IgA Antiphospholipid Antibodies in Antiphospholipid Syndrome and Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Results
3. Discussion
4. Subjects and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nasonov, E.L. (Ed.) Antiphospholipid Syndrome; Litterra: Moscow, Russia, 2004; 424p. (In Russian) [Google Scholar]
- Reshetnyak, T.M. ANTIPHOSPHOLIPID SYNDROME: DIAGNOSIS AND CLINICAL MANIFESTATIONS (A LECTURE). Rheumatol. Sci. Pract. 2014, 52, 56–71. (In Russian) [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus ery-thematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.-M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, T.M.; Cheldieva, F.A.; Nurbaeva, K.S.; Lila, A.M.; Nasonov, E.L. Antiphospholipid syndrome: Diagnosis, mechanism of development, issues of therapy. Thromb. Hemost. Rheol. 2020, 4, 4–21. (In Russian) [Google Scholar] [CrossRef]
- Sciascia, S.; Amigo, M.-C.; Roccatello, D.; Khamashta, M. Diagnosing antiphospholipid syndrome: ‘extra-criteria’ manifestations and technical advances. Nat. Rev. Rheumatol. 2017, 13, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Pierangeli, S.; De Groot, P.; Dlott, J.; Favaloro, E.; Harris, E.; Lakos, G.; Ortel, T.; Meroni, P.L.; Otomo, K.; Pengo, V.; et al. ‘Criteria’ aPL tests: Report of a Task Force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, Texas, April 2010. Lupus 2011, 20, 182–190. [Google Scholar] [CrossRef]
- Hu, C.; Li, X.; Zhao, J.; Wang, Q.; Li, M.; Tian, X.; Zeng, X. Immunoglobulin A Isotype of Antiphospholipid Antibodies Does Not Provide Added Value for the Diagnosis of Antiphospholipid Syndrome in a Chinese Population. Front. Immunol. 2020, 11, 568503. [Google Scholar] [CrossRef]
- Ahmed, E.; Stegmayr, B.; Trifunovic, J.; Weinehall, L.; Hallmans, G.; Lefvert, A.K. Anticardiolipin Antibodies Are Not an Independent Risk Factor for Stroke. Stroke 2000, 31, 1289–1293. [Google Scholar] [CrossRef] [Green Version]
- Gonzales-Portillo, F.; McIntyre, J.A.; Wagenknecht, D.R.; Williams, L.S.; Bruno, A.; Biller, J. Spectrum of antiphospholipid antibodies (aPL) in patients with cerebrovascular disease. J. Stroke Cerebrovasc. Dis. 2001, 10, 222–226. [Google Scholar] [CrossRef]
- Palomo, I.; Pereira, J.; Alarcón, M.; Vasquez, M.; Pinochet, C.; Vélez, M.T.; Sandoval, J.; Icaza, G.; Pierangeli, S. Prevalence and isotype distribution of antiphospholipid antibodies in unselected Chilean patients with venous and arterial thrombosis. Clin. Rheumatol. 2004, 23, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kahles, T.; Humpich, M.; Steinmetz, H.; Sitzer, M.; Lindhoff-Last, E. Phosphatidylserine IgG and beta-2-glycoprotein I IgA antibodies may be a risk factor for ischaemic stroke. Rheumatology 2005, 44, 1161–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, K.; Knöbl, P.; Rintelen, C.; Kyrle, P.A.; Quehenberger, P.; Bialonczyk, C.; Partsch, H.; Lechner, K.; Pabinger, I. Is the determination of anti-beta2 glycoprotein I antibodies useful in patients with venous thromboembolism without the antiphospholipid syndrome? Br. J. Haematol. 2003, 123, 490–495. [Google Scholar] [CrossRef]
- Veres, K.; Lakos, G.; Kerényi, A.; Szekanecz, Z.; Szegedi, G.; Shoenfeld, Y.; Soltész, P. Antiphospholipid antibodies in acute coronary syndrome. Lupus 2004, 13, 423–427. [Google Scholar] [CrossRef]
- Ünlü, O.; Erkan, D.; Barbhaiya, M.; Andrade, D.; Nascimento, I.; Rosa, R.; Banzato, A.; Pengo, V.; Ugarte, A.; Gerosa, M.; et al. The Impact of Systemic Lupus Erythematosus on the Clinical Phenotype of Antiphospholipid Antibody–Positive Patients: Results From the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Clinical Database and Repository. Arthritis Care Res. 2019, 71, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frodlund, M.; Vikerfors, A.; Grosso, G.; Skogh, T.; Wetterö, J.; Elvin, K.; Gunnarsson, I.; Kastbom, A.; Dahlström, Ö.; Rönnelid, J.; et al. Immunoglobulin A anti-phospholipid antibodies in Swedish cases of systemic lupus erythematosus: Associations with disease phenotypes, vascular events and damage accrual. Clin. Exp. Immunol. 2018, 194, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolaccini, M.L.; Atsumi, T.; Escudero Contreras, A.; Khamashta, M.A.; Hughes, G.R. The value of IgA antiphospholipid testing for diagnosis of antiphospholipid (Hughes) syndrome in systemic lupus erythematosus. J. Rheumatol. 2001, 28, 2637–2643. [Google Scholar]
- Vlagea, A.; Pascual-Salcedo, D.; Doforno, R.; Lavilla, P.; Diez, J.; Merlano, B.P.; Cuesta, M.V.; Gil, A. IgA anti-β2 glycoprotein I antibodies: Experience from a large center. Thromb. Res. 2018, 162, 38–43. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Matsuura, E.; Ichikawa, K.; Fujisaku, A.; Mukai, M.; Koike, T. IgA class anti-beta2-glycoprotein I in patients with systemic lupus erythematosus. J. Rheumatol. 1998, 25, 74–78. [Google Scholar]
- Shen, Y.; Lee, R.; Frenkel, E.; Sarode, R. IgA antiphospholipid antibodies are an independent risk factor for thromboses. Lupus 2008, 17, 996–1003. [Google Scholar] [CrossRef]
- Lakos, G.; Kiss, E.; Regéczy, N.; Tarján, P.; Soltész, P.; Zeher, M.; Bodolay, E.; Szakony, S.; Sipka, S.; Szegedi, G. Isotype distribution and clinical relevance of anti-β2-glycoprotein I (β2-GPI) antibodies: Importance of IgA isotype. Clin. Exp. Immunol. 1999, 117, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Cho, M.L.; Joo, Y.S.; Kim, W.U.; Hong, Y.S.; Min, J.K.; Park, S.H.; Cho, C.S.; Kim, H.Y. Isotypes of anti-beta2-glycoprotein I antibodies: Association with thrombosis in patients with systemic lupus erythematosus. J. Rheumatol. 2001, 28, 520–524. [Google Scholar]
- Ruiz-García, R.; Serrano, M.; Martínez-Flores, J.; Mora, S.; Morillas, L.; Martín-Mola, M.; Morales, J.M.; Paz-Artal, E.; Serrano, A. Isolated IgA Anti-β2 Glycoprotein I Antibodies in Patients with Clinical Criteria for Antiphospholipid Syndrome. J. Immunol. Res. 2014, 2014, 704395. [Google Scholar] [CrossRef] [Green Version]
- Mehrani, T.; Petri, M. Association of IgA Anti-ß2 Glycoprotein I with Clinical and Laboratory Manifestations of Systemic Lupus Erythematosus. J. Rheumatol. 2011, 38, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; Branch, D.; Silver, R.M. Immunoglobulin A anti–β2-glycoprotein antibodies in women who experience unexplained recurrent spontaneous abortion and unexplained fetal death. Am. J. Obstet. Gynecol. 2001, 185, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, J.L.; Huang, Z.C.; Su, Z.Z.; Li, M.T.; Zeng, X.F.; Hu, C.J. Value of IgA antiphospholipid antibodies in diagnosis of the antiphospholipid syndrome. Zhonghua Yi Xue Za Zhi 2021, 101, 3404–3410, In Chinese. [Google Scholar] [CrossRef]
- Liu, T.; Gu, J.; Wan, L.; Hu, Q.; Teng, J.; Liu, H.; Cheng, X.; Ye, J.; Su, Y.; Sun, Y.; et al. “Non-criteria” antiphospholipid antibodies add value to antiphospholipid syndrome diagnoses in a large Chinese cohort. Arthritis Res. Ther. 2020, 22, 33. [Google Scholar] [CrossRef] [Green Version]
- Da Rosa, G.P.; Bettencourt, P.; Rodríguez-Pintó, I.; Cervera, R.; Espinosa, G. “Non-criteria” antiphospholipid syndrome: A nomenclature proposal. Autoimmun. Rev. 2020, 19, 102689. [Google Scholar] [CrossRef]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Rebrova, O.Y. Statistical Analysis of Medical Data—Application of a Package of Applied Programs STATISTICA; MediaSphere: Moscow, Russia, 2002; 312p. (In Russian) [Google Scholar]
- Morozov, S.P.; Vladzimirsky, A.V.; Kljashtorny, V.G. Clinical Trials of Software Based on Intelligent Technologies (Radiation Diagnostics); Preprint No. CDT-2019-1; Series “Best Practices in Radiation and Instrumental Diagnostics”; Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies of the Moscow Health Care Department: Moscow, Russia, 2019; 51p. (In Russian) [Google Scholar]

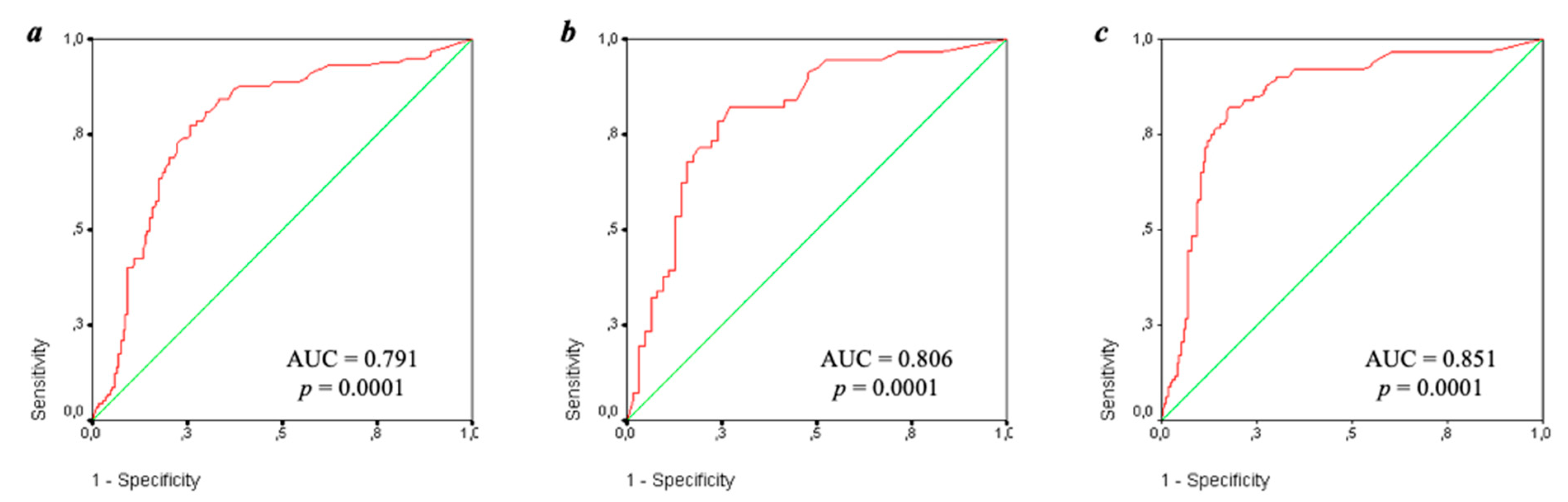
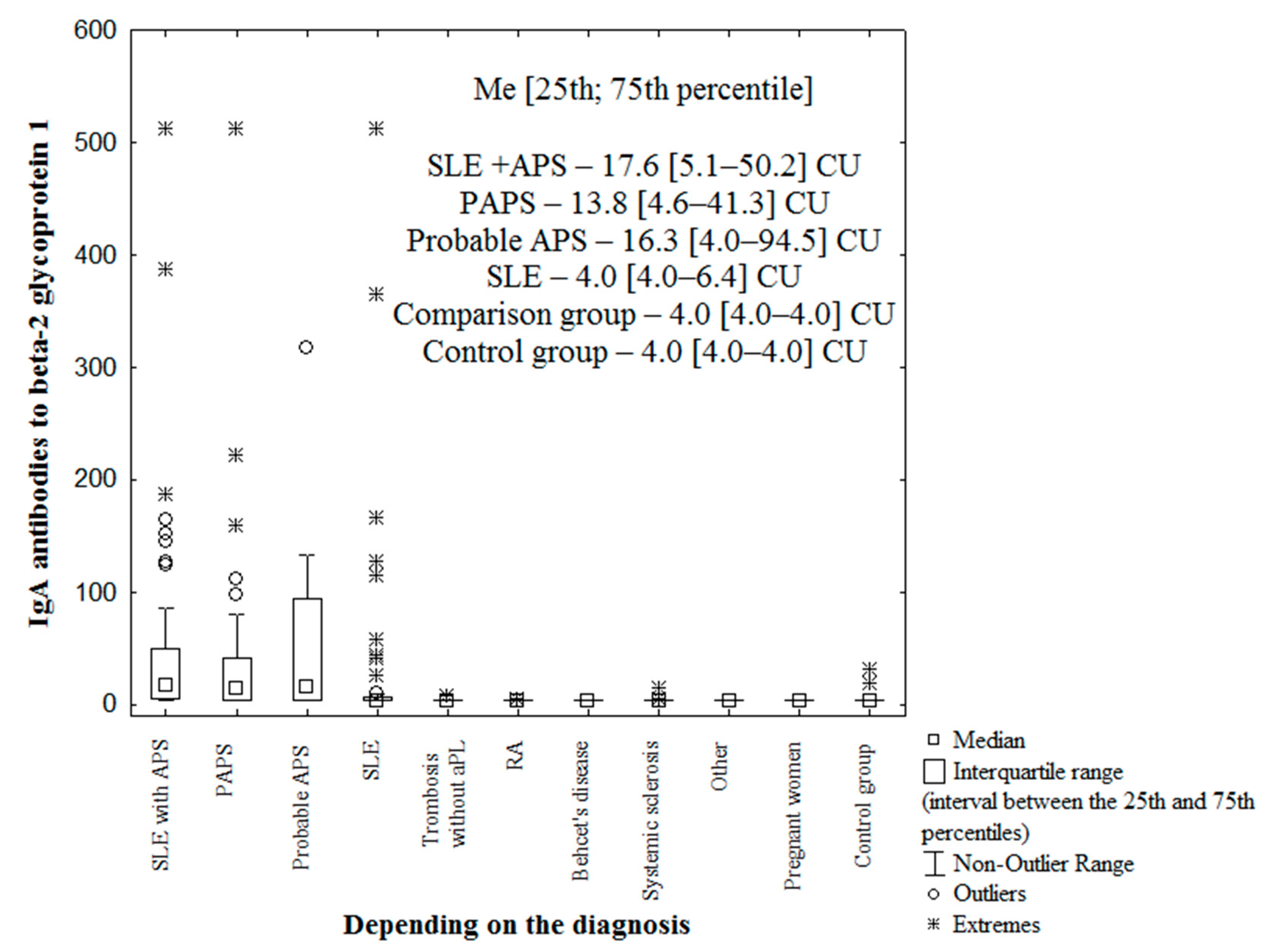

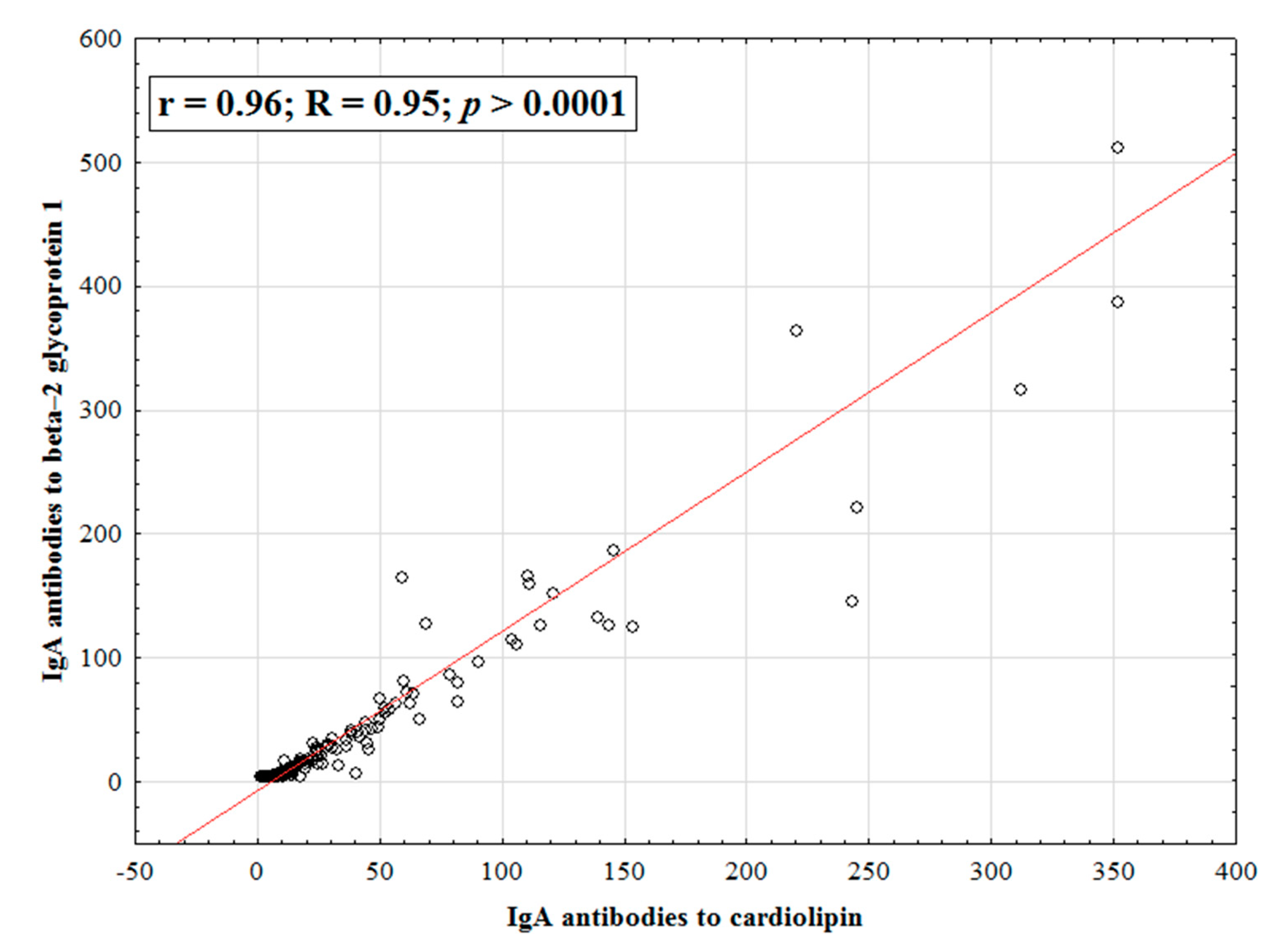
| Parameter | IgA aCL (+), n (%) | IgA aCL (−), n (%) | χ2; p OR [95% CI] | |
|---|---|---|---|---|
| APS | yes | 61 (81) | 51 (45) | 23.96; <0.0001 5.26[2.63–11.11] |
| no | 14 (19) | 61 (55) | ||
| Thrombosis (total) | yes | 53 (71) | 61 (54) | 4.96; 0.02 2.04 [1.08–3.84] |
| no | 22 (29) | 51 (46) | ||
| Arterial | yes | 29 (39) | 29 (26) | 3.43; 0.06 1.81 [0.97–3.44] |
| no | 46 (61) | 83 (74) | ||
| Venous | yes | 36 (48) | 45 (40) | 1.12; 0.28 1.38 [0.76–2.50] |
| no | 39 (52) | 67 (60) | ||
| Pregnancy morbidity * | yes | n = 25 20 (80) | n = 45 34 (75) | 0.02; 0.88 1.29 [0.39–4.34] |
| no | 5 (20) | 11 (25) | ||
| Parameter | IgA anti-β2-GP1 Positive, n (%) | IgA anti-β2-GP1 Negative, n (%) | χ2; p OR [95% CI] | |
|---|---|---|---|---|
| APS | yes | 50 (79) | 62 (50) | 15.00; 0.0001 3.84 [1.92–8.33] |
| no | 13 (21) | 62 (50) | ||
| Thrombosis (total) | yes | 45 (71) | 69 (56) | 4.37; 0.04 2.00 [1.04–3.84] |
| no | 18 (29) | 55 (44) | ||
| Arterial | yes | 26 (41) | 32 (26) | 4.67; 0.03 2.04 [1.06–3.84] |
| no | 37 (59) | 92 (74) | ||
| Venous | yes | 31 (49) | 50 (40) | 1.34; 0.24 1.44 [0.78–2.70] |
| no | 32 (51) | 74 (60) | ||
| Pregnancy morbidity * | yes | n = 21; 18 (86) | n = 49; 36 (73) | 0.02; 0.88 1.31 [0.40–4.34] |
| no | 5 (14) | 13 (27) | ||
| Parameter | PAPS, n = 53 | Probable APS, n = 12 | SLE + APS, n = 59 | SLE, n = 63 | Total, n = 187 | Comparison Group, n = 49 | Control Group, n = 100 |
|---|---|---|---|---|---|---|---|
| Average age, Me [25;75 percentile], years | 38.0 [32.0–43.0] | 34.0 [29.5–44.5] | 40.0 [33.0–47.0] | 31.0 [24.0–41.0] | 39.0 [35.0–48.0] | 39.0 [35.0–48.0] | 41.0 [30.0–54.0] |
| Duration of the disease, Me [25;75 percentile], years | 8.4 [3.1–13.5] | 0.9 [0.3–2.1] | 15.0 [7.3–21.0] | 4.0 [1.5–8.0] | 7.0 [2.0–15.0] | 6.0 [1.6–13.0] | - |
| Sex: male/female, abs | 30/23 | 10/2 | 47/12 | 55/8 | 142/45 | 33/16 | 86/14 |
| History of thrombosis, abs (%) | 48 (91) | 1 (8) *** | 51 (86) | 14 (22) | 19 (39) | 19 (39) | 1 (1) |
| Obstetric pathology *, n (%)/n | 20 (95)/21 | 1 (50) ****/2 | 26 (81)/32 | 7 (44)/16 | 5 (15)/33 | 5 (15)/33 | 2 (4); n = 51 |
| Thrombocytopenia for the entire period of the disease, n (%) | 7 (13) | 5 (42) | 19 (32) | 15 (24) | 0 (0) | 0 (0) | 0 (0) |
| Livedo reticularis, n (%) | 10 (19) | 2 (17) | 19 (32) | 5 (8) | 0 (0) | 0 (0) | 0 (0) |
| IgG aCL, n (%) | 42 (79) | 6 (50) | 52 (88) | 13 (21) | 0 (0) | 0 (0) | 0 (0) |
| IgM aCL, n (%) | 19 (36) | 5 (42) | 18 (30) | 10 (16) | 2 (4) | 2 (4) | 4 (4) |
| IgG anti-β2-GP1, n (%) | 41 (77) | 6 (50) | 52 (88) | 18 (29) | 6 (12) | 6 (12) | 1 (1) |
| IgM anti-β2-GP1, n (%) | 19 (36) | 8 (67) | 18 (30) | 12 (19) | 3 (6) | 3 (6) | 2 (2) |
| Lupus anticoagulant **, n (%)/n | 7 (87.5)/8 | 8 (89)/9 | 10 (71)/14 | 19 (79)/24 | 0 (0); n = 1 | 0 (0); n = 1 | - |
| Therapy: | |||||||
| Glucocorticoids | 4 (7) | 2 (17) | 47 (80) | 53 (84) | 106 (57) | 31 (63) | - |
| Hydroxychloroquine | 31 (58) | 4 (33) | 52 (88) | 54 (86) | 141 (75) | 8 (16) | |
| Basic anti-inflammatory drugs | 0 (0) | 0 (0) | 7 (12) | 18 (29) | 25 (13) | 26 (53) | |
| Aspirin | 17 (32) | 3 (25) | 24 (41) | 18 (29) | 62 (33) | 8 (16) | |
| Low-molecular-weight heparin | 7 (13) | 1 (8) | 10 (17) | 14 (22) | 32 (17) | 8 (16) | |
| Direct Oral anticoagulants | 27 (51) | 1 (8) | 18 (31) | 2 (3) | 48 (26) | 8 (16) | |
| Warfarin | 11 (21) | 0 (0) | 14 (24) | 2 (3) | 27 (15) | 1 (2) | |
| Without anticoagulant therapy | 8 (15) | 10 (84) | 16 (27) | 45 (72) | 79 (42) | 32 (66) | |
| Parameters | SLE with APS (n = 59) n (%) | SLE without APS (n = 63) n (%) | Total (n = 122) n (%) |
|---|---|---|---|
| Erythema of the zygomatic arches | 19 (32) | 26 (41) | 45 (37) |
| Discoid rash | 3 (5) | 2 (3) | 5 (4) |
| Photosensitization | 11 (19) | 14 (22) | 25 (20) |
| Oral ulcers | 8 (13) | 18 (28) | 26 (21) |
| Arthritis | 29 (49) | 39 (62) | 68 (56) |
| Serositis | 27 (46) | 32 (51) | 59 (48) |
| Renal damage | 22 (37) | 27 (43) | 49 (40) |
| Central nervous system damage | 9 (15) * | 1 (2) | 10 (8) |
| Hematological disorders | 38 (64) | 44 (70) | 82 (67) |
| Immunological disorders | 57 (97) | 62 (98) | 119 (97) |
| Positive antinuclear factor | 59 (100) | 63 (100) | 122 (100) |
| IgA anti-β2-GP1 positivity | 28 (47) ** | 9 (14) | 37 (30) |
| IgA aCL positivity | 35 (59) ** | 9 (14) | 44 (36) |
| SLEDAI | |||
|---|---|---|---|
| SLE with APS (n = 59) n (%) | SLE without APS (n = 63) n (%) | Total (n = 122) n (%) | |
| No activity | 7 (12) | 7 (11) | 14 (11) |
| Low activity | 30 (49) | 23 (36) | 53 (43) |
| Medium activity | 14 (25) | 12 (19) | 26(22) |
| High activity | 7 (12) | 12 (19) | 19 (16) |
| Very high activity | 1 (2) | 9 (14) | 10 (8) |
| SLEDAI activity index (points; median, 25th and 75th percentiles) | 4.0 [2.0–8.0] | 6.0 [2.0–14.0] * | 4.0 [2.0–10.0] |
| SLICC Damage index | |||
| No damage | 22 (37) | 42 (67) | 64 (57) |
| Low DI | 16 (27) | 8 (13) | 24 (17) |
| Medium DI | 19 (32) | 11 (17) | 30 (25) |
| High DI | 2 (3) | 2 (3) | 4 (3) |
| SLICC Damage index (Points; median, 25th and 75th percentiles) | 1.0 [0.0–3.0] ** | 0.0 [0.0–1.0] | 0.0 [0.0–2.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshetnyak, T.; Cheldieva, F.; Cherkasova, M.; Lila, A.; Nasonov, E. IgA Antiphospholipid Antibodies in Antiphospholipid Syndrome and Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2022, 23, 9432. https://doi.org/10.3390/ijms23169432
Reshetnyak T, Cheldieva F, Cherkasova M, Lila A, Nasonov E. IgA Antiphospholipid Antibodies in Antiphospholipid Syndrome and Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2022; 23(16):9432. https://doi.org/10.3390/ijms23169432
Chicago/Turabian StyleReshetnyak, Tatiana, Fariza Cheldieva, Maria Cherkasova, Alexander Lila, and Evgeny Nasonov. 2022. "IgA Antiphospholipid Antibodies in Antiphospholipid Syndrome and Systemic Lupus Erythematosus" International Journal of Molecular Sciences 23, no. 16: 9432. https://doi.org/10.3390/ijms23169432
APA StyleReshetnyak, T., Cheldieva, F., Cherkasova, M., Lila, A., & Nasonov, E. (2022). IgA Antiphospholipid Antibodies in Antiphospholipid Syndrome and Systemic Lupus Erythematosus. International Journal of Molecular Sciences, 23(16), 9432. https://doi.org/10.3390/ijms23169432







