scholar] [CrossRef]
- Michelis, R.; Kristal, B.; Snitkovsky, T.; Sela, S. Oxidative Modifications Impair Albumin Quantification. Biochem. Biophys. Res. Commun. 2010, 401, 137–142. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance (2020); World Health Organization: Geneva, Switzerland, 2020. Available online: https://apps.who.int/iris/handle/10665/331446 (accessed on 7 July 2022).
- Hanasand, M.; Omdal, R.; Norheim, K.B.; Gøransson, L.G.; Brede, C.; Jonsson, G. Improved Detection of Advanced Oxidation Protein Products in Plasma. Clin. Chim. Acta 2012, 413, 901–906. [Google Scholar] [CrossRef] [PubMed]
| Parameters (Units) | Median | Interquartile Rang | Reference Values | Albumin | AOPPs | AOPPs/Albumin | |||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||||
| Age (years) | 64.5 | 51–72 | −0.260 | 0.035 | −0.021 | 0.868 | 0.068 | 0.585 | |
| Symptoms (days) | 7 | 5–10 | −0.142 | 0.256 | 0.318 | 0.009 | 0.295 | 0.016 | |
| WBC (103/µL) | 6.8 | 5.1–9.6 | 4.0–10.0 | −0.214 | 0.084 | −0.035 | 0.778 | 0.055 | 0.661 |
| Neutrophils (103/µL) | 4.8 | 3.6–7.5 | 2.5–5.0 | −0.192 | 0.125 | 0.005 | 0.969 | 0.075 | 0.553 |
| Lymphocytes (103/µL) | 0.9 | 0.7–1.2 | 1.5–3.5 | −0.031 | 0.805 | −0.115 | 0.363 | −0.072 | 0.571 |
| RBC (106/µL) | 4.45 | 4.2–4.8 | 4.5–5.5 | 0.194 | 0.118 | −0.162 | 0.195 | −0.201 | 0.106 |
| Hgb (g/dL) | 13.7 | 12.7–14.5 | 14.0–18.0 | 0.113 | 0.365 | −0.172 | 0.168 | −0.181 | 0.146 |
| PLT (103/µL) | 211 | 172–292 | 130–350 | −0.163 | 0.192 | −0.012 | 0.923 | 0.048 | 0.702 |
| CRP (mg/L) | 84 | 42–138 | <5.0 | −0.235 | 0.058 | 0.323 | 0.008 | 0.357 | 0.003 |
| Procalcitonin (ng/mL) | 0.08 | 0.05–0.15 | <0.05 | −0.480 | <0.001 | 0.309 | 0.012 | 0.388 | 0.001 |
| LDH (U/L) | 645 | 543–885 | 225–450 | −0.437 | <0.001 | 0.362 | 0.003 | 0.459 | <0.001 |
| D-Dimers (ng/mL) | 941 | 735–1574 | <500 | −0.477 | <0.001 | 0.453 | <0.001 | 0.534 | <0.001 |
| Troponin (ng/L) | 10.6 | 6.2–21 | <19.0 | −0.268 | 0.030 | 0.238 | 0.054 | 0.309 | 0.012 |
| Creatinine (mg/dL) | 0.97 | 0.87–1.14 | 0.8–1.3 | −0.067 | 0.591 | 0.162 | 0.195 | 0.177 | 0.155 |
| CPK (U/L) | 142 | 76–231 | 25–200 | 0.008 | 0.952 | 0.147 | 0.238 | 0.120 | 0.336 |
| AST (U/L) | 53 | 36–70 | <37 | −0.307 | 0.012 | 0.362 | 0.003 | 0.417 | <0.001 |
| ALT (U/L) | 43 | 30–66 | <40 | −0.193 | 0.120 | 0.245 | 0.047 | 0.282 | 0.022 |
| IL-6 (pg/mL) | 13.1 | 4.8–36 | <7.0 | −0.009 | 0.942 | −0.171 | 0.177 | −0.166 | 0.189 |
| HRCT score | 0.25 | 0.14–0.41 | −0.423 | <0.001 | 0.348 | 0.004 | 0.433 | <0.001 | |
| Albumin (g/L) | 34 | 31–37 | 39–51 | 1.000 | - | −0.323 | 0.008 | −0.593 | <0.001 |
| AOPPs (µM) | 13.5 | 11.4–16.3 | −0.323 | 0.008 | 1.000 | - | 0.932 | <0.001 | |
| AOPPs/Albumin (µM/g) | 0.39 | 0.33–0.50 | −0.593 | <0.001 | 0.932 | <0.001 | 1.000 | - | |
| A | 1W | 6M | 12M | |
|---|---|---|---|---|
| A | <0.001 | <0.001 | 0.016 | |
| 1W | <0.001 | <0.001 | 0.004 | |
| 6M | <0.001 | <0.001 | 0.055 | |
| 12M | 0.016 | 0.004 | 0.055 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wybranowski, T.; Napiórkowska, M.; Bosek, M.; Pyskir, J.; Ziomkowska, B.; Cyrankiewicz, M.; Pyskir, M.; Pilaczyńska-Cemel, M.; Rogańska, M.; Kruszewski, S.; et al. Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences. Int. J. Mol. Sci. 2022, 23, 10103. https://doi.org/10.3390/ijms231710103
Wybranowski T, Napiórkowska M, Bosek M, Pyskir J, Ziomkowska B, Cyrankiewicz M, Pyskir M, Pilaczyńska-Cemel M, Rogańska M, Kruszewski S, et al. Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences. International Journal of Molecular Sciences. 2022; 23(17):10103. https://doi.org/10.3390/ijms231710103
Chicago/Turabian StyleWybranowski, Tomasz, Marta Napiórkowska, Maciej Bosek, Jerzy Pyskir, Blanka Ziomkowska, Michał Cyrankiewicz, Małgorzata Pyskir, Marta Pilaczyńska-Cemel, Milena Rogańska, Stefan Kruszewski, and et al. 2022. "Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences" International Journal of Molecular Sciences 23, no. 17: 10103. https://doi.org/10.3390/ijms231710103
APA StyleWybranowski, T., Napiórkowska, M., Bosek, M., Pyskir, J., Ziomkowska, B., Cyrankiewicz, M., Pyskir, M., Pilaczyńska-Cemel, M., Rogańska, M., Kruszewski, S., & Przybylski, G. (2022). Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences. International Journal of Molecular Sciences, 23(17), 10103. https://doi.org/10.3390/ijms231710103





