“Universal” Antimicrobial Combination of Bacitracin and His6-OPH with Lactonase Activity, Acting against Various Bacterial and Yeast Cells
Abstract
:1. Introduction
2. Results
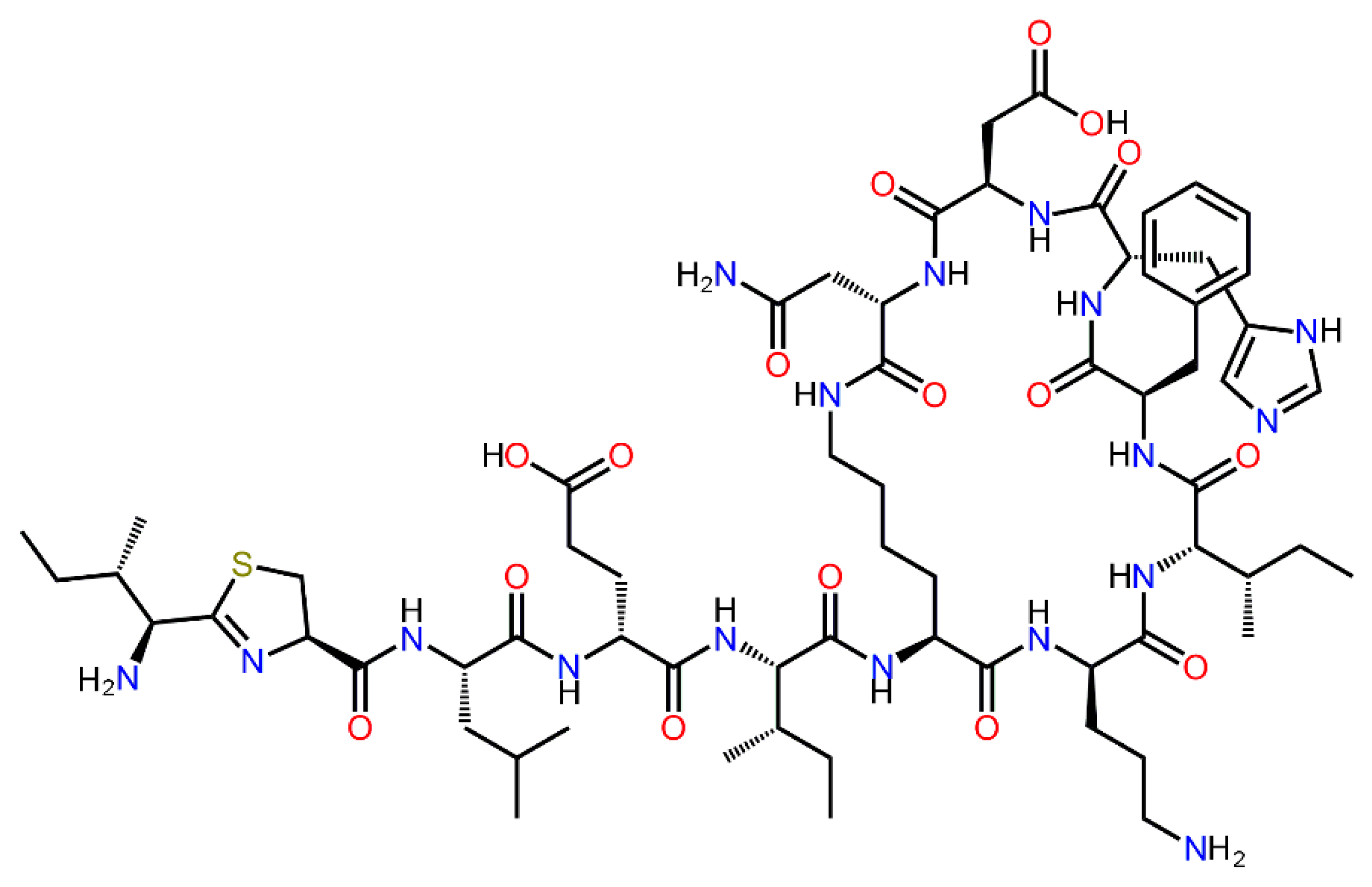
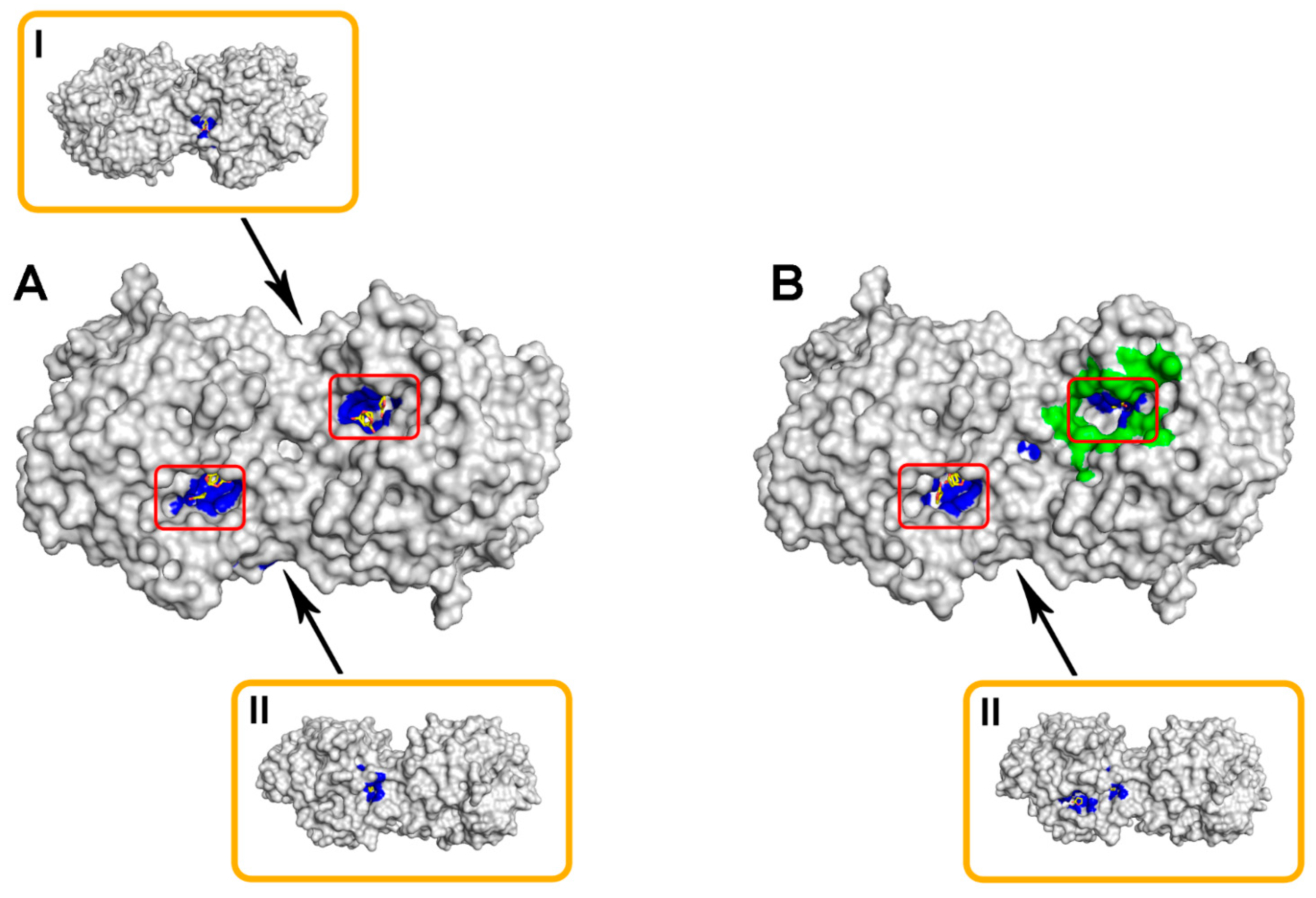
2.1. Computational Modeling of His6-OPH/Bacitracin Interactions
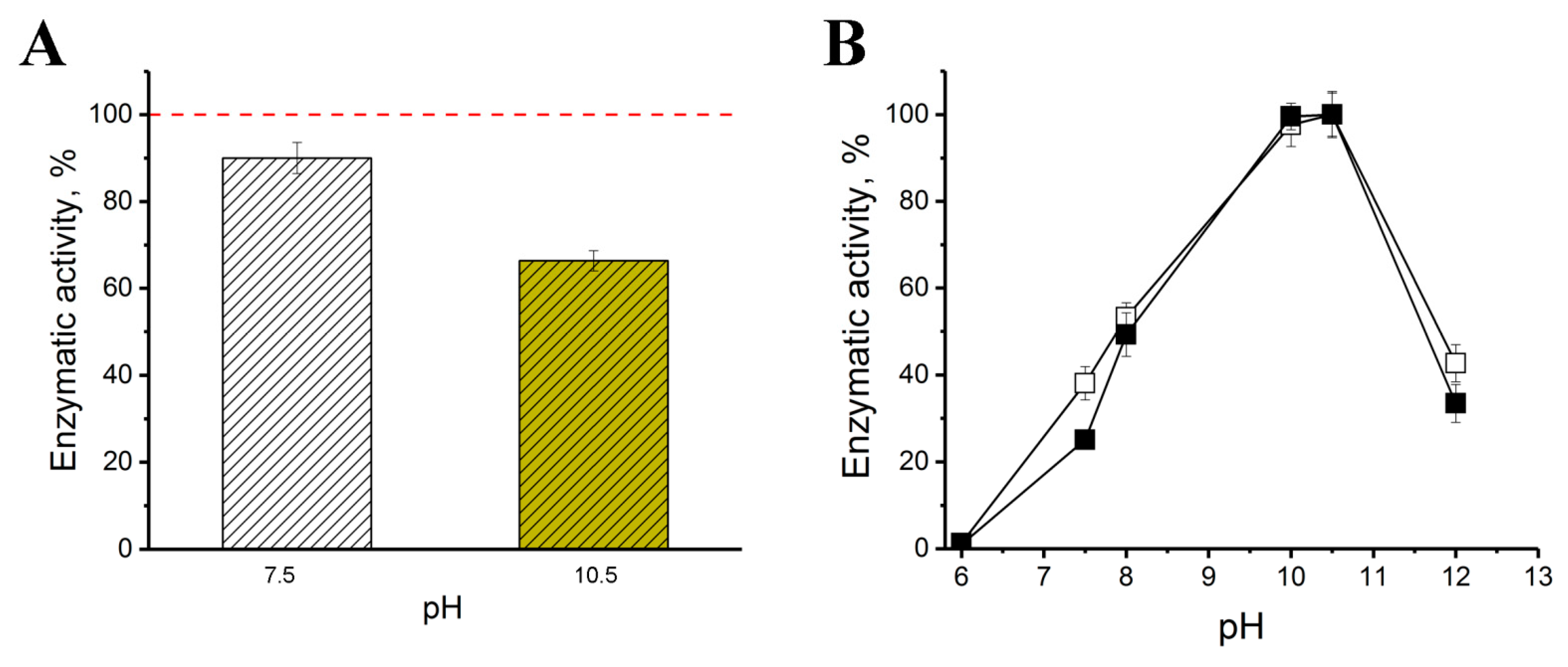
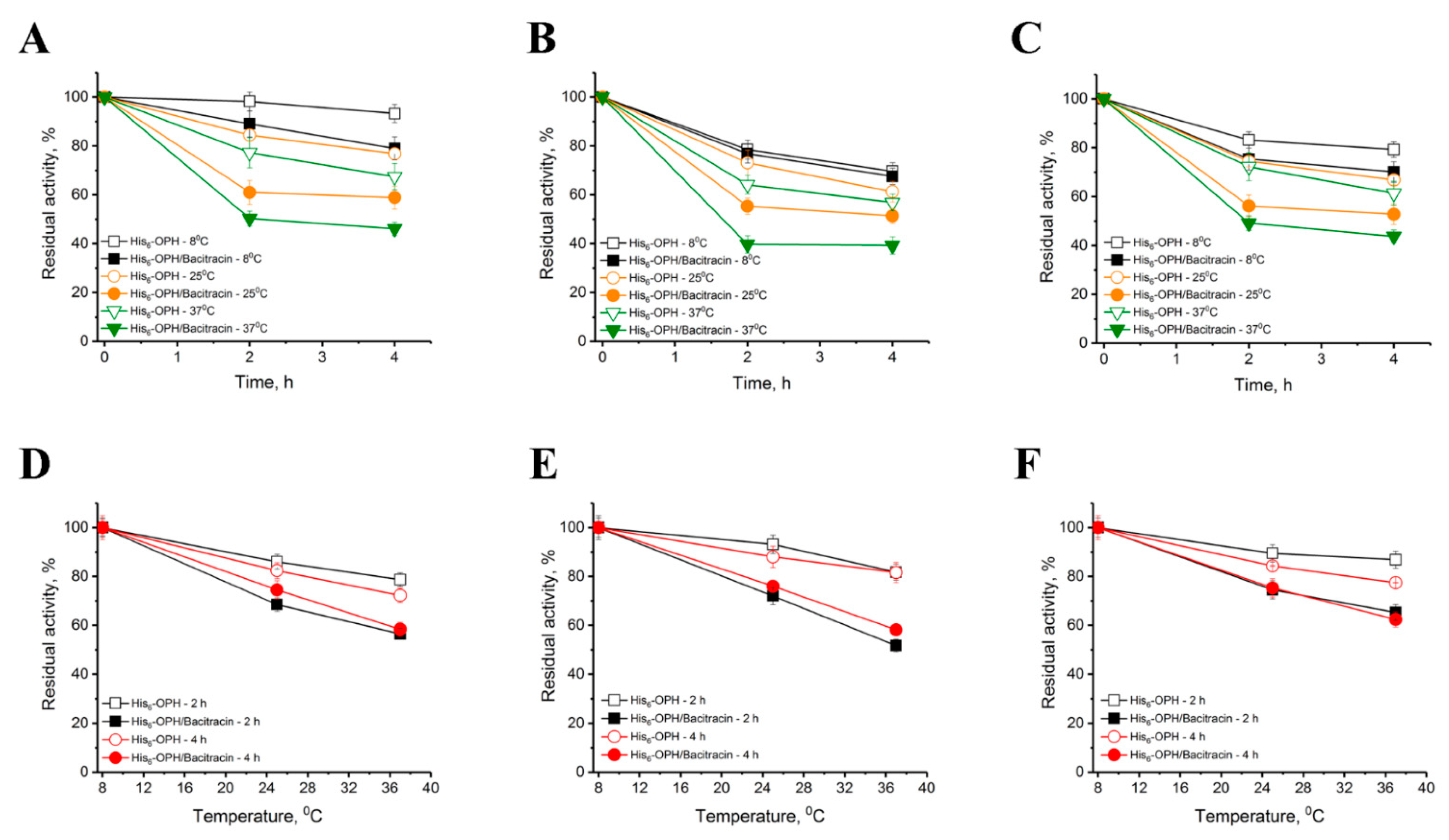
2.2. Catalytic and Physical-Chemical Characteristics of His6-OPH in the Absence and Presence of Bacitracin
2.3. Antimicrobial Activity of Bacitracin alone or in Combination with His6-OPH
2.4. In Silico Estimation of Possible Hydrolysis Catalyzed by His6-OPH of Lactone-Containing Molecules Synthesyzed by Fungous (Including Yeast) Cells
3. Discussion
4. Materials and Methods
4.1. Computational Methods
4.2. Enzyme Preparation and Determination of Its Activity
4.3. Determination of Antimicrobial Effect of Bacitracin and Its Combination with His6-OPH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobias, N.J.; Brehm, J.; Kresovic, D.; Brameyer, S.; Bode, H.B.; Heermann, R. New vocabulary for bacterial communication. ChemBioChem 2020, 21, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to quorum quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Charges’ interaction in polyelectrolyte (nano)complexing of His6-OPH with peptides: Unpredictable results due to imperfect or useless concept? Int. J. Biol. Macromol. 2019, 140, 368–376. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Stepanov, N.; Presnov, D.; Efremenko, E. Bacterial cellulose containing combinations of antimicrobial peptides with various QQ enzymes as a prototype of an “enhanced antibacterial” dressing: In silico and in vitro data. Pharmaceutics 2020, 12, 1155. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 2559. [Google Scholar] [CrossRef]
- Bellotto, O.; Semeraro, S.; Bandiera, A.; Tramer, F.; Pavan, N.; Marchesan, S. Polymer conjugates of antimicrobial peptides (AMPs) with D-amino acids (d-aa): State of the art and future opportunities. Pharmaceutics 2022, 14, 446. [Google Scholar] [CrossRef]
- Hong, W.; Gao, X.; Qiu, P.; Yang, J.; Qiao, M.; Shi, H.; Zhang, D.; Tian, C.; Niu, S.; Liu, M. Synthesis, construction, and evaluation of self-assembled nano-bacitracin A as an efficient antibacterial agent in vitro and in vivo. Int. J. Nanomed. 2017, 12, 4691–4708. [Google Scholar] [CrossRef] [Green Version]
- Ming, L.J.; Epperson, J.D. Metal binding and structure-activity relationship of the metalloantibiotic peptide bacitracin. J. Inorg. Biochem. 2002, 91, 46–58. [Google Scholar] [CrossRef]
- Meng, L.; Deresinski, S.; Holubar, M. Intraoperative bacitracin irrigations for the prevention of surgical site infections—consider the alternatives. Infect. Control Hosp. Epidemiol. 2020, 41, 831–832. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Haas, B.; Gottschalk, M.; Grenier, D. Antimicrobial potential of bacteriocins in poultry and swine production. Vet. Res. 2017, 48, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, M.K. Antibiotics that inhibit cell wall synthesis. In Chemistry of Antibiotics and Related Drugs; Bhattacharjee, M.K., Ed.; Springer: Cham, Switzerland, 2016; pp. 49–94. [Google Scholar] [CrossRef]
- Stone, K.J.; Strominger, J.L. Mechanism of action of bacitracin: Complexation with metal ion and C55-isoprenyl pyrophosphate. PNAS 1971, 68, 3223–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, B.P.; Martin, W.F.; Carbone, V.; Duin, E.C.; Cronin, B.; Sauter, J.; Schofield, L.R.; Sutherland-Smith, A.J.; Ronimus, R.S. Archaeal pseudomurein and bacterial murein cell wall biosynthesis share a common evolutionary ancestry. FEMS Microbes 2021, 2, xtab012. [Google Scholar] [CrossRef]
- Dridi, B.; Fardeau, M.L.; Ollivier, B.; Raoult, D.; Drancourt, M. The antimicrobial resistance pattern of cultured human methanogens reflects the unique phylogenetic position of archaea. J. Antimicrob. Chemother. 2011, 66, 2038–2044. [Google Scholar] [CrossRef]
- Efremenko, E.; Lyagin, I.; Votchitseva, Y.; Sirotkina, M.; Varfolomeyev, S. Polyhistidine-containing organophosphorus hydrolase with outstanding properties. Biocatal. Biotransformation 2007, 25, 103–108. [Google Scholar] [CrossRef]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: A review. Molecules 2019, 24, 1950. [Google Scholar] [CrossRef] [Green Version]
- Krzyczkowska, J.; Phan-Thi, H.; Waché, Y. Lactone formation in yeast and fungi. In Fungal Metabolites. Reference Series in Phytochemistry, 1st ed.; Merillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–39. [Google Scholar] [CrossRef]
- Schimmel, T.G.; Coffman, A.D.; Parsons, S.J. Effect of butyrolactone I on the producing fungus Aspergillus terreus. Appl. Environ. Microbiol. 1998, 64, 3707–3712. [Google Scholar] [CrossRef] [Green Version]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbial. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, e2362. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, A.; Avitabile, C.; Cirillo, A.; Moretta, A.; Merlino, A.; Paduano, L.; Duilio, A.; Romanelli, A. The antimicrobial peptide Temporin L impairs E. coli cell division by interacting with FtsZ and the divisome complex. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129606. [Google Scholar] [CrossRef]
- Andrault, P.M.; Samsonov, S.A.; Weber, G.; Coquet, L.; Nazmi, K.; Bolscher, J.G.; Lalmanach, A.C.; Jouenne, T.; Brömme, D.; Pisabarro, M.T.; et al. Antimicrobial peptide LL-37 is both a substrate of cathepsins S and K and a selective inhibitor of cathepsin L. Biochemistry 2015, 54, 2785–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Chen, F.A.; Wang, L.F.; Hsu, J.L. Discovery and study of novel antihypertensive peptides derived from Cassia obtusifolia seeds. J. Agric. Food Chem. 2019, 67, 7810–7820. [Google Scholar] [CrossRef]
- Tivari, S.R.; Kokate, S.V.; Sobhia, E.M.; Kumar, S.G.; Shelar, U.B.; Jadeja, Y.S. A series of novel bioactive cyclic peptides: Synthesis by head-to-tail cyclization approach, antimicrobial activity and molecular docking studies. Chem. Sel. 2022, 7, e202201481. [Google Scholar] [CrossRef]
- Cartotto, R. Topical antimicrobial agents for pediatric burns. Burn Trauma 2017, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, J.P.; Friedman, J.K.; Wang, Y.; McLachlan, J.B.; Sammarco, M.C.; Morici, L.A.; Roy, C.J. In situ treatment with novel microbiocide inhibits methicillin resistant Staphylococcus aureus in a murine wound infection model. Front. Microbiol. 2020, 10, 3106. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burns Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Senko, O.; Stepanov, N.; Mareev, N.; Volikov, A.; Perminova, I. Suppression of methane generation during methanogenesis by chemically modified humic compounds. Antioxidants 2020, 9, 1140. [Google Scholar] [CrossRef]
- Varnava, K.G.; Ronimus, R.S.; Sarojini, V. A review on comparative mechanistic studies of antimicrobial peptides against archaea. Biotechnol. Bioeng. 2017, 114, 2457–2473. [Google Scholar] [CrossRef]
- Ismayilov, I.T.; Stepanov, N.A.; Efremenko, E.N.; Abbasov, V.M. Evaluation of biocidal properties of vegetable oil-based corrosion inhibitors using bioluminescent enzymatic method. Moscow Univ. Chem. Bull. 2015, 70, 197–201. [Google Scholar] [CrossRef]
- Batmanghelich, F.; Li, L.; Seo, Y. Influence of multispecies biofilms of Pseudomonas aeruginosa and Desulfovibrio vulgaris on the corrosion of cast iron. Corros. Sci. 2017, 121, 94–104. [Google Scholar] [CrossRef]
- Haiko, J.; Saeedi, B.; Bagger, G.; Karpati, F.; Özenci, V. Coexistence of Candida species and bacteria in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1071–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Q.; Yuan, S.; Zhao, S.; Fu, D.; Chen, Y.; Zhou, Y.; Cao, Y.; Gao, Y.; Xu, X.; Zhou, X.; et al. Coexistence of Candida albicans and Enterococcus faecalis increases biofilm virulence and periapical lesions in rats. Biofouling 2021, 37, 964–974. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA. 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35 (Suppl. 2), W522–W525. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and Auto-DockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Efremenko, E.; Votchitseva, Y.; Plieva, F.; Galaev, I.; Mattiasson, B. Purification of His6-organophosphate hydrolase using monolithic supermacroporous polyacrylamide cryogels developed for immobilized metal affinity chromatography. Appl. Microbiol. Biotechnol. 2006, 70, 558–563. [Google Scholar] [CrossRef]
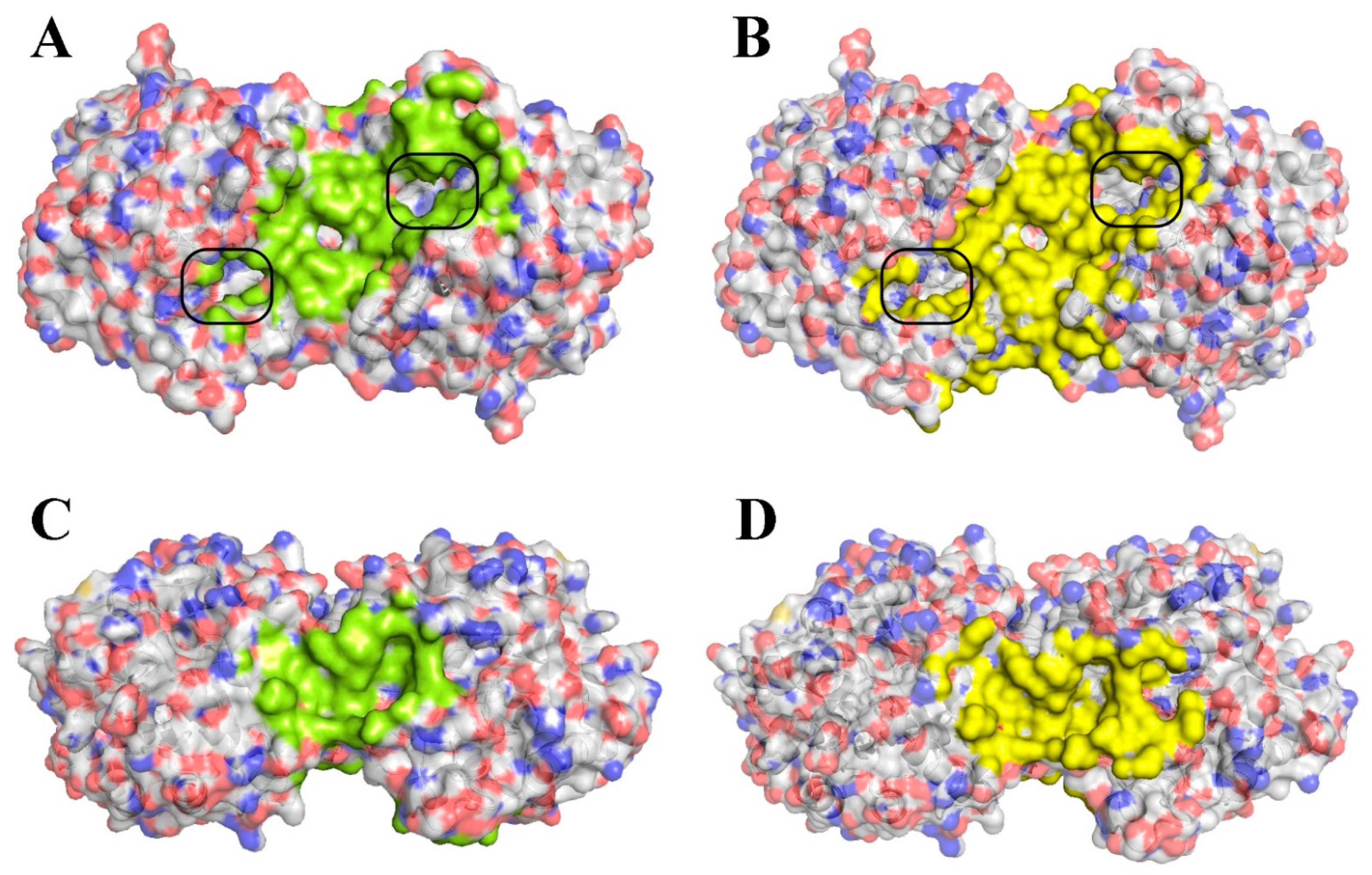
| pH | Net Charge | Affinity (kJ/mol) | Area (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Upper (Lower) Bounds | p Value | Near Active Sites | Total | ||
| 7.5 | −3.8 | −24.1 | −24.3 ± 1.2 | −23.0 (−25.0) | <0.001 | 0.1 | 11.4 |
| 10.5 | −7.4 | −27.6 | −27.2 ± 1.2 | −26.8 (−28.3) | 0.1 | 14.2 | |
| Enzyme or Complex | Km (μM) | Vmax/E0 (1/s) | Vmax/(E0 × Km) (1 × 106/(M × s) |
|---|---|---|---|
| His6-OPH | 10.5 ± 2.2 | 5040 ± 340 | 480 ± 154 |
| His6–OPH/Bacitracin | 15.1 ± 3.3 | 6632 ± 856 | 449 ± 217 |
| Microorganism | w/o His6-OPH | with His6-OPH | Decrease of IC50 Due to the Use of His6-OPH/Bacitracin Compared to the Bacitracin Alone (Times) |
|---|---|---|---|
| Bacterial cells | |||
| Escherichia coli (G(−)) ** | 1.94 ± 0.12 | 1.56 ± 0.18 | 1.3 |
| Pseudomonas sp. (G(−)) | 52.23 ± 3.01 | 16.5 ± 1.46 | 3.2 |
| Agrobacterium sp. (G(−)) | 21.19 ± 0.22 | 6.41 ± 0.21 | 3.3 |
| Rhodococcus sp. (G(+)) | 3.05 ± 0.28 | 3.5 ± 0.31 | 0.9 |
| Bacillus subtilis (G(+)) | 0.11 ± 0.01 | 0.11 ± 0.01 | 1 |
| Yeast cells | |||
| Candida sp. | 17.83 ± 2.41 | 14.83 ± 1.51 | 1.2 |
| Saccharomyces cerevisiae | 14.52 ± 1.4 | 9.85 ± 0.61 | 1.5 |
| Cryptococcus albidus | 2.8 ± 0.15 | 2.89 ± 0.15 | 1 |
| Pachysolen tannophilus | 14.92 ± 1.01 | 1.75 ± 0.21 | 8.5 |
| Kluyveromyces marxianus | 10.21 ± 0.71 | 2.6 ± 0.16 | 3.9 |
| Torulopsis spp. | 31.47 ± 2.04 | 5.76 ± 0.05 | 5.5 |
| Trichosporon beigelii | 15.65 ± 0.75 | 3.98 ± 0.06 | 3.9 |
| Lactones | His6-OPH without Bacitracin | His6-OPH with Bacitracin | ||||
|---|---|---|---|---|---|---|
| Area, % | Affinity, (kJ/mol) | Area, % | Affinity, (kJ/mol) | |||
| Near Active Site | Total | Median | Near Active Site | Total | Median | |
| γ-butyrolactone | 1.1 | 3 | −16.3 ± 0.8 | 1 | 3.7 | −16.1 ± 0.9 |
| γ-heptalactone | 0.8 | 3.1 | −20.7 ± 1.3 | 0.6 | 3.5 | −20.7 ± 2.0 |
| γ-decalactone | 0.6 | 4.9 | −21.1 ± 1.6 | 0.4 | 3.3 | −22.0 ± 1.5 |
| Gluconolactone | 0.1 | 6 | −24.1 ± 0.8 | 0.1 | 4.6 | −23.2 ± 1.2 |
| Butyrolactone I | 0.2 | 9.3 | −30.3 ± 1.4 | 0.1 | 9.1 | −29.5 ± 1.6 |
| Multicolanic acid | 0.6 | 6.6 | −22.6 ± 1.5 | 0.1 | 4.8 | −21.7 ± 1.3 |
| Multicolosic acid | 0.1 | 14.9 | −21.3 ± 2.1 | 0.1 | 7.8 | −20.7 ± 1.7 |
| Multicolic acid | 0.1 | 6.9 | −23.6 ± 1.2 | 0.3 | 5.9 | −22.2 ± 0.5 |
| Enzyme; [Reference] | Peptide | * Interaction with the Active Site Residues | Effect on Catalytic Activity |
|---|---|---|---|
| Guanosine triphosphatase (GTPase) FtsZ; [22] | Temporin L | + | competitive inhibition |
| Cathepsin L (CatL); [23] | LL-37 | + | competitive inhibition |
| Myeloperoxidase (MPO); [24] | Antioxidative peptide TEFHLL | + | inhibition |
| Angiotensin I-converting enzyme (ACE); [25] | Antihypertensive peptide FHAPWK | + | competitive inhibition |
| DNA gyrase and Lanosterol 14-alpha demethylase; [26] | Cyclo (Thr-Arg-Pro-D-Val-Leu) | + | competitive inhibition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. “Universal” Antimicrobial Combination of Bacitracin and His6-OPH with Lactonase Activity, Acting against Various Bacterial and Yeast Cells. Int. J. Mol. Sci. 2022, 23, 9400. https://doi.org/10.3390/ijms23169400
Aslanli A, Domnin M, Stepanov N, Efremenko E. “Universal” Antimicrobial Combination of Bacitracin and His6-OPH with Lactonase Activity, Acting against Various Bacterial and Yeast Cells. International Journal of Molecular Sciences. 2022; 23(16):9400. https://doi.org/10.3390/ijms23169400
Chicago/Turabian StyleAslanli, Aysel, Maksim Domnin, Nikolay Stepanov, and Elena Efremenko. 2022. "“Universal” Antimicrobial Combination of Bacitracin and His6-OPH with Lactonase Activity, Acting against Various Bacterial and Yeast Cells" International Journal of Molecular Sciences 23, no. 16: 9400. https://doi.org/10.3390/ijms23169400
APA StyleAslanli, A., Domnin, M., Stepanov, N., & Efremenko, E. (2022). “Universal” Antimicrobial Combination of Bacitracin and His6-OPH with Lactonase Activity, Acting against Various Bacterial and Yeast Cells. International Journal of Molecular Sciences, 23(16), 9400. https://doi.org/10.3390/ijms23169400







