Copper Foam as Active Catalysts for the Borylation of α, β-Unsaturated Compounds
Abstract
:1. Introduction
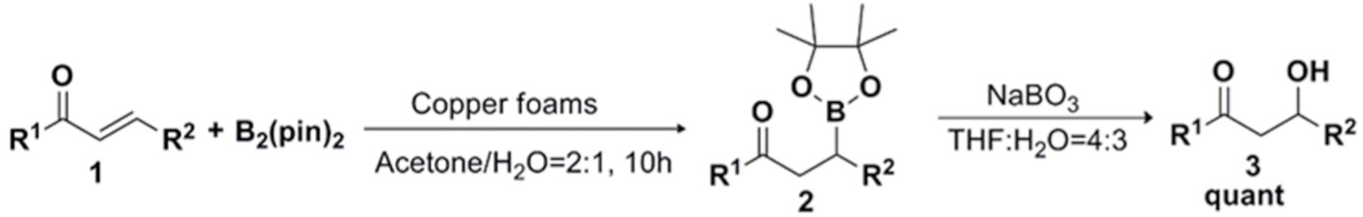
2. Results and Discussion
2.1. Optimization of Reaction Conditions
2.2. Substrate Expansion under Optimal Reaction Conditions
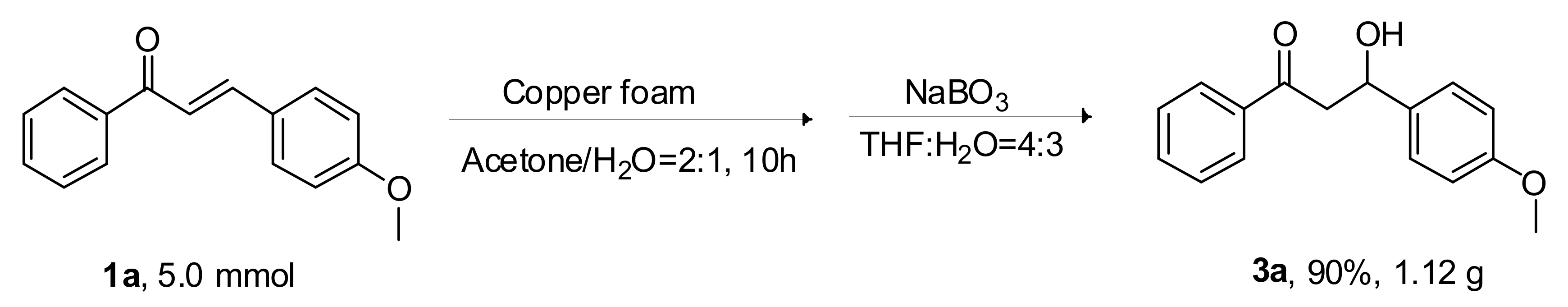
2.3. Gram-Scale Synthesis Reaction of 1a
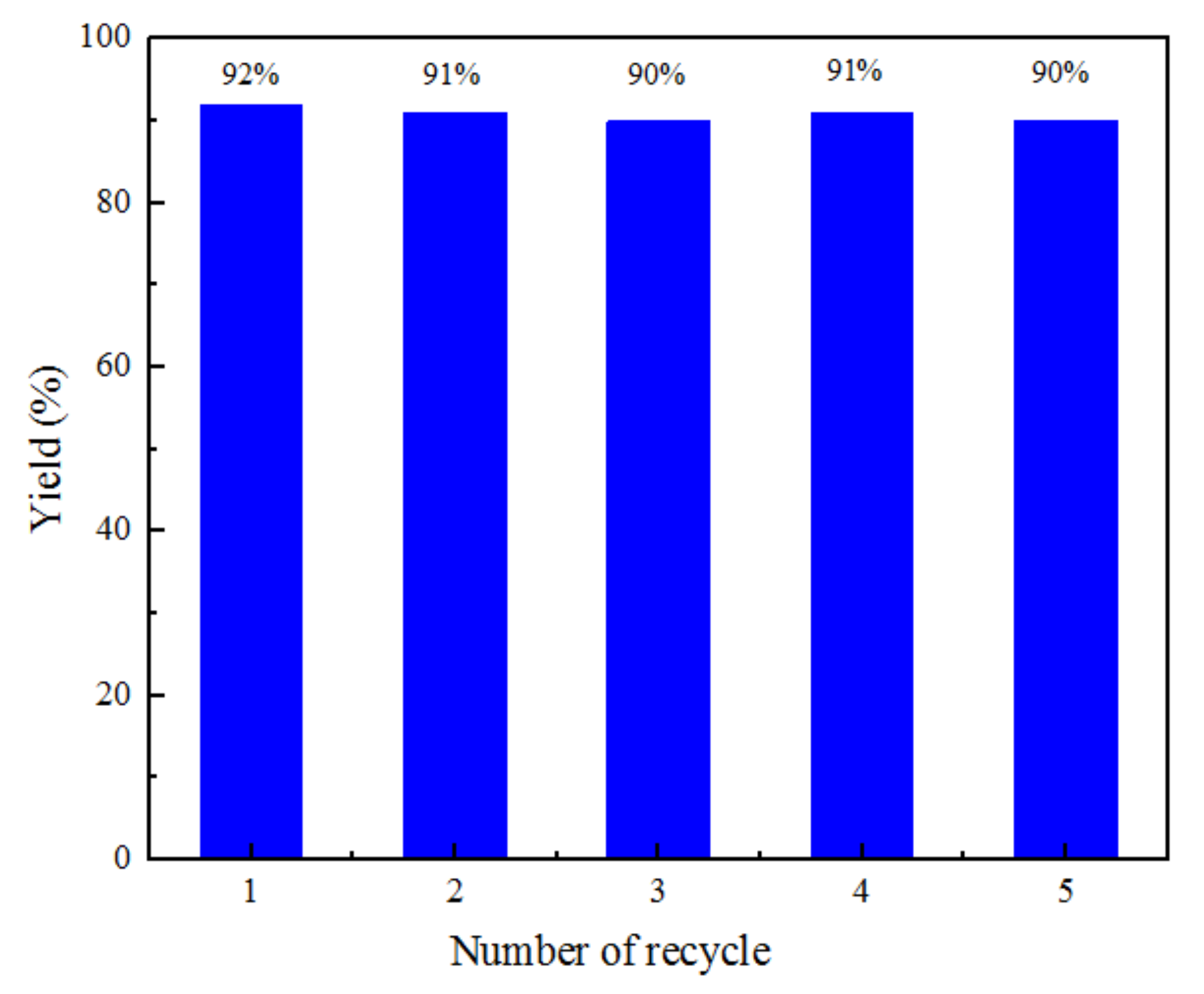
2.4. Catalyst Reuse
2.5. Characterization of Catalytic Materials and Mechanistic Studies
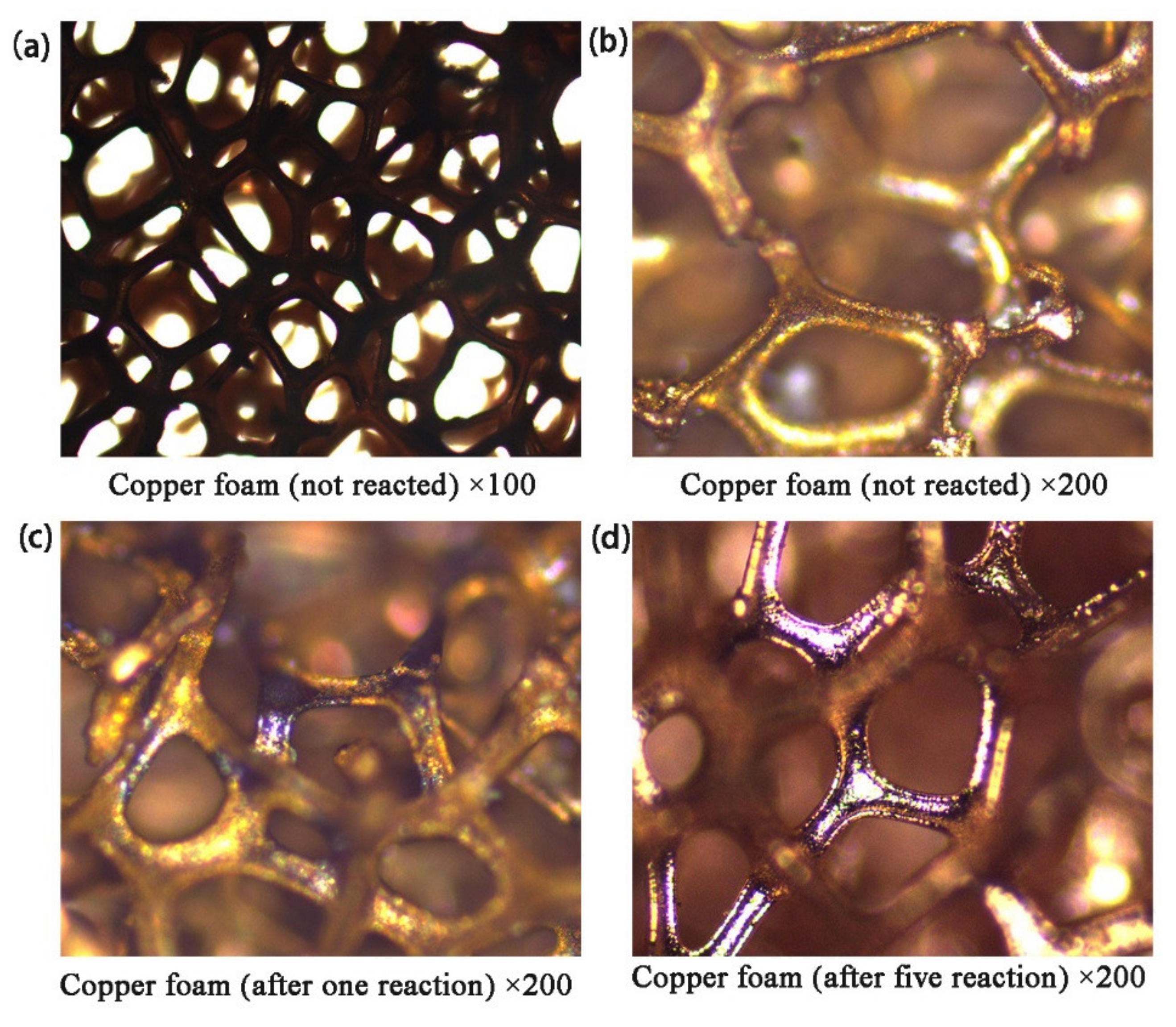
2.5.1. Polarizing Microscope Analysis
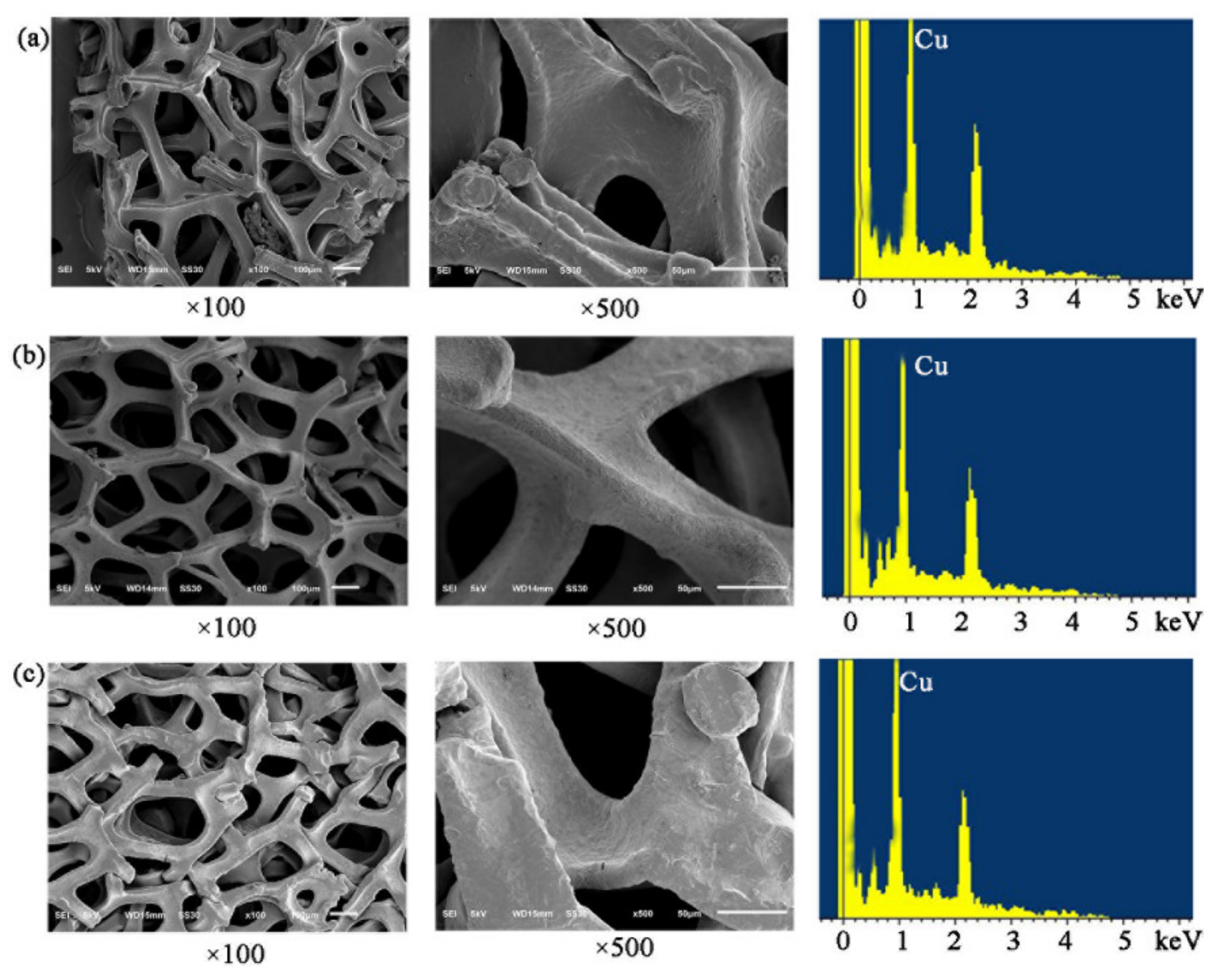
2.5.2. SEM-EDS Analysis
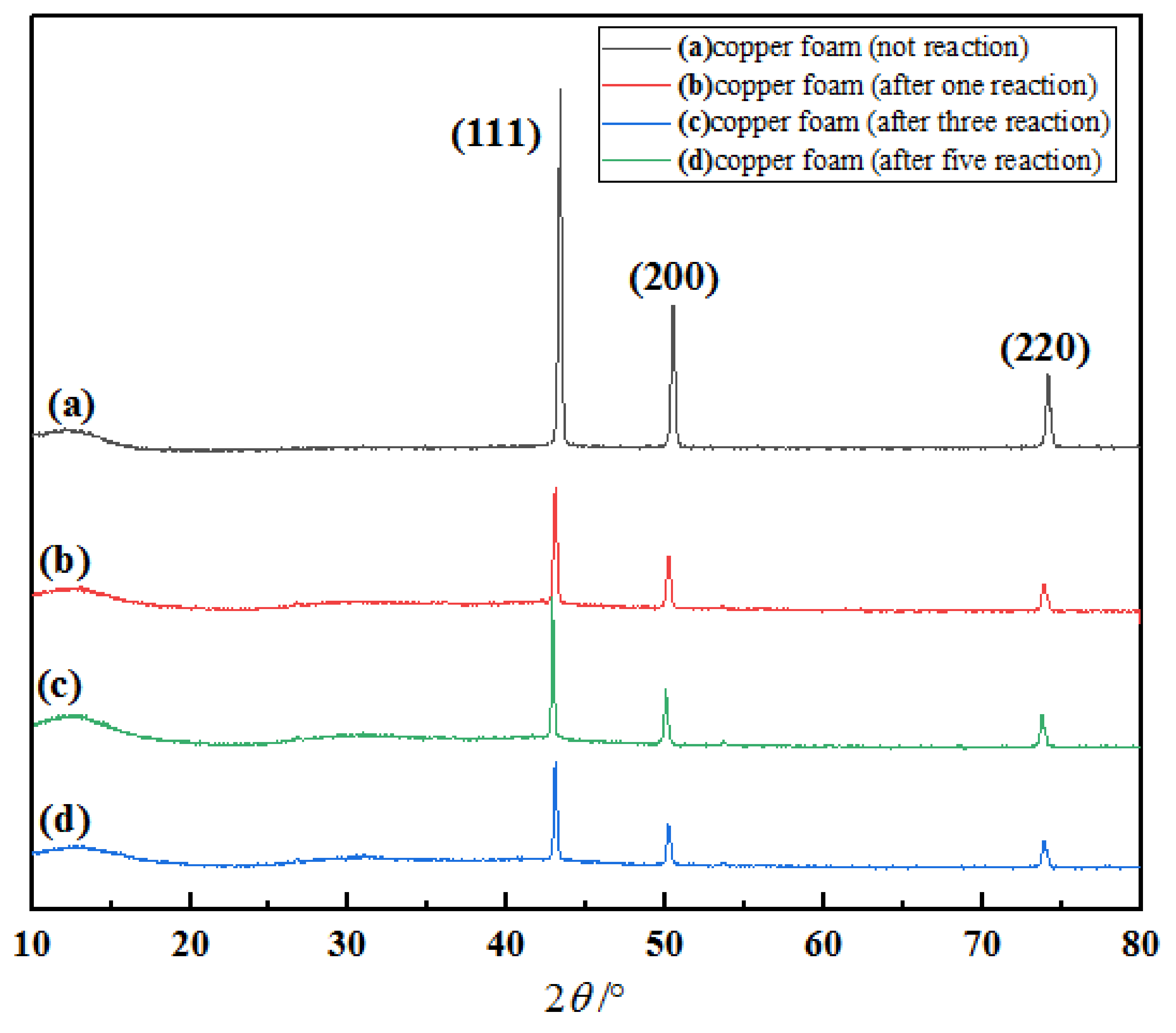
2.5.3. XRD Analysis
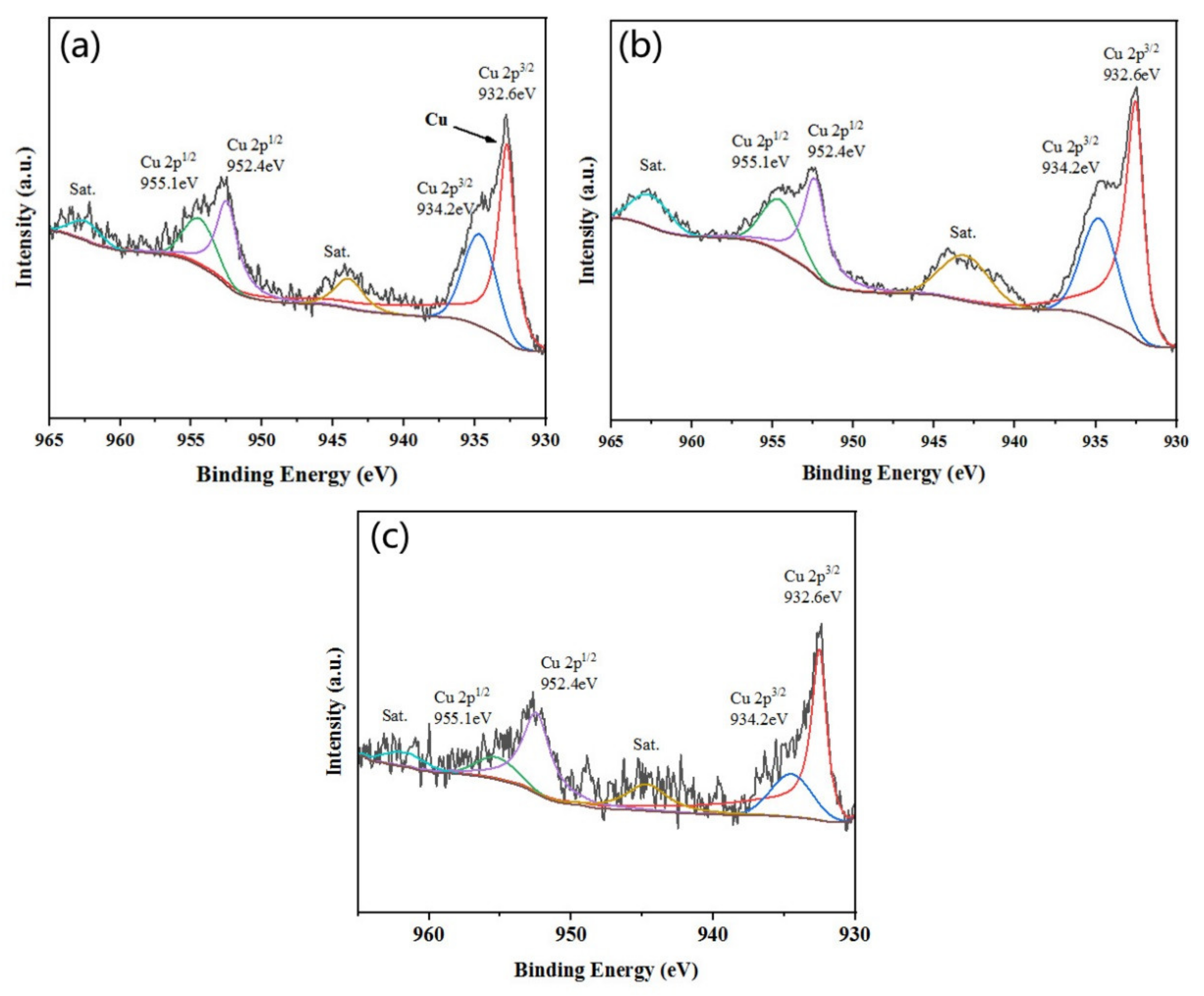
2.5.4. XPS Analysis
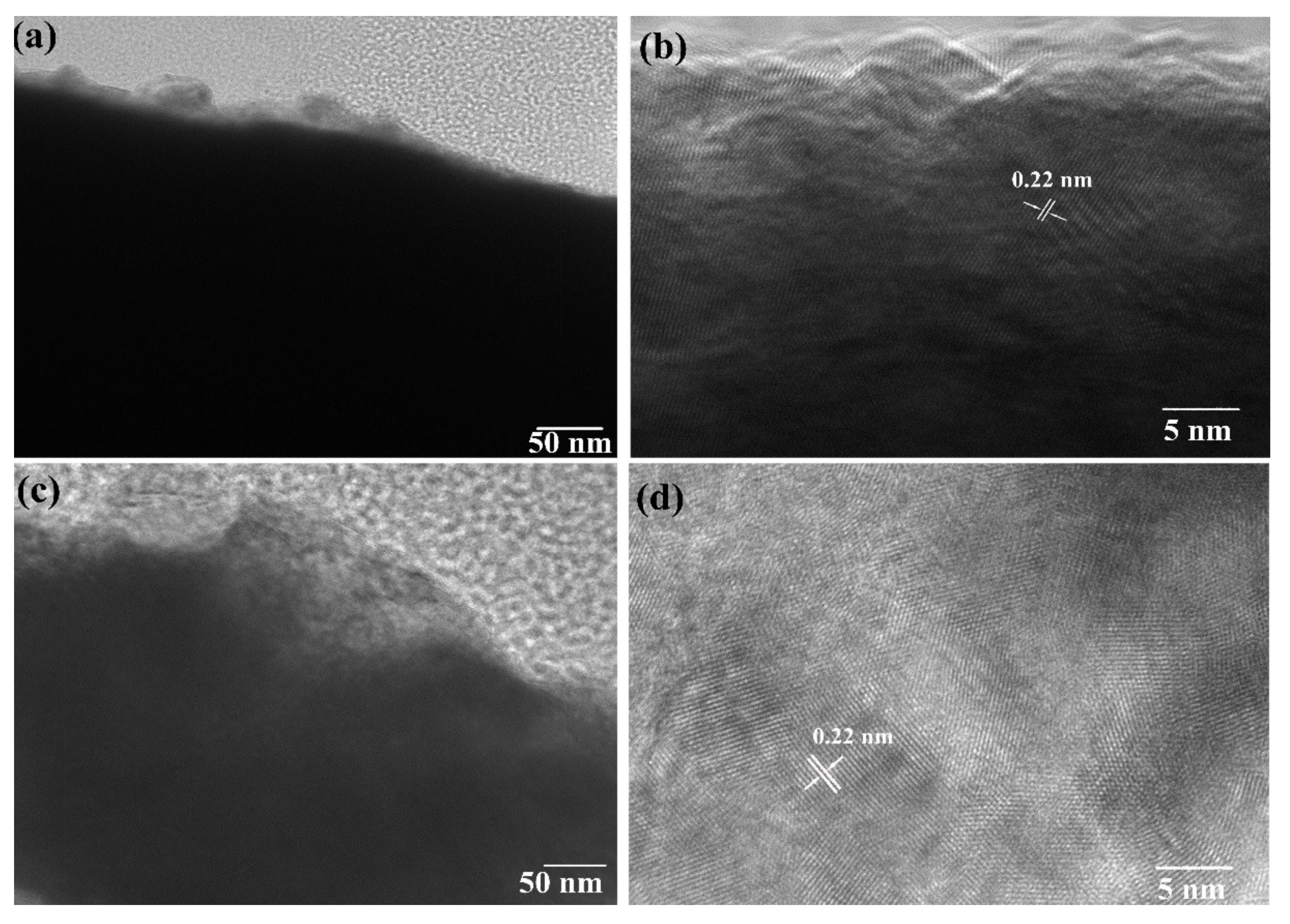
2.5.5. TEM Analysis
2.5.6. Isotopic Effects of Reactions
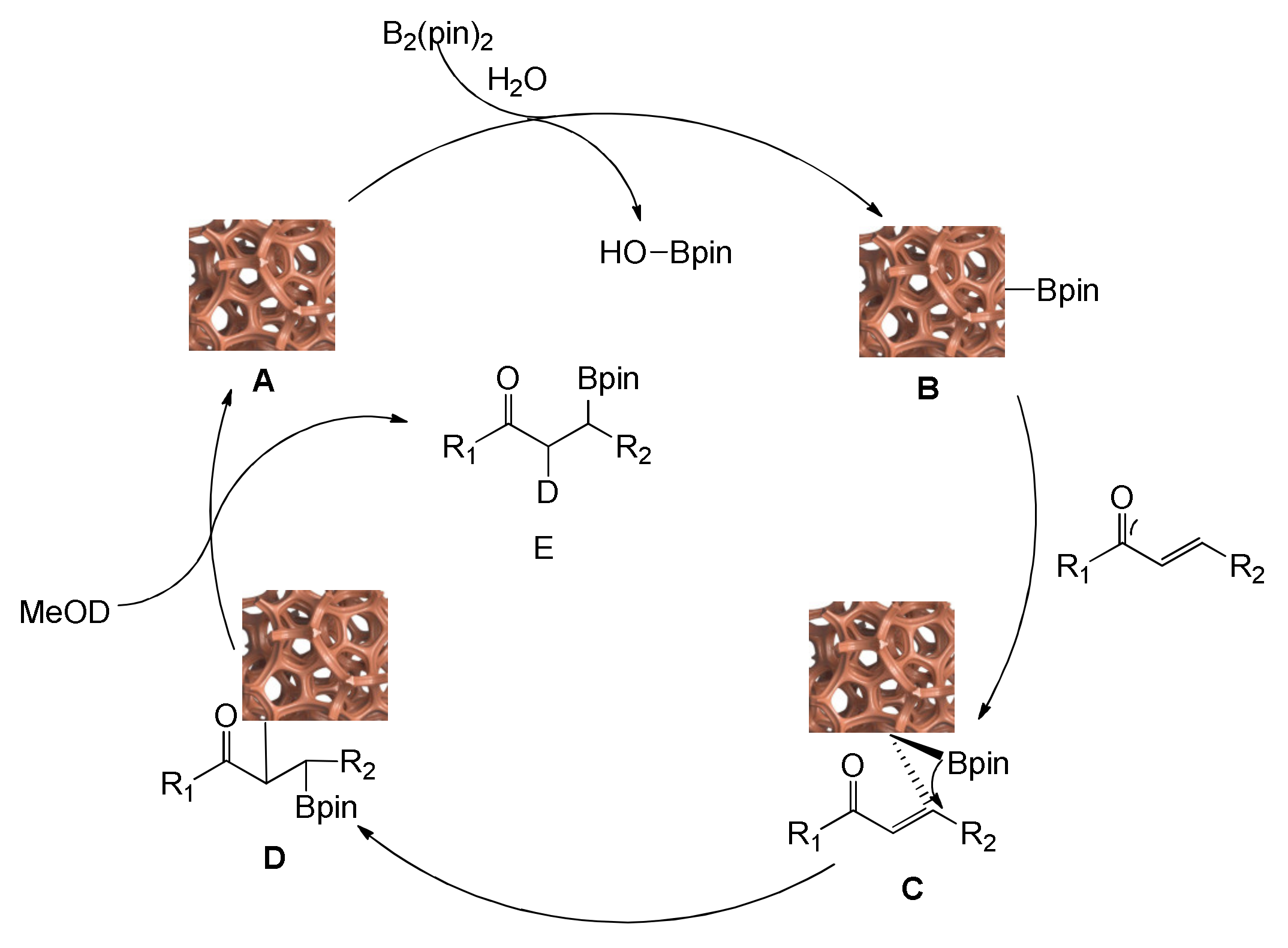
2.6. Mechanistic Studies
2.7. Comparison of the Catalytic Performance of Different Copper Catalytic Materials
3. Materials and Methods
3.1. Materials
3.2. Arylboronic Acid Self-Coupling Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, N.; Suzuki, T.; Ota, Y.; Nakano, T.; Kurihara, M.; Okuda, H.; Yamori, T.; Tsumoto, H.; Nakagawa, H.; Miyata, N. Design, synthesis, and biological activity of boronic acid-based histone deacetylase inhibitors. J. Med. Chem. 2009, 52, 2909–2922. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Komarovska-Porokhnyavets, O.; Misterkiewicz, B.; Novikov, V.P.; Sporzyński, A. Biological activity of selected boronic acids and their derivatives. Appl. Organomet. Chem. 2012, 26, 390–393. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Strongly enhanced antibacterial action of copper oxide nanoparticles with boronic acid surface functionality. ACS Appl. Mater. Interfaces 2019, 11, 12232–12243. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N. Organoboron compounds. Cross-Coupling React. 2002, 29, 11–59. [Google Scholar]
- Ishiyama, T.; Miyaura, N. Metal-catalyzed reactions of diborons for synthesis of organoboron compounds. Chem. Rec. 2004, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Chudinov, M.V. Progress in the medical chemistry of organoboron compounds. Russ. Chem. Rev. 2021, 90, 451. [Google Scholar] [CrossRef]
- Manna, S.; Das, K.K.; Nandy, S.; Aich, D.; Paul, S.; Panda, S. A new avenue for the preparation of organoboron compounds via nickel catalysis. Coord. Chem. Rev. 2021, 448, 214165. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C−H activation for the construction of C−B bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Jin, S.; Dang, H.T.; Haug, G.C.; He, R.; Nguyen, V.D.; Nguyen, V.T.; Arman, H.D.; Schanze, K.S.; Larionov, O.V. Visible light-induced borylation of C–O, C–N, and C–X bonds. J. Am. Chem. Soc. 2020, 142, 1603–1613. [Google Scholar] [CrossRef]
- Lee, K.-S.; Zhugralin, A.; Hoveyda, A. NHC-Catalyzed Boron Conjugate Additions to α,β-Unsaturated Carbonyl Compounds. Synfacts 2009, 2009, 0898. [Google Scholar]
- Gao, M.; Thorpe, S.; Kleeberg, C.; Slebodnick, C.; Marder, T.; Santos, W. Catalytic Regioselective Boration of α, β-Unsaturated Compounds. Synfacts 2011, 2011, 0995. [Google Scholar]
- Shilpa, T.; Neetha, M.; Anilkumar, G. Recent Trends and Prospects in the Copper-Catalysed “on Water” Reactions. Adv. Synth. Catal. 2021, 363, 1559–1582. [Google Scholar] [CrossRef]
- Tsuda, T.; Choi, S.-M.; Shintani, R. Palladium-catalyzed synthesis of dibenzosilepin derivatives via 1, n-Palladium migration coupled with anti-Carbopalladation of alkyne. J. Am. Chem. Soc. 2021, 143, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Männig, D.; Nöth, H. Catalytic hydroboration with rhodium complexes. Angew. Chem. Int. Ed. Engl. 1985, 24, 878–879. [Google Scholar] [CrossRef]
- Burgess, K.; Ohlmeyer, M.J. Enantioselective hydroboration mediated by homochiral rhodium catalysts. J. Org. Chem. 1988, 53, 5178–5179. [Google Scholar] [CrossRef]
- Burgess, K.; Van der Donk, W.A.; Jarstfer, M.B.; Ohlmeyer, M.J. Further evidence for the role of d. pi.-p. pi. bonding in rhodium-mediated hydroborations. J. Am. Chem. Soc. 1991, 113, 6139–6144. [Google Scholar] [CrossRef]
- Burgess, K.; van der Donk, W.A.; Ohlmeyer, M.J. Enantioselective hydroborations catalyzed by rhodium (+1) complexes. Tetrahedron Asymmetry 1991, 2, 613–621. [Google Scholar] [CrossRef]
- Suginome, M.; Yamamoto, A.; Murakami, M. Palladium-Catalyzed Addition of Cyanoboranes to Alkynes: Regio-and Stereoselective Synthesis of α,β-Unsaturated β-Boryl Nitriles. Angew. Chem. Int. Ed. 2005, 44, 2380–2382. [Google Scholar] [CrossRef]
- Dang, L.; Lin, Z.; Marder, T.B. DFT studies on the borylation of α,β-unsaturated carbonyl compounds catalyzed by phosphine copper (I) boryl complexes and observations on the interconversions between O-and C-bound enolates of Cu, B, and Si. Organometallics 2008, 27, 4443–4454. [Google Scholar] [CrossRef]
- Fleming, W.J.; Müller-Bunz, H.; Lillo, V.; Fernández, E.; Guiry, P.J. Axially chiral PN ligands for the copper catalyzed β-borylation of α,β-unsaturated esters. Org. Biomol. Chem. 2009, 7, 2520–2524. [Google Scholar] [CrossRef]
- Thorpe, S.B.; Guo, X.; Santos, W.L. Regio-and stereoselective copper-catalyzed β-borylation of allenoates by a preactivated diboron. Chem. Commun. 2011, 47, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.B.; Calderone, J.A.; Santos, W.L. Unexpected copper (II) catalysis: Catalytic amine base promoted β-borylation of α,β-unsaturated carbonyl compounds in water. Org. Lett. 2012, 14, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Shegavi, M.L.; Saini, S.; Bhawar, R.; Vishwantha, M.D.; Bose, S.K. Recyclable Copper Nanoparticles-Catalyzed Hydroboration of Alkenes and α,β-Borylation of α,β-Unsaturated Carbonyl Compounds with Bis (Pinacolato) Diboron. Adv. Synth. Catal. 2021, 363, 2408–2416. [Google Scholar] [CrossRef]
- Neely, J.M.; Rovis, T. Rh (III)-Catalyzed regioselective synthesis of pyridines from alkenes and α,β-unsaturated oxime esters. J. Am. Chem. Soc. 2013, 135, 66–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heravi, M.M.; Dehghani, M.; Zadsirjan, V. Rh-catalyzed asymmetric 1,4-addition reactions to α,β-unsaturated carbonyl and related compounds: An update. Tetrahedron Asymmetry 2016, 27, 513–588. [Google Scholar] [CrossRef] [Green Version]
- Romano, C. Transition Metal-Catalyzed Olefin Isomerization for Remote Functionalization Strategies. Ph.D. Thesis, University of Geneva, Geneva, Switzerland, 2018. [Google Scholar]
- Dembitsky, V.M.; Ali, H.A.; Srebnik, M. Recent chemistry of the diboron compounds. Adv. Organomet. Chem. 2004, 51, 193–250. [Google Scholar]
- Lawson, Y.; Norman, N.; Rice, C.; Marder, T. Platinum catalysed 1, 4-diboration of α,β-unsaturated ketones. Chem. Commun. 1997, 21, 2051–2052. [Google Scholar] [CrossRef]
- Cox, A.J. Metal Catalysed Diboration of α,β-Unsaturated Carbonyl Compounds. Master’s Thesis, Durham University, Durham, UK, 2002. [Google Scholar]
- Kurahashi, T. Ni-Catalyzed C–C Bond Formation with α,β-Unsaturated Carbonyl Compounds and Alkynes. Bull. Chem. Soc. Jpn. 2014, 87, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Zaramello, L.; Albuquerque, B.L.; Domingos, J.B.; Philippot, K. Kinetic investigation into the chemoselective hydrogenation of α,β-unsaturated carbonyl compounds catalyzed by Ni (0) nanoparticles. Dalton Trans. 2017, 46, 5082–5090. [Google Scholar] [CrossRef]
- Miwa, Y.; Kamimura, T.; Sato, K.; Shishido, D.; Yoshida, K. Chiral Bicyclic NHC/Cu Complexes for Catalytic Asymmetric Borylation of α,β-Unsaturated Esters. J. Org. Chem. 2019, 84, 14291–14296. [Google Scholar] [CrossRef]
- Che, F.; Wang, M.; Yu, C.; Sun, X.; Xie, D.; Wang, Z.; Zhang, Y. Cu2O-catalyzed selective 1,2-addition of acetonitrile to α,β-unsaturated aldehydes. Org. Chem. Front. 2020, 7, 868–872. [Google Scholar] [CrossRef]
- Ito, H.; Yamanaka, H.; Tateiwa, J.-I.; Hosomi, A. Boration of an α,β-enone using a diboron promoted by a copper (I)–phosphine mixture catalyst. Tetrahedron Lett. 2000, 41, 6821–6825. [Google Scholar] [CrossRef]
- Lee, J.E.; Yun, J. Catalytic Asymmetric Boration of Acyclic α,β-Unsaturated Esters and Nitriles. Angew. Chem. 2008, 120, 151–153. [Google Scholar] [CrossRef]
- Ding, W.; Song, Q. Chemoselective catalytic reduction of conjugated α,β-unsaturated ketones to saturated ketones via a hydroboration/protodeboronation strategy. Org. Chem. Front. 2016, 3, 14–18. [Google Scholar] [CrossRef]
- Kobayashi, S.; Xu, P.; Endo, T.; Ueno, M.; Kitanosono, T. Chiral Copper (II)-Catalyzed Enantioselective Boron Conjugate Additions to α,β-Unsaturated Carbonyl Compounds in Water. Angew. Chem. Int. Ed. 2012, 51, 12763–12766. [Google Scholar] [CrossRef]
- Stavber, G.; Časar, Z. Basic CuCO3/ligand as a new catalyst for ‘on water’ borylation of Michael acceptors, alkenes and alkynes: Application to the efficient asymmetric synthesis of β-alcohol type sitagliptin side chain. Appl. Organomet. Chem. 2013, 27, 159–165. [Google Scholar] [CrossRef]
- Deng, F.; Li, S.; Zhou, M.; Zhu, Y.; Qiu, S.; Li, K.; Ma, F.; Jiang, J. A biochar modified nickel-foam cathode with iron-foam catalyst in electro-Fenton for sulfamerazine degradation. Appl. Catal. B Environ. 2019, 256, 117796. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Lin, Y.; Chen, L.; Chu, X.; Zheng, T.; Wan, S.; Lin, J. Novel copper foam with ordered hole arrays as catalyst support for methanol steam reforming microreactor. Appl. Energy 2019, 246, 24–37. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Dong, S.; Wang, P. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ. 2019, 647, 352–361. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, Z.; Mei, D. A thermally autonomous methanol steam reforming microreactor with porous copper foam as catalyst support for hydrogen production. Int. J. Hydrogen Energy 2021, 46, 6734–6744. [Google Scholar] [CrossRef]
- Yan, F.; Zhou, L.; Han, B.; Zhang, Y.; Li, B.; Wang, L.; Zhu, L. Zeolite Immobilized Copper Catalyzed Conjugate Borylation of alpha, beta-Unsaturated Compounds in Aqueous Media. Chin. J. Org. Chem. 2021, 41, 2074–2081. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.; Huang, C.; Zheng, K.; Luo, Y.; Dong, Y.; Shen, Z.; Li, W.; Qin, C. Preparation of Modified Chitosan Microsphere-Supported Copper Catalysts for the Borylation of α,β-Unsaturated Compounds. Polymers 2019, 11, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, W.; Han, B.; Yan, F.; Ding, L.; Li, B.; Wang, L.; Zhu, L. Borylation of α,β-Unsaturated Acceptors by Chitosan Composite Film Supported Copper Nanoparticles. Nanomaterials 2018, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Han, B.; Zhang, Y.; Li, B.; Wang, L.; Wang, J.; Wang, X.; Zhu, L. Cellulosic CuI Nanoparticles as a Heterogeneous, Recyclable Catalyst for the Borylation of α,β-Unsaturated Acceptors in Aqueous Media. Catal. Lett. 2021, 151, 3220–3229. [Google Scholar] [CrossRef]
- Yang, Z.; Tuo, Y.; Lu, Q.; Chen, C.; Liu, M.; Liu, B.; Duan, X.; Zhou, Y.; Zhang, J. Hierarchical Cu3P-based nanoarrays on nickel foam as efficient electrocatalysts for overall water splitting. Green Energy Environ. 2020, 7, 236–245. [Google Scholar] [CrossRef]
- Xu, P.; Li, B.; Wang, L.; Qin, C.; Zhu, L. A green and recyclable chitosan supported catalyst for the borylation of α,β-unsaturated acceptors in water. Catal. Commun. 2016, 86, 23–26. [Google Scholar] [CrossRef]
- Zhu, L.; Kitanosono, T.; Xu, P.; Kobayashi, S. A Cu (II)-based strategy for catalytic enantioselective β-borylation of α,β-unsaturated acceptors. Chem. Commun. 2015, 51, 11685–11688. [Google Scholar] [CrossRef]
- Zhou, X.F.; Sun, Y.Y.; Wu, Y.D.; Dai, J.J.; Xu, J.; Huang, Y.; Xu, H.J. Borylation and selective reduction of α,β-unsaturated ketones under mild conditions catalyzed by Cu nanoparticles. Tetrahedron 2016, 72, 5691–5698. [Google Scholar] [CrossRef]
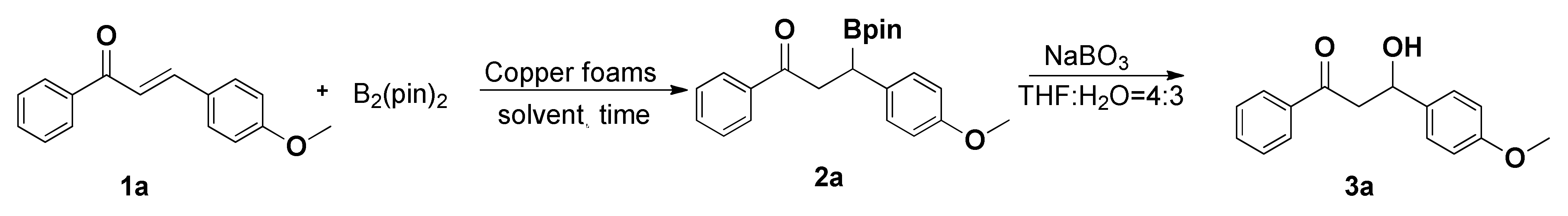
 | ||||
|---|---|---|---|---|
| Entry | Copper Foam (mg) | Solvent (2 mL) | Time (h) | Yield (%) |
| 1 | 10 | MeOH | 12 | 67 |
| 2 | 10 | EtOH | 12 | 53 |
| 3 | 10 | i-PrOH | 12 | 24 |
| 4 | 10 | DMF | 12 | <5% |
| 5 | 10 | H2O | 12 | 8% |
| 6 | 10 | Et2O | 12 | 10% |
| 7 | 10 | Toluene | 12 | 8% |
| 8 | 10 | DCM | 12 | 17% |
| 9 | 10 | THF | 12 | 15% |
| 10 | 10 | acetone | 12 | 63% |
| 11 | 10 | MeOH: H2O (1:1) | 12 | 87% |
| 12 | 10 | acetone:H2O (1:1) | 12 | 89% |
| 13 | 10 | acetone:H2O (2:1) | 12 | 92% |
| 14 | 10 | acetone:H2O (1:2) | 12 | 86% |
| 15 | 10 | acetone:H2O (3:1) | 12 | 91% |
| 16 | - | acetone:H2O (2:1) | 12 | - |
| 17 | 6 | acetone:H2O (2:1) | 12 | 85% |
| 18 | 8 | acetone:H2O (2:1) | 12 | 92% |
| 19 | 8 | acetone:H2O (2:1) | 6 | 81% |
| 20 | 8 | acetone:H2O (2:1) | 8 | 91% |
| 21 | 8 | acetone:H2O (2:1) | 10 | 92% |
| 22 | 8 | acetone:H2O (2:1) | 14 | 91% |
 |
 |
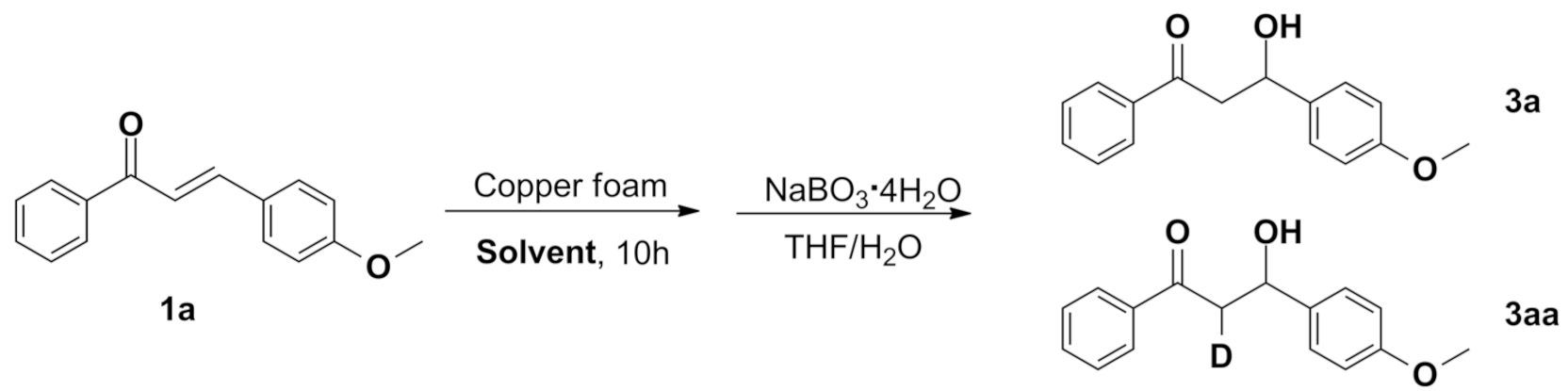 | ||
|---|---|---|
| Solvent | Product | Yield |
| CD3OD/H2O | 3a | 87% |
| CD3OH/D2O | 3a | 81% |
| CD3OD/D2O | 3aa | 63% |
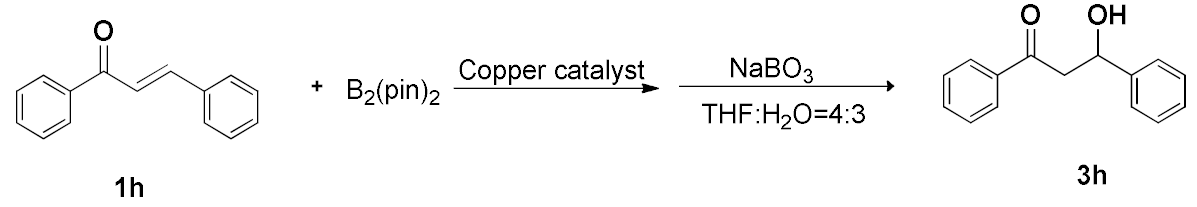 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Support | Ligand | Yield (%) |
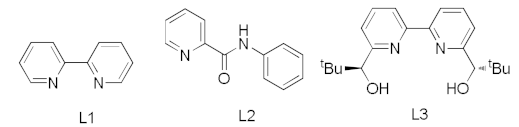
| 1 [48] | Cu(OH)2 | - | L1 | 93% |
| 2 [48] | Cu(OH)2 | Chitosan | L1 | 90% |
| 3 [48] | Cu(OH)2 | Chitosan | L2 | 100% |
| 4 [48] | Cu(OH)2 | Chitosan | - | 90% |
| 5 [49] | CuO | - | L3 | 96% |
| 6 [49] | Cu(OAc)2 | Chitosan | - | 91% |
| 7 [46] | CuI | Cellulosic | - | 96% |
| 8 [50] | Cu2O | - | - | NR |
| 9 [50] | Cu | Carbon black | - | 95% |
| 10 [44] | Cu | Chitosan | - | 93% |
| 11 [45] | Cu | Chitosan/PVA | - | 87% |
| 12 | copper foam | - | - | 92% |
 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Liu, M.; Meng, Z.; Xiao, Z.; Zhong, F.; Wang, W.; Qin, C. Copper Foam as Active Catalysts for the Borylation of α, β-Unsaturated Compounds. Int. J. Mol. Sci. 2022, 23, 8403. https://doi.org/10.3390/ijms23158403
Zheng K, Liu M, Meng Z, Xiao Z, Zhong F, Wang W, Qin C. Copper Foam as Active Catalysts for the Borylation of α, β-Unsaturated Compounds. International Journal of Molecular Sciences. 2022; 23(15):8403. https://doi.org/10.3390/ijms23158403
Chicago/Turabian StyleZheng, Kewang, Miao Liu, Zhifei Meng, Zufeng Xiao, Fei Zhong, Wei Wang, and Caiqin Qin. 2022. "Copper Foam as Active Catalysts for the Borylation of α, β-Unsaturated Compounds" International Journal of Molecular Sciences 23, no. 15: 8403. https://doi.org/10.3390/ijms23158403
APA StyleZheng, K., Liu, M., Meng, Z., Xiao, Z., Zhong, F., Wang, W., & Qin, C. (2022). Copper Foam as Active Catalysts for the Borylation of α, β-Unsaturated Compounds. International Journal of Molecular Sciences, 23(15), 8403. https://doi.org/10.3390/ijms23158403







