Differences of Uric Acid Transporters Carrying Extracellular Vesicles in the Urine from Uric Acid and Calcium Stone Formers and Non-Stone Formers
Abstract
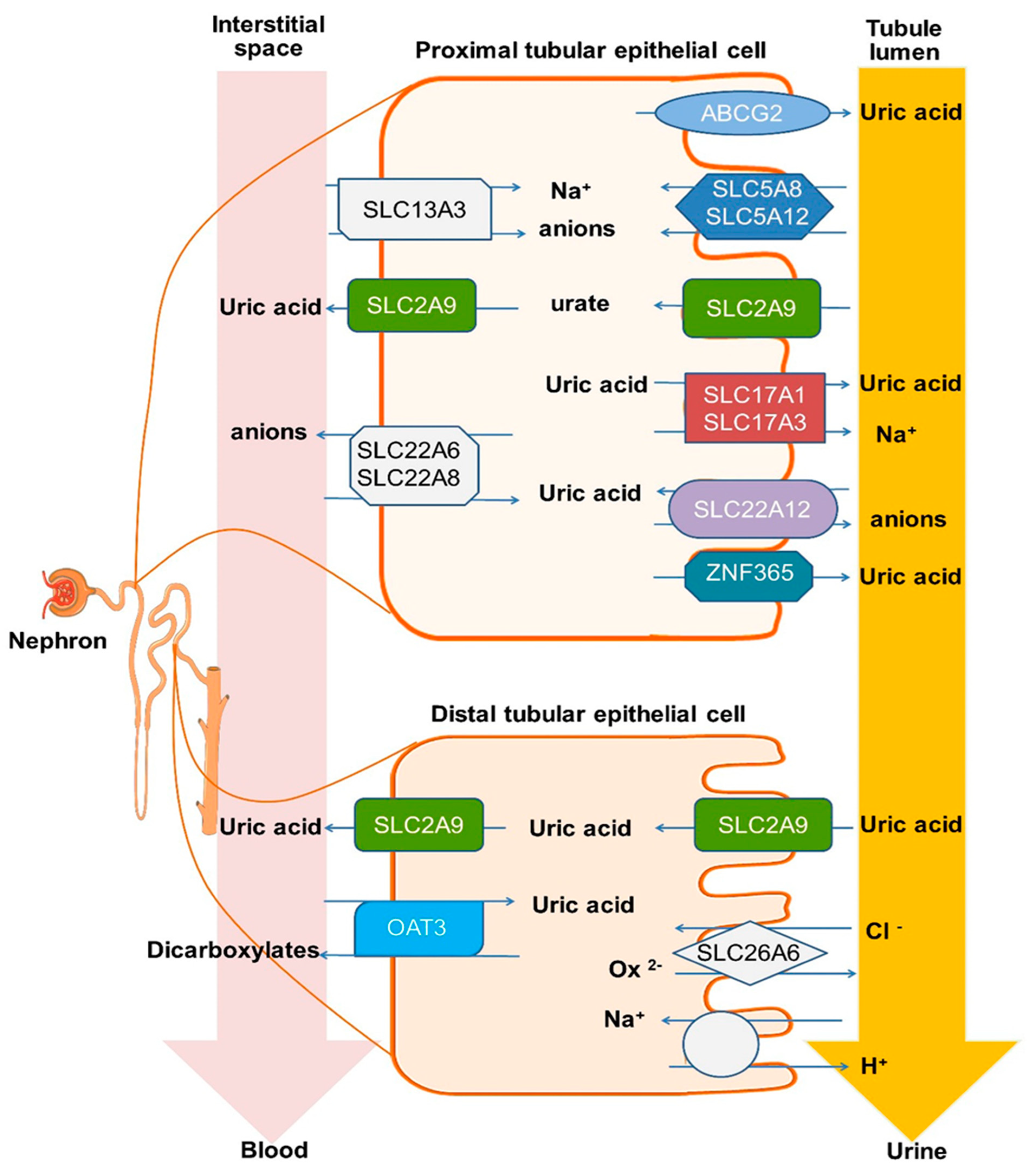
1. Introduction
2. Methods
2.1. Study Participants
2.2. Laboratory Measurements
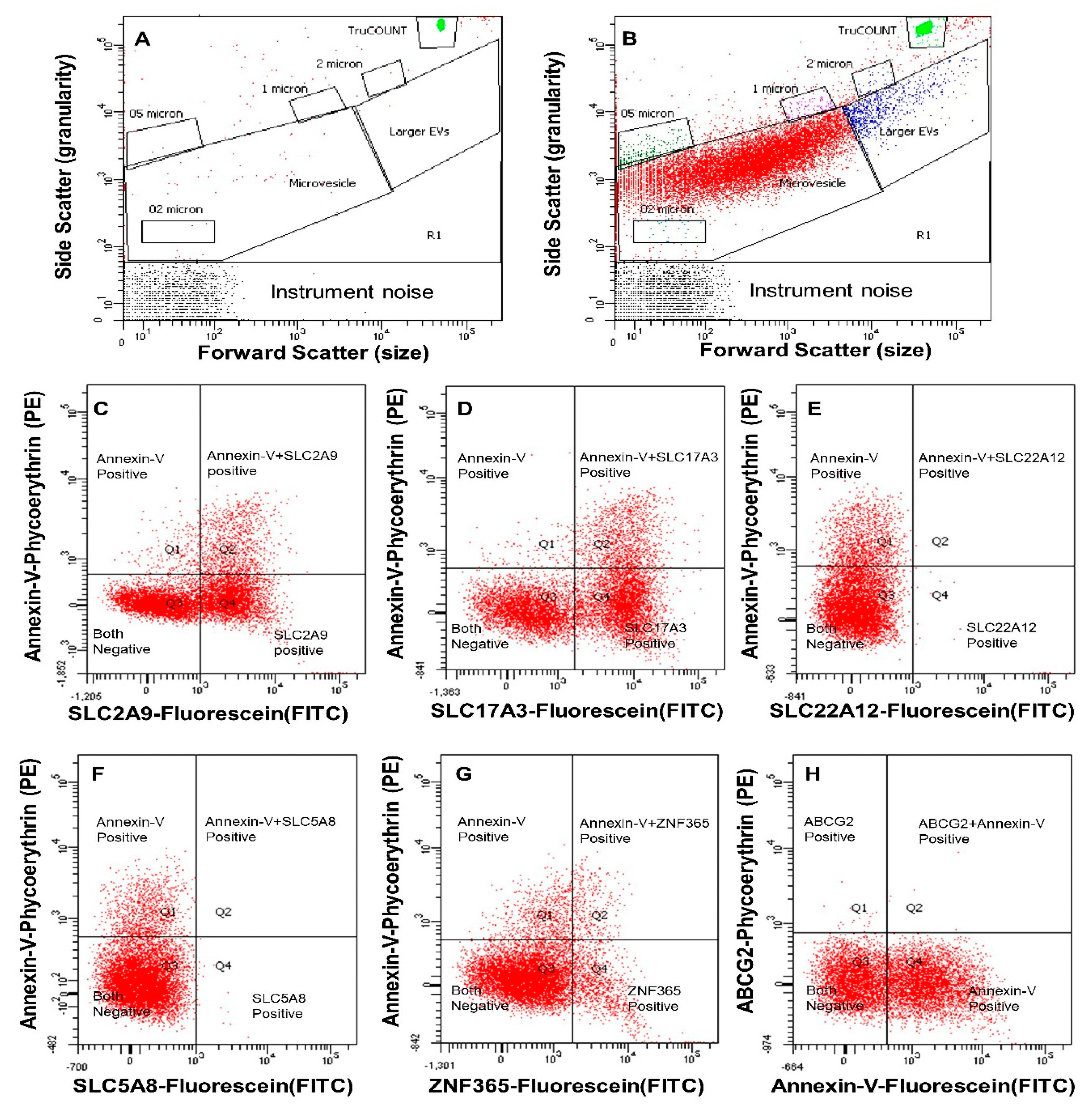
2.3. Quantification of Extracellular Vesicles (EVs) by Digital Flow Cytometer
2.4. Chemicals, Reagents, and Antibodies
2.5. Data Analysis
3. Results
Baseline Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-binding cassette transporter G2 |
| CSFs | calcium stone formers |
| eGFR | estimated glomerular filtration rate |
| EVs | extracellular vesicles |
| FITC | Fluorescein isothiocyanate |
| NSFs | non-stone formers |
| PE | phycoerythrin |
| SLC2A9 | solute carrier family 2, glucose transporter, member 9 |
| SLC5A8 | solute carrier family 5, member 8 |
| SLC17A3 | solute carrier family 17, organic anion transporter, member 3 |
| SLC22A12 | solute carrier family 22, organic anion/cation trans-porter, member 12 |
| UA | uric acid |
| UASD | stone disease |
| UASFs | UA stone formers |
| URAT1 | urate transporter 1 |
| USD | urinary stone disease |
| ZNF365 | zinc finger protein 365 |
| COM | calcium oxalate monohydrate |
| CKD | chronic kidney disease |
References
- Kittanamongkolchai, W.; Vaughan, L.E.; Enders, F.T.; Dhondup, T.; Mehta, R.A.; Krambeck, A.E.; McCollough, C.H.; Vrtiska, T.J.; Lieske, J.C.; Rule, A.D. The Changing Incidence and Presentation of Urinary Stones Over 3 Decades. Mayo. Clin. Proc. 2018, 93, 291–299. [Google Scholar] [CrossRef]
- Mandel, N.S.; Mandel, G.S. Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. J. Urol. 1989, 142, 1516–1521. [Google Scholar] [CrossRef]
- Lieske, J.C.; Rule, A.D.; Krambeck, A.E.; Williams, J.C.; Bergstralh, E.J.; Mehta, R.A.; Moyer, T.P. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. 2014, 9, 2141–2146. [Google Scholar] [CrossRef]
- Moe, O.W. Uric acid nephrolithiasis: Proton titration of an essential molecule? Curr. Opin. Nephrol. Hypertens. 2006, 15, 366–373. [Google Scholar] [CrossRef]
- Wiederkehr, M.R.; Moe, O.W. Uric Acid Nephrolithiasis: A Systemic Metabolic Disorder. Clin. Rev. Bone Miner. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A.; Gomila, I.; Ramis, M.; Garcia-Raja, A.; Prieto, R.M. Urinary pH and renal lithiasis. Urol. Res. 2012, 40, 41–46. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Moe, O.W. Renal transport of uric acid: Evolving concepts and uncertainties. Adv. Chronic Kidney Dis. 2012, 19, 358–371. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Zhuang, S.; Liu, N. Recent advances on uric acid transporters. Oncotarget 2017, 8, 100852–100862. [Google Scholar] [CrossRef]
- Karns, R.; Zhang, G.; Sun, G.; Rao Indugula, S.; Cheng, H.; Havas-Augustin, D.; Novokmet, N.; Rudan, D.; Durakovic, Z.; Missoni, S.; et al. Genome-wide association of serum uric acid concentration: Replication of sequence variants in an island population of the Adriatic coast of Croatia. Ann. Hum. Genet. 2012, 76, 121–127. [Google Scholar] [CrossRef]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar]
- Merriman, T.R. An update on the genetic architecture of hyperuricemia and gout. Arthritis Res. Ther. 2015, 17, 98. [Google Scholar] [CrossRef]
- Jutabha, P.; Anzai, N.; Kitamura, K.; Taniguchi, A.; Kaneko, S.; Yan, K.; Yamada, H.; Shimada, H.; Kimura, T.; Katada, T.; et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J. Biol. Chem. 2010, 285, 35123–35132. [Google Scholar] [CrossRef]
- Srivastava, S.; Nakagawa, K.; He, X.; Kimura, T.; Fukutomi, T.; Miyauchi, S.; Sakurai, H.; Anzai, N. Identification of the multivalent PDZ protein PDZK1 as a binding partner of sodium-coupled monocarboxylate transporter SMCT1 (SLC5A8) and SMCT2 (SLC5A12). J. Physiol. Sci. 2019, 69, 399–408. [Google Scholar] [CrossRef]
- Gianfrancesco, F.; Esposito, T.; Casu, G.; Maninchedda, G.; Roberto, R.; Pirastu, M. Emergence of Talanin protein associated with human uric acid nephrolithiasis in the Hominidae lineage. Gene 2004, 339, 131–138. [Google Scholar] [CrossRef]
- Medina-Escobedo, M.; Gonzalez-Herrera, L.; Villanueva-Jorge, S.; Martin-Soberanis, G. Metabolic abnormalities and polymorphisms of the vitamin D receptor (VDR) and ZNF365 genes in children with urolithiasis. Urolithiasis 2014, 42, 395–400. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R434–R444. [Google Scholar] [CrossRef]
- Lv, L.L.; Feng, Y.; Tang, T.T.; Liu, B.C. New insight into the role of extracellular vesicles in kidney disease. J. Cell Mol. Med. 2019, 23, 731–739. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Gai, C.; Bussolati, B.; Camussi, G. Extracellular Vesicles in Renal Pathophysiology. Front. Mol. Biosci. 2017, 4, 37. [Google Scholar] [CrossRef]
- Murakami, T.; Oakes, M.; Ogura, M.; Tovar, V.; Yamamoto, C.; Mitsuhashi, M. Development of glomerulus-, tubule-, and collecting duct-specific mRNA assay in human urinary exosomes and microvesicles. PLoS ONE 2014, 9, e109074. [Google Scholar]
- Jayachandran, M.; Lugo, G.; Heiling, H.; Miller, V.M.; Rule, A.D.; Lieske, J.C. Extracellular vesicles in urine of women with but not without kidney stones manifest patterns similar to men: A case control study. Biol. Sex. Differ. 2015, 6, 2. [Google Scholar] [CrossRef]
- Chirackal, R.S.; Jayachandran, M.; Wang, X.; Edeh, S.; Haskic, Z.; Perinpam, M.; Halling, T.M.; Mehta, R.; Rivera, M.E.; Lieske, J.C. Urinary extracellular vesicles associated MCP-1 and NGAL derived from specific nephron segments differ between calcium oxalate stone formers and controls. Am. J. Physiol. Renal. Physiol. 2019, 317, F1475–F1482. [Google Scholar]
- Werness, P.G.; Brown, C.M.; Smith, L.H.; Finlayson, B. EQUIL2: A BASIC computer program for the calculation of urinary saturation. J. Urol. 1985, 134, 1242–1244. [Google Scholar] [CrossRef]
- Jayachandran, M.; Miller, V.M.; Heit, J.A.; Owen, W.G. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J. Immunol. Methods 2012, 375, 207–214. [Google Scholar]
- Turco, A.E.; Lam, W.; Rule, A.D.; Denic, A.; Lieske, J.C.; Miller, V.M.; Larson, J.J.; Kremers, W.K.; Jayachandran, M. Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J. Extracell. Vesicles 2016, 5, 29642. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Jayachandran, M.; Yuzhakov, S.V.; Kumar, S.; Larson, N.B.; Enders, F.T.; Milliner, D.S.; Rule, A.D.; Lieske, J.C. Correction to: Specific populations of urinary extracellular vesicles and proteins differentiate type 1 primary hyperoxaluria patients without and with nephrocalcinosis or kidney stones. Orphanet. J. Rare Dis. 2021, 16, 91. [Google Scholar] [CrossRef]
- Zhang, J.; Kumar, S.; Jayachandran, M.; Herrera Hernandez, L.P.; Wang, S.; Wilson, E.M.; Lieske, J.C. Excretion of urine extracellular vesicles bearing markers of activated immune cells and calcium/phosphorus physiology differ between calcium kidney stone formers and non-stone formers. BMC Nephrol. 2021, 22, 204. [Google Scholar] [CrossRef]
- Blijdorp, C.J.; Tutakhel, O.A.Z.; Hartjes, T.A.; van den Bosch, T.P.P.; van Heugten, M.H.; Rigalli, J.P.; Willemsen, R.; Musterd-Bhaggoe, U.M.; Barros, E.R.; Carles-Fontana, R.; et al. Comparing Approaches to Normalize, Quantify, and Characterize Urinary Extracellular Vesicles. J. Am. Soc. Nephrol. 2021, 32, 1210–1226. [Google Scholar] [CrossRef]
- Kanbara, A.; Miura, Y.; Hyogo, H.; Chayama, K.; Seyama, I. Effect of urine pH changed by dietary intervention on uric acid clearance mechanism of pH-dependent excretion of urinary uric acid. Nutr. J. 2012, 11, 39. [Google Scholar]
- Bobulescu, I.A.; Park, S.K.; Xu, L.H.R.; Blanco, F.; Poindexter, J.; Adams-Huet, B.; Davidson, T.L.; Sakhaee, K.; Maalouf, N.M.; Moe, O.W. Net Acid Excretion and Urinary Organic Anions in Idiopathic Uric Acid Nephrolithiasis. Clin. J. Am. Soc. Nephrol. 2019, 14, 411–420. [Google Scholar] [CrossRef]
- Viers, B.R.; Lieske, J.C.; Vrtiska, T.J.; Herrera Hernandez, L.P.; Vaughan, L.E.; Mehta, R.A.; Bergstralh, E.J.; Rule, A.D.; Holmes, D.R., 3rd; Krambeck, A.E. Endoscopic and histologic findings in a cohort of uric acid and calcium oxalate stone formers. Urology 2015, 85, 771–776. [Google Scholar] [CrossRef]
- Emmerson, B.T.; Cross, M.; Osborne, J.M.; Axelsen, R.A. Reaction of MDCK cells to crystals of monosodium urate monohydrate and uric acid. Kidney Int. 1990, 37, 36–43. [Google Scholar] [CrossRef]
- Koka, R.M.; Huang, E.; Lieske, J.C. Adhesion of uric acid crystals to the surface of renal epithelial cells. Am. J. Physiol. Renal. Physiol. 2000, 278, F989–F998. [Google Scholar] [CrossRef]
- Umekawa, T.; Chegini, N.; Khan, S.R. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol. Dial. Transplant. 2003, 18, 664–669. [Google Scholar] [CrossRef]
- Jalal, D.I.; Chonchol, M.; Chen, W.; Targher, G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 2013, 61, 134–146. [Google Scholar] [CrossRef]
- Tang, X.; Lieske, J.C. Acute and chronic kidney injury in nephrolithiasis. Curr. Opin. Nephrol. Hypertens. 2014, 23, 385–390. [Google Scholar] [CrossRef][Green Version]
| Clinical Parameters | NSFs (n = 65) | UASFs (n = 18) | CSFs (n = 26) |
|---|---|---|---|
| Age (years) | 63 (56, 73) | 64 (55, 74) | 65 (59, 74) |
| Female, n [%] | 26 [40] | 7 [39] | 10 [38] |
| Body mass index (kg/m2) | 27 (24, 30) | 32 (27, 42) * | 29 (26, 34) |
| Systolic blood pressure (mm Hg) | 126 (111, 138) | 130 (112, 143) | 127 (113, 136) |
| Diastolic blood pressure (mm Hg) | 73 (66, 82) | 69 (58, 72) * | 70 (61, 78) |
| Malabsorption syndrome, n [%] | 3 [5] | 2 [11] | 2 [8] |
| Hypertension, n [%] | 18 [28] | 9 [50] | 12 [46] |
| Diabetic mellitus, n [%] | 11 [17] | 9 [50] | 8 [31] |
| Estimated GFR (mL/min/1.73 m2) | 78 (62, 91) | 67 (46, 76) * | 62 (51, 76) * |
| Blood biochemistry | |||
| Serum uric acid (mg/dL) | 5.0 (4.0, 6.2) | 6.7 (5.6, 7.0) * | 5.8 (4.8, 6.5) * |
| Serum phosphorous (mg/dL) | 3.3 (3.0, 3.7) | 3.3 (2.9, 3.6) | 3.6 (3.2, 3.8) * |
| Serum calcium (mg/dL) | 9.3 (8.9, 9.6) | 9.3 (9.2, 9.8) | 9.5 (9.3, 9.7) |
| Serum creatinine (mg/dL) | 0.9 (0.69, 1.1) | 1.1 (0.9, 1.4) * | 1.0 (0.9, 1.2) * |
| Urine biochemistry | |||
| Urine pH | 6.0 (6.0, 7.0) | 5.7 (5.4, 6.1) * | 6.0 (5.5, 6.2) * |
| Osmolality (mOsm) | 528 (388, 763) | 495 (346, 726) | 409 (241, 580) * |
| Urine volume (mL/24 h) | 1955 (1402, 2634) | 2167 (1518, 2544) | 2064 (1443, 2680) |
| Protein (mg/24 h) | 26 (10, 42) | 71.5 (22, 303) * | 33 (16, 61) |
| Uric acid (mg/24 h) | 376 (295, 581) | 580 (431, 710) * | 584 (456, 775) * |
| Phosphorus (mg/24 h) | 516 (353, 826) | 872 (714,1406) * | 942 (713,1304) * |
| Calcium (mg/24 h) | 139 (99, 200) | 140 (105, 236) | 255 (168, 327) *,† |
| Creatinine (mg/24 h) | 939 (644, 1325) | 1384 (1117, 1715) * | 1573 (1235, 1977) * |
| CaOx (supersaturation, dG) | 1.2 (0.8, 1.9) | 1.5 (0.9, 1.9) | 1.8 (1.2, 2.3) * |
| Uric acid (supersaturation, dG) | −0.7 (−6, 0.2) | 0.8 (0, 3.9) * | 0.8 (−1, 1.9) * |
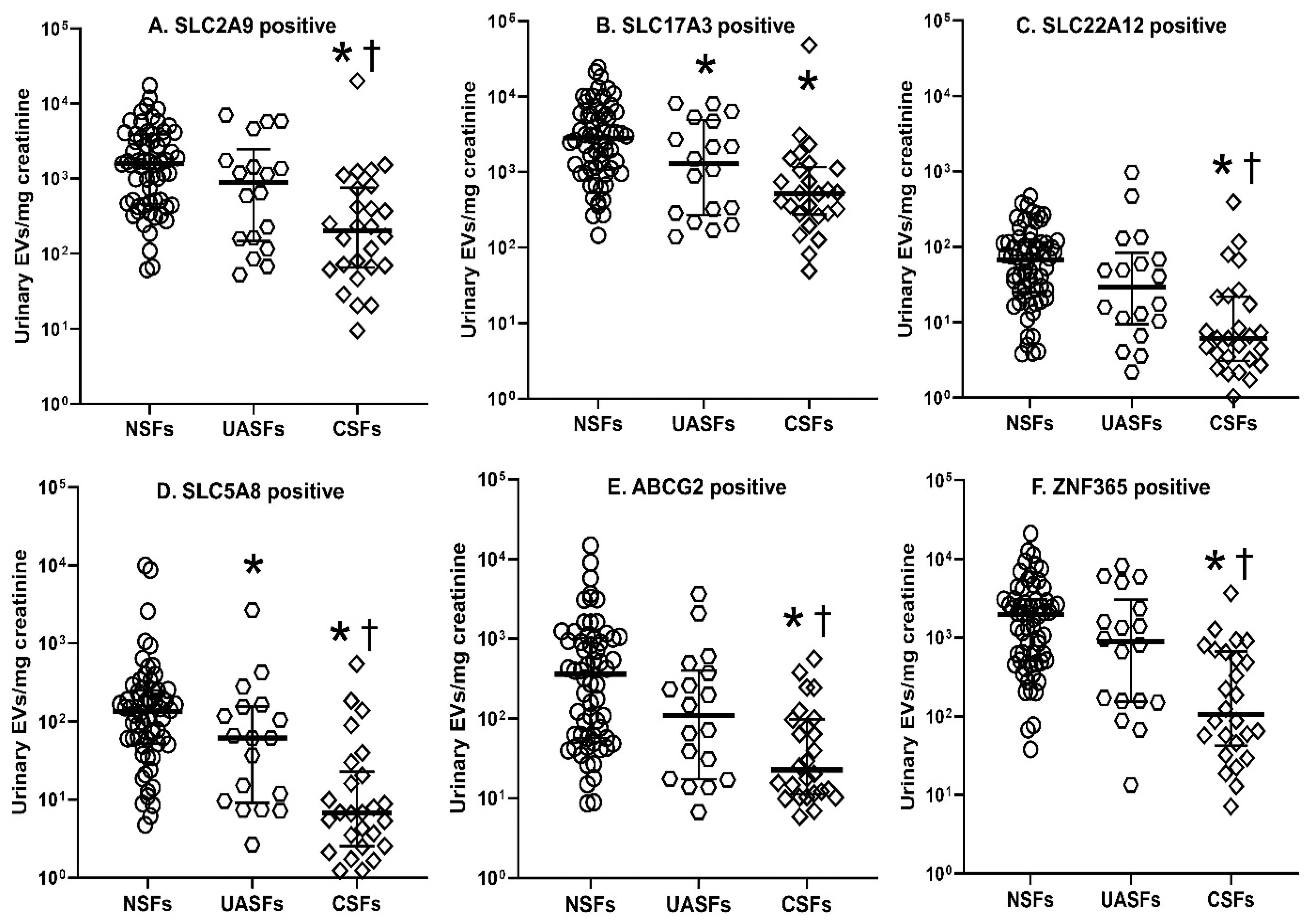
| Uric Acid Transporter-Positive EVs Excreted (106 per 24 h) | NSFs (n = 65) | UASFs (n = 18) | CSFs (n = 26) |
|---|---|---|---|
| SLC2A9 positive | 1477 (477, 2872) | 963 (342, 3089) | 399 (167, 1450) * |
| SLC17A3 positive | 2786 (1047, 4549) | 2148 (507, 4977) | 1305 (587, 2343) * |
| SLC22A12 positive | 52 (24, 102) | 41 (14, 119) | 13 (6, 22) *,† |
| SLC5A8 positive | 98 (48, 188) | 129 (14, 257) | 13 (7, 28) *,† |
| ABCG2 positive | 183 (66, 763) | 228 (31, 612) | 47 (25, 187) * |
| ZNF365 positive | 1404 (519, 2968) | 1448 (336, 3401) | 316 (71, 1146) *,† |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Jayachandran, M.; Haskic, Z.; Kumar, S.; Lieske, J.C. Differences of Uric Acid Transporters Carrying Extracellular Vesicles in the Urine from Uric Acid and Calcium Stone Formers and Non-Stone Formers. Int. J. Mol. Sci. 2022, 23, 10010. https://doi.org/10.3390/ijms231710010
Lin Z, Jayachandran M, Haskic Z, Kumar S, Lieske JC. Differences of Uric Acid Transporters Carrying Extracellular Vesicles in the Urine from Uric Acid and Calcium Stone Formers and Non-Stone Formers. International Journal of Molecular Sciences. 2022; 23(17):10010. https://doi.org/10.3390/ijms231710010
Chicago/Turabian StyleLin, Zhijian, Muthuvel Jayachandran, Zejfa Haskic, Sanjay Kumar, and John C. Lieske. 2022. "Differences of Uric Acid Transporters Carrying Extracellular Vesicles in the Urine from Uric Acid and Calcium Stone Formers and Non-Stone Formers" International Journal of Molecular Sciences 23, no. 17: 10010. https://doi.org/10.3390/ijms231710010
APA StyleLin, Z., Jayachandran, M., Haskic, Z., Kumar, S., & Lieske, J. C. (2022). Differences of Uric Acid Transporters Carrying Extracellular Vesicles in the Urine from Uric Acid and Calcium Stone Formers and Non-Stone Formers. International Journal of Molecular Sciences, 23(17), 10010. https://doi.org/10.3390/ijms231710010






