General Construction of Amine via Reduction of N=X (X = C, O, H) Bonds Mediated by Supported Nickel Boride Nanoclusters
Abstract
:1. Introduction
2. Results and Discussion
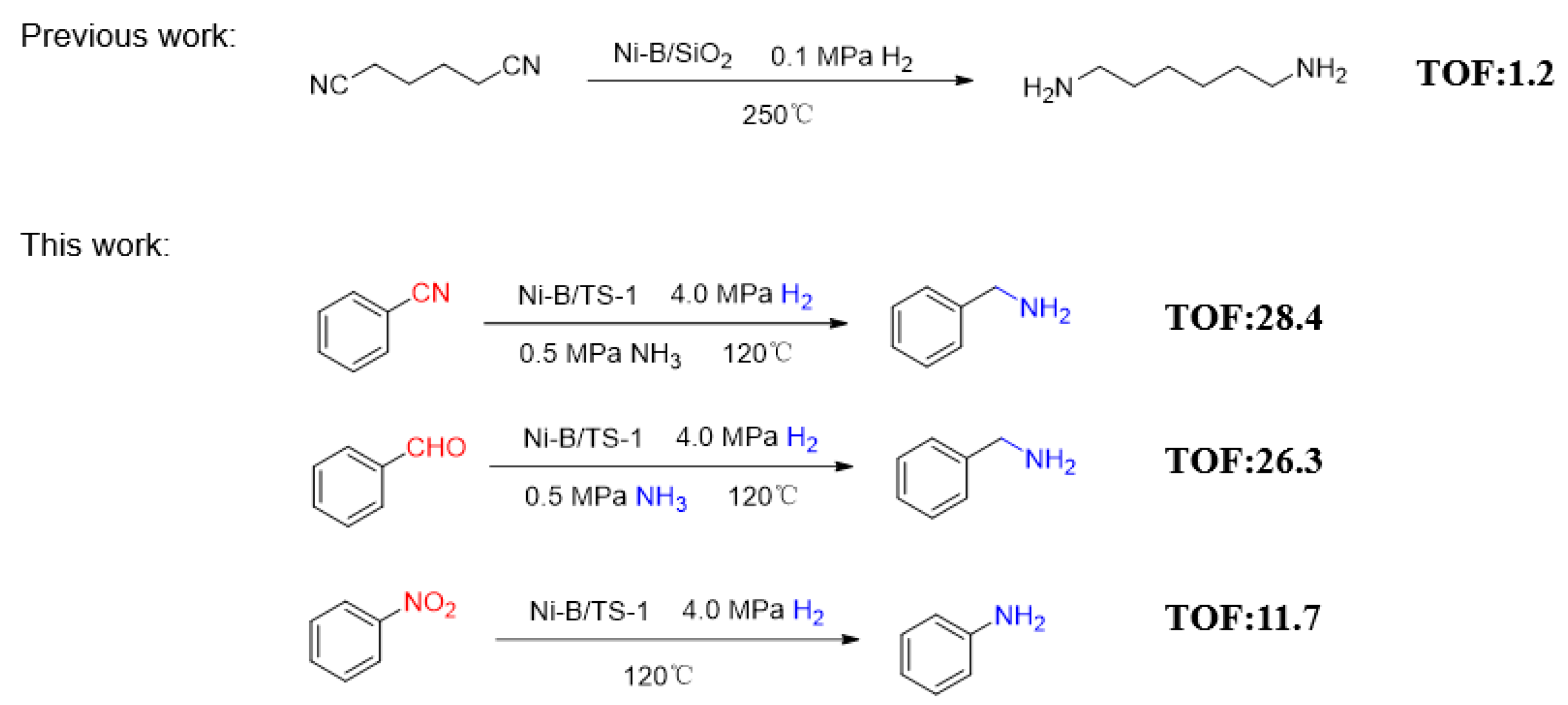
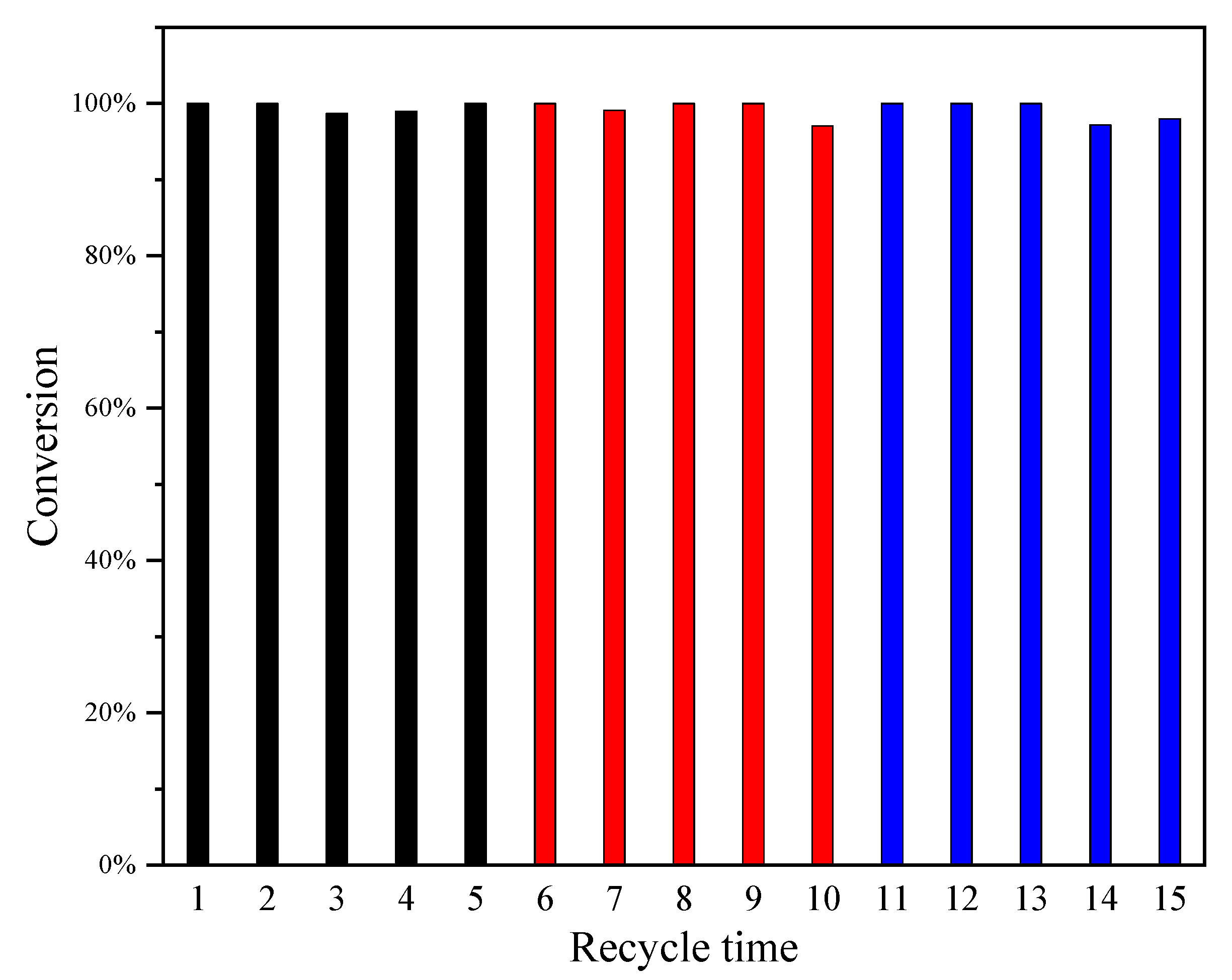
2.1. Catalyst Evaluation
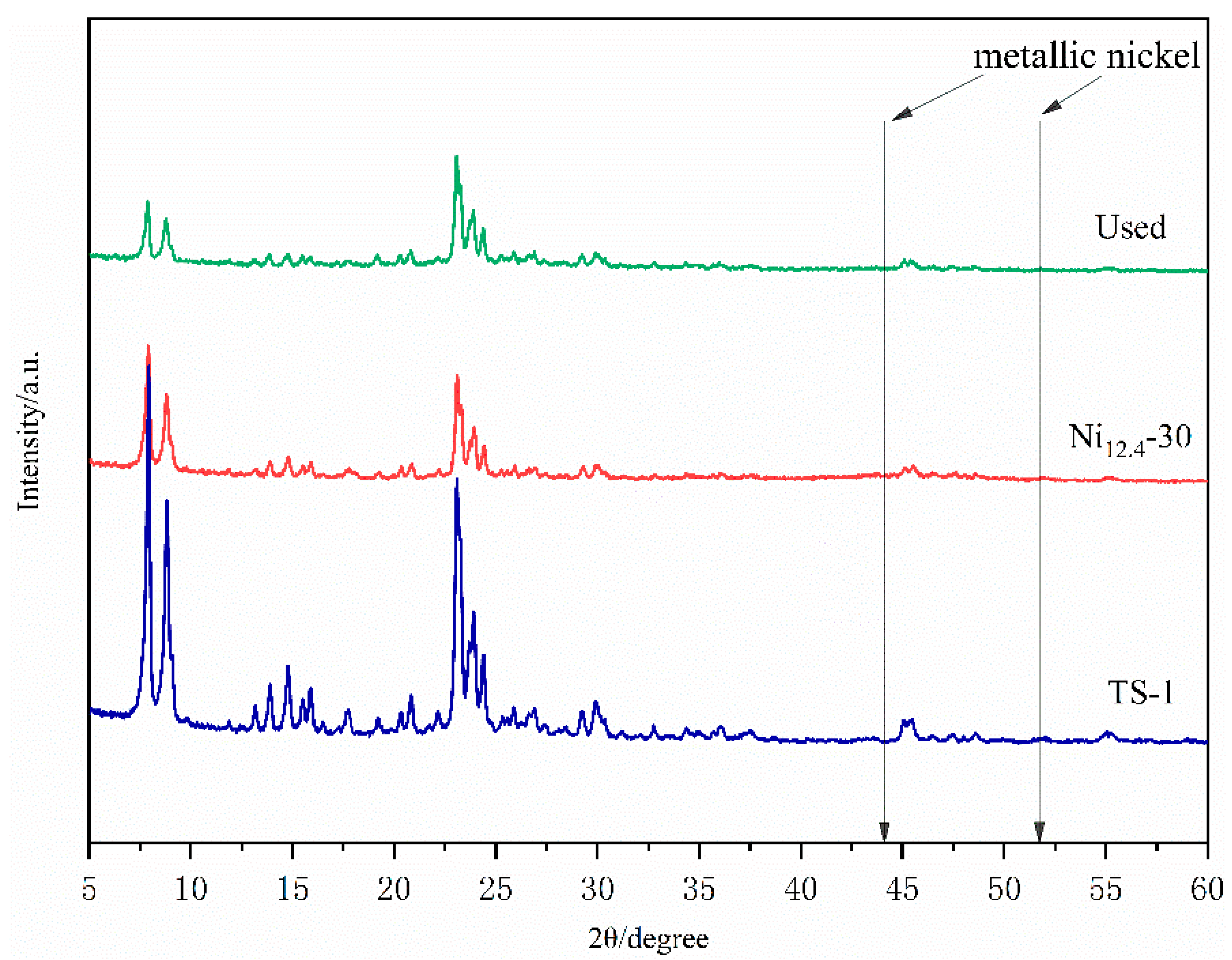
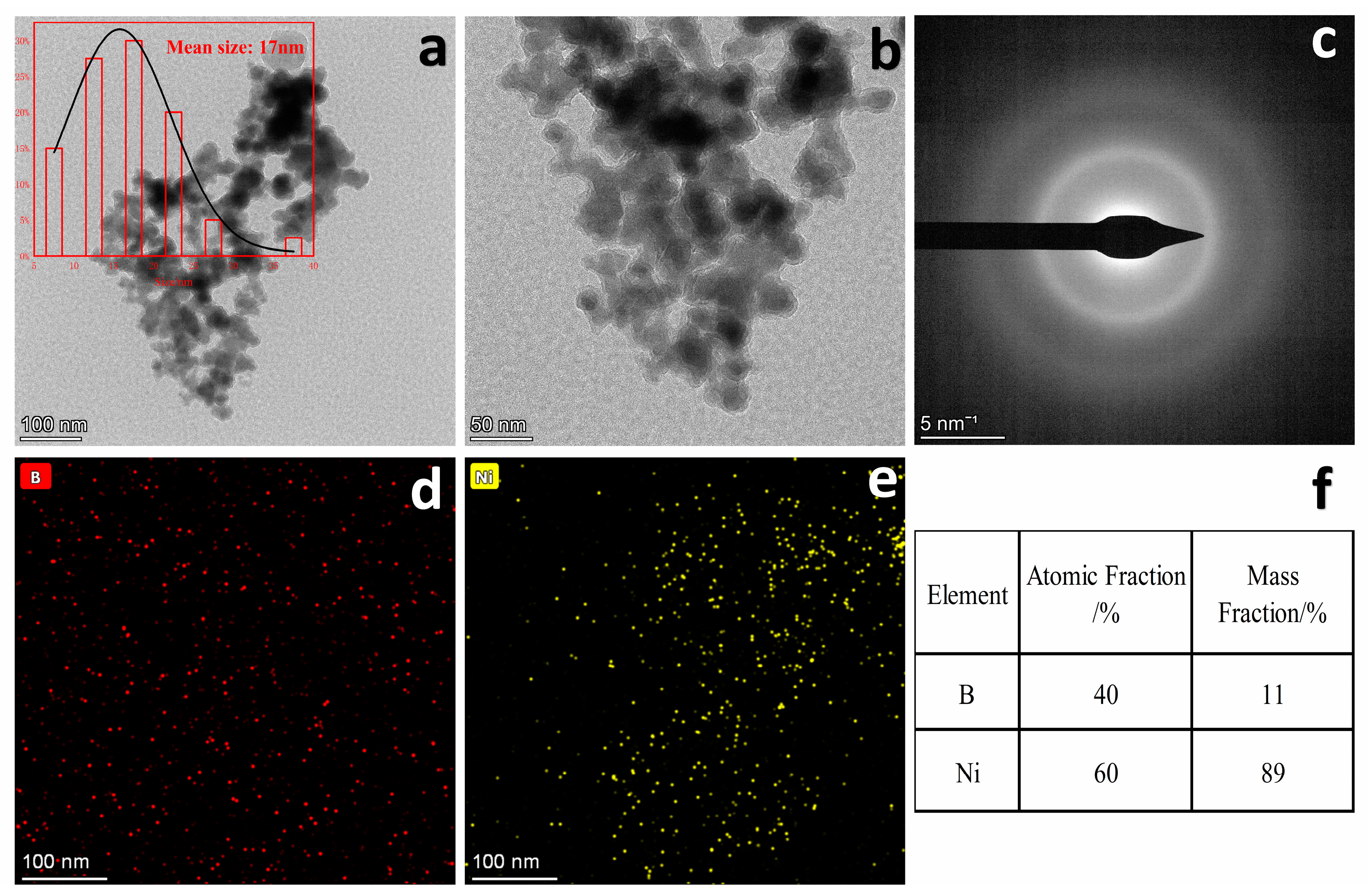
2.2. Characterization of Ni12.4-30
2.3. The Reduction of Nitrile
2.4. The Reduction of Nitro Compounds
2.5. The Reduction for Aldehyde and Ammonia
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. 2018, 351, e1800141. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, T.; Mohammadi Ziarani, G.; Bahar, S.; Badiei, A. Domino synthesis of quinoxaline derivatives using SBA-Pr-NH2 as a nanoreactor and their spectrophotometric complexation studies with some metals ions. J. Iran. Chem. Soc. 2018, 15, 1153–1161. [Google Scholar] [CrossRef]
- Vo, N.B.; Nguyen, L.A.; Pham, T.L.; Doan, D.T.; Nguyen, T.B.; Ngo, Q.A. Straightforward access to new vinca-alkaloids via selective reduction of a nitrile containing anhydrovinblastine derivative. Tetrahedron Lett. 2017, 58, 2503–2506. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Yemul, O.; Imae, T. Synthesis and characterization of poly(ethyleneimine) dendrimers. Colloid Polym. Sci. 2008, 286, 747–752. [Google Scholar] [CrossRef]
- Garduño, J.A.; García, J.J. Non-Pincer Mn(I) Organometallics for the Selective Catalytic Hydrogenation of Nitriles to Primary Amines. ACS Catal. 2018, 9, 392–401. [Google Scholar] [CrossRef]
- Mastalir, M.; Stöger, B.; Pittenauer, E.; Puchberger, M.; Allmaier, G.; Kirchner, K. Air Stable Iron(II) PNP Pincer Complexes as Efficient Catalysts for the Selective Alkylation of Amines with Alcohols. Adv. Synth. Catal. 2016, 358, 3824–3831. [Google Scholar] [CrossRef]
- Baumann, W.; Spannenberg, A.; Pfeffer, J.; Haas, T.; Kockritz, A.; Martin, A.; Deutsch, J. Utilization of common ligands for the ruthenium-catalyzed amination of alcohols. Chem. Eur. J. 2013, 19, 17702–17706. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Zhong, S.; Ma, L. Selective catalysis for the reductive amination of furfural toward furfurylamine by graphene-co-shelled cobalt nanoparticles. Green Chem. 2022, 24, 271–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Chi, Q.; Zhang, Z. Nitrogen-Doped Carbon-Supported Nickel Nanoparticles: A Robust Catalyst to Bridge the Hydrogenation of Nitriles and the Reductive Amination of Carbonyl Compounds for the Synthesis of Primary Amines. ChemSusChem 2019, 12, 1246–1255. [Google Scholar] [CrossRef]
- Hahn, G.; Kunnas, P.; de Jonge, N.; Kempe, R. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst. Nat. Catal. 2018, 2, 71–77. [Google Scholar] [CrossRef]
- Coeck, R.; De Vos, D.E. One-pot reductive amination of carboxylic acids: A sustainable method for primary amine synthesis. Green Chem. 2020, 22, 5105–5114. [Google Scholar] [CrossRef]
- Citoler, J.; Derrington, S.R.; Galman, J.L.; Bevinakatti, H.; Turner, N.J. A biocatalytic cascade for the conversion of fatty acids to fatty amines. Green Chem. 2019, 21, 4932–4935. [Google Scholar] [CrossRef]
- Antil, N.; Kumar, A.; Akhtar, N.; Newar, R.; Begum, W.; Dwivedi, A.; Manna, K. Aluminum Metal–Organic Framework-Ligated Single-Site Nickel(II)-Hydride for Heterogeneous Chemoselective Catalysis. ACS Catal. 2021, 11, 3943–3957. [Google Scholar] [CrossRef]
- Wang, C.; Jia, Z.; Zhen, B.; Han, M. Supported Ni Catalyst for Liquid Phase Hydrogenation of Adiponitrile to 6-Aminocapronitrile and Hexamethyenediamine. Molecules 2018, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Konnerth, H.; Prechtl, M.H.G. Nitrile hydrogenation using nickel nanocatalysts in ionic liquids. New J. Chem. 2017, 41, 9594–9597. [Google Scholar] [CrossRef]
- Cheng, H.; Meng, X.; Wu, C.; Shan, X.; Yu, Y.; Zhao, F. Selective hydrogenation of benzonitrile in multiphase reaction systems including compressed carbon dioxide over Ni/Al2O3 catalyst. J. Mol. Catal. A Chem. 2013, 379, 72–79. [Google Scholar] [CrossRef]
- Segobia, D.J.; Trasarti, A.F.; Apesteguía, C.R. Hydrogenation of nitriles to primary amines on metal-supported catalysts: Highly selective conversion of butyronitrile to n-butylamine. Appl. Catal. A Gen. 2012, 445–446, 69–75. [Google Scholar] [CrossRef]
- Liu, Y.; He, S.; Quan, Z.; Cai, H.; Zhao, Y.; Wang, B. Mild palladium-catalysed highly efficient hydrogenation of C–N, C–NO2, and C–O bonds using H2 of 1 atm in H2O. Green Chem. 2019, 21, 830–838. [Google Scholar] [CrossRef]
- Martina, K.; Baricco, F.; Tagliapietra, S.; Moran, M.J.; Cravotto, G.; Cintas, P. Highly efficient nitrobenzene and alkyl/aryl azide reduction in stainless steel jars without catalyst addition. New J. Chem. 2018, 42, 18881–18888. [Google Scholar] [CrossRef]
- Göksu, H.; Ho, S.F.; Metin, Ö.; Korkmaz, K.; Mendoza Garcia, A.; Gültekin, M.S.; Sun, S. Tandem Dehydrogenation of Ammonia Borane and Hydrogenation of Nitro/Nitrile Compounds Catalyzed by Graphene-Supported NiPd Alloy Nanoparticles. ACS Catal. 2014, 4, 1777–1782. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Zhang, W.; Luo, M. A Convenient and General Reduction of Amides to Amines with Low-Valent Titanium. Adv. Synth. Catal. 2013, 355, 2775–2780. [Google Scholar] [CrossRef]
- Amberchan, G.; Snelling, R.A.; Moya, E.; Landi, M.; Lutz, K.; Gatihi, R.; Singaram, B. Reaction of Diisobutylaluminum Borohydride, a Binary Hydride, with Selected Organic Compounds Containing Representative Functional Groups. J. Org. Chem. 2021, 86, 6207–6227. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.-F.; Fu, Z.-C.; Liang, F.; Xu, Y.; Yang, D.-D.; Yang, Z.; Gan, X.; Lin, Z.-S.; Chen, Y.; Fu, W.-F. Robust Hydrogenation of Nitrile and Nitro Groups to Primary Amines Using Ni2P as a Catalyst and Ammonia Borane under Ambient Conditions. Asian J. Org. Chem. 2017, 6, 1589–1593. [Google Scholar] [CrossRef]
- Maddani, M.R.; Moorthy, S.K.; Prabhu, K.R. Chemoselective reduction of azides catalyzed by molybdenum xanthate by using phenylsilane as the hydride source. Tetrahedron 2010, 66, 329–333. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Mousavi, H.; Mohammad Aminzadeh, F. A hassle-free and cost-effective transfer hydrogenation strategy for the chemoselective reduction of arylnitriles to primary amines through in situ-generated nickelII dihydride intermediate in water. J. Mol. Struct. 2022, 1255. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Ai, Y.; Liu, Y.; Xiong, J.; Wang, H.; Qiao, Y.; Liu, W.; Tan, S.; Feng, S.; et al. A ppm level Rh-based composite as an ecofriendly catalyst for transfer hydrogenation of nitriles: Triple guarantee of selectivity for primary amines. Green Chem. 2019, 21, 1390–1395. [Google Scholar] [CrossRef]
- Podyacheva, E.; Afanasyev, O.I.; Vasilyev, D.V.; Chusov, D. Borrowing Hydrogen Amination Reactions: A Complex Analysis of Trends and Correlations of the Various Reaction Parameters. ACS Catal. 2022, 12, 7142–7198. [Google Scholar] [CrossRef]
- Lévay, K.; Hegedűs, L. Recent Achievements in the Hydrogenation of Nitriles Catalyzed by Transitional Metals. Curr. Org. Chem. 2019, 23, 1881–1900. [Google Scholar] [CrossRef]
- Debellefon, C.; Fouilloux, P. Homogeneous and heterogeneous hydrogenation of nitriles in a liquid-phase—Chemical, mechanistic, and catalytic aspects. Catal. Rev. 1994, 36, 459–506. [Google Scholar] [CrossRef]
- Chandrashekhar, V.G.; Senthamarai, T.; Kadam, R.G.; Malina, O.; Kašlík, J.; Zbořil, R.; Gawande, M.B.; Jagadeesh, R.V.; Beller, M. Silica-supported Fe/Fe–O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives. Nat. Catal. 2021, 5, 20–29. [Google Scholar] [CrossRef]
- Chakraborty, S.; Milstein, D. Selective Hydrogenation of Nitriles to Secondary Imines Catalyzed by an Iron Pincer Complex. ACS Catal. 2017, 7, 3968–3972. [Google Scholar] [CrossRef]
- Lange, S.; Elangovan, S.; Cordes, C.; Spannenberg, A.; Jiao, H.; Junge, H.; Bachmann, S.; Scalone, M.; Topf, C.; Junge, K.; et al. Selective catalytic hydrogenation of nitriles to primary amines using iron pincer complexes. Catal. Sci. Technol. 2016, 6, 4768–4772. [Google Scholar] [CrossRef]
- Chakraborty, S.; Leitus, G.; Milstein, D. Selective hydrogenation of nitriles to primary amines catalyzed by a novel iron complex. Chem. Commun. 2016, 52, 1812–1815. [Google Scholar] [CrossRef]
- Mérel, D.S.; Do, M.L.T.; Gaillard, S.; Dupau, P.; Renaud, J.-L. Iron-catalyzed reduction of carboxylic and carbonic acid derivatives. Coordin. Chem. Rev. 2015, 288, 50–68. [Google Scholar] [CrossRef]
- Bornschein, C.; Werkmeister, S.; Wendt, B.; Jiao, H.; Alberico, E.; Baumann, W.; Junge, H.; Junge, K.; Beller, M. Mild and selective hydrogenation of aromatic and aliphatic (di)nitriles with a well-defined iron pincer complex. Nat. Commun. 2014, 5, 4111. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Yamaguchi, S.; Nakata, A.; Yamazoe, S.; Nakajima, K.; Yamasaki, J.; Mizugaki, T.; Mitsudome, T. Hydrotalcite-Supported Cobalt Phosphide Nanorods as a Highly Active and Reusable Heterogeneous Catalyst for Ammonia-Free Selective Hydrogenation of Nitriles to Primary Amines. ACS Sustain. Chem. Eng. 2021, 9, 11238–11246. [Google Scholar] [CrossRef]
- Mitsudome, T.; Sheng, M.; Nakata, A.; Yamasaki, J.; Mizugaki, T.; Jitsukawa, K. A cobalt phosphide catalyst for the hydrogenation of nitriles. Chem. Sci. 2020, 11, 6682–6689. [Google Scholar] [CrossRef]
- Formenti, D.; Mocci, R.; Atia, H.; Dastgir, S.; Anwar, M.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. A State-of-the-Art Heterogeneous Catalyst for Efficient and General Nitrile Hydrogenation. Chem. Eur. J. 2020, 26, 15589–15595. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Sohail, M.; Alshammari, A.S.; Pohl, M.M.; Beller, M.; Jagadeesh, R.V. Cobalt-based nanoparticles prepared from MOF-carbon templates as efficient hydrogenation catalysts. Chem. Sci. 2018, 9, 8553–8560. [Google Scholar] [CrossRef]
- Ferraccioli, R.; Borovika, D.; Surkus, A.-E.; Kreyenschulte, C.; Topf, C.; Beller, M. Synthesis of cobalt nanoparticles by pyrolysis of vitamin B12: A non-noble-metal catalyst for efficient hydrogenation of nitriles. Catal. Sci. Technol. 2018, 8, 499–507. [Google Scholar] [CrossRef]
- Dai, H.; Guan, H. Switching the Selectivity of Cobalt-Catalyzed Hydrogenation of Nitriles. ACS Catal. 2018, 8, 9125–9130. [Google Scholar] [CrossRef]
- Tokmic, K.; Jackson, B.J.; Salazar, A.; Woods, T.J.; Fout, A.R. Cobalt-Catalyzed and Lewis Acid-Assisted Nitrile Hydrogenation to Primary Amines: A Combined Effort. J. Am. Chem. Soc. 2017, 139, 13554–13561. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Bheeter, C.B.; Cabrero-Antonino, J.R.; Junge, K.; Jackstell, R.; Beller, M. Selective Hydrogenation of Nitriles to Primary Amines by using a Cobalt Phosphine Catalyst. ChemSusChem 2017, 10, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Fu, S.; Wei, M.; Zhou, S.; Liu, Q. Mild and Selective Cobalt-Catalyzed Chemodivergent Transfer Hydrogenation of Nitriles. Angew. Chem. Int. Ed. 2016, 55, 14653–14657. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Topf, C.; Radnik, J.; Kreyenschulte, C.; Lund, H.; Schneider, M.; Surkus, A.E.; He, L.; Junge, K.; Beller, M. Stable and Inert Cobalt Catalysts for Highly Selective and Practical Hydrogenation of C≡N and C=O Bonds. J. Am. Chem. Soc. 2016, 138, 8781–8788. [Google Scholar] [CrossRef]
- Mukherjee, A.; Srimani, D.; Chakraborty, S.; Ben-David, Y.; Milstein, D. Selective Hydrogenation of Nitriles to Primary Amines Catalyzed by a Cobalt Pincer Complex. J. Am. Chem. Soc. 2015, 137, 8888–8891. [Google Scholar] [CrossRef]
- Segobia, D.J.; Trasarti, A.F.; Apesteguía, C.R. Chemoselective hydrogenation of unsaturated nitriles to unsaturated primary amines: Conversion of cinnamonitrile on metal-supported catalysts. Appl. Catal. A-Gen. 2015, 494, 41–47. [Google Scholar] [CrossRef]
- van der Waals, D.; Pettman, A.; Williams, J.M.J. Copper-catalysed reductive amination of nitriles and organic-group reductions using dimethylamine borane. RSC Adv. 2014, 4, 51845–51849. [Google Scholar] [CrossRef]
- Lv, Y.; Hao, F.; Liu, P.; Xiong, S.; Luo, H. Liquid phase hydrogenation of adiponitrile over acid-activated sepiolite supported K–La–Ni trimetallic catalysts. React. Kinet. Mech. Cat. 2016, 119, 555–568. [Google Scholar] [CrossRef]
- Konnerth, H.; Prechtl, M.H. Selective partial hydrogenation of alkynes to (Z)-alkenes with ionic liquid-doped nickel nanocatalysts at near ambient conditions. Chem. Commun. 2016, 52, 9129–9132. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhen, B.; Han, M.; Wang, C. Liquid phase hydrogenation of adiponitrile over directly reduced Ni/SiO2 catalyst. Catal. Commun. 2016, 73, 80–83. [Google Scholar] [CrossRef]
- Cao, Y.; Niu, L.; Wen, X.; Feng, W.; Huo, L.; Bai, G. Novel layered double hydroxide/oxide-coated nickel-based core–shell nanocomposites for benzonitrile selective hydrogenation: An interesting water switch. J. Catal. 2016, 339, 9–13. [Google Scholar] [CrossRef]
- Weber, S.; Stoger, B.; Kirchner, K. Hydrogenation of Nitriles and Ketones Catalyzed by an Air-Stable Bisphosphine Mn(I) Complex. Org. Lett. 2018, 20, 7212–7215. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Topf, C.; Fischer, S.; Jiao, H.; Spannenberg, A.; Baumann, W.; Ludwig, R.; Junge, K.; Beller, M. Selective Catalytic Hydrogenations of Nitriles, Ketones, and Aldehydes by Well-Defined Manganese Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 8809–8814. [Google Scholar] [CrossRef]
- Ma, K.; Liao, W.; Shi, W.; Xu, F.; Zhou, Y.; Tang, C.; Lu, J.; Shen, W.; Zhang, Z. Ceria-supported Pd catalysts with different size regimes ranging from single atoms to nanoparticles for the oxidation of CO. J. Catal. 2022, 407, 104–114. [Google Scholar] [CrossRef]
- Yoshimura, M.; Komatsu, A.; Niimura, M.; Takagi, Y.; Takahashi, T.; Ueda, S.; Ichikawa, T.; Kobayashi, Y.; Okami, H.; Hattori, T.; et al. Selective Synthesis of Primary Amines from Nitriles under Hydrogenation Conditions. Adv. Synth. Catal. 2018, 360, 1726–1732. [Google Scholar] [CrossRef]
- Saito, Y.; Ishitani, H.; Ueno, M.; Kobayashi, S. Selective Hydrogenation of Nitriles to Primary Amines Catalyzed by a Polysilane/SiO2-Supported Palladium Catalyst under Continuous-Flow Conditions. ChemistryOpen 2017, 6, 211–215. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Cao, X.; Li, X.; Gu, H. Selective synthesis of secondary amines from nitriles using Pt nanowires as a catalyst. Chem. Commun. 2014, 50, 3512–3515. [Google Scholar] [CrossRef]
- Muratsugu, S.; Kityakarn, S.; Wang, F.; Ishiguro, N.; Kamachi, T.; Yoshizawa, K.; Sekizawa, O.; Uruga, T.; Tada, M. Formation and nitrile hydrogenation performance of Ru nanoparticles on a K-doped Al2O3 surface. Phys. Chem. Chem. Phys. 2015, 17, 24791–24802. [Google Scholar] [CrossRef] [PubMed]
- Segobia, D.J.; Trasarti, A.F.; Apesteguia, C.R. Conversion of butyronitrile to butylamines on noble metals: Effect of the solvent on catalyst activity and selectivity. Catal. Sci. Technol. 2014, 4, 4075–4083. [Google Scholar] [CrossRef]
- Xie, X.F.; Liotta, C.L.; Eckert, C.A. CO2-protected amine formation from nitrile and imine hydrogenation in gas-expanded liquids. Ind. Eng. Chem. Res. 2004, 43, 7907–7911. [Google Scholar] [CrossRef]
- Rajesh, K.; Dudle, B.; Blacque, O.; Berke, H. Homogeneous Hydrogenations of Nitriles Catalyzed by Rhenium Complexes. Adv. Synth. Catal. 2011, 353, 1479–1484. [Google Scholar] [CrossRef]
- Chatterjee, M.; Sato, M.; Kawanami, H.; Yokoyama, T.; Suzuki, T.; Ishizaka, T. An Efficient Hydrogenation of Dinitrile to Aminonitrile in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2010, 352, 2394–2398. [Google Scholar] [CrossRef]
- Monguchi, Y.; Mizuno, M.; Ichikawa, T.; Fujita, Y.; Murakami, E.; Hattori, T.; Maegawa, T.; Sawama, Y.; Sajiki, H. Catalyst-Dependent Selective Hydrogenation of Nitriles: Selective Synthesis of Tertiary and Secondary Amines. J. Org. Chem. 2017, 82, 10939–10944. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Sautier, B.; Spain, M.; Procter, D.J. Electron transfer reduction of nitriles using SmI2-Et3N-H2O: Synthetic utility and mechanism. Org. Lett. 2014, 16, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Lopez-De Jesus, Y.M.; Johnson, C.E.; Monnier, J.R.; Williams, C.T. Selective Hydrogenation of Benzonitrile by Alumina-Supported Ir-Pd Catalysts. Top. Catal. 2010, 53, 1132–1137. [Google Scholar] [CrossRef]
- Molnar, A.; Smith, G.V.; Bartok, M. New catalytic materials from amorphous metal-alloys. Adv. Catal. 1989, 36, 329–383. [Google Scholar]
- Li, H.; Xu, Y.; Li, H.; Deng, J.F. Gas-phase hydrogenation of adiponitrile with high selectivity to primary amine over supported Ni-B amorphous catalysts. Appl. Catal. A-Gen. 2001, 216, 51–58. [Google Scholar] [CrossRef]
- Wang, W.-J.; Qiao, M.-H.; Li, H.-X.; Deng, J.-F. Partial Hydrogenation of Cyclopentadiene over Amorphous NiB Alloy on a-Alumina and Titania-Modifiedd a-Alumina. J. Chem. Technol. Biotechnol. 1998, 72, 280–284. [Google Scholar] [CrossRef]
- Chiang, S.-J.; Yang, C.-H.; Chen, Y.-Z.; Liaw, B.-J. High-active nickel catalyst of NiB/SiO2 for citral hydrogenation at low temperature. Appl. Catal. A-Gen. 2007, 326, 180–188. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Wang, Y.; Chai, Y.; Liu, C. Preparation, mechanism and applications of oriented MFI zeolite membranes: A review. Microporous Mesoporous Mater. 2021, 312, 110790. [Google Scholar] [CrossRef]
- Huybrechts, D.R.C.; Debruycker, L.; Jacobs, P.A. Oxyfunctionalization of alkanes with hydrogen-peroxide on titanium silicalite. Nature 1990, 345, 240–242. [Google Scholar] [CrossRef]
- Lu, J.-Q.; Li, N.; Pan, X.-R.; Zhang, C.; Luo, M.-F. Direct propylene epoxidation with H2 and O2 over in modified Au/TS-1 catalysts. Catal. Commun. 2012, 28, 179–182. [Google Scholar] [CrossRef]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; J. Wiley: New York, NY, USA, 2001. [Google Scholar]
- Liu, Y.; Zhou, K.; Lu, M.; Wang, L.; Wei, Z.; Li, X. Acidic/Basic Oxides-Supported Cobalt Catalysts for One-Pot Synthesis of Isophorone Diamine from Hydroamination of Isophorone Nitrile. Ind. Eng. Chem. Res. 2015, 54, 9124–9132. [Google Scholar] [CrossRef]
- Chojecki, A.; Veprek-Heijman, M.; Müller, T.E.; Schärringer, P.; Veprek, S.; Lercher, J.A. Tailoring Raney-catalysts for the selective hydrogenation of butyronitrile to n-butylamine. J. Catal. 2007, 245, 237–248. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Mărginean, P.; Stănescu, U. Effect of supports on the activity of nickel catalysts in acetonitrile hydrogenation. Appl. Catal. A-Gen. 2005, 294, 208–214. [Google Scholar] [CrossRef]
- Huang, J.; Han, J.; Wang, R.; Zhang, Y.; Wang, X.; Zhang, X.; Zhang, Z.; Zhang, Y.; Song, B.; Jin, S. Improving Electrocatalysts for Oxygen Evolution Using NixFe3−xO4/Ni Hybrid Nanostructures Formed by Solvothermal Synthesis. ACS Energy Lett. 2018, 3, 1698–1707. [Google Scholar] [CrossRef]
- Li, H.; Li, H.X.; Dai, W.L.; Wang, W.J.; Fang, Z.G.; Deng, J.F. XPS studies on surface electronic characteristics of Ni-B and Ni-P amorphous alloy and its correlation to their catalytic properties. Appl. Surf. Sci. 1999, 152, 25–34. [Google Scholar] [CrossRef]
- Jiang, W.J.; Niu, S.; Tang, T.; Zhang, Q.H.; Liu, X.Z.; Zhang, Y.; Chen, Y.Y.; Li, J.H.; Gu, L.; Wan, L.J.; et al. Crystallinity-Modulated Electrocatalytic Activity of a Nickel(II) Borate Thin Layer on Ni3 B for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2017, 56, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Zhang, M.; Tao, K. The interactions between the NiB amorphous alloy and TiO2 support in the NiB/TiO2 amorphous catalysts. Appl. Catal. A-Gen. 2004, 259, 185–190. [Google Scholar] [CrossRef]

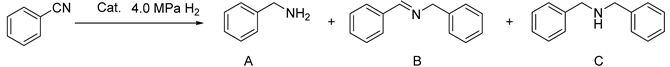 | ||||
| Catalyst (mg) | Conversion (%) b | Yield (%) c | TOF (h−1) d | |
| 1 | Ni6.2-30 (100) | 99 | 74 | 23.4 |
| 2 | Ni6.2-50 (100) | 100 | 71 | 21.9 |
| 3 | Ni6.2-70 (100) | 99 | 66 | 18.8 |
| 4 | Ni6.2-100 (100) | 100 | 60 | 17.2 |
| 5 | Ni2.5-30 (250) | 100 | 68 | 19.0 |
| 6 | Ni12.4-30 (50) | 100 | 77 | 28.4 |
| 7 | Ni18.6-30 (33) | 77 | 51 | 22.2 |
| 8 | Ni24.8-30 (25) | 71 | 47 | 12.7 |
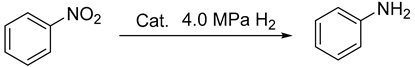 | ||||
| Catalyst (mg) | Conversion (%) b | Yield (%) c | TOF (h−1) d | |
| 1 | Ni6.2-30 (100) | 100 | 96 | 5.9 |
| 2 | Ni6.2-50 (100) | 100 | 95 | 5.6 |
| 3 | Ni6.2-70 (100) | 100 | 95 | 5.1 |
| 4 | Ni6.2-100 (100) | 100 | 94 | 4.3 |
| 5 | Ni2.5-30 (250) | 85 | 81 | 2.9 |
| 6 | Ni12.4-30 (50) | 100 | 97 | 9.5 |
| 7 | Ni18.6-30 (33) | 100 | 95 | 11.7 |
| 8 | Ni24.8-30 (25) | 100 | 94 | 10.5 |
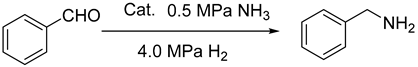 | ||||
| Catalyst (mg) | Conversion (%) b | Yield (%) c | TOF (h−1) d | |
| 1 | Ni6.2-30 (100) | 100 | 92 | 21.5 |
| 2 | Ni6.2-50 (100) | 100 | 97 | 19.0 |
| 3 | Ni6.2-70 (100) | 100 | 96 | 15.8 |
| 4 | Ni6.2-100 (100) | 100 | 95 | 14.8 |
| 5 | Ni2.5-30 (250) | 100 | 93 | 19.0 |
| 6 | Ni12.4-30 (50) | 100 | 96 | 23.7 |
| 7 | Ni18.6-30 (33) | 100 | 96 | 26.3 |
| 8 | Ni24.8-30 (25) | 100 | 95 | 22.6 |
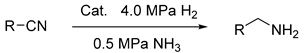 | ||||
| Product | Time (h) | Conversion (%) a | Yield (%) b | |
| 1 | Ph-CH2NH2 | 3.5 | 100 | 97 |
| 2 | 4-CH3-Ph-CH2NH2 | 4.0 | 100 | 94 |
| 3 | 4-CH3O-Ph-CH2NH2 | 2.5 | 100 | 95 |
| 4 | 4-Cl-Ph-CH2NH2 | 3.5 | 100 | 95 |
| 5 | 4-NH2-Ph-CH2NH2 | 4.5 | 100 | 97 |
| 6 | 3-CH3-Ph-CH2NH2 | 4.5 | 100 | 96 |
| 7 | 3-CH3O-Ph-CH2NH2 | 2.5 | 100 | 93 |
| 8 | 3-Cl-Ph-CH2NH2 | 1.5 | 100 | 95 |
| 9 | 3-NH2-Ph-CH2NH2 | 3.0 | 100 | 95 |
| 10 | 2-CH3-Ph-CH2NH2 | 4.0 | 100 | 96 |
| 11 | 2-CH3O-Ph-CH2NH2 | 4.5 | 100 | 92 |
| 12 | 2-Cl-Ph-CH2NH2 | 5.0 | 100 | 96 |
| 13 | 2-NH2-Ph-CH2NH2 | 3.0 | 100 | 95 |
| 14 | 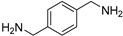 | 5.0 | 74.9 | 69 |
| 15 c | 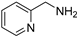 | 8.0 | 22.1 | 17 |
| 16 d | 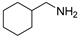 | 4.0 | 63.9 | 55 |
| 17 | 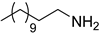 | 8.0 | 100 | 97 |
| 18 | 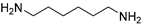 | 9.0 | 68.3 | 63 |
| 19 e | 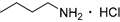 | 6.0 | 40.3 | 35 |
| 20 |  | 5.0 | <5 | - |
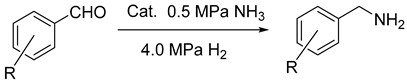 | ||||
| Product (R) | Time (h) | Conversion (%) a | Yield (%) b | |
| 1 | H | 5.0 | 100 | 97 |
| 2 | 4-CH3 | 6.0 | 100 | 95 |
| 3 | 4-F | 6.5 | 100 | 93 |
| 4 | 3-F | 6.0 | 100 | 95 |
| 5 | 4-Cl | 6.5 | 100 | 94 |
| 6 | 4-Br | 7.0 | 100 | 93 |
| 7 c | 4-OH | 5.5 | 100 | 94 |
| 8 | 4-NH2 | 7.5 | 100 | 96 |
 | ||||
| Product | Time (h) | Conversion (%) a | Yield (%) b | |
| 1 | Ph-CH2NH2 | 2.0 | 100 | 96 |
| 2 | 4-CH3-Ph-CH2NH2 | 3.0 | 100 | 95 |
| 3 | 4-Cl-Ph-CH2NH2 | 3.5 | 100 | 95 |
| 4 | 4-Br-Ph-CH2NH2 | 1.5 | 100 | 95 |
| 5 | 4-OH-Ph-CH2NH2 | 2.5 | 100 | 92 |
| 6 | 3-CH3-Ph-CH2NH2 | 2.0 | 100 | 98 |
| 7 | 3-Cl-Ph-CH2NH2 | 3.5 | 100 | 92 |
| 8 | 3-Br-Ph-CH2NH2 | 2.0 | 100 | 96 |
| 9 | 3-OH-Ph-CH2NH2 | 1.5 | 100 | 92 |
| 10 | 2-CH3-Ph-CH2NH2 | 2.5 | 100 | 98 |
| 11 | 2-Cl-Ph-CH2NH2 | 2.0 | 100 | 97 |
| 12 | 2-Br-Ph-CH2NH2 | 1.0 | 100 | 97 |
| 13 | 2-OH-Ph-CH2NH2 | 2.0 | 100 | 91 |
| 14 | 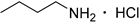 | 4.0 | 100 | >99 c |
| Catalyst | Time (h) | Conversion (%) | Yield (%) | |
|---|---|---|---|---|
| Benzonitrile | Ni12.4-30 | 3.5 | 100 | 77 |
| Raney Ni | 4 | 100 | 26 | |
| Nitrobenzene | Ni12.4-30 | 5 | 100 | 97 |
| Raney Ni | 2 | 100 | 97 | |
| Benzaldehyde | Ni12.4-30 | 2 | 100 | 96 |
| Raney Ni | 3.5 | 100 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, D.; Zhou, S. General Construction of Amine via Reduction of N=X (X = C, O, H) Bonds Mediated by Supported Nickel Boride Nanoclusters. Int. J. Mol. Sci. 2022, 23, 9337. https://doi.org/10.3390/ijms23169337
Ke D, Zhou S. General Construction of Amine via Reduction of N=X (X = C, O, H) Bonds Mediated by Supported Nickel Boride Nanoclusters. International Journal of Molecular Sciences. 2022; 23(16):9337. https://doi.org/10.3390/ijms23169337
Chicago/Turabian StyleKe, Da, and Shaodong Zhou. 2022. "General Construction of Amine via Reduction of N=X (X = C, O, H) Bonds Mediated by Supported Nickel Boride Nanoclusters" International Journal of Molecular Sciences 23, no. 16: 9337. https://doi.org/10.3390/ijms23169337
APA StyleKe, D., & Zhou, S. (2022). General Construction of Amine via Reduction of N=X (X = C, O, H) Bonds Mediated by Supported Nickel Boride Nanoclusters. International Journal of Molecular Sciences, 23(16), 9337. https://doi.org/10.3390/ijms23169337







