The Possible Positive Mechanisms of Pirenoxine in Cataract Formation
Abstract
1. Introduction
2. Effects of Pirenoxine on Age-Related Cataract: Evidence from In Vitro, Ex Vivo, In Vivo, and Clinical Studies
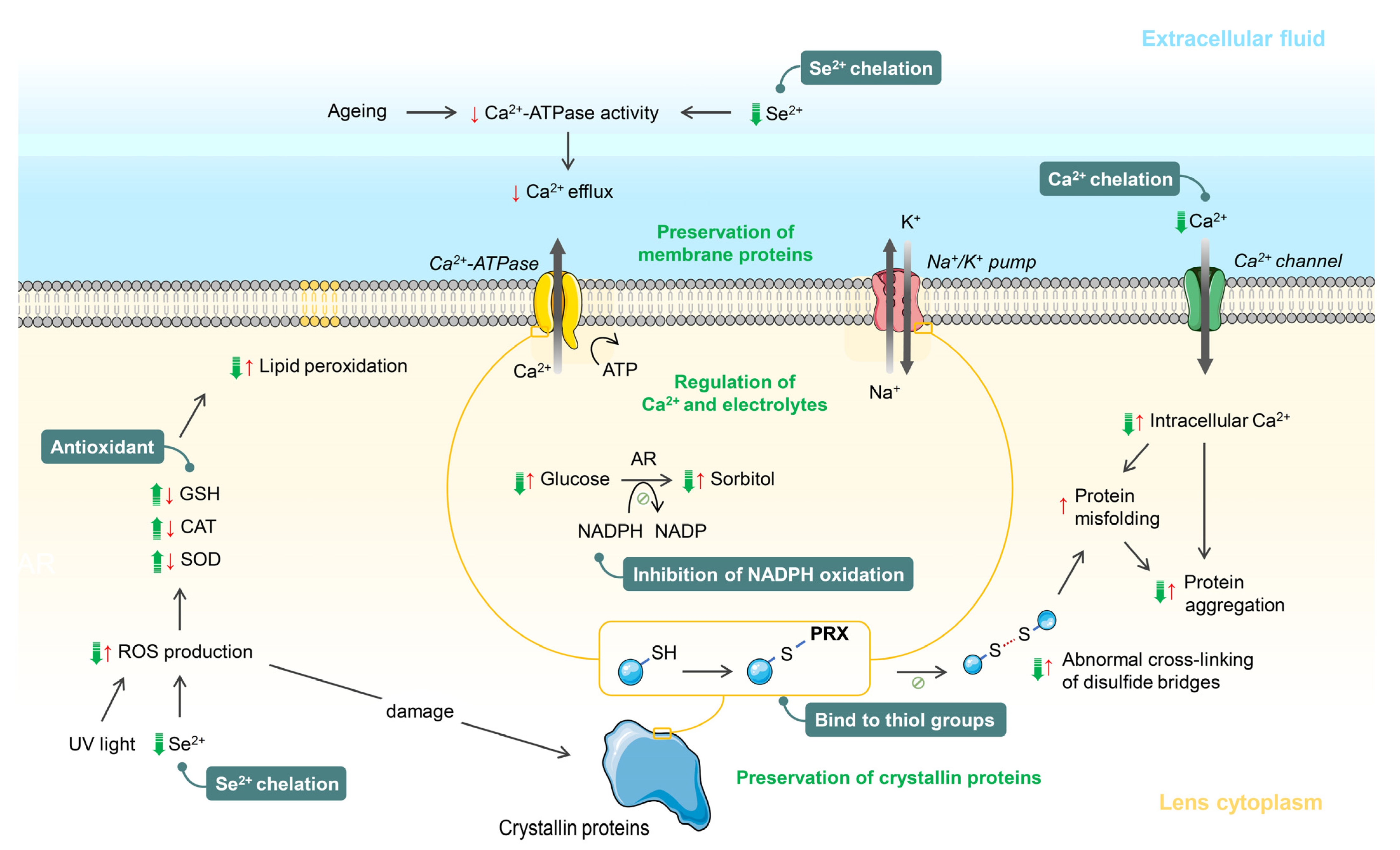
2.1. Effects of Pirenoxine on Calcium Dysregulation-Induced Age-Related Cataract
2.2. Effects of Pirenoxine on Oxidative Stress-Induced Age-Related Cataract
2.3. Effects of Pirenoxine on Selenium-Induced Age-Related Cataract
2.4. Effects of Pirenoxine on Ultraviolet (UV) Radiation-Induced Age-Related Cataract
2.5. Effects of Pirenoxine on Quinone-Induced Age-Related Cataracts
2.6. Effects of Pirenoxine on the Natural Progression of Cataract
3. Effects of Pirenoxine on Diabetic Cataract
4. Effects of Pirenoxine on Congenital Cataract
5. Safety of Pirenoxine on the Eyes
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet. Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision Beyond 2020. Lancet. Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Gupta, V.B.; Rajagopala, M.; Ravishankar, B. Etiopathogenesis of Cataract: An Appraisal. Indian J. Ophthalmol. 2014, 62, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Harris, F.; Dennison, S.; Singh, J.P.; Phoenix, D. Calpains: Enzymes Of Vision? Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2005, 11, RA301–RA310. [Google Scholar]
- Truscott, R.J. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Kisic, B.; Miric, D.; Zoric, L.; Ilic, A. Role of Lipid Peroxidation in the Pathogenesis of Age-Related Cataract. Lipid Peroxidation 2012, 66, 371–375. [Google Scholar]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and Vision: Structure, Stability And Function Of Lens Crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-Related Changes In The Kinetics Of Human Lenses: Prevention of the Cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [CrossRef]
- Wang, B.; Hom, G.; Zhou, S.; Guo, M.; Li, B.; Yang, J.; Monnier, V.M.; Fan, X. The Oxidized Thiol Proteome in Aging and Cataractous Mouse and Human Lens Revealed by ICAT labeling. Aging Cell 2017, 16, 244–261. [Google Scholar] [CrossRef]
- Lee, C.M.; Afshari, N.A. The Global State of Cataract Blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef]
- Chan, E.; Mahroo, O.A.; Spalton, D.J. Complications of Cataract Surgery. Clin. Exp. Optom. J. Aust. Optom. Assoc. 2010, 93, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kociecki, J.; Załecki, K.; Wasiewicz-Rager, J.; Pecold, K. Evaluation of Effectiveness of Catalin Eyedrops in Patients with Presenile and Senile Cataract. Klin. Ocz. 2004, 106, 778–782. [Google Scholar]
- Heruye, S.H.; Maffofou Nkenyi, L.N.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.F.; Ohia, S.E.; Opere, C.A. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Rupenthal, I.D.; Rasch, S.S.; Lim, J.C.; Morton, J.D.; Bunt, C.R. Drug Delivery to the Lens for the Management of Cataracts. Adv. Drug Deliv. Rev. 2018, 126, 185–194. [Google Scholar] [CrossRef]
- Hu, C.C.; Liao, J.H.; Hsu, K.Y.; Lin, I.L.; Tsai, M.H.; Wu, W.H.; Wei, T.T.; Huang, Y.S.; Chiu, S.J.; Chen, H.Y.; et al. Role of Pirenoxine in the Effects of Catalin on In Vitro Ultraviolet-Induced Lens Protein Turbidity and Selenite-Induced Cataractogenesis In Vivo. Mol. Vis. 2011, 17, 1862–1870. [Google Scholar]
- Liao, J.H.; Chen, C.S.; Hu, C.C.; Chen, W.T.; Wang, S.P.; Lin, I.L.; Huang, Y.H.; Tsai, M.H.; Wu, T.H.; Huang, F.Y.; et al. Ditopic Complexation of Selenite Anions or Calcium Cations by Pirenoxine: An Implication for Anti-Cataractogenesis. Inorg. Chem. 2011, 50, 365–377. [Google Scholar] [CrossRef]
- Ciuffi, M.; Neri, S.; Franchi-Micheli, S.; Failli, P.; Zilletti, L.; Moncelli, M.R.; Guidelli, R. Protective Effect of Pirenoxine and U74389F on Induced Lipid Peroxidation in Mammalian Lenses. An In Vitro, Ex Vivo And In Vivo Study. Exp. Eye Res. 1999, 68, 347–359. [Google Scholar] [CrossRef]
- Drago, F.; D’Agata, V.; Marino, V.; Marino, A.; Blasco, G. Biochemical Changes Induced by Pyrphenoxone in the Lens of Rabbits and Rats. Pharmacol. Res. 1995, 31, 325–329. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, P.; Zhao, C.; Zhang, Y.; Liu, H.; Hu, L.; Gao, X.; Zhang, D. Prevention Effect in Selenite-Induced Cataract In Vivo and Antioxidative Effects In Vitro of Crataegus Pinnatifida Leaves. Biol. Trace Elem. Res. 2011, 142, 106–116. [Google Scholar] [CrossRef]
- Ogino, S. Studies on the Metabolism of Crystalline Lens. Nippon. Ganka Gakkai Zasshi 1955, 59, 666–709. (In Japanese) [Google Scholar]
- Ogino, S. Etiology and Treatment of Cataract. Jpn. Med. J. 1957, 1732, 13–22. (In Japanese) [Google Scholar]
- Ogino, S. Studies on Pharmacological Treatment of Cataract. Rinsyo Ganka 1957, 11, 272–281. (In Japanese) [Google Scholar]
- Nishizaki, K.; Inoue, K. Clinical Experiments with High Concentration Catalin Ophthalmic Solution. Folia Ophthalmol Jpn. 1975, 26, 1087–1090. (In Japanese) [Google Scholar]
- Sekimoto, M.; Imanaka, Y.; Kitano, N.; Ishizaki, T.; Takahashi, O. Why are Physicians Not Persuaded by Scientific Evidence? A Grounded Theory Interview Study. BMC Health Serv. Res. 2006, 6, 92. [Google Scholar] [CrossRef][Green Version]
- Ito, Y.; Nagai, N.; Cai, H.; Takeda, M.; Koizumi, Y. Preventive Effect of Eye Drops of Liposomes Containing Disulfiram and Cefmetazole on Selenite-Induced Cataract in Rat Pups. J. Oleo Sci. 2006, 55, 15–22. [Google Scholar] [CrossRef]
- Testa, M.; Iuliano, G.; Morton, P.; Longoni, A. Topical Benzyl Alcohol Reduces Cataract Surgery Need: Two Long-Term Double Blind Studies. J. Ocul. Pharm. 1987, 3, 211–225. [Google Scholar] [CrossRef]
- Asakura, S.; Ohta, M.; Takimoto, Y.; Hara, K.; Nishi, M. Effect of Pirenoxine Ophthalmic Solution on Senile Incipient Cataract in Dogs. J. Jpn. Vet. Med. Assoc. 1993, 46, 952–957. [Google Scholar] [CrossRef][Green Version]
- Nakamura, K.; Nomoto, K.; Kariya, K.; Nakajima, Y.; Nishimoto, H.; Uga, S.; Miyata, M.; Osawa, T.; Kawakishi, S.; Kakimoto, N. Prevention and Reversible Solubilization of Advanced Glycation and Products (AGE) By Organic Germanium Compounds as Derivatives of Amino Acids. Amino Acids 1991, 1, 263–278. [Google Scholar] [CrossRef]
- Angra, S.K.; Mohan, M.; Saini, J.S. Medical Therapy of Cataract (Evaluation of Catalin). Indian J. Ophthalmol. 1983, 31, 5–8. [Google Scholar]
- Cekic, S.; Zlatanovic, G.; Cvetkovic, T.; Petrovic, B. Oxidative Stress in Cataractogenesis. Bosn. J. Basic Med. Sci. 2010, 10, 265–269. [Google Scholar] [CrossRef]
- Fang, H.; Hu, X.; Wang, M.; Wan, W.; Yang, Q.; Sun, X.; Gu, Q.; Gao, X.; Wang, Z.; Gu, L.; et al. Anti-Osmotic and Antioxidant Activities of Gigantol From Dendrobium Aurantiacum Var. Denneanum Against Cataractogenesis in Galactosemic Rats. J. Ethnopharmacol. 2015, 172, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Albal, M.V.; Chandorkar, A.G.; Bulakh, P.M. Evaluation of Catalin, Succus Cineraria Maritima and Catobell in Goat Lens Cultures. Indian J. Ophthalmol. 1981, 29, 147–149. [Google Scholar]
- Korte, I.; Hockwin, O.; Ohrloff, C. Influence of Catalin® (1-Hydroxy-pyrido-(3,2α)-5-Phenoxazone-3-Carboxylic Acid) on the Sorbitol Content of Incubated Bovine Lenses. Ophthalmic Res. 1979, 11, 123–125. [Google Scholar] [CrossRef]
- Noma, T.; Okubo, Y.; Yamamoto, Y.; Ikemoto, F.; Iwata, S. Effects of Sodium Salt of Catalin Against Alloxan Diabetic Cataract in Rabbit. Senju Res. Lab. 1974, 328, 1–3. [Google Scholar]
- Bulakh, P.M.; Chandorkar, A.G.; Balsara, J.J.; Ranade, S.M.; Albal, M.V. Effect of ‘Catalin’ an Anticataract Agent on Alloxan Induced Hyperglycaemia and Diabetic Cataract in Rats. Indian J. Ophthalmol. 1980, 28, 1–3. [Google Scholar]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic Acid Extracted from Herba dendrobii Prevents Diabetic Cataract Pathogenesis by Inhibiting Aldose Reductase Activity. Evid. Based Complementary Altern. Med. Ecam. 2012, 2012, 426537. [Google Scholar] [CrossRef] [PubMed]
- Cantore, M.; Siano, S.; Coronnello, M.; Mazzetti, L.; Franchi-Micheli, S.; Boldrini, E.; Ciuffi, M.; Failli, P. Pirenoxine Prevents Oxidative Effects of Argon Fluoride Excimer Laser Irradiation in Rabbit Corneas: Biochemical, Histological and Cytofluorimetric Evaluations. J. Photochem. Photobiol. B Biol. 2005, 78, 35–42. [Google Scholar] [CrossRef]
- Abdelkawi, S.; Ghoneim, D.; Atoat, W.; Badr, Y.A. 193 nm ArF Excimer Laser and the Potential Risk for Cataract Formation. J. Appl. Sci. Res. 2010, 6, 796–805. [Google Scholar]
- Mansour, A.M.; Ghabra, M. Cataractogenesis after Repeat Laser in situ Keratomileusis. Case Rep. Ophthalmol. 2012, 3, 262–265. [Google Scholar] [CrossRef]
- Kornilosvskiy, I.M. Factors of Cataractogenesis in Laser Corneal Refractive Surgery. Ophthalmol. Russ. 2019, 16, 112–117. [Google Scholar] [CrossRef]
- Saba, S.; Ghahramani, M.; Yousefi, R. A Comparative Study of the Impact of Calcium Ion on Structure, Aggregation and Chaperone Function of Human alphaA-crystallin and its Cataract-Causing R12C Mutant. Protein Pept. Lett. 2017, 24, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Anbaraki, A.; Khoshaman, K.; Ghasemi, Y.; Yousefi, R. Preventive Role of Lens Antioxidant Defense Mechanism against Riboflavin-Mediated Sunlight Damaging of Lens Crystallins. Int. J. Biol. Macromol. 2016, 91, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castineiras, S. Iron, the Retina And the Lens: A Focused Review. Exp. Eye Res. 2010, 90, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Tweeddale, H.J.; Hawkins, C.L.; Janmie, J.F.; Truscott, R.J.; Davies, M.J. Cross-Linking of Lens Crystallin Proteins Induced by Tryptophan Metabolites and Metal Ions: Implications for Cataract Development. Free. Radic. Res. 2016, 50, 1116–1130. [Google Scholar] [CrossRef]
- Chasovnikova, L.V.; Formazyuk, V.E.; Sergienko, V.I.; Boldyrev, A.A.; Severin, S.E. The Antioxidative Properties of Carnosine and Other Drugs. Biochem. Int. 1990, 20, 1097–1103. [Google Scholar]
- Jones, G.M.; Vale, J.A. Mechanisms of Toxicity, Clinical Features, and Management of Diquat Poisoning: A Review. J. Toxicol. Clin. Toxicol. 2000, 38, 123–128. [Google Scholar] [CrossRef]
- Marino, A.; Drago, F.; Marino, V.; Villareale, G.; Paulick, B.; Cerilli, C. Effects of Pyrphenoxone on Lens Protein Denaturation and Metabolism in Rabbits and Rats. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2208. [Google Scholar]
- Ciuffi, M.; Pisanello, M.; Pagliai, G.; Raimondi, L.; Franchi-Micheli, S.; Cantore, M.; Mazzetti, L.; Failli, P. Antioxidant Protection in Cultured Corneal Cells and Whole Corneas Submitted to UV-B Exposure. J. Photochem. Photobiol. B Biol. 2003, 71, 59–68. [Google Scholar] [CrossRef][Green Version]
- Ikemoto, F.; Fukui, S.; Iwata, S. Studies on Transparency Mechanism of the Lens. Report 1: Capsular Function of the Lens and Its Inhibition; Japanese Pharmacological Society: Kanto, Japan, 1974. [Google Scholar]
- Sakthivel, M.; Elanchezhian, R.; Thomas, P.A.; Geraldine, P. Alterations in Lenticular Proteins During Ageing and Selenite-Induced Cataractogenesis in Wistar Rats. Mol. Vis. 2010, 16, 445–453. [Google Scholar]
- Flohe, L. Selenium, Selenoproteins and Vision. Dev. Ophthalmol. 2005, 38, 89–102. [Google Scholar] [CrossRef]
- Seko, Y.; Imura, N. Active Oxygen Generation as a Possible Mechanism of Selenium Toxicity. Biomed. Env. Sci. 1997, 10, 333–339. [Google Scholar]
- Vaajanen, A.; Vapaatalo, H. A Single Drop in the Eye-Effects on the Whole Body? Open Ophthalmol. J. 2017, 11, 305–314. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant Defenses in the Ocular Surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Drago, F. Pirenoxine for the Topical Treatment of Inflammatory Conditions. Patent EP0885612A1, 23 December 1998. [Google Scholar]
- Enrico, B.; Banchetti, M. Use of Pirenoxine for the Protection of Corneal Tissues in Photokeratectomy. Patent A61P 27/02, 27 December 2006. [Google Scholar]
- Baig, A.M.; Katyara, P.; Khaleeq, A.; Nazim, F. Repurposing drugs: Ca(2+) Ion Dependency That Can be Exploited to Treat Keratitis Caused by Acanthamoeba Castellanii. Eye 2019, 33, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Sevin, G.; Kerry, Z.; Sozer, N.; Ozsarlak-Sozer, G. Taurine Supplementation Protects Lens Against Glutathione Depletion. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4520–4526. [Google Scholar] [CrossRef]
- Maclean, H. The Melbourne Catalin Trial Current Protocol and Some Early Results. Aust. J. Opthalmology 1977, 5, 183–187. [Google Scholar] [CrossRef]
- Kleber, E.; Kroner, R.; Elstner, E.F. Cataract Induction By 1,2-Naphthoquinone. I. Studies on the Redox Properties of Bovine Lens Proteins. Z. Für Nat. C 1991, 46, 280–284. [Google Scholar] [CrossRef]
- Polunin, G.S.; Makarova, I.A.; Bubnova, I.A. Efficacy of Catalin Eyed Drops in Age-Related Cataract Agents. Vestn. Oftalmol. 2010, 126, 36–39. [Google Scholar]
- Pollreisz, A.; Schmidt-Erfurth, U. Diabetic Cataract-Pathogenesis, Epidemiology and Treatment. J. Ophthalmol. 2010, 2010, 608751. [Google Scholar] [CrossRef]
- Harding, J.J. Viewing Molecular Mechanisms of Ageing Through A Lens. Ageing Res. Rev. 2002, 1, 465–479. [Google Scholar] [CrossRef]
- Korte, I.; Hockwin, O.; Tullius, H.; Diederich, D. Proceedings: Effect of Catalin (1-Hydroxy-(3,2alpha)-5-Phenoxazone-3-Carboxylic Acid) on the Reduced Coenzymes NADH And NADPH. Exp. Eye Res. 1975, 20, 180. [Google Scholar] [CrossRef]
- Chandorkar, A.G.; Albal, M.V.; Bulakh, P.M.; Jain, P.K. Hypoglycaemic Effect of ‘Catalin’ an Anti-Cataract Agent In Rabbits, (a Preliminary Study). Indian J. Ophthalmol. 1978, 26, 6–8. [Google Scholar] [PubMed]
- Knopfel, E.B.; Vilches, C.; Camargo, S.M.R.; Errasti-Murugarren, E.; Staubli, A.; Mayayo, C.; Munier, F.L.; Miroshnikova, N.; Poncet, N.; Junza, A.; et al. Dysfunctional LAT2 Amino Acid Transporter Is Associated With Cataract in Mouse and Humans. Front. Physiol. 2019, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.R.; Mongre, P.K.; Das, G.K.; Sen, S.; Angra, S.K.; Vajpayee, R.B. Animal Study on the Effects of Catalin on Aftercataract and Posterior Capsule Opacification. Ophthalmic Res. 1999, 31, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, Y.; Higuchi, A.; Negishi, K.; Tsubota, K. Suppression of Presbyopia Progression with Pirenoxine Eye Drops: Experiments on Rats and Non-Blinded, Randomized Clinical Trial of Efficacy. Sci. Rep. 2017, 7, 6819. [Google Scholar] [CrossRef] [PubMed]
- Niiya, A.; Yokoi, N.; Matsumoto, Y.; Komuro, A.; Ishibashi, T.; Tomii, S.; He, J.; Kinoshita, S. Effect of Beta-Blocker Eyedrops on Corneal Epithelial Barrier Function. Ophthalmologica 2000, 214, 332–336. [Google Scholar] [CrossRef]
- Inui, S.; Ozawa, K.; Song, M.; Itami, S.; Katayama, I. Contact Dermatitis Due to Pirfenoxone. Contact Dermat. 2004, 50, 375–376. [Google Scholar] [CrossRef]
| Induction of Cataract | Source of Lens | Name/Dose/Route/Duration of PRX | Major Findings | Interpretation | Ref | ||
|---|---|---|---|---|---|---|---|
| Lens Opacity | Oxidative Stress | Others | |||||
| Ca or selenite (10 mM) | Pig lens homogenate | Pure PRX/0.03, 0.1, and 0.3 μM/ 0–4 d | ↓ | PRX decelerated Ca- and selenite-induced lens opacification. | [15] | ||
| Ca or selenite (10 mM) | Pig lens homogenate | PRX/ 1 μM/5 d | ↓ | PRX decelerated Ca- and selenite-induced lens opacification. | [16] | ||
| Selenite (10 mM) | SD-rat pup lens homogenate | Catalin/0.016, 0.032, 0.080, and 0.1 μM/ 0–4 d Only cataV in Catalin/ 0–4 d | 0.016 μM: ⟷ 0.032, 0.080, and 0.1 μM: ↓ (only at d1) ⟷ | ↓ degradation of water-insoluble lens proteins | High dose PRX decelerated early selenite-induced lens opacification by a deceleration of degradation of water-insoluble lens proteins. CataV in Catalin had no effect on selenite-induced lens opacification. | [15] | |
| Fe3+ (10 μM)/ascorbate | Rat lens homogenate | Catalin/ 0.1–1000 μM/2 h | ↓ TBA ↓ lipid hydroperoxide | Catalin prevented ROS damage of the lens after induction with Fe3+/ascorbate. | [17] | ||
| Fe3+/ascorbate, Hb (10 μM), fMLP-stimulated macrophages (10 nM) | Rat whole lens | Catalin/ 0.1–1000 μM/2 h | ↓ TBA ↓ lipid hydroperoxide | Catalin prevented ROS damage of the lens after an induction with either Fe3+/ascorbate, Hb, or stimulated macrophages. | [17] | ||
| X (600 μM)/ XO (0.1 U/mL) | Rat whole lens | Catalin/ 0.1–1000 μM/2 h | ↓ lipid peroxidation ⟷ Superoxide ⟷ Urate | Catalin prevented ROS damage of the lens with mechanisms other than inhibition of X/XO system. | [17] | ||
| UVC (4 h) | Pig lens homogenate | Pure PRX/ 0.1, 1, 10, 100, and 1000 μM/ 0–4 h | PRX (1000 μM): ↓ PRX (<1000 μM): ⟷ | Pure PRX and cataV provided comparable benefits in decelerating lens protein opacity via the deceleration of lens degradation. The combination therapy provided greater efficacy than the monotherapy. | [15] | ||
| Catalin/ 16, 32, 80, and 100 μM PRX + cataV/ 0–4 h Only cataV in Catalin/ 0–4 h | ↓ ↓ | ↓ degradation of γ-crystallins ↓ degradation of γ-crystallins | |||||
| m-calpain activated by Ca | Pig lens homogenate | Catalin/ 0, 32, 80, and 100 μM Pure PRX/100 μM | ⟷ degradation of β- and α-crystallins | Catalin failed to decelerated proteolysis of lens induced by m-calpain. | [15] | ||
| UVB (6 h) | Pig lens homogenate | Catalin/ 0.1, 1, 10, and 100 μM/2 h | ⟷ | Catalin had no protective effect against UVB-induced cataract. | [15] | ||
| Study Types | Models | Induction of Cataract | Name/Dose/Route/Duration of PRX | Major Findings | Interpretation | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Lens Opacity/ Evaluation Time | Oxidative Stress | Others | ||||||
| Ex vivo | Rabbit lens | Fe3+/ ascorbate | Catalin/0.005%, 2 drops q 1 h/topical/8 h daily (total 2 d) before incubation with FeCl3 | ↓ conjugated- dienes ↓ lipid soluble fluorescent compound | Catalin decreased oxidative degradation of lipids in the lens after induction with Fe3+. | [17] | ||
| In vivo | Rabbit | IVT 50 µM, 100 µM Hb at 2, 4, 6, and 8 d | Catalin/0.005%, 2 drops q 1 h/topical/8 h daily (total 4 d) before IVT Hb | ↓ conjugated- dienes ↓ lipid soluble fluorescent compound | Catalin decreased oxidative degradation of lipids in the lens after induction with IVT Hb. | [17] | ||
| In vivo | Rabbit | IVT diquat (300 μM) | Catalin/0.005%, 2 drops q 1 h/topical/8 h daily (total 4 d) before IVT diquat | ↓ conjugated- dienes ↓ lipid soluble fluorescent compound | Catalin decreased oxidative degradation of lipids in the lens after induction with IVT diquat. | [17] | ||
| In vivo | Wistar rat | A single dose of 19 µmol/kg of selenite via SC route at d3 | PRX/0.8 mg/15 mL, tid/topical/7 d | Serum: ↑ SOD ↑ CAT ↓ MDA Lens: ↑ SOD ↑ CAT ↑ GSH | PRX increased antioxidative enzymes in both lens and serum after induction with selenite. PRX decreased oxidative degradation of lipids in serum. | [19] | ||
| In vivo | Mouse | Senescence-accelerated inbred | Catalin/0.005%, qid/120 d | ↓ progression ↓ wedge opacity formation | PRX decelerated progression of age-related cataract. | [28] | ||
| In vivo | Dog with age-related incipient cataract | None | PRX/0.05%, 1–2 drops, 3–5 times/d/average 8 mo | ↓ opacity or ↓ progression: 72.2% % improvement: Cortical type: 62% Cortical and nuclear type: 30% | PRX reversed opacity and retarded progression of age-related cataract particularly at the cortical region of the lens. | [27] | ||
| In vivo | SD-rat pup | A single dose of 19 µmoL/kg of selenite via SC route | Catalin/2.5 and 5 mg/kg single dose/SC/3 d before selenite injection | 2.5 mg/kg: ⟷/d 3 ⟷/d 4 5 mg/kg ↓/d 3 ⟷/d 4 | Pretreatment with high-dose subcutaneous Catalin only prevented early gross lens opacity in selenite-induced cataract. IVT Catalin also failed to decelerate gross lens opacity. | [15] | ||
| Catalin/ 2 mg/mL single dose/IVT/after selenite injection | ⟷/d 5 | |||||||
| In vivo | Wistar rat | A single dose of 19 µmol/kg of selenite via SC route | Catalin solution/0.03%/topical/1 time 1.5 h before selenite injection and qid for 1 wk after selenite injection Catalin liposome/ 0.24 mg/mL (particle size 100 nm)/topical/1 time 1.5 h before selenite injection and qid for 1 wk after selenite injection | By Scheimpflug camera/d 0–7: ⟷ By slit-lamp microscope/ d 1–4: ⟷ | Neither solution or liposomal forms of Catalin could prevent or decelerated selenite-induced cataract. | [25] | ||
| By Scheimpflug camera/d 0–7: ⟷ By slit-lamp microscope/ d 1–4: ⟷ | ||||||||
| In vivo | Wistar rat lens homogenate | A single dose of 19 µmol/kg of selenite via SC route | Catalin solution/0.03%/topical/1 time 1.5 h before selenite injection and qid for 1 wk after selenite injection | ⟷ GSH | ⟷ Na/K ratio ⟷ Ca | Neither soluble or liposomal forms of Catalin changed GSH, Na, K, or Ca level of the lens exposed to selenite. | [25] | |
| Catalin liposome/ 0.24 mg/mL (particle size 100 nm)/topical/1 time 1.5 h before selenite injection and qid for 1 wk after selenite injection | ⟷ GSH | ⟷ Na/K ratio ⟷ Ca | ||||||
| Clinical | Patients aged > 40 yr with initial cortical cataract | None | Catalin/24 mo | By slit-lamp microscope: ↓ opacity and ↓ progression/mo3, 6, 12, 18, and 24 (especially in age <59 years and after 18 mo use) % increased opacification • Catalin: 1.425 • Control: 9.228 | Catalin decelerated lens opacity and slowed progression of cortical type of presenile and aged-related cataract. The change was more obvious in those younger than 59 years. Peak effect was observed after 18 months of treatment. | [12] | ||
| Clinical (double blinded RCT) | Patients with early idiopathic cataract, mean age 60.3 yr (PRX vs. BA, BA vs. control) | None | Catalin/ q 8 h/topical/ 22 mo | % decrease/ q 1 mo (mo 1–14), mo 18, mo 22: • Catalin: none • BA: high • Control: none | VA/1, 2 mo: • Catalin ↓ • BA ↑ • Control ↓ % operated-eyes/22 mo • Catalin: high • BA: low • Control: high | In age-related cataract BA decelerated or reversed lens opacity, and VA more extensively than PRX. BA also had greater impact on the reduction of the number of cataract operations. From the raw data, PRX seemed not to have effects on lens opacity, VA, and number of cataract operation. (No direct comparison between PRX and control.) | [26] | |
| Clinical (double blinded RCT) | Patients with age-related cataract (<50% extension), age ≥ 40 yr | None | Catalin/6 times/day/topical/24 mo | ⟷ progression | ⟷ VA | PRX had no effect on early age-related cataract. | [29] | |
| Study Types | Source of Lens | Induction of Cataract | Name/Dose/Route/Duration of PRX | Major Findings of the Lens | Interpretation | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Opacity | GSH | Water-Soluble Protein | S-Containing Amino Acids | Others | ||||||
| 1. Diabetic Cataract | ||||||||||
| In vitro | SD rat lens | Hypergalactosemic diet (50% galactose + 50% standard food) | PRX/10−7 M, 10−6 M, 10−5 M, or 10−4 M/11–96 h | 10−7 M: ⟷ 10−6 to 10−4 M: ↑ | 10−7 M and 10−6 M: ⟷ 10−5 M and 10−4 M: ↑ | 10−6 to 10−5 M: ⟷ 10−4 M: ↑ | Only a high concentration of PRX increased GSH and preserved lens protein by binding to sulfhydryl group. | [16] | ||
| In vitro | Wistar rat whole lens | D-galactose (250 mmol/L) | Pure PRX/0.0053%/ 6–24 h | ↓ opacity ↓ progression of lens opacity | PRX delayed progression and improved lens transparency. | [29] | ||||
| In vitro | Rat lens | D-galactose (250 mM) | Catalin/100 μL/24 h | ↓ opacity (h 12, h 18, and h 24) | PRX improved lens transparency. | [30] | ||||
| In vitro | Goat whole lens | Glucose or galactose: 50,100, and 200% over the normal glucose concentration at 0.99 g/L | Catalin/0.001% and 0.01%/7 d | ↓ onset of opacity by 12–24 h (effect of 0.001% PRX = 0.01% PRX) ↓ opacity at 12–18 h (effect of 0.001% PRX = 0.01% PRX) | 0.001% and 0.01% Catalin delayed the onset of opacity and improved lens transparency. | [31] | ||||
| In vitro | Cow lens | Sorbitol | Catalin/ 60 µM/48 h | ↓ sorbitol | Catalin decreased sorbitol content in lens | [32] | ||||
| In vivo | SD rat | Hypergalactosemic diet (50% galactose + 50% standard food) | PRX/0.005%, 0.01, or 2%, 2 drops tid/topical/30 d simultaneously with galactose administration | ↓ incidence of cataract by 40% | ↑ | ↑ | ↑ | PRX increased GSH and preserved lens protein by binding to the sulfhydryl group. PRX prevented diabetic cataract. | [16] | |
| In vivo | Rat | 10 mL/kg of 50% D-galactose bid/IP/90 d + 10% D-galactose water and food/oral/90 d | Catalin/ 0.8 mg/15 mL/topical/3 drops tid/90 d simultaneously with galactose administration | ↓ opacity (d 20, d 30, d40, d60 and d90) | PRX improved lens transparency of diabetic cataract. | [30] | ||||
| In vivo | Wistar rat | 10 mL/kg of 50% D-galactose bid/IP/30 d + 10% D-galactose water/oral/ 30 d | Pure PRX/0.0053% tid/topical/60 d after d30 of galactose administration | ↓ opacity (10 d–90 d) ↓ progression of lens opacity (10 d–90 d) | PRX delayed progression and reversed lens opacification of diabetic cataract. | [29] | ||||
| In vivo | Rabbit | Alloxan | Catalin | ↓ opacity | ↓ Na ↑ K | PRX prevented and delayed lens transparency of diabetic cataract by controlling electrolytes. | [33] | |||
| In vivo | Rat | Alloxan 50 mg/kg IV | Catalin/20 mg/kg/IP/daily for 6 wk | 100% delayed onset of opacity 81.6% had no lens opacity (wk 5 and wk 6 | PRX delayed onset and progression of diabetic cataract. | [65] | ||||
| In vitro | Rat lens | Glucose 55.5 mM/5 d | PRX/5 d | ↑ | ↑ | PRX preserved lens protein by binding to the sulfhydryl group. | [52] | |||
| In vivo | Rat | Hypergalactosemic diet | PRX/20 d | ↑ | ↓ aldose reductase activity | PRX increased GSH and decreased aldose reductase activity. | [52] | |||
| 2. Congenital Cataract | ||||||||||
| In vivo | Pigmented rabbit | Tryptophan-free diet (30 d) | PRX/0.005%, 0.01 or 2%, 2 drops tid/topical/30 d | ↓ incidence of cataract by 40%/d 30 | ↑ | ↑ | ↑ | PRX prevented cataract. PRX increased GSH and preserved lens protein by binding to the sulfhydryl group. | [16] | |
| In vivo | Rabbit | Tryptophan-free diet | PRX/20 d | ↑ | ↑ | ↑ | ↓ | PRX increased GSH and preserved lens protein by binding to the sulfhydryl group. PRX decreased aldose reductase activity. | [52] | |
| Clinical (double blinded RCT) | Patients with congenital cataract (age 6–8 wk) | None | Catalin/ 6 times/day/topical/16 wk | ⟷ progression | PRX had no effect on congenital cataract. | [27] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upaphong, P.; Thonusin, C.; Choovuthayakorn, J.; Chattipakorn, N.; Chattipakorn, S.C. The Possible Positive Mechanisms of Pirenoxine in Cataract Formation. Int. J. Mol. Sci. 2022, 23, 9431. https://doi.org/10.3390/ijms23169431
Upaphong P, Thonusin C, Choovuthayakorn J, Chattipakorn N, Chattipakorn SC. The Possible Positive Mechanisms of Pirenoxine in Cataract Formation. International Journal of Molecular Sciences. 2022; 23(16):9431. https://doi.org/10.3390/ijms23169431
Chicago/Turabian StyleUpaphong, Phit, Chanisa Thonusin, Janejit Choovuthayakorn, Nipon Chattipakorn, and Siriporn C. Chattipakorn. 2022. "The Possible Positive Mechanisms of Pirenoxine in Cataract Formation" International Journal of Molecular Sciences 23, no. 16: 9431. https://doi.org/10.3390/ijms23169431
APA StyleUpaphong, P., Thonusin, C., Choovuthayakorn, J., Chattipakorn, N., & Chattipakorn, S. C. (2022). The Possible Positive Mechanisms of Pirenoxine in Cataract Formation. International Journal of Molecular Sciences, 23(16), 9431. https://doi.org/10.3390/ijms23169431






