Asymmetric Dimethylarginine Is a Marker of Endothelial Dysfunction in Thrombotic Antiphospholipid Syndrome Patients
Abstract
1. Introduction
2. Results
2.1. Clinical and Laboratory Characteristics
2.2. Brachial Artery Measurements
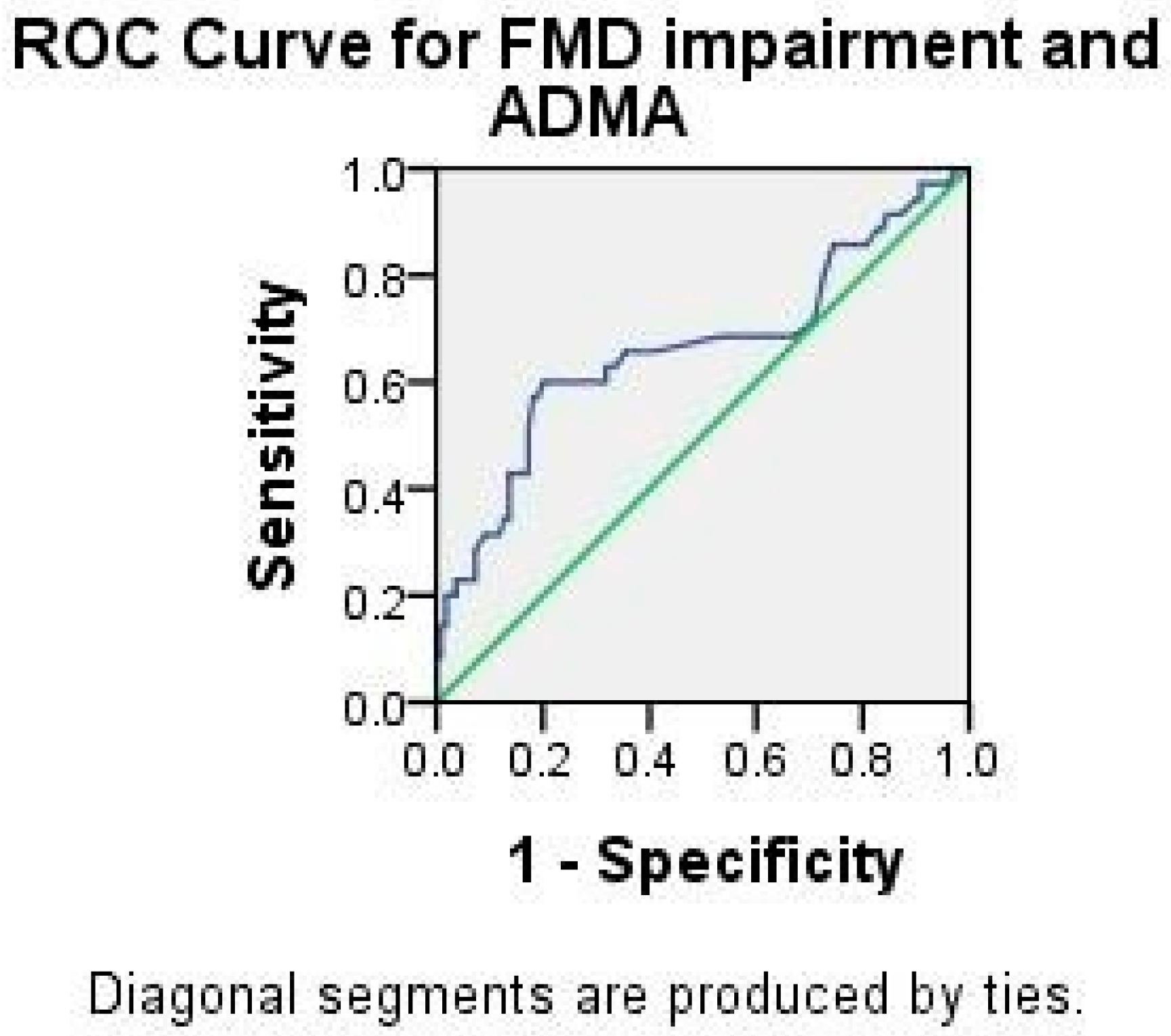
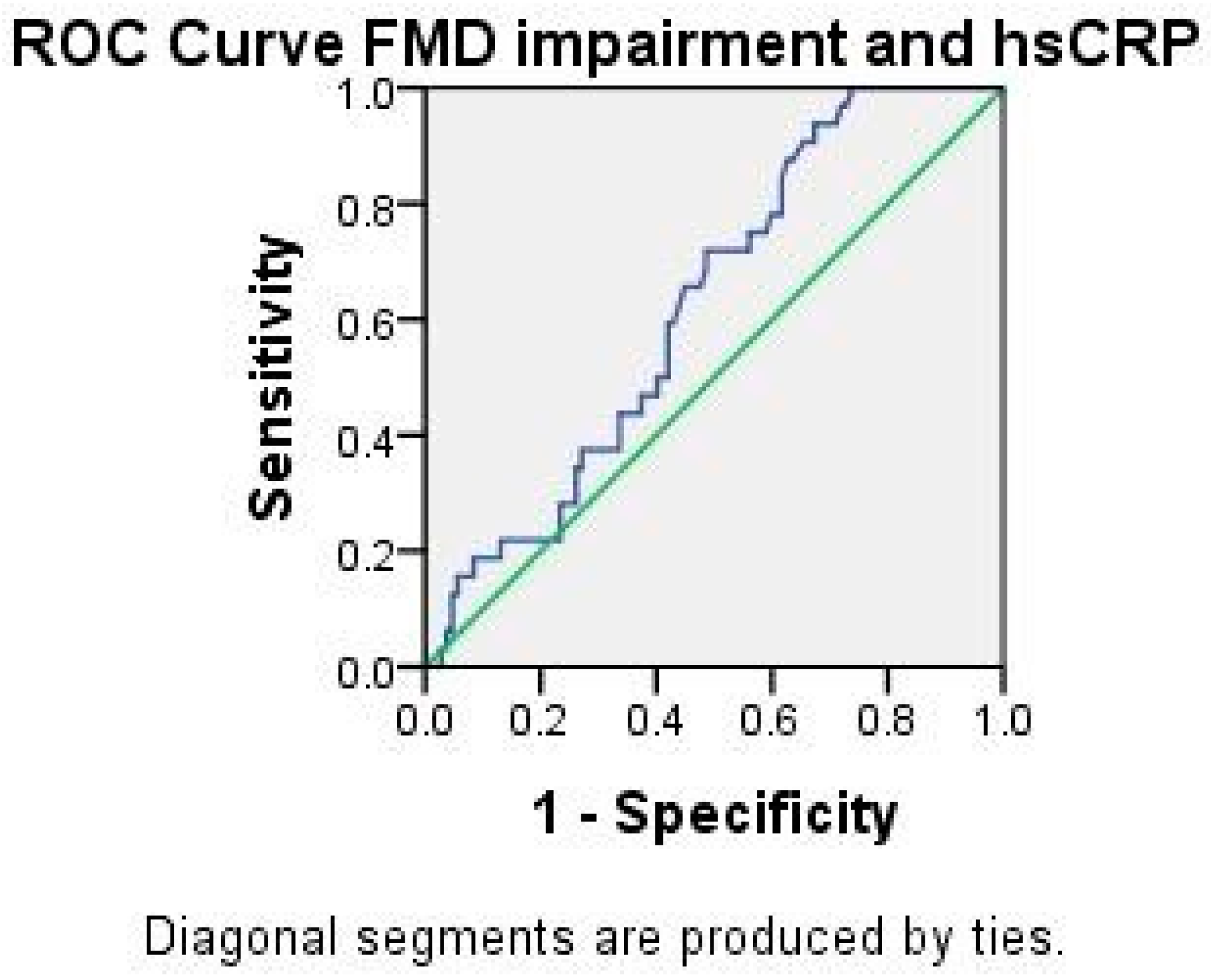
2.3. Analysis of ADMA and hsCRP
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; De Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Cyr, R.; Huckaby, V.; Shiva, S.; Zuckerbraun, S. Nitric Oxide and Endothelial Dysfunction. Crit. Care. Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.; Batuca, J.R.; Ciampa, A.; Iannaccone, L.; Alves, J.D. Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome. J. Rheumatol. 2010, 37, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J. ADMA: Its role in vascular disease. Vas. Med. 2005, 10, S11–S17. [Google Scholar] [CrossRef]
- Zafari, P.; Zarifian, A.; Alizadeh-Navaei, R.; Taghadosi, M.; Rafiei, A.; Samimi, Z.; Niksolat, F. Asymmetric and symmetric dimethylarginine concentration as an indicator of cardiovascular diseases in rheumatoid arthritis patients: A systematic review and meta-analysis of case-control studies. Clin. Rheumatol. 2020, 39, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Perna, M.; Roman, M.J.; Alpert, D.R.; Crow, M.K.; Lockshin, M.D.; Sammaritano, L.; Devereux, R.B.; Cooke, J.P.; Salmon, J.E. Relationship of asymmetric dimethylarginine and homocysteine to vascular aging in systemic lupus erythematosus patients. Arthritis Care Res. 2010, 62, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1–executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef]
- Verma, S.; Wang, C.H.; Li, S.H.; Dumont, A.S.; Fedak, P.W.; Badiwala, M.V.; Dhillon, B.; Weisel, R.D.; Li, R.-K.; Mickle, D.A.G.; et al. A self-fulling prophecy: C-reactive protein attenuates nitric oxyde production and inhibits angiogenesis. Circulation 2002, 106, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Vienna, J.L.; Khamashata, M.A.; Ordi-Ros, J. Comparison of the primary and secondary antiphospholipid syndrome: A Eu-ropean multicenter study of 114 patients. Am. J. Med. 1994, 96, 3–9. [Google Scholar] [CrossRef]
- Day, H.M.; Thiagarajan, P.; Ahn, C.; Reveille, J.D.; Tinker, K.F.; Arnett, F.C. Autoantibodies to beta2-glycoprotein I in systemic lupus erythematosus and primary antiphospholipid antibody syndrome: Clinical correlations in comparison with other antiphospholipid antibody tests. J. Rheumatol. 1998, 25, 667–674. [Google Scholar] [PubMed]
- Sahin, M.; Duzgun, N.; Ercan-Tunc, S.; Tutkak, H. Antibodies to β2-glycoprotien I: Relation of anticardiolipin antibodies with clinical and laboratory parameters in patients with systemic lupus erythematosus. Clin. Biochem. 2007, 40, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Luciani, D.; Bertolini, G.; Barbui, T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: A systematic review of the literature. Blood 2003, 1, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Stojanovich, L.; Djokovic, A.; Kontic, M. Antiphospholipid-mediated thrombosis: Interplay between type of antibodies and lo-calisation of lung, and cardiovascular incidences in primary antiphospholipid syndrome. Clin. Exp. Rheumatol. 2015, 33, 531–536. [Google Scholar]
- Neville, C.; Rauch, J.; Kassis, J.; Chang, E.R.; Joseph, L.; Le Comte, M.; Fortin, P.R. Thromboembolic risk in patients with high levels anticardiolipin and multiple antiphospho-lipid antibodies. Thromb. Haemost. 2003, 90, 108–115. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Patrick Vallance Internal brachial artery reactivity task force. Guidelines for the ultrasound as-sessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International brachial artery reactivity task force. J. Am. Coll. Cardiol. 2002, 39, 257–267. [Google Scholar] [CrossRef]
- Stanisavljevic, N.; Stojanovich, L.; Marisavljevic, D.; Djokovic, A.; Dopsaj, V.; Kotur-Stevuljevic, J.; Martinovic, J.; Memon, L.; Radovanovic, S.; Todic, B.; et al. Lipid peroxidation as risk factor for endothelial dysfunction in antiphospholipid syndrome patients. Clin. Rheumatol. 2016, 35, 2485–2493. [Google Scholar] [CrossRef]
- Benincasa, G.; Coscioni, E.; Napoli, C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: Novel opportunities for primary prevention. Biochem. Pharmacol. 2022, 202, 115108. [Google Scholar] [CrossRef]
- Felmeden, D.C.; Spencer, C.G.; Blann, A.D.; Beevers, D.G.; Lip, G.Y. Low-Density Lipoprotein Subfractions and Cardiovascular Risk in Hypertension. Hypertension 2003, 41, 528–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perticone, F.; Sciacqua, A.; Maio, R.; Perticone, M.; Maas, R.; Boger, R.H.; Tripepi, G.; Sesti, G.; Zoccali, C. Asymmetric Dimethylarginine, L-Arginine, and Endothelial Dysfunction in Essential Hypertension. J. Am. Coll. Cardiol. 2005, 46, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.J.; Miyake, Y.; Grahame-Clarke, C.; Di Tullio, M.R.; Sciacca, R.R.; Boden-Albala, B.; Sacco, R.L.; Homma, S. Relation of Plasma Glucose and Endothelial Function in a Population-Based Multiethnic Sample of Subjects without Diabetes Mellitus. Am. J. Cardiol. 2005, 96, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Arrebola-Moreno, A.; Laclaustra, M.; Kaski, J. Noninvasive assesment of endothelial function in clinical practice. Rev. esp. Cardiol. 2012, 65, 80–90. [Google Scholar] [CrossRef]
- Migliacci, R.; Falcinelli, F.; Imperiali, P.; Floridi, A.; Nenci, G.G.; Gresele, P. Endothelial dysfunction in patients with kidney failure and vascular risk factors: Acute effects of hemodialysis. Ital. Heart J. Off. J. Ital. Fed. Cardiol. 2004, 5, 371–377. [Google Scholar]
- Kiss, E.; Soltesz, P.; Der, H.; Kocsis, Z.; Tarr, T.; Bhattoa, H.; Shoenfeld, Y.; Szegedi, G. Reduced flow-mediated vasodilation as a marker for cardiovascular complications in lupus patients. J. Autoimmun. 2006, 27, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Štalc, M.; Poredoš, P.; Peternel, P.; Tomšič, M.; Šebeštjen, M.; Kveder, T. Endothelial function is impaired in patients with primary antiphospholipid syndrome. Thromb. Res. 2006, 118, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bilora, F.; Sartori, M. Flow-mediated arterial dilation in primary antiphospholipid syndrome. Angiology 2008, 59, 717–720. [Google Scholar] [CrossRef]
- Pierangeli, S.S.; Espinola, R.G.; Liu, X.; Harris, E.N. Thrombogenic Effects of Antiphospholipid Antibodies Are Mediated by Intercellular Cell Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1, and P-Selectin. Circ. Res. 2001, 88, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef]
- Velásquez, M.; Peláez, L.F.; Rojas, M.; Narváez-Sánchez, R.; Velásquez, J.A.; Escudero, C.; San Martín, S.; Cadavid, A.P. Differences in Endothelial Activation and Dysfunction Induced by Antiphospholipid Antibodies among Groups of Patients with Thrombotic, Refractory, and Non-refractory Antiphospholipid Syndrome. Front. Physiol. 2021, 12, 764702. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, M.; Rojas, M.; Abrahams, V.M.; Escudero, C.; Cadavid, Á.P. Mechanisms of Endothelial Dysfunction in Antiphospholipid Syndrome: Association With Clinical Manifestations. Front. Physiol. 2018, 9, 1840. [Google Scholar] [CrossRef]
- Kiani, A.N.; Mahoney, J.A.; Petri, M. Asymetric dimethylarginine is a marker of poor prognosis and coronary calcium in systemic lupus erythematosus. J. Rheumatol. 2007, 34, 1502–1505. [Google Scholar] [PubMed]
- Mayer-Pickel, K.; Kolovetsiou-Kreiner, V.; Mörtl, M.G.; Schlembach, D.; Eberhard, K.; Obermayer-Pietsch, B.; Lang, U.; Cervar-Zivkovic, M. Endothelin 1, ADMA and SDMA in pregnancies with obstetric and thrombotic antiphospholipid syndrome. J. Reprod. Immun. 2016, 116, 86–92. [Google Scholar] [CrossRef]
- Bećarević, M.; Majkić-Singh, N. High-sensitivity C-reactive protein: Discriminator between patients with primary and secondary antiphospholipid syndrome. Clin. Biochem. 2008, 41, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Rezaieyazdi, Z.; Sahebari, M.; Hatef, M.R.; Abbasi, B.; Rafatpanah, H.; Afshari, J.T.; Esmaily, H. Is there any correlation between high sensitive CRP and disease activity in systemic lupus erythematosus? Lupus 2011, 20, 1494–1500. [Google Scholar] [CrossRef]
- Ames, P.R.J.; Antinolfi, I.; Ciampa, A.; Batuca, J.; Scenna, G.; Lopez, L.R.; Delgado Alves, J.; Iannaccone, L.; Matsuura, E. Primary antiphospholipid syndrome: A low-grade auto-inflammatory disease? Rheumatology 2008, 47, 1832–1837. [Google Scholar] [CrossRef][Green Version]
- Fischetti, F.; Durigutto, P.; Pellis, V.; Debeus, A.; Macor, P.; Bulla, R.; Bossi, F.; Ziller, F.; Sblattero, D.; Meroni, P.; et al. Thrombus formation induced by antibodies to B2glycoprotein Iis complement dependent and requires priming factor. Blood 2005, 106, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Care Res. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Tripodi, A.; Reber, G.; Rand, J.H.; Ortel, T.L.; Galli, M.; DeGroot, P.G. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. J. Thromb. Haemost. 2009, 7, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Biasiolo, A.; Pegoraro, C.; Cucchini, U.; Noventa, F.; Iliceto, S.; Pengo, V. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb. Haemost. 2005, 93, 1147–1152. [Google Scholar] [CrossRef] [PubMed]

| Variables | PAPS N = 90 | SAPS N = 50 | Control Group N = 40 | p |
|---|---|---|---|---|
| BMI | 24.73 ± 4.96 | 25.44 ± 4.23 | 25.14 ± 5,42 | 0.424 |
| Hypertension, n (%) | 23 (38.9%) | 14 (30%) | 11 (27.5%) | 0.473 |
| Diabetes, n (%) | 2 (3.4%) | 2 (4.3%) | 1 (2,5%) | 0.902 |
| Smoking, n (%) | 17 (28.8%) | 9 (30.4%) | 7 (17.5%) | 0.902 |
| Triglycerides, (mmol/L) | 1.28 ± 1.12 | 1.24 ± 0.55 | 1.26 ± 0.58 | 0.426 |
| Total cholesterol, (mmol/L) | 5.39 ± 1.20 | 5.28 ± 1.0 | 5.46 ± 0.91 | 0.733 |
| HDL-C, (mmol/L) | 1.52 ± 0.48 | 1.45 ± 0.36 | 1.51 ± 0.41 | 0.921 |
| LDL-C, (mmol/L) | 3.32 ± 1.12 | 3.25 ± 0.88 | 3.37 ± 0.88 | 0.567 |
| PAPS N (%) | SAPS N (%) | p Value | |
|---|---|---|---|
| aCL IgM median—U/mL; IQR | 29 (49.2%) 12.1 (26.8) | 31 (67.4%) 24.6 (42.4) | 0.051 0.068 * |
| aCL IgG median—U/mL; IQR | 25 (42.4%) 8.9 (26.4) | 31 (67.4%) 22.6 (36.5) | 0.011 0.014 * |
| ß2GPI IgM median—U/mL; IQR | 23 (39%) 5.7 (16.7) | 23 (50%) 11.1 (41.2) | 0.259 0.130 * |
| ß2GPI IgG median—U/mL; IQR | 18 (30.5%) 5.7 (18.5) | 26 (56.5%) 17.8 (37.5) | 0.007 0.031 * |
| LA | 24 (40.7%) | 24 (52.2%) | 0.241 |
| Category IIa | 6 (10.2%) | 2 (4.3%) | 0.265 |
| Category IIb | 12 (20.3%) | 5 (10.9%) | 0.191 |
| Category IIc | 7 (11.9%) | 3 (6.5%) | 0.355 |
| Category I | 34 (57.6%) | 38 (82.6%) | 0.006 |
| Triple positivity | 12 (20.3%) | 18 (39.1%) | 0.034 |
| Parameter | PAPS | SAPS | Control | p * | p ** |
|---|---|---|---|---|---|
| Mean ADMA μmol/L (min–max) | 0.71 (0.35–1.38) | 1.96 (0.55–5.81) | 0.56 (0.22–0.97) | <0.001 | <0.001 |
| Mean hsCRP mg/L (min–max) | 2.98 (0.05–53.9) | 7.94 (0.16–62.6) | 1.61 (0.05–9.44) | 0.006 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanisavljevic, N.; Stojanovich, L.; Djokovic, A.; Todic, B.; Dopsaj, V.; Saponjski, J.; Saponjski, D.; Markovic, O.; Belizna, C.; Zdravkovic, M.; et al. Asymmetric Dimethylarginine Is a Marker of Endothelial Dysfunction in Thrombotic Antiphospholipid Syndrome Patients. Int. J. Mol. Sci. 2022, 23, 12309. https://doi.org/10.3390/ijms232012309
Stanisavljevic N, Stojanovich L, Djokovic A, Todic B, Dopsaj V, Saponjski J, Saponjski D, Markovic O, Belizna C, Zdravkovic M, et al. Asymmetric Dimethylarginine Is a Marker of Endothelial Dysfunction in Thrombotic Antiphospholipid Syndrome Patients. International Journal of Molecular Sciences. 2022; 23(20):12309. https://doi.org/10.3390/ijms232012309
Chicago/Turabian StyleStanisavljevic, Natasa, Ljudmila Stojanovich, Aleksandra Djokovic, Brankica Todic, Violeta Dopsaj, Jovica Saponjski, Dusan Saponjski, Olivera Markovic, Cristina Belizna, Marija Zdravkovic, and et al. 2022. "Asymmetric Dimethylarginine Is a Marker of Endothelial Dysfunction in Thrombotic Antiphospholipid Syndrome Patients" International Journal of Molecular Sciences 23, no. 20: 12309. https://doi.org/10.3390/ijms232012309
APA StyleStanisavljevic, N., Stojanovich, L., Djokovic, A., Todic, B., Dopsaj, V., Saponjski, J., Saponjski, D., Markovic, O., Belizna, C., Zdravkovic, M., & Marisavljevic, D. (2022). Asymmetric Dimethylarginine Is a Marker of Endothelial Dysfunction in Thrombotic Antiphospholipid Syndrome Patients. International Journal of Molecular Sciences, 23(20), 12309. https://doi.org/10.3390/ijms232012309








