PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of [68Ga]Ga-NODAGA-AMBA and [44Sc]Sc-NODAGA-AMBA
Abstract
1. Introduction
2. Results
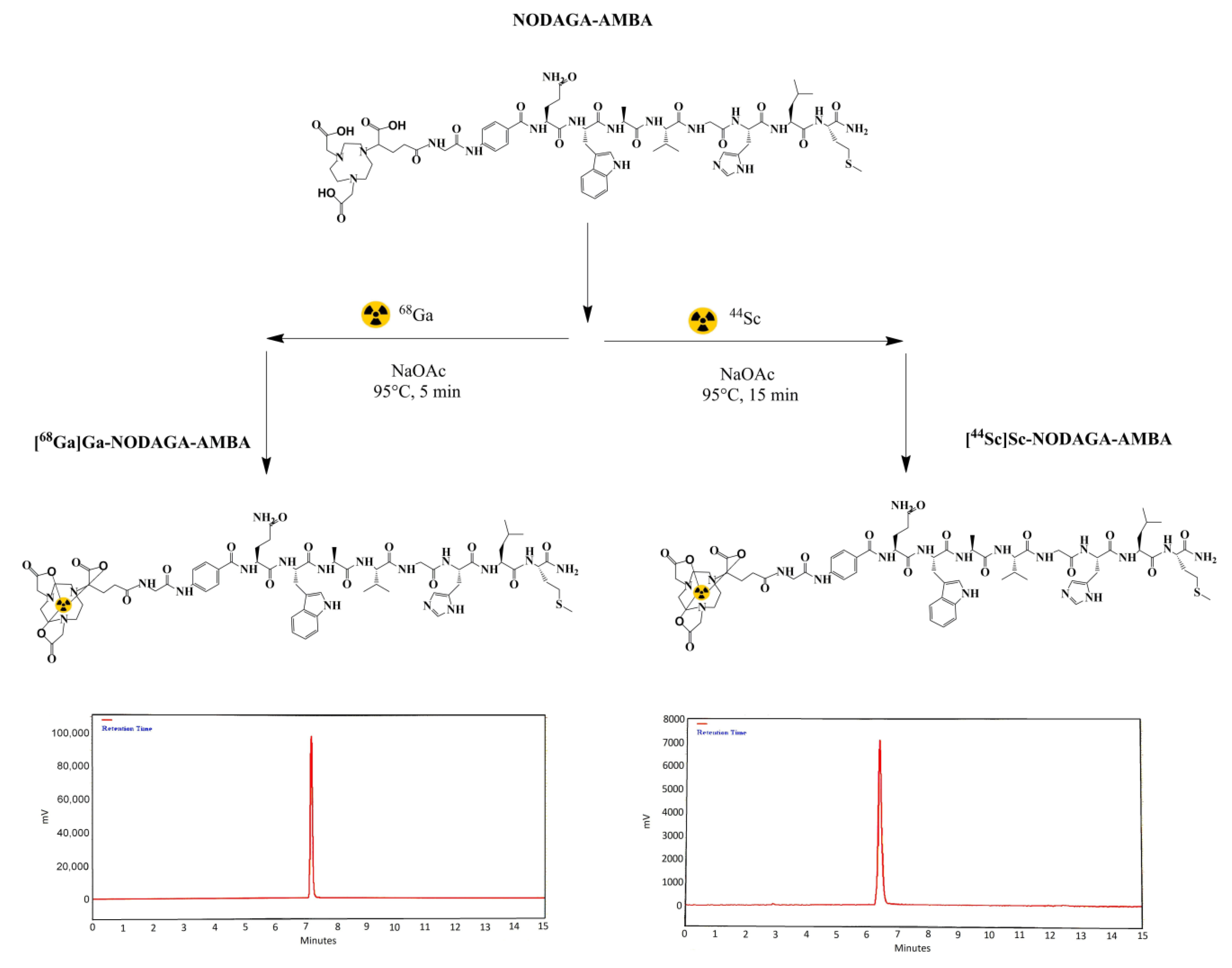
2.1. Radiochemistry
2.2. LogP and Serum Stability Measurements
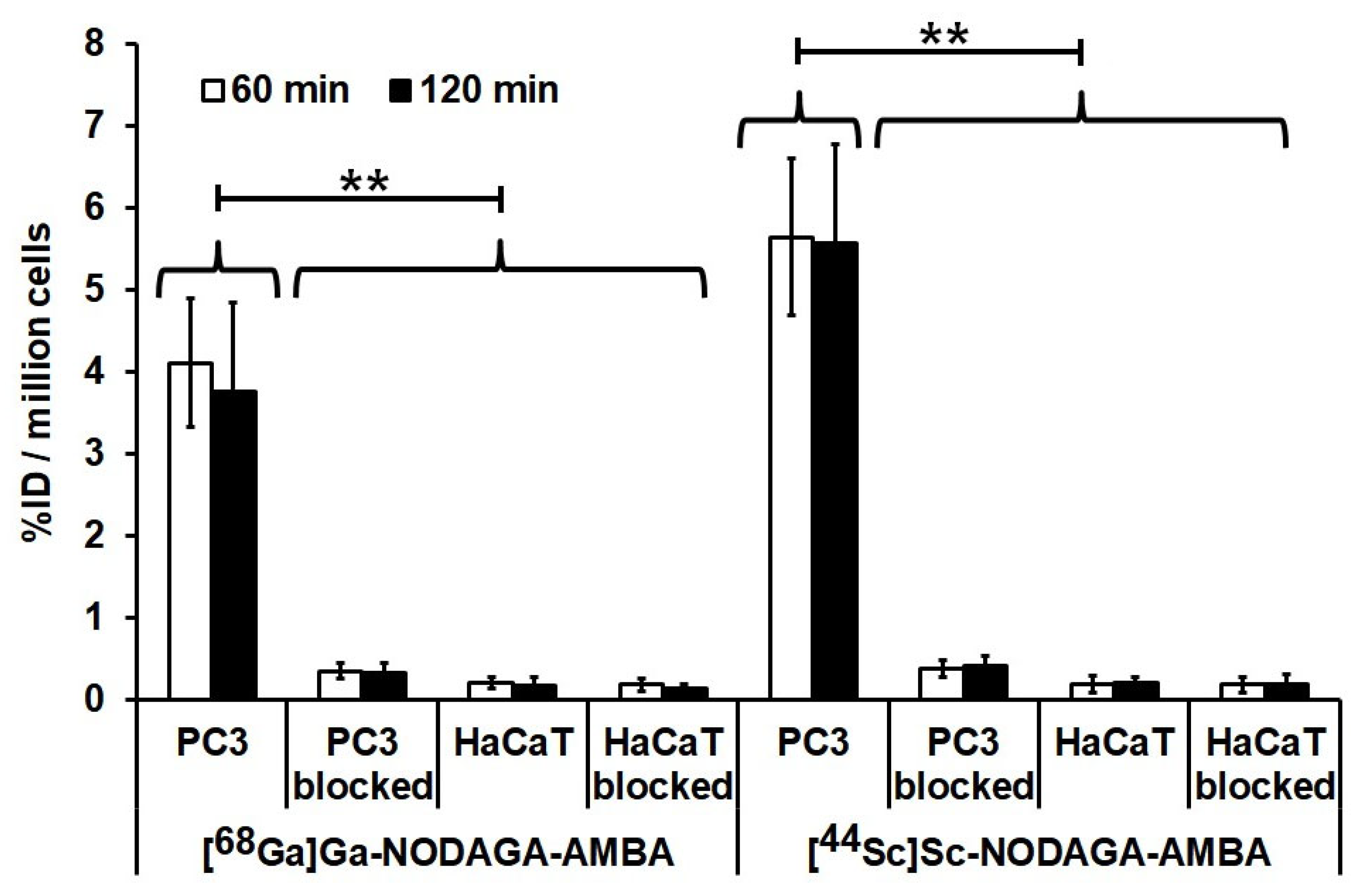
2.3. In Vitro Cellular Uptake Studies
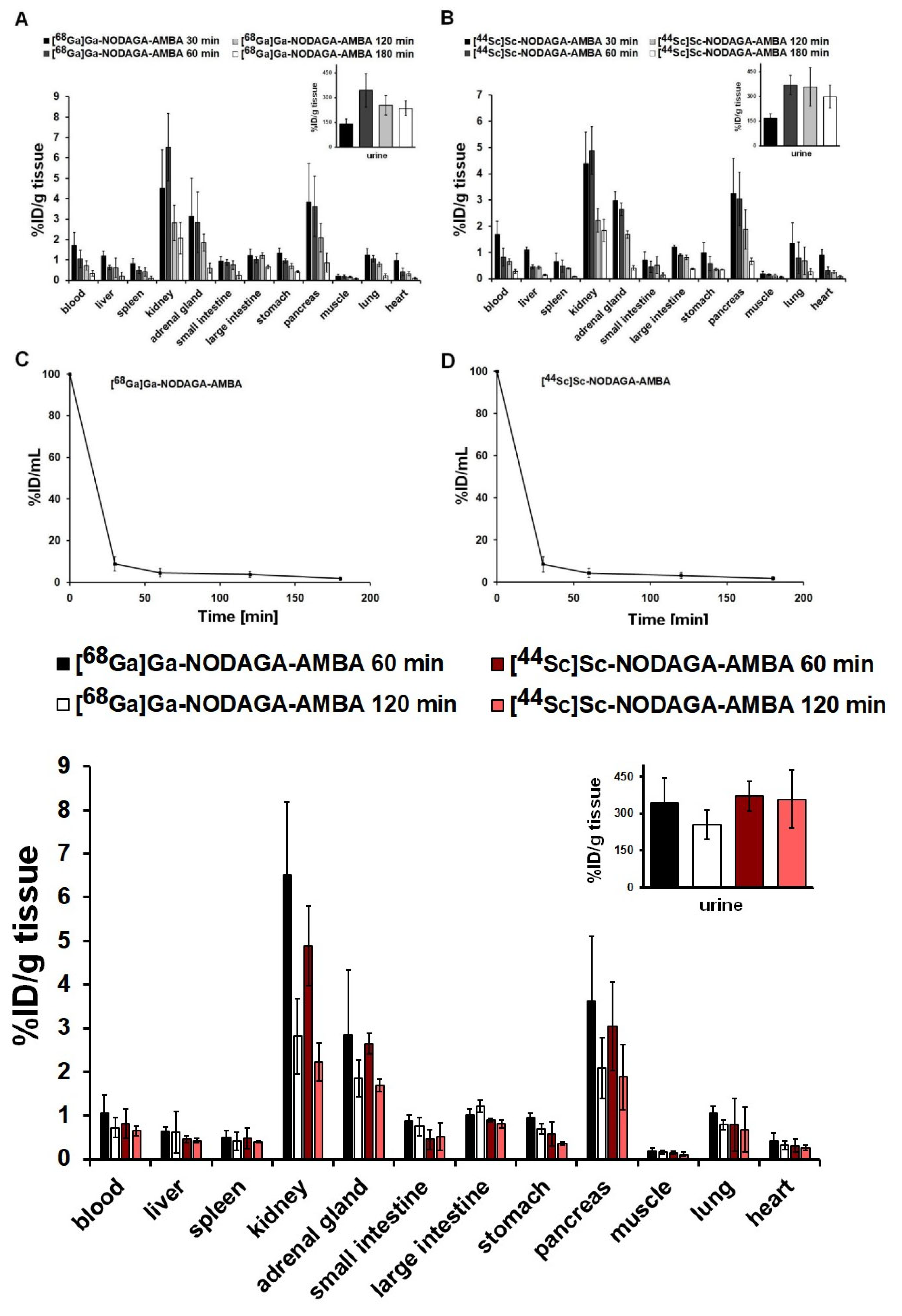
2.4. Biodistribution and Pharmacokinetic Studies in Healthy Mice
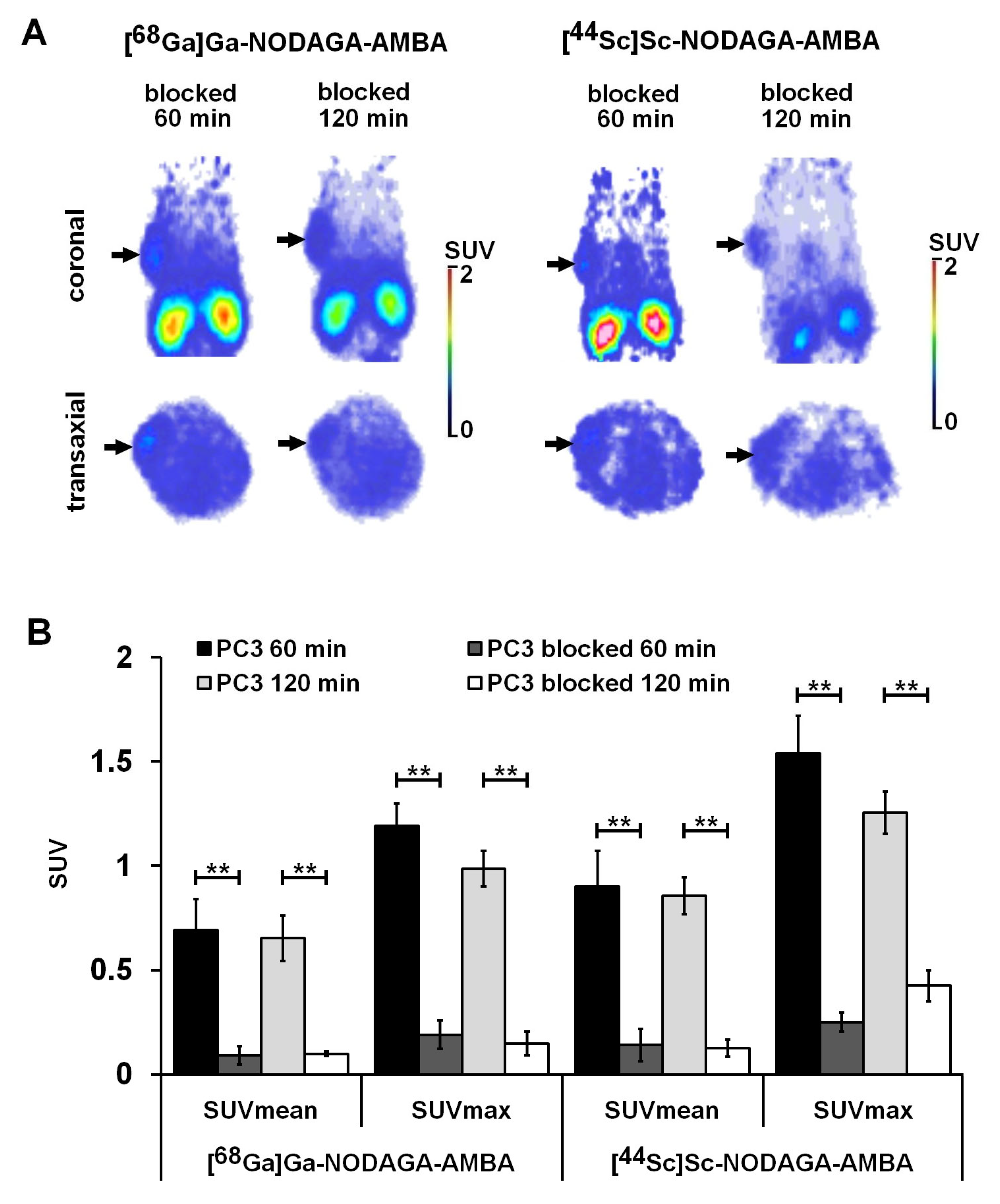
2.5. PET Imaging and Ex Vivo Biodistribution Studies of PC-3 Tumor-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. 68Ga-Labelling of NODAGA-AMBA
4.3. 44Sc-Labelling of NODAGA-AMBA
4.4. Determination of Partition Coefficient and Metabolic Stability of 68Ga- and 44Sc-Labelled NODAGA-AMBA
4.5. Cell Lines
4.6. Cellular Uptake Studies
4.7. In Vivo Tumor Model
4.8. In Vivo PET Imaging
4.9. Ex Vivo Biodistribution Studies
4.10. Pharmacokinetic Studies and In Vivo Stability
4.11. Blocking Experiments
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMBA | aminobenzoic-acid |
| AR | androgen receptor |
| BBN | bombesin |
| BB2 | bombesin type 2 receptor |
| BFC | bifunctional chelator |
| DOTA | tetraazacyclododecane-tetrayl-tetraacetic acid |
| DTPA | diethylenetriamine-pentaacetic acid |
| 18F-FCH | 18F-fluorocholine |
| GRP | gastrin-releasing peptide |
| GRPR | gastrin-releasing peptide receptor |
| NET | neuroendocrine |
| NODAGA | aminoethylamino-carboxy-oxobutyl-triazonane-diyldiacetic acid |
| NOTA | triazacyclononane-triyl-triacetic acid |
| PCa | prostate cancer |
| PET | positron emission tomography |
| SUV | standardized uptake values |
| T/M | Tumor-to-muscle |
| VOI | volumes of interest |
References
- Grant, K.; Lindenberg, M.L.; Shebel, H.; Pang, Y.; Agarwal, H.K.; Bernardo, M.; Kurdziel, K.A.; Turkbey, B.; Choyke, P.L. Functional and Molecular Imaging of Localized and Recurrent Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, X.; Liu, H.; Bu, L.; Ma, X.; Cheng, K.; Li, J.; Tian, M.; Zhang, H.; Cheng, Z. A Comparative Study of Radiolabeled Bombesin Analogs for the PET Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 2132–2138. [Google Scholar] [CrossRef]
- Faviana, P.; Boldrini, L.; Erba, P.A.; Di Stefano, I.; Manassero, F.; Bartoletti, R.; Galli, L.; Gentile, C.; Bardi, M. Gastrin-Releasing Peptide Receptor in Low Grade Prostate Cancer: Can It Be a Better Predictor Than Prostate-Specific Membrane Antigen? Front. Oncol. 2021, 11, 650249. [Google Scholar] [CrossRef] [PubMed]
- Markwalder, R.; Reubi, J.C. Gastrin-Releasing Peptide Receptors in the Human Prostate: Relation to Neoplastic Transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar] [PubMed]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian Bombesin Receptors: Nomenclature, Distribution, Pharmacology, Signaling, and Functions in Normal and Disease States. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of High-Affinity Receptors for Bombesin/Gastrin Releasing Peptide on the Human Prostate Cancer Cell Lines PC-3 and DU-145: Internalization of Receptor Bound125I-(Tyr4) Bombesin by Tumor Cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; Iori, M.; Capponi, P.C.; Atti, G.; Rubagotti, S.; Martin, R.; Brennauer, A.; Müller, M.; Bergmann, R.; Erba, P.A.; et al. Influence of Different Chelators on the Radiochemical Properties of a 68-Gallium Labelled Bombesin Analogue. Nucl. Med. Biol. 2014, 41, 24–35. [Google Scholar] [CrossRef]
- Graham, M.M.; Menda, Y. Radiopeptide Imaging and Therapy in the United States. J. Nucl. Med. 2011, 52, 56S–63S. [Google Scholar] [CrossRef]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.-L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1,4,7,10-Tetraazacyclododecane-1,4,7,10-Tetraacetic Acid–Conjugated Bombesin-Based Radioantagonist for the Labeling with Single-Photon Emission Computed Tomography, Positron Emission Tomography, and Therapeutic Radionuclides. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar] [CrossRef]
- Lantry, L.E.; Cappelletti, E.; Maddalena, M.E.; Fox, J.S.; Feng, W.; Chen, J.; Thomas, R.; Eaton, S.M.; Bogdan, N.J.; Arunachalam, T.; et al. 177Lu-AMBA: Synthesis and Characterization of a Selective 177Lu-Labeled GRP-R Agonist for Systemic Radiotherapy of Prostate Cancer. J. Nucl. Med. 2006, 47, 1144–1152. [Google Scholar]
- Anderson, C.J.; Ferdani, R. Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical and Clinical Research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Makris, G.; Shegani, A.; Kankanamalage, P.H.A.; Kuchuk, M.; Bandari, R.P.; Smith, C.J.; Hennkens, H.M. Preclinical Evaluation of Novel 64 Cu-Labeled Gastrin-Releasing Peptide Receptor Bioconjugates for PET Imaging of Prostate Cancer. Bioconjug. Chem. 2021, 32, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A Powerful Generator-Based Alternative to Cyclotron-Based PET Radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; De Pietri, G.; Fraternali, A.; Grassi, E.; Sghedoni, R.; Fioroni, F.; Roesch, F.; Versari, A.; Salvo, D. Validation of 68Ge/68Ga Generator Processing by Chemical Purification for Routine Clinical Application of 68Ga-DOTATOC. Nucl. Med. Biol. 2008, 35, 721–724. [Google Scholar] [CrossRef]
- Schroeder, R.P.J.; Müller, C.; Reneman, S.; Melis, M.L.; Breeman, W.A.P.; de Blois, E.; Bangma, C.H.; Krenning, E.P.; van Weerden, W.M.; de Jong, M. A Standardised Study to Compare Prostate Cancer Targeting Efficacy of Five Radiolabelled Bombesin Analogues. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1386–1396. [Google Scholar] [CrossRef]
- Schroeder, R.P.J.; van Weerden, W.M.; Krenning, E.P.; Bangma, C.H.; Berndsen, S.; Grievink-de Ligt, C.H.; Groen, H.C.; Reneman, S.; de Blois, E.; Breeman, W.A.P.; et al. Gastrin-Releasing Peptide Receptor-Based Targeting Using Bombesin Analogues Is Superior to Metabolism-Based Targeting Using Choline for in Vivo Imaging of Human Prostate Cancer Xenografts. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1257–1266. [Google Scholar] [CrossRef][Green Version]
- Bratanovic, I.J.; Zhang, C.; Zhang, Z.; Kuo, H.; Colpo, N.; Zeisler, J.; Merkens, H.; Uribe, C.; Lin, K.; Bénard, F. A Radiotracer for Molecular Imaging and Therapy of Gastrin-Releasing Peptide Receptor–Positive Prostate Cancer. J. Nucl. Med. 2022, 63, 424–430. [Google Scholar] [CrossRef]
- Forgács, V.; Fekete, A.; Gyuricza, B.; Szücs, D.; Trencsényi, G.; Szikra, D. Methods for the Determination of Transition Metal Impurities in Cyclotron-Produced Radiometals. Pharmaceuticals 2022, 15, 147. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Reber, J.; Fischer, C.; Zhernosekov, K.; Türler, A.; Schibli, R. Promises of Cyclotron-Produced 44 Sc as a Diagnostic Match for Trivalent β − -Emitters: In Vitro and In Vivo Study of a 44 Sc-DOTA-Folate Conjugate. J. Nucl. Med. 2013, 54, 2168–2174. [Google Scholar] [CrossRef]
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 44Sc for Labeling of DOTA- and NODAGA-Functionalized Peptides: Preclinical in Vitro and in Vivo Investigations. EJNMMI Radiopharm. Chem. 2017, 1, 8. [Google Scholar] [CrossRef]
- Nagy, G.; Dénes, N.; Kis, A.; Szabó, J.P.; Berényi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsényi, G. Preclinical Evaluation of Melanocortin-1 Receptor (MC1-R) Specific 68Ga- and 44Sc-Labeled DOTA-NAPamide in Melanoma Imaging. Eur. J. Pharm. Sci. 2017, 106, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Szikra, D.; Trencsényi, G.; Fekete, A.; Garai, I.; Giani, A.M.; Negri, R.; Masciocchi, N.; Maiocchi, A.; Uggeri, F.; et al. AAZTA: An Ideal Chelating Agent for the Development of 44 Sc PET Imaging Agents. Angew. Chemie Int. Ed. 2017, 56, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Szücs, D.; Csupász, T.; Szabó, J.P.; Kis, A.; Gyuricza, B.; Arató, V.; Forgács, V.; Vágner, A.; Nagy, G.; Garai, I.; et al. Synthesis, Physicochemical, Labeling and In Vivo Characterization of 44Sc-Labeled DO3AM-NI as a Hypoxia-Sensitive PET Probe. Pharmaceuticals 2022, 15, 666. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for Radiotheragnostics in Tandem with 177Lu-PSMA-617—Preclinical Investigations in Comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Maecke, H.R. PET of Somatostatin Receptor–Positive Tumors Using 64 Cu- and 68 Ga-Somatostatin Antagonists: The Chelate Makes the Difference. J. Nucl. Med. 2011, 52, 1110–1118. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Wester, H.-J. Comparative Gallium-68 Labeling of TRAP-, NOTA-, and DOTA-Peptides: Practical Consequences for the Future of Gallium-68-PET. EJNMMI Res. 2012, 2, 28. [Google Scholar] [CrossRef]
- Ananias, H.; de Jong, I.; Dierckx, R.; de Wiele, C.; Helfrich, W.; Elsinga, P. Nuclear Imaging of Prostate Cancer with Gastrin-Releasing-Peptide- Receptor Targeted Radiopharmaceuticals. Curr. Pharm. Des. 2008, 14, 3033–3047. [Google Scholar] [CrossRef]
- Schroeder, R.P.J.; van Weerden, W.M.; Bangma, C.; Krenning, E.P.; de Jong, M. Peptide Receptor Imaging of Prostate Cancer with Radiolabelled Bombesin Analogues. Methods 2009, 48, 200–204. [Google Scholar] [CrossRef]
- Bologna, M.; Festuccia, C.; Muzi, P.; Biordi, L.; Ciomei, M. Bombesin Stimulates Growth of Human Prostatic Cancer Cellsin Vitro. Cancer 1990, 63, 1714–1720. [Google Scholar] [CrossRef]
- Pinski, J.; Schally, A.V.; Halmos, G.; Szepeshazi, K. Effect of Somatostatin Analog RC-160 and Bombesin/Gastrin Releasing Peptide Antagonist RC-3095 on Growth of PC-3 Human Prostate-Cancer Xenografts in Nude Mice. Int. J. Cancer 1993, 55, 963–967. [Google Scholar] [CrossRef]
- Liolios, C.C.; Xanthopoulos, S.; Loudos, G.; Varvarigou, A.D.; Sivolapenko, G.B. Co-Administration of Succinylated Gelatine with a 99m Tc-Bombesin Analogue, Effects on Pharmacokinetics and Tumor Uptake. Nucl. Med. Biol. 2016, 43, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, J. Effects of Bombesin and Its Antibody on Growth of Human Prostatic Carcinoma Cell Lines. Jpn. J. Urol. 1992, 83, 1459–1468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang-Yin, J.; Provost, C.; Cancel-Tassin, G.; Rusu, T.; Penent, M.; Radulescu, C.; Comperat, E.; Cussenot, O.; Montravers, F.; Renard-Penna, R.; et al. A Comparative Study of Peptide-Based Imaging Agents [68Ga]Ga-PSMA-11, [68Ga]Ga-AMBA, [68Ga]Ga-NODAGA-RGD and [68Ga]Ga-DOTA-NT-20.3 in Preclinical Prostate Tumour Models. Nucl. Med. Biol. 2020, 84–85, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.-F.; Thisgaard, H. In Vivo Evaluation of a Bombesin Analogue Labeled with Ga-68 and Co-55/57. Mol. Imaging Biol. 2016, 18, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Bunka, M.; Müller, C.; Vermeulen, C.; Haller, S.; Türler, A.; Schibli, R.; van der Meulen, N.P. Imaging Quality of 44Sc in Comparison with Five Other PET Radionuclides Using Derenzo Phantoms and Preclinical PET. Appl. Radiat. Isot. 2016, 110, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with 44 Sc-Labeled Cetuximab Fab Fragment. Bioconjug. Chem. 2014, 25, 2197–2204. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.A.; Woo, S.-K.; Lee, K.C.; An, G.I.; Kim, B.S.; Kim, K.I.; Lee, T.S.; Kim, C.W.; Kim, K.M.; et al. Evaluation of a 64Cu-Labeled 1,4,7-Triazacyclononane, 1-Glutaric Acid-4,7 Acetic Acid (NODAGA)-Galactose-Bombesin Analogue as a PET Imaging Probe in a Gastrin-Releasing Peptide Receptor-Expressing Prostate Cancer Xenograft Model. Int. J. Oncol. 2015, 46, 1159–1168. [Google Scholar] [CrossRef][Green Version]
- Kilgore, W.R.; Mantyh, P.W.; Mantyh, C.R.; McVey, D.C.; Vigna, S.R. Bombesin/GRP-Preferring and Neuromedin B-Preferring Receptors in the Rat Urogenital System. Neuropeptides 1993, 24, 43–52. [Google Scholar] [CrossRef]
- Fournier, P.; Dumulon-Perreault, V.; Ait-Mohand, S.; Tremblay, S.; Bénard, F.; Lecomte, R.; Guérin, B. Novel Radiolabeled Peptides for Breast and Prostate Tumor PET Imaging: 64 Cu/and 68 Ga/NOTA-PEG-[ d -Tyr 6,ΒAla 11,Thi 13,Nle 14 ]BBN(6–14). Bioconjug. Chem. 2012, 23, 1687–1693. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, J.; Hampton, L.L.; Weber, H.C. The Human Gastrin-Releasing Peptide Receptor Gene Structure, Its Tissue Expression and Promoter. Gene 2001, 264, 95–103. [Google Scholar] [CrossRef]
- Jensen, R.T.; Moody, T.; Pert, C.; Rivier, J.E.; Gardner, J.D. Interaction of Bombesin and Litorin with Specific Membrane Receptors on Pancreatic Acinar Cells. Proc. Natl. Acad. Sci. USA 1978, 75, 6139–6143. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Georgieff, M.K. Pulmonary Neuroendocrine Cells: Their Secretory Products and Their Potential Roles in Health and Chronic Lung Disease in Infancy. Am. Rev. Respir. Dis. 1989, 140, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Wharton, J.; Polak, J.M.; Bloom, S.R.; Ghatei, M.A.; Solcia, E.; Brown, M.R.; Pearse, A.G.E. Bombesin-like Immunoreactivity in the Lung. Nature 1978, 273, 769–770. [Google Scholar] [CrossRef]
- Sunday, M.E.; Hua, J.; Dai, H.B.; Nusrat, A.; Torday, J.S. Bombesin Increases Fetal Lung Growth and Maturation In Utero and in Organ Culture. Am. J. Respir. Cell Mol. Biol. 1990, 3, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.C. Regulation and Signaling of Human Bombesin Receptors and Their Biological Effects. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kertész, I.; Vida, A.; Nagy, G.; Emri, M.; Farkas, A.; Kis, A.; Angyal, J.; Dénes, N.; Szabó, J.P.; Kovács, T.; et al. In Vivo Imaging of Experimental Melanoma Tumors using the Novel Radiotracer 68Ga-NODAGA-Procainamide (PCA). J. Cancer 2017, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Jószai, I.; Enyedi, K.N.; Lakatos, S.; Garai, I.; Mező, G.; Kertész, I.; et al. In Vivo Assessment of Aminopeptidase N (APN/CD13) Specificity of Different 68Ga-Labelled NGR Derivatives Using PET/MRI Imaging. Int. J. Pharm. 2020, 589, 119881. [Google Scholar] [CrossRef] [PubMed]

| Tumor | [68Ga]Ga-NODAGA-AMBA | [44Sc]Sc-NODAGA-AMBA | ||
|---|---|---|---|---|
| 60 min | 120 min | 60 min | 120 min | |
| PC3 | 3.78 ± 0.93 ** | 3.29 ± 1.20 ** | 4.56 ± 0.45 ** | 4.14 ± 0.47 ** |
| PC3 blocked | 0.60 ± 0.22 | 0.48 ± 0.14 | 0.79 ± 0.16 | 0.69 ± 0.17 |
| PC3 T/M | 13.21 ± 2.47 ** | 21.64 ± 3.78 ** | 16.88 ± 1.96 ** | 27.57 ± 2.88 ** |
| PC3 T/M blocked | 1.97 ± 0.22 | 2.86 ± 0.47 | 2.19 ± 0.36 | 3.04 ± 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kálmán-Szabó, I.; Szabó, J.P.; Arató, V.; Dénes, N.; Opposits, G.; Jószai, I.; Kertész, I.; Képes, Z.; Fekete, A.; Szikra, D.; et al. PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of [68Ga]Ga-NODAGA-AMBA and [44Sc]Sc-NODAGA-AMBA. Int. J. Mol. Sci. 2022, 23, 10061. https://doi.org/10.3390/ijms231710061
Kálmán-Szabó I, Szabó JP, Arató V, Dénes N, Opposits G, Jószai I, Kertész I, Képes Z, Fekete A, Szikra D, et al. PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of [68Ga]Ga-NODAGA-AMBA and [44Sc]Sc-NODAGA-AMBA. International Journal of Molecular Sciences. 2022; 23(17):10061. https://doi.org/10.3390/ijms231710061
Chicago/Turabian StyleKálmán-Szabó, Ibolya, Judit P. Szabó, Viktória Arató, Noémi Dénes, Gábor Opposits, István Jószai, István Kertész, Zita Képes, Anikó Fekete, Dezső Szikra, and et al. 2022. "PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of [68Ga]Ga-NODAGA-AMBA and [44Sc]Sc-NODAGA-AMBA" International Journal of Molecular Sciences 23, no. 17: 10061. https://doi.org/10.3390/ijms231710061
APA StyleKálmán-Szabó, I., Szabó, J. P., Arató, V., Dénes, N., Opposits, G., Jószai, I., Kertész, I., Képes, Z., Fekete, A., Szikra, D., Hajdu, I., & Trencsényi, G. (2022). PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of [68Ga]Ga-NODAGA-AMBA and [44Sc]Sc-NODAGA-AMBA. International Journal of Molecular Sciences, 23(17), 10061. https://doi.org/10.3390/ijms231710061







