Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure–Activity Relationship
Abstract
:1. Introduction
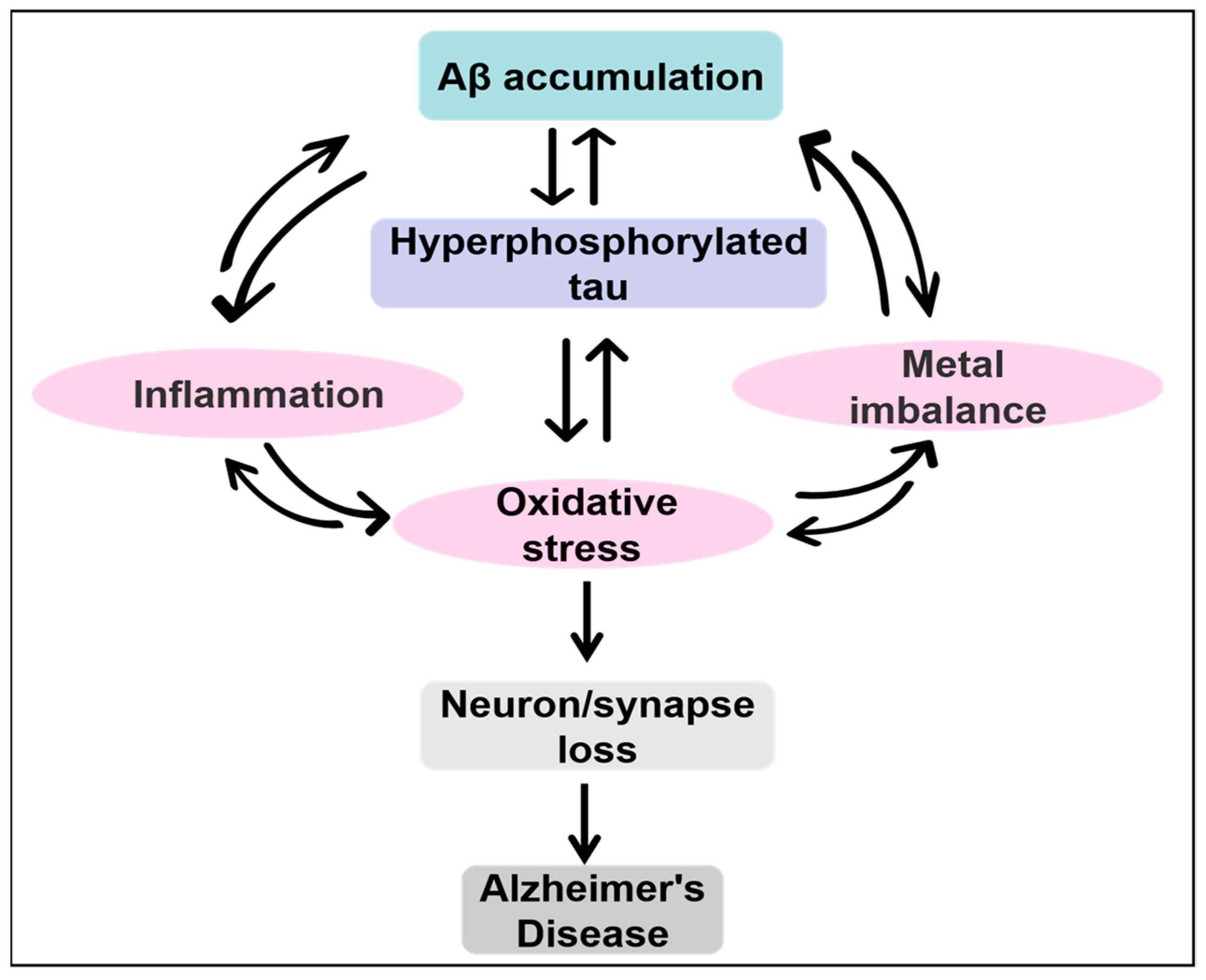
2. AD and Current Hypotheses on AD
3. Amyloid Cascade Hypothesis
4. Tau Hypothesis
5. Oxidative Stress Hypothesis
6. Inflammation Hypothesis
7. Cholinergic Hypothesis
8. Metal Ion Hypothesis
9. New Pathway of AD—The Infectious Theory
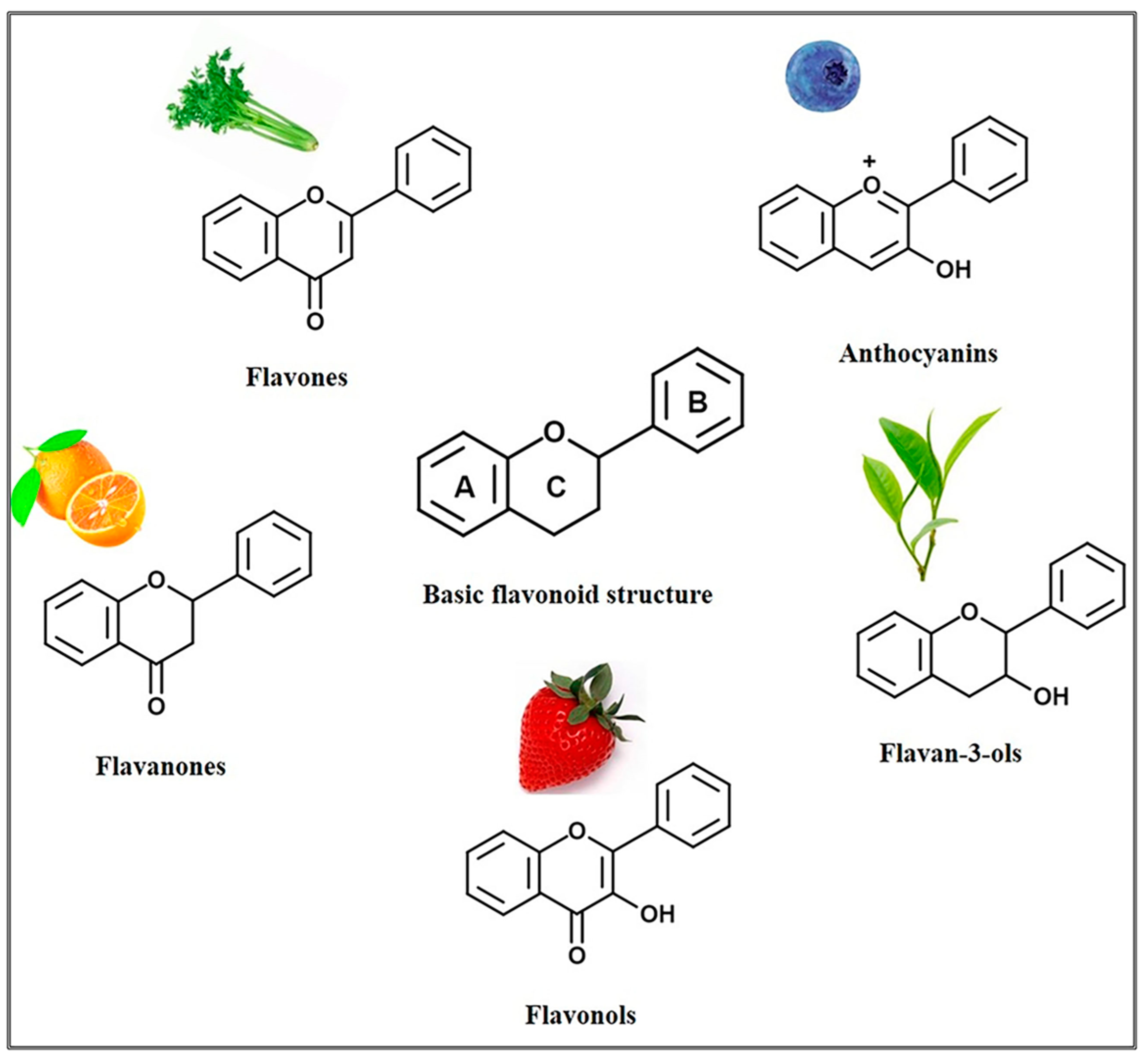
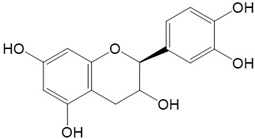
10. Flavonoids
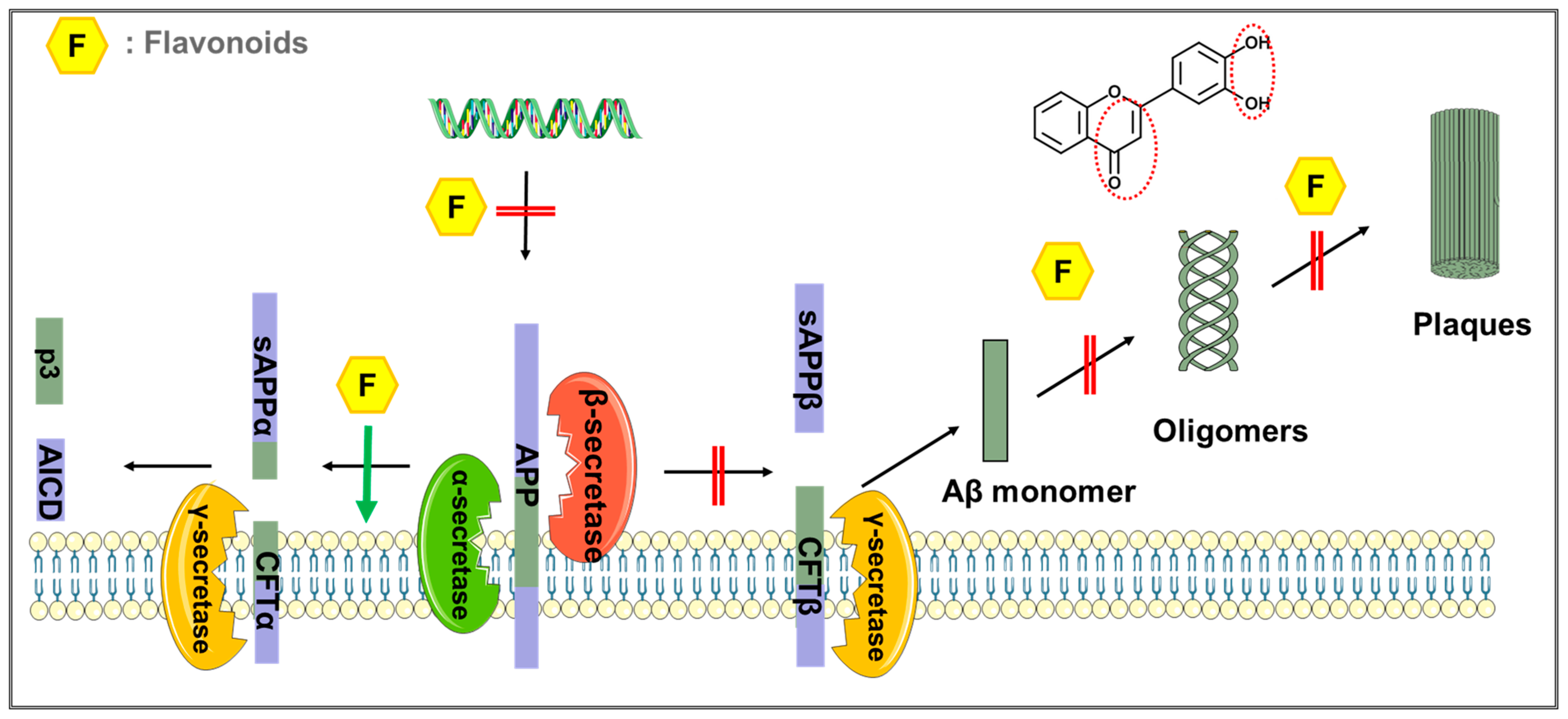
11. Flavonoids Exert Anti-AD Effects by Affecting Aβ
12. Flavonoids Exhibit Anti-AD Effects through Anti-Inflammatory Activity
13. Flavonoids Exert Anti-AD Effects through Antioxidant Activity
14. Flavonoids Exert Anti-AD Effects by Acting as Metal-Ion-Chelating Agents
15. Flavonoids Exert Anti-AD Effects by Inhibiting AChE
16. Flavonoids Exert Anti-AD Effects by Inhibiting Bacteria and Viruses
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.; Qin, W.; Zhao, L.; Jin, H.; Xu, H.; Wang, F.; et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Beking, K.; Vieira, A. Flavonoid intake and disability-adjusted life years due to Alzheimer’s and related dementias: A population-based study involving twenty-three developed countries. Public Health Nutr. 2010, 13, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.-F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- de Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxid. Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef]
- Hole, K.L.; Williams, R.J. Flavonoids as an Intervention for Alzheimer’s Disease: Progress and Hurdles Towards Defining a Mechanism of Action. Brain Plast. 2021, 6, 167–192. [Google Scholar] [CrossRef]
- Uddin, S.; Kabir, T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.; Sobarzo-Sánchez, E.; Ashraf, G.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef]
- Xia, H. Extensive metabolism of flavonoids relevant to their potential efficacy on Alzheimer’s disease. Drug Metab. Rev. 2021, 53, 563–591. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s disease: Past, present, and future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- O’Brien, C. Science History: Auguste D. and Alzheimer’s Disease. Science 1996, 273, 28. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- De Strooper, B. Proteases and Proteolysis in Alzheimer Disease: A Multifactorial View on the Disease Process. Physiol. Rev. 2010, 90, 465–494. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., III. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Sambamurti, K.; Greig, N.H.; Lahiri, D.K. Advances in the Cellular and Molecular Biology of the Beta-Amyloid Protein in Alzheimer ‘s Disease. NeuroMolecular Med. 2002, 1, 1–32. [Google Scholar] [CrossRef]
- De Felice, F.G.; Ferreira, S.T. β-Amyloid Production, Aggregation, and Clearance as Targets for Therapy in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2002, 22, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Struble, R.G.; Ala, T.; Patrylo, P.R.; Brewer, G.J.; Yan, X.-X. Is Brain Amyloid Production a Cause or a Result of Dementia of The Alzheimer’s Type? J. Alzheimer’s Dis. 2010, 22, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Varnum, M.M.; Ikezu, T. The Classification of Microglial Activation Phenotypes on Neurodegeneration and Regeneration in Alzheimer’s Disease Brain. Arch. Immunol. Et Ther. Exp. 2012, 60, 251–266. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer Disease: Mechanistic Understanding Predicts Novel Therapies. Ann. Intern. Med. 2004, 140, 627–638. [Google Scholar] [CrossRef]
- Soria-Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology, 3rd ed.; DeKosky, S.T., Asthana, S., Eds.; Elsevier: Boston, MA, USA, 2019; Volume 167, pp. 231–255. [Google Scholar] [CrossRef]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef]
- Pirscoveanu, D.F.V.; Pirici, I.; Tudorica, V.; Balseanu, T.A.; Albu, V.C.; Bondari, S.; Bumbea, A.M.; Pirscoveanu, M. Tau protein in neurodegenerative diseases–A review. Rom. J. Morphol. Embryol. 2017, 58, 1141–1150. [Google Scholar]
- Bejanin, A.; Schonhaut, D.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Pratico, M. Evidence of Oxidative Stress in Alzheimer’s Disease Brain and Antioxidant Therapy. Ann. New York Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [CrossRef]
- Sokoloff, L. Energetics of Functional Activation in Neural Tissues. Neurochem. Res. 1999, 24, 321–329. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Butterfield, D.A. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of Oxidative Stress in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, M.; Gearing, A.J.; Miller, K.M. A central role for astrocytes in the inflammatory response to β-amyloid; chemokines, cytokines and reactive oxygen species are produced. J. Neuroimmunol. 1999, 93, 182–193. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of Amyloid β-Peptide-Associated Oxidative Stress and Brain Protein Modifications in the Pathogenesis of Alzheimer’s Disease and Mild Cognitive Impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef]
- Zhang, B.; Gaiteri, C.; Bodea, L.-G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated Systems Approach Identifies Genetic Nodes and Networks in Late-Onset Alzheimer’s Disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef]
- Song, W.; Hooli, B.; Mullin, K.; Jin, S.C.; Cella, M.; Ulland, T.K.; Wang, Y.; Tanzi, R.E.; Colonna, M. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Bolos, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fillit, H.; Ding, W.; Buee, L.; Kalman, J.; Altstiel, L.; Lawlor, B.; Wolf-Klein, G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 1991, 129, 318–320. [Google Scholar] [CrossRef]
- Bauer, J.; Strauss, S.; Schreiter-Gasser, U.; Ganter, U.; Schlegel, P.; Witt, I.; Yolk, B.; Berger, M. Interleukin-6 and α-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991, 285, 111–114. [Google Scholar] [CrossRef]
- Stamouli, E.; Politis, A. Pro-inflammatory cytokines in Alzheimer’s disease. Psychiatriki 2016, 27, 264–275. [Google Scholar] [CrossRef]
- Taipa, R.; das Neves, S.P.; Sousa, A.L.; Fernandes, J.; Pinto, C.; Correia, A.P.; Santos, E.; Pinto, P.S.; Carneiro, P.; Costa, P.; et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 2019, 76, 125–132. [Google Scholar] [CrossRef]
- Onyango, I.; Jauregui, G.; Čarná, M.; Bennett, J.; Stokin, G. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef]
- Casal, C.; Serratosa, J.; Tusell, J.M. Effects of β-AP peptides on activation of the transcription factor NF-κB and in cell proliferation in glial cell cultures. Neurosci. Res. 2004, 48, 315–323. [Google Scholar] [CrossRef]
- Sastre, M.; Dewachter, I.; Landreth, G.E.; Willson, T.M.; Klockgether, T.; Van Leuven, F.; Heneka, M.T. Nonsteroidal Anti-Inflammatory Drugs and Peroxisome Proliferator-Activated Receptor-γ Agonists Modulate Immunostimulated Processing of Amyloid Precursor Protein through Regulation of β-Secretase. J. Neurosci. 2003, 23, 9796–9804. [Google Scholar] [CrossRef]
- Chen, C.-H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2011, 15, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-T.; Yu, J.; Grass, D.; De Beer, F.C.; Kindy, M.S. Inflammation-Dependent Cerebral Deposition of Serum Amyloid A Protein in a Mouse Model of Amyloidosis. J. Neurosci. 2002, 22, 5900–5909. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Valdovinos, M.G.; Williams, D.C. Relevance of Donepezil in Enhancing Learning and Memory in Special Populations: A Review of the Literature. J. Autism Dev. Disord. 2007, 37, 1883–1901. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Anderson, B.P.; Bower, J.M. Cholinergic modulation of cortical associative memory function. J. Neurophysiol. 1992, 67, 1230–1246. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bruno, J.P. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res. Rev. 1997, 23, 28–46. [Google Scholar] [CrossRef]
- Brinkman, S.D.; Gershon, S. Measurement of cholinergic drug effects on memory in alzheimer’s disease. Neurobiol. Aging 1983, 4, 139–145. [Google Scholar] [CrossRef]
- Summers, W.K.; Majovski, L.V.; Marsh, G.M.; Tachiki, K.; Kling, A. Oral Tetrahydroaminoacridine in Long-Term Treatment of Senile Dementia, Alzheimer Type. N Engl. J. Med. 1986, 315, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Akıncıoğlu, H.; Gulcin, I. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini-Rev. Med. Chem. 2020, 20, 703–715. [Google Scholar] [CrossRef]
- Lovell, M.; Robertson, J.; Teesdale, W.; Campbell, J.; Markesbery, W. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- James, S.A.; Churches, Q.I.; de Jonge, M.D.; Birchall, I.E.; Streltsov, V.; McColl, G.; Adlard, P.A.; Hare, D.J. Iron, Copper, and Zinc Concentration in Aβ Plaques in the APP/PS1 Mouse Model of Alzheimer’s Disease Correlates with Metal Levels in the Surrounding Neuropil. ACS Chem. Neurosci. 2016, 8, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Accounts Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Bearer, E.L.; Wu, C. Herpes Simplex Virus, Alzheimer’s Disease and a Possible Role for Rab GTPases. Front. Cell Dev. Biol. 2019, 7, 134. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Miklossy, J.; Khalili, K.; Gern, L.; Ericson, R.L.; Darekar, P.; Bolle, L.; Hurlimann, J.; Paster, B.J. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J. Alzheimer’s Dis. 2005, 6, 639–649; discussion 673–681. [Google Scholar] [CrossRef]
- Bishop, G.M.; Robinson, S.R. The amyloid hypothesis: Let sleeping dogmas lie? Neurobiol. Aging 2002, 23, 1101–1105. [Google Scholar] [CrossRef]
- Robinson, S.R.; Bishop, G.M. Aβ as a bioflocculant: Implications for the amyloid hypothesis of Alzheimer’s disease. Neurobiol. Aging 2002, 23, 1051–1072. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Mee, A.P.; Itzhaki, R.F. Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J. Pathol. 2009, 217, 131–138. [Google Scholar] [CrossRef]
- Santana, S.; Recuero, M.; Bullido, M.J.; Valdivieso, F.; Aldudo, J. Herpes simplex virus type I induces the accumulation of intracellular β-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol. Aging 2012, 33, 430.e19–430.e33. [Google Scholar] [CrossRef]
- Miklossy, J. Bacterial Amyloid and DNA are Important Constituents of Senile Plaques: Further Evidence of the Spirochetal and Biofilm Nature of Senile Plaques. J. Alzheimer’s Dis. 2016, 53, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Krut, J.J.; Zetterberg, H.; Blennow, K.; Cinque, P.; Hagberg, L.; Price, R.W.; Studahl, M.; Gisslén, M. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J. Neurol. 2012, 260, 620–626. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Marcocci, M.E.; Civitelli, L.; Argnani, R.; Piacentini, R.; Ripoli, C.; Manservigi, R.; Grassi, C.; Garaci, E.; Palamara, A.T. APP Processing Induced by Herpes Simplex Virus Type 1 (HSV-1) Yields Several APP Fragments in Human and Rat Neuronal Cells. PLoS ONE 2010, 5, e13989. [Google Scholar] [CrossRef] [PubMed]
- Bourgade, K.; Le Page, A.; Bocti, C.; Witkowski, J.M.; Dupuis, G.; Frost, E.H.; Fülöp, T., Jr. Protective Effect of Amyloid-β Peptides Against Herpes Simplex Virus-1 Infection in a Neuronal Cell Culture Model. J. Alzheimer’s Dis. 2016, 50, 1227–1241. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- De Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; Silva, C.H.T.D.P.D.; Rosa, J.M.C.; Cruz, R.A.S.; Hage-Melim, L.I.D.S. Antioxidant Effect of Flavonoids Present in Euterpe oleracea Martius and Neurodegenerative Diseases: A Literature Review. Central Nerv. Syst. Agents Med. Chem. 2019, 19, 75–99. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, T. The Significance of the Study about the Biological Effects of Solar Ultraviolet Radiation using the Exposed Facility on the International Space Station. Biol. Sci. Space 2004, 18, 255–260. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of Flavonoids in Plants. Int. J. Pharm. Sci. Tech. 2011, 6, 11–35. [Google Scholar]
- Dixon, R.A.; Pasinetti, G. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef]
- Ali, B.; Jamal, Q.M.; Shams, S.; Al-Wabel, N.A.; Siddiqui, M.U.; Alzohairy, M.A.; Al Karaawi, M.A.; Kesari, K.K.; Mushtaq, G.; Kamal, M.A. In Silico Analysis of Green Tea Polyphenols as Inhibitors of AChE and BChE Enzymes in Alzheimer’s Disease Treatment. CNS Neurol. Disord.-Drug Targets 2016, 15, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.K.; Choudhury, S. Tea polyphenols as multi-target therapeutics for Alzheimer’s disease: An in silico study. Med Hypotheses 2019, 125, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Karimi, A.; Ebadi, A. The potential of natural product vs neurodegenerative disorders: In silico study of artoflavanocoumarin as BACE-1 inhibitor. Comput. Biol. Chem. 2018, 77, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Rasheed, M.A.; Jamil, F.; Ibrahim, M.; Kanwal, S. In Silico Identification of Novel Apolipoprotein E4 Inhibitor for Alzheimer’s Disease Therapy. Curr. Comput. Aided-Drug Des. 2019, 15, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-W.; Wang, Y.; Guo, Z.-F.; Du, K.-C.; Meng, D.-L. Systems Pharmacological Approach to Investigate the Mechanism of Ohwia caudata for Application to Alzheimer’s Disease. Molecules 2019, 24, 1499. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef]
- Adewole, K.E.; Gyebi, G.A.; Ibrahim, I.M. Amyloid β fibrils disruption by kolaviron: Molecular docking and extended molecular dynamics simulation studies. Comput. Biol. Chem. 2021, 94, 107557. [Google Scholar] [CrossRef]
- Gupta, M.K.; Vadde, R. In silico identification of natural product inhibitors for γ-secretase activating protein, a therapeutic target for Alzheimer’s disease. J. Cell. Biochem. 2019, 120, 10323–10336. [Google Scholar] [CrossRef]
- Youn, K.; Jun, M. Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda. Nutrients 2019, 11, 662. [Google Scholar] [CrossRef]
- Duan, S.; Guan, X.; Lin, R.; Liu, X.; Yan, Y.; Lin, R.; Zhang, T.; Chen, X.; Huang, J.; Sun, X.; et al. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: A dual-target drug for the treatment of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1792–1807. [Google Scholar] [CrossRef]
- Rehman, K.; Chohan, T.A.; Waheed, I.; Gilani, Z.; Akash, M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: Evidence from an in vivo and in silico studies. J. Cell. Biochem. 2019, 120, 425–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 819–825. [Google Scholar] [CrossRef]
- Espargaró, A.; Ginex, T.; Vadell, M.D.M.; Busquets, M.A.; Estelrich, J.; Muñoz-Torrero, D.; Luque, F.J.; Sabate, R. Combined in Vitro Cell-Based/in Silico Screening of Naturally Occurring Flavonoids and Phenolic Compounds as Potential Anti-Alzheimer Drugs. J. Nat. Prod. 2017, 80, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ji, Y.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Baicalein as a Potential Inhibitor against BACE1 and AChE: Mechanistic Comprehension through In Vitro and Computational Approaches. Nutrients 2019, 11, 2694. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Shafreen, R.B.; Pandian, S.K.; Devi, K.P. Cholinesterase inhibitory, anti-amyloidogenic and neuroprotective effect of the medicinal plant Grewia tiliaefolia–An in vitro and in silico study. Pharm. Biol. 2017, 55, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, J.; Wang, Y.; Cui, K.; Cao, Y.; Wang, L.; Wu, Y. Linarin improves the dyskinesia recovery in Alzheimer’s disease zebrafish by inhibiting the acetylcholinesterase activity. Life Sci. 2019, 222, 112–116. [Google Scholar] [CrossRef]
- Atalar, M.N.; Aras, A.; Türkan, F.; Barlak, N.; Yildiko, Ü.; Karatas, O.F.; Alma, M.H. The effects of Daucus carota extract against PC3, PNT1a prostate cells, acetylcholinesterase, glutathione S-transferase, and α-glycosidase; an in vitro–in silico study. J. Food Biochem. 2021, 45, e13975. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Issa, M.Y.; Ebrahim, H.S.; Alaaeldin, R.; Elrehany, M.A.; El-Kadder, E.M.A.; Abdelmohsen, U.R. Anti-Alzheimer chemical constituents of Morus macroura Miq.: Chemical profiling, in silico and in vitro investigations. Food Funct. 2021, 12, 8078–8089. [Google Scholar] [CrossRef]
- Singh, S.K.; Gaur, R.; Kumar, A.; Fatima, R.; Mishra, L.; Srikrishna, S. The Flavonoid Derivative 2-(4′ Benzyloxyphenyl)-3-hydroxy-chromen-4-one Protects Against Aβ42-Induced Neurodegeneration in Transgenic Drosophila: Insights from In Silico and In Vivo Studies. Neurotox. Res. 2014, 26, 331–350. [Google Scholar] [CrossRef]
- Cui, Z.; Sheng, Z.; Yan, X.; Cao, Z.; Tang, K. In Silico Insight into Potential Anti-Alzheimer’s Disease Mechanisms of Icariin. Int. J. Mol. Sci. 2016, 17, 113. [Google Scholar] [CrossRef]
- Lemkul, J.A.; Bevan, D.R. Destabilizing Alzheimer’s Aβ42 Protofibrils with Morin: Mechanistic Insights from Molecular Dynamics Simulations. Biochemistry 2010, 49, 3935–3946. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Zaman, A.; Jahan, I.; Chakravorty, R.; Chakraborty, S. In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer’s disease. J. Young-Pharm. 2013, 5, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Katalinić, M.; Rusak, G.; Barović, J.D.; Šinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef]
- Youn, K.; Park, J.-H.; Lee, S.; Lee, S.; Lee, J.; Yun, E.-Y.; Jeong, W.-S.; Jun, M. BACE1 Inhibition by Genistein: Biological Evaluation, Kinetic Analysis, and Molecular Docking Simulation. J. Med. Food 2018, 21, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Castillo, W.O.; Palomino, N.V.; Takahashi, C.S.; Giuliatti, S. Genistein and Galantamine Combinations Decrease β-Amyloid Peptide (1–42)–Induced Genotoxicity and Cell Death in SH-SY5Y Cell Line: An In Vitro and In Silico Approach for Mimic of Alzheimer’s Disease. Neurotox. Res. 2020, 38, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, X.-L.; Liu, G.-Q.; Zhou, T.; Yang, X.-L.; Ma, B.-X. The in silico and in vivo evaluation of puerarin against Alzheimer’s disease. Food Funct. 2019, 10, 799–813. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, T.; Chen, D.; Liu, R.; Qin, H.-H.; Min, Z.-L.; Liu, G.-Q.; Cao, X.-L. In silico-determined compound from the root of Pueraria lobate alleviates synaptic plasticity injury induced by Alzheimer’s disease via the p38MAPK-CREB signaling pathway. Food Funct. 2021, 12, 1039–1050. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Kuhad, A.; Chopra, K. Naringin ameliorates memory deficits in experimental paradigm of Alzheimer’s disease by attenuating mitochondrial dysfunction. Pharmacol. Biochem. Behav. 2014, 127, 101–110. [Google Scholar] [CrossRef]
- Meng, X.; Fu, M.; Wang, S.; Chen, W.; Wang, J.; Zhang, N. Naringin ameliorates memory deficits and exerts neuroprotective effects in a mouse model of Alzheimer’s disease by regulating multiple metabolic pathways. Mol. Med. Rep. 2021, 23, 1–13. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-κB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug Des. Dev. Ther. 2021, ume 15, 2013–2024. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Y.; Zhu, S.; Lu, Y.; Zhu, L.; Wang, Y.; Wang, X. Inhibition of Aβ aggregates in Alzheimer’s disease by epigallocatechin and epicatechin-3-gallate from green tea. Bioorg. Chem. 2020, 105, 104382. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.J.; Choudhry, F.; Peacey, E.; Perkinton, M.S.; Richardson, J.C.; Howlett, D.R.; Lichtenthaler, S.F.; Francis, P.T.; Williams, R.J. Dietary (−)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing. Neurobiol. Aging 2015, 36, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Hole, K.L.; Staniaszek, L.E.; Balan, G.M.; Mason, J.M.; Brown, J.T.; Williams, R.J. Oral (−)-Epicatechin Inhibits Progressive Tau Pathology in rTg4510 Mice Independent of Direct Actions at GSK3β. Front. Neurosci. 2021, 15, 697319. [Google Scholar] [CrossRef]
- Lim, H.J.; Shim, S.B.; Jee, S.W.; Lee, S.H.; Lim, C.J.; Hong, J.T.; Sheen, Y.Y.; Hwang, D.Y. Green tea catechin leads to global improvement among Alzheimer’s disease-related phenotypes in NSE/hAPP-C105 Tg mice. J. Nutr. Biochem. 2013, 24, 1302–1313. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Jyoti, S.; Naz, F.; Ashafaq, M.; Shahid, M.; Siddique, Y.H. Therapeutic potential of luteolin in transgenic Drosophila model of Alzheimer’s disease. Neurosci. Lett. 2019, 692, 90–99. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.-J.; Nam, Y.; Kim, H.-C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- Kimura, J.; Shimizu, K.; Kajima, K.; Yokosuka, A.; Mimaki, Y.; Oku, N.; Ohizumi, Y. Nobiletin Reduces Intracellular and Extracellular β-Amyloid in iPS Cell-Derived Alzheimer’s Disease Model Neurons. Biol. Pharm. Bull. 2018, 41, 451–457. [Google Scholar] [CrossRef]
- Sawmiller, D.; Habib, A.; Li, S.; Darlington, D.; Hou, H.; Tian, J.; Shytle, R.D.; Smith, A.; Giunta, B.; Mori, T.; et al. Diosmin reduces cerebral Aβ levels, tau hyperphosphorylation, neuroinflammation, and cognitive impairment in the 3xTg-AD mice. J. Neuroimmunol. 2016, 299, 98–106. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.; De Almeida, M.M.A.; Da Silva, A.B.; dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; Da Silva, J.S.; Souza, D.O.; Costa, M.D.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Alsadat, A.M.; Nikbakht, F.; Nia, H.H.; Golab, F.; Khadem, Y.; Barati, M.; Vazifekhah, S. GSK-3β as a target for apigenin-induced neuroprotection against Aβ 25–35 in a rat model of Alzheimer’s disease. Neuropeptides 2021, 90, 102200. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-S.; Yu, Y.-C.; Wu, C.-H.; Lin, J.-Y. Protective Effects of Wogonin against Alzheimer’s Disease by Inhibition of Amyloidogenic Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 3545169. [Google Scholar] [CrossRef]
- Vedagiri, A.; Thangarajan, S. Mitigating effect of chrysin loaded solid lipid nanoparticles against Amyloid β25–35 induced oxidative stress in rat hippocampal region: An efficient formulation approach for Alzheimer’s disease. Neuropeptides 2016, 58, 111–125. [Google Scholar] [CrossRef]
- Giacomeli, R.; de Gomes, M.G.; Reolon, J.B.; Haas, S.E.; Colomé, L.M.; Jesse, C.R. Chrysin loaded lipid-core nanocapsules ameliorates neurobehavioral alterations induced by β-amyloid1-42 in aged female mice. Behav. Brain Res. 2020, 390, 112696. [Google Scholar] [CrossRef]
- Beg, T.; Jyoti, S.; Naz, F.; Rahul; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord.-Drug Targets 2018, 17, 421–429. [Google Scholar] [CrossRef]
- Paula, P.-C.; Maria, S.-G.A.; Luis, C.-H.; Patricia, C.-G.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef]
- Mohammadi, N.; Asle-Rousta, M.; Rahnema, M.; Amini, R. Morin attenuates memory deficits in a rat model of Alzheimer’s disease by ameliorating oxidative stress and neuroinflammation. Eur. J. Pharmacol. 2021, 910, 174506. [Google Scholar] [CrossRef]
- Huang, L.; Lin, M.; Zhong, X.; Yang, H.; Deng, M. Galangin decreases p-tau, Aβ42 and β-secretase levels, and suppresses autophagy in okadaic acid-induced PC12 cells via an Akt/GSK3β/mTOR signaling-dependent mechanism. Mol. Med. Rep. 2019, 19, 1767–1774. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Jumnongprakhon, P.; Suksamrarn, A.; Tocharus, J. Cyanidin attenuates Aβ25-35-induced neuroinflammation by suppressing NF-κB activity downstream of TLR4/NOX4 in human neuroblastoma cells. Acta Pharmacol. Sin. 2018, 39, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Sohanaki, H.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Pelargonidin improves memory deficit in amyloid β25-35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016, 83, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Alisavari, N.; Soleimani-Asl, S.; Zarei, M.; Hashemi-Firouzi, N. Protective effect of chronic administration of pelargonidin on neuronal apoptosis and memory process in amyloid-beta-treated rats. Avicenna J. Phytomed. 2021, 11, 407–416. [Google Scholar] [CrossRef]
- Bonet-Costa, V.; Herranz-Pérez, V.; Blanco-Gandía, M.; Mas-Bargues, C.; Inglés, M.; Garcia-Tarraga, P.; Rodriguez-Arias, M.; Miñarro, J.; Borras, C.; Garcia-Verdugo, J.M.; et al. Clearing Amyloid-β through PPARγ/ApoE Activation by Genistein is a Treatment of Experimental Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 51, 701–711. [Google Scholar] [CrossRef]
- Gutierrez-Zepeda, A.; Santell, R.; Wu, Z.; Brown, M.; Wu, Y.; Khan, I.; Link, C.D.; Zhao, B.; Luo, Y. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci. 2005, 6, 54. [Google Scholar] [CrossRef]
- Wei, J.; Yang, F.; Gong, C.; Shi, X.; Wang, G. Protective effect of daidzein against streptozotocin-induced Alzheimer’s disease via improving cognitive dysfunction and oxidative stress in rat model. J. Biochem. Mol. Toxicol. 2019, 33, e22319. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Lin, S.-H.; Hidayah, K.; Lin, C.-I. Equol Pretreatment Protection of SH-SY5Y Cells against Aβ (25–35)-Induced Cytotoxicity and Cell-Cycle Reentry via Sustaining Estrogen Receptor Alpha Expression. Nutrients 2019, 11, 2356. [Google Scholar] [CrossRef]
- Kim, S.-K.; Ko, Y.-H.; Lee, Y.; Lee, S.-Y.; Jang, C.-G. Antineuroinflammatory Effects of 7,3’,4’-Trihydroxyisoflavone in Lipopolysaccharide-Stimulated BV2 Microglial Cells through MAPK and NF-κB Signaling Suppression. Biomol. Ther. 2021, 29, 127–134. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-Derived Polyphenolics Prevent A Oligomerization and Attenuate Cognitive Deterioration in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s Disease Mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Rimbach, G.; Augustin, K.; Schliebs, R.; Wolffram, S.; Cermak, R. Effect of a short- and long-term treatment with Ginkgo biloba extract on Amyloid Precursor Protein Levels in a transgenic mouse model relevant to Alzheimer’s disease. Arch. Biochem. Biophys. 2009, 481, 177–182. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green Tea Epigallocatechin-3-Gallate (EGCG) Modulates Amyloid Precursor Protein Cleavage and Reduces Cerebral Amyloidosis in Alzheimer Transgenic Mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef] [PubMed]
- E Ehrnhoefer, D.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; E Wanker, E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef]
- Hirohata, M.; Hasegawa, K.; Tsutsumi-Yasuhara, S.; Ohhashi, Y.; Ookoshi, T.; Ono, K.; Yamada, M.; Naiki, H. The Anti-Amyloidogenic Effect Is Exerted against Alzheimer’s β-Amyloid Fibrils in Vitro by Preferential and Reversible Binding of Flavonoids to the Amyloid Fibril Structure. Biochemistry 2007, 46, 1888–1899. [Google Scholar] [CrossRef]
- Ushikubo, H.; Watanabe, S.; Tanimoto, Y.; Abe, K.; Hiza, A.; Ogawa, T.; Asakawa, T.; Kan, T.; Akaishi, T. 3,3′,4′,5,5′-Pentahydroxyflavone is a potent inhibitor of amyloid β fibril formation. Neurosci. Lett. 2012, 513, 51–56. [Google Scholar] [CrossRef]
- Murakami, K.; Irie, K. Three Structural Features of Functional Food Components and Herbal Medicine with Amyloid β42 Anti-Aggregation Properties. Molecules 2019, 24, 2125. [Google Scholar] [CrossRef]
- Rios, M.A.M.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.; Puddey, I.; Mori, T.; Burke, V.; Baker, R.; Beilin, L. Effects of regular ingestion of black tea on haemostasis and cell adhesion molecules in humans. Eur. J. Clin. Nutr. 2001, 55, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; A Francis, M.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar] [CrossRef]
- Steptoe, A.; Gibson, L.; Vuononvirta, R.; Hamer, M.; Wardle, J.; Rycroft, J.A.; Martin, J.F.; Erusalimsky, J. The effects of chronic tea intake on platelet activation and inflammation: A double-blind placebo controlled trial. Atherosclerosis 2007, 193, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Sacanella, E.; Badia, E.; Antúnez, E.; Nicolás, J.M.; Fernández-Solá, J.; Rotilio, D.; de Gaetano, G.; Rubin, E.; Urbano-Márquez, A. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: A prospective randomized crossover trial: Effects of wine on inflammatory markers. Atherosclerosis 2004, 175, 117–123. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Higgins, A.J.; Lees, P. The acute inflammatory process, arachidonic acid metabolism and the mode of action of anti-inflammatory drugs. Equine Vet. J. 1984, 16, 163–175. [Google Scholar] [CrossRef]
- Madeswaran, A. In-silico docking studies of cyclooxygenase inhibitory activity of commercially available flavonoids. Asian J. Pharm. Life Sci. 2013, 174–181. [Google Scholar]
- Madeswaran, A.; Umamaheswari, M.; Asokkumar, K.; Sivashanmugam, T.; SubhadraDevi, V.; Jagannath, P. In silico docking studies of phosphodiesterase inhibitory activity of commercially available flavonoids. Orient. Pharm. Exp. Med. 2012, 12, 301–306. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.; Porto, G.; Cabrita, E.J.; Marques, M.M.B.; Fernandes, E. Inhibition of LOX by flavonoids: A structure–activity relationship study. Eur. J. Med. Chem. 2014, 72, 137–145. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Istituto Super. Sanita 2007, 43, 394–405. [Google Scholar]
- Geng, Y.; Zhang, B.; Lotz, M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J. Immunol. 1993, 151, 6692–6700. [Google Scholar]
- Cho, J.Y.; Kim, P.S.; Park, J.; Yoo, E.S.; Baik, K.U.; Kim, Y.-K.; Park, M.H. Inhibitor of tumor necrosis factor-α production in lipopolysaccharide-stimulated RAW264.7 cells from Amorpha fruticosa. J. Ethnopharmacol. 2000, 70, 127–133. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; McDonald, T.L.; Koop, D.R. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signaling pathways involved in the release of tumor necrosis factor-α. Biochem. Pharmacol. 2001, 62, 963–974. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. [36] Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 343–355. [Google Scholar] [CrossRef]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chiang, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer. Res. 1993, 13, 2165–2170. [Google Scholar]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.A.J.; Berghe, D.V. Structure−Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef] [PubMed]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Ferenczyova, K.; Kalocayova, B.; Bartekova, M.; Tothova, L.; Radosinska, J. Beneficial Effect of Quercetin on Erythrocyte Properties in Type 2 Diabetic Rats. Molecules 2021, 26, 4868. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef] [PubMed]
- Chlebda, E.; Magdalan, J.; Merwid-Ląd, A.; Trocha, M.; Kopacz, M.; Kuźniar, A.; Nowak, D.; Szeląg, A. Influence of water-soluble flavonoids, quercetin-5′-sulfonic acid sodium salt and morin-5′-sulfonic acid sodium salt, on antioxidant parameters in the subacute cadmium intoxication mouse model. Exp. Toxicol. Pathol. 2010, 62, 105–108. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.; Alhazza, I.; Rhodes, C.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Wang, Z.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Jo, S.H.; Kim, J.S.; Kang, S.S.; Lee, J.S.; Park, D.H.; Hyun, J.W. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ. Toxicol. Pharmacol. 2010, 29, 12–18. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, A.A.; Fahim, H.I.; Zaky, M.Y. Quercetin and naringenin abate diethylnitrosamine/acetylaminofluorene-induced hepatocarcinogenesis in Wistar rats: The roles of oxidative stress, inflammation and cell apoptosis. Drug Chem. Toxicol. 2019, 45, 262–273. [Google Scholar] [CrossRef]
- Goudarzi, M.; Kalantar, M.; Sadeghi, E.; Karamallah, M.H.; Kalantar, H. Protective effects of apigenin on altered lipid peroxidation, inflammation, and antioxidant factors in methotrexate-induced hepatotoxicity. Naunyn-Schmiedebergs Arch. fur Exp. Pathol. und Pharmakol. 2021, 394, 523–531. [Google Scholar] [CrossRef]
- DeToma, A.S.; Choi, J.-S.; Braymer, J.J.; Lim, M.H. Myricetin: A Naturally Occurring Regulator of Metal-Induced Amyloid-β Aggregation and Neurotoxicity. ChemBioChem 2011, 12, 1198–1201. [Google Scholar] [CrossRef]
- Hyung, S.-J.; DeToma, A.S.; Brender, J.R.; Lee, S.; Vivekanandan, S.; Kochi, A.; Choi, J.-S.; Ramamoorthy, A.; Ruotolo, B.T.; Lim, M.H. Insights into antiamyloidogenic properties of the green tea extract (−)-epigallocatechin-3-gallate toward metal-associated amyloid-β species. Proc. Natl. Acad. Sci. USA 2013, 110, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- DeToma, A.S.; Krishnamoorthy, J.; Nam, Y.; Lee, H.J.; Brender, J.R.; Kochi, A.; Lee, D.; Onnis, V.; Congiu, C.; Manfredini, S.; et al. Interaction and reactivity of synthetic aminoisoflavones with metal-free and metal-associated amyloid-β. Chem. Sci. 2014, 5, 4851–4862. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Den Berg, D.-J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Perry, E.K.; E Tomlinson, B.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. BMJ 1978, 2, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Noui, A.; Boudiar, T.; Boulebd, H.; Gali, L.; Contreras, M.D.M.; Segura-Carretero, A.; Nieto, G.; Akkal, S. HPLC–DAD–ESI/MS profiles of bioactive compounds, antioxidant and anticholinesterase activities of Ephedra alata subsp. alenda growing in Algeria. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Khare, N.; Maheshwari, S.K.; Jha, A.K. Screening and identification of secondary metabolites in the bark of Bauhinia variegata to treat Alzheimer’s disease by using molecular docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2021, 39, 5988–5998. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Orhan, I.; Şenol, F.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Interactions 2009, 181, 383–389. [Google Scholar] [CrossRef]
- Zhao, L.; Shu-Ping, X.U.; Zong-Yang, L.I.; Zhang, L.; Zhang, Z.S.; Pan, R.L. Study on the antioxidant and antiacetylcholinesterase activities of myricitrin and its structure-similar compounds. Sci. Technol. Food Ind. 2012, 33, 56–58+62. [Google Scholar]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef]
- Sun, C.; Yin, Z.; Chen, J.; Wang, W.; Zheng, G.; Li, J.; Chen, L.; Zhang, Q. Dihydromyricetin Improves Cognitive Impairments in d -Galactose-Induced Aging Mice through Regulating Oxidative Stress and Inhibition of Acetylcholinesterase. Mol. Nutr. Food Res. 2022, 66, e2101002. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Laskar, M.A.; Sarker, S.D.; Choudhury, M.D.; Choudhury, P.R.; Mitra, A.; Jamil, S.; Lathiff, S.M.A.; Abdullah, S.A.; Basar, N.; et al. Prediction of Anti-Alzheimer’s Activity of Flavonoids Targeting Acetylcholinesterase in silico. Phytochem. Anal. 2017, 28, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch. Pharmacal Res. 2008, 31, 640–644. [Google Scholar] [CrossRef]
- Grenier, D.; Chen, H.; Ben Lagha, A.; Fournier-Larente, J.; Morin, M.-P. Dual Action of Myricetin on Porphyromonas gingivalis and the Inflammatory Response of Host Cells: A Promising Therapeutic Molecule for Periodontal Diseases. PLoS ONE 2015, 10, e0131758. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Nakane, H.; Fukushima, M.; Chermann, J.-C.; Barre-Sinoussi, F. Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. JBIC J. Biol. Inorg. Chem. 1990, 190, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, R.I.; Stoermer, M.; Fairlie, D. Flavones are inhibitors of HIV-1 proteinase. Biochem. Biophys. Res. Commun. 1992, 188, 631–637. [Google Scholar] [CrossRef]
- Fesen, M.R.; Pommier, Y.; Leteurtre, F.; Hiroguchi, S.; Yung, J.; Kohn, K.W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem. Pharmacol. 1994, 48, 595–608. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, E.-R.; Shin, A.C.-G.; Park, H. A New Flavonol Glycoside Gallate Ester from Acer okamotoanum and Its Inhibitory Activity against Human Immunodeficiency Virus-1 (HIV-1) Integrase. J. Nat. Prod. 1998, 61, 145–148. [Google Scholar] [CrossRef]
- Kaul, T.N.; Middleton, E.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2)in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
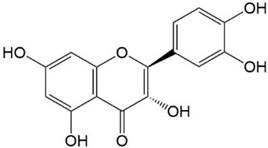 |
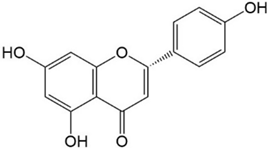 |
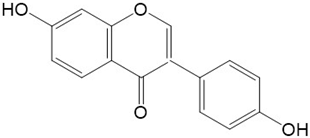 |
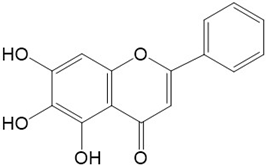 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
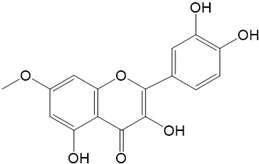 |
 |
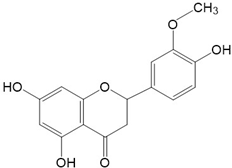 |
 |
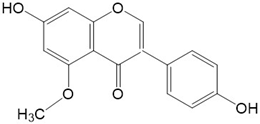 |
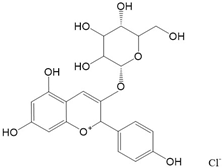 |
| Class | Flavonoids | AD Targets | Ligand–Receptor Interactions | References |
|---|---|---|---|---|
| Flavanols | (−)-Epigallocatechin (EGC) | AChE | Hydrogen bonding | [82,83] |
| BChE | ||||
| GSK-3β | ||||
| γ-secretase | [83] | |||
| BACE-1 | ||||
| Epicatechin-3-O-gallate | AChE | Hydrogen bonding | [82] | |
| BChE | ||||
| (−)-Epicatechin (EPC) | AChE | Hydrogen bonding | [83] | |
| BChE | ||||
| GSK-3β | ||||
| γ-secretase | ||||
| BACE-1 | ||||
| (−)-Epigallocatechin gallate (EGCG) | AChE | Hydrogen bonding | [82,83] | |
| Flavanols | (−)-Epigallocatechin gallate (EGCG) | BChE | ||
| GSK-3β | [83] | |||
| γ-secretase | ||||
| BACE-1 | ||||
| (+)-Catechin (CAT) | AChE | Hydrogen bonding | [82,83] | |
| BChE | ||||
| GSK-3β | ||||
| γ-secretase | [83] | |||
| BACE-1 | ||||
| Artoflavanocoumarin | BACE-1 | Hydrogen bonding; hydrophobic interactions | [84] | |
| Flavanols | Epicatechin Gallate(ECG) | ApoE4 | Hydrogen bonding; hydrophobic interactions | [85] |
| Flavanones | Cudraflavanone B | PTGS2 | Hydrogen bonding | [86] |
| Hesperidin | BACE-1 | Hydrogen bonding | [87] | |
| AChE | ||||
| BChE | ||||
| Kolaviron | Aβ42 fibrils | Hydrogen bonding; hydrophobic interactions | [88] | |
| Macaflavanone C | GSAP | Hydrogen bonding; hydrophobic interactions | [89] | |
| Naringenin | AChE | Hydrogen bonding | [87] | |
| Pinocembrin | BACE-1 | Hydrogen bonding | [90] | |
| Flavanones | Pinostrobin | BACE-1 | Hydrogen bonding | [90] |
| Silibinin | AChE | Hydrogen bonding; π-π and π-H interaction | [91] | |
| Aβ42 | Hydrophobic interactions | |||
| Taxifolin | α-amylase | Hydrogen bonding; π-π interaction | [92] | |
| Flavones | Apigenin | BACE-1 | Hydrogen bonding | [93] |
| Aβ42 fibrils | [94] | |||
| Baicalein | BACE-1 | Hydrogen bonding; hydrophobic interactions | [95] | |
| AChE | ||||
| Isovitexin | AChE | Hydrogen bonding | [96] | |
| Flavones | Linarin | AChE | Hydrogen bonding; π-π interaction | [97] |
| Vitexin | AChE | Hydrogen bonding | [96] | |
| Vitexin-4-O-glucoside | AChE | Hydrogen bonding | [96] | |
| Chrysin | AChE | Hydrogen bonding; π-π interaction; hydrophobic interactions | [98] | |
| BACE-1 | Hydrogen bonding; hydrophobic interactions | [99] | ||
| MAO-B | ||||
| Flavonols | 2-(4′ Benzyloxyphenyl)-3-hydroxychromen-4-one | β-amyloid fibril | Hydrogen bonding | [100] |
| Flavonols | 2-(4′ Benzyloxyphenyl)-3-hydroxychromen-4-one | β-amyloid | Hydrogen bonding; hydrophobic interactions | [100] |
| 8-Prenylkaempferol | PTGS2 | Hydrogen bonding | [86] | |
| Icariin | AChE | - | [101] | |
| NMDAR | - | |||
| PDE5 | - | |||
| Kaempherol | BACE-1 | Hydrogen bonding | [93] | |
| Morin | Aβ42 protofibril | Hydrogen bonding; hydrophobic interactions; aromatic stacking interactions | [102] | |
| Flavonols | Morin | BACE-1 | Hydrogen bonding | [93] |
| Flavonols | Myricetin | BACE-1 | Hydrogen bonding | [93] |
| Quercetin | AChE | Hydrogen bonding | [103] | |
| BACE-1 | [93] | |||
| Aβ42 fibrils | [94] | |||
| Fisetin | AChE | Hydrogen bonding | [104] | |
| Isoflavones | Genistein | BACE-1 | Hydrogen bonding | [105] |
| Isoflavones | Genistein | AChE | Hydrogen bonding; hydrophobic interactions | [106] |
| BChE | ||||
| NMDAR | ||||
| Puerarin | AChE | Hydrogen bonding | [107] | |
| COX-2 | ||||
| C3 | ||||
| CaMK IIα | [108] |
| Class | Flavonoids | Effects | Model | References |
|---|---|---|---|---|
| Flavanones | Naringin | Attenuates oxido-nitrosative stress and inflammation | ICV-STZ-induced rats | [109] |
| Regulates multiple pathways, including amyloid β metabolism, tau protein hyperphosphorylation, acetylcholinergic system, glutamate receptor system, oxidative stress, and cell apoptosis | Hydrocortisone-induced mice | [110] | ||
| Hesperetin | Multipotent effect, involving the inhibition of oxidative stress, and neuroinflammation | C57BL/6N mice treated with Aβ1–42 | [111] | |
| Eriodictyol | Attenuates neuroinflammation and amyloidogenesis | LPS-induced C57BL/6J mice model and BV2 microglial cells | [112] | |
| Flavanols | Epigallocatechin-3-gallate | Decreased the hyperphosphorylation of tau, suppressed BACE1 expression and activity as well as the expression of Aβ1–42, and promoted Ach content by diminishing the activity of AchE | AD rat models through an injection with Aβ 25–35 solution | [113] |
| Epigallocatechin | Alleviate Aβ40 aggregation and diminish ROS production, reduce the Aβ plaques in the brain | Neuroblastoma cells treated with Aβ40/APP/PS1 mouse | [114] | |
| (−)-Epicatechin | Reduces Aβ levels by inhibiting β, γ-secretase | TASTPM transgenic mouse model | [115] | |
| Inhibits tau phosphorylation | rTg4510 mouse model | [116] | ||
| Flavanols | Catechins | Decrease Aβ42 production, APP-C99/89 expression, γ-secretase component and Wnt protein levels, and γ-secretase activity, and increases the levels of APP-C83 protein and enzyme activities (α-secretase, neprilysin and Pin1) | NSE/hAPP-C105 Tg mice | [117] |
| Flavones | Luteolin | Decrease in the expression of Aβ42 aggregated, the oxidative stress, and apoptotic markers | Transgenic flies expressing human Aβ42 peptides | [118] |
| Nobiletin | Improves cognitive impairment and reduces soluble Aβ levels | 3xTg-AD mice model | [119] | |
| Reduces intracellular and extracellular β-Amyloid | iPS cells | [120] | ||
| Flavones | Diosmin | Reduces cerebral Aβ levels, tau hyperphosphorylation, and neuroinflammation | 3xTg-AD mice model | [121] |
| Apigenin | Preserves neuron and astrocyte morphology and reduces inflammation by regulating the expression of inflammatory mediators | LPS induced neuron/glial cells or neuron/glial cells treated with Aβ1–42 | [122] | |
| Decreases the expression of GSK-3β with the consequence of lowering the hyperphosphorylation of tau protein and suppresses BACE1 expression | Wistar rats treated with Aβ 25–35 | [123] | ||
| Flavones | Wogonin | Attenuates amyloidogenic pathway by decreasing the levels of BACE1, APP β-C-terminal fragment, Aβ-aggregation, and phosphorylated tau | 3xTg-AD mice model | [124] |
| Chrysin | Attenuated Aβ-induced memory impairment through the reduction of lipid peroxidation levels and the elevation of antioxidant molecules | Sprague–Dawley rats treated with Aβ25–35 | [125] | |
| Reverse learning impairment, reduced neuroinflammation induced by Aβ by lowering the expressions of IL-1, IL-10, and TNF-1 in the brain | Swiss mice treated with Aβ1–42 | [126] | ||
| Flavonols | Kaempferol | Reduced the oxidative stress and acetylcholinesterase activity | Transgenic flies expressing human Aβ42 peptides | [127] |
| Flavonols | Quercetin | Reduces Aβ protein and tauopathy in hippocampus and amygdala | 3xTg-AD mice model | [128] |
| Morin | Ameliorates oxidative stress and neuroinflammation | Wistar rats treated with Aβ1–42 | [129] | |
| Galangin | Decreases β-secretase, Aβ42, and p-tau levels; suppresses Beclin-1 and p-GSK3β expression; promotes p-Akt and p-mTOR expression | Okadaic-acid-induced PC12 cell | [130] | |
| Fisetin | Decreased the accumulation of Aβ, BACE-1 expression, and hyperphosphorylation of tau protein; increased the levels of both presynaptic and postsynaptic proteins | C57BL/6N mice treated with Aβ1–42 | [131] | |
| Anthocyanins | Cyanidin | Attenuates Aβ25–35-induced neuroinflammation | SK-N-SH cells (human neuroblastoma cell line) treated with Aβ25–35 | [132] |
| Pelargonidin | Inhibits of glial activation, cholinesterase, and oxidative stress | Wistar rats treated with Aβ25–35 | [133] | |
| Decreases neuronal apoptosis | Wistar rats treated with Aβ25–35 | [134] | ||
| Isoflavones | Genistein | Clears amyloid-β through PPARγ/ApoE activation | APPswe/PS1dE9 mice model | [135] |
| Glycitein | Inhibits Abeta deposition and decreases oxidative stress | Caenorhabditis elegans (CL2006 and CL4176) | [136] | |
| Isoflavones | Daidzein | Improves cognitive dysfunction and oxidative stress | ICV-STZ-induced rats | [137] |
| Equol | Reduces Aβ-induced neurotoxicity via sustaining estrogen receptor alpha expression | SH-SY5Y cells treated with Aβ25–35 | [138] | |
| 7,3′,4′-Trihydroxyisoflavone | Suppresses the production of the proinflammatory mediators NO, iNOS, and COX-2 as well as of the proinflammatory cytokineIL-6 and inhibits reactive ROS generation | LPS-induced BV2 microglial cells | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, M.; Cui, X.; Li, C. Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure–Activity Relationship. Int. J. Mol. Sci. 2022, 23, 10020. https://doi.org/10.3390/ijms231710020
Li J, Sun M, Cui X, Li C. Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure–Activity Relationship. International Journal of Molecular Sciences. 2022; 23(17):10020. https://doi.org/10.3390/ijms231710020
Chicago/Turabian StyleLi, Jiao, Min Sun, Xiaodong Cui, and Chen Li. 2022. "Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure–Activity Relationship" International Journal of Molecular Sciences 23, no. 17: 10020. https://doi.org/10.3390/ijms231710020
APA StyleLi, J., Sun, M., Cui, X., & Li, C. (2022). Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure–Activity Relationship. International Journal of Molecular Sciences, 23(17), 10020. https://doi.org/10.3390/ijms231710020







