New Derivatives of 5-((1-Methyl-Pyrrol-2-yl) Methyl)-4-(Naphthalen-1-yl)-1,2,4-Triazoline-3-Thione and Its Coordination Compounds with Anticancer Activity
Abstract
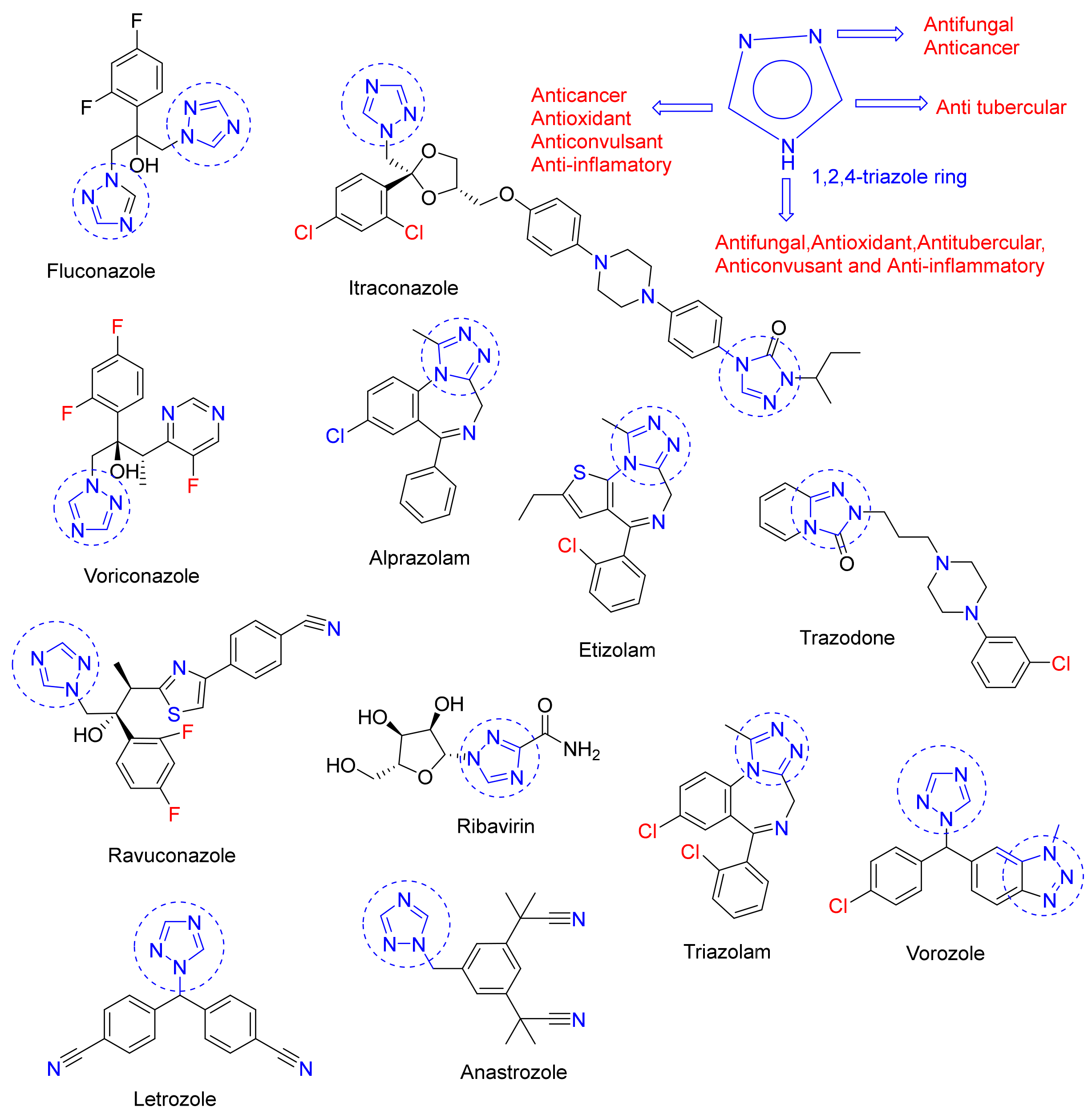
:1. Introduction
2. Results and Discussion
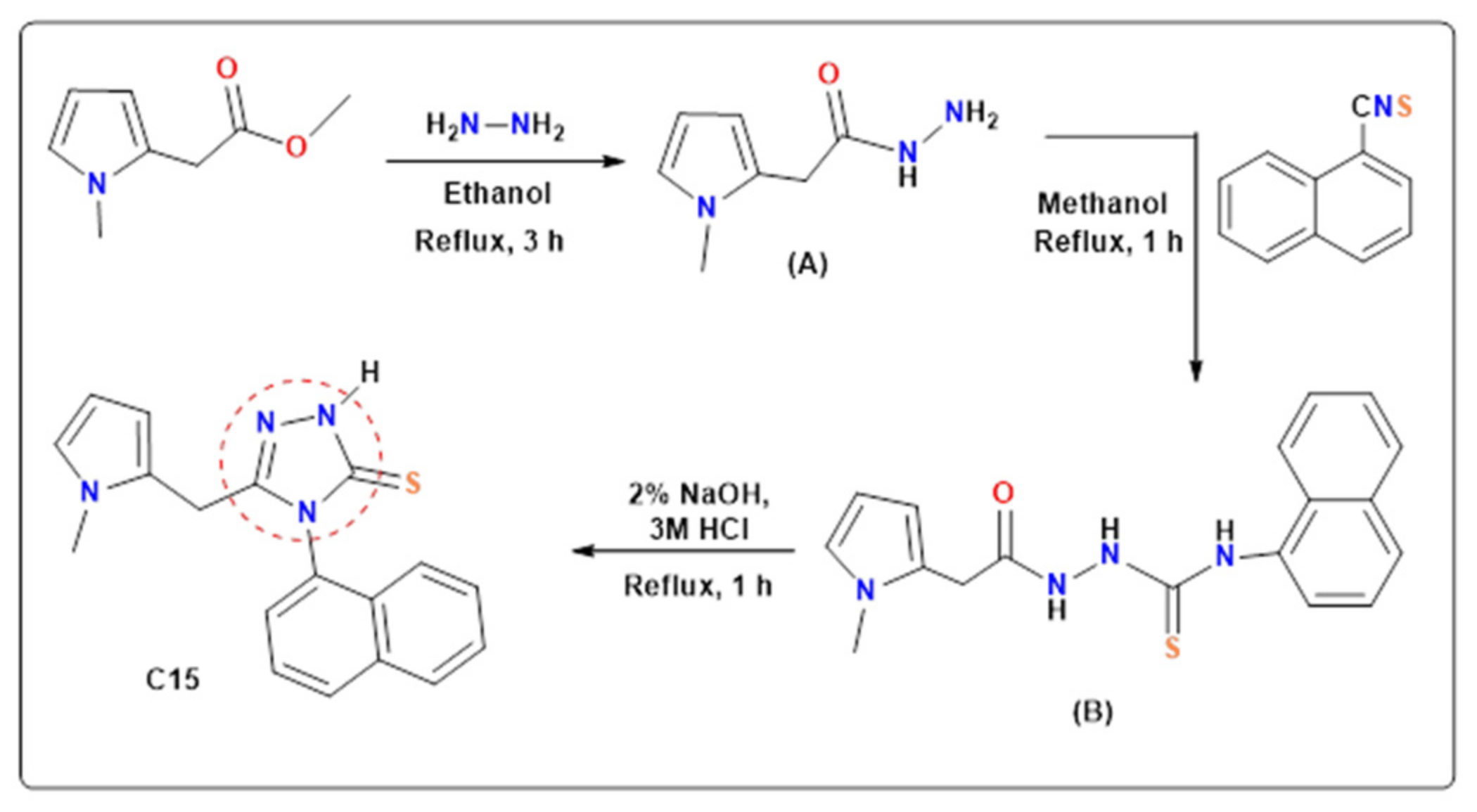
2.1. Synthesis
2.2. FT-IR Spectra
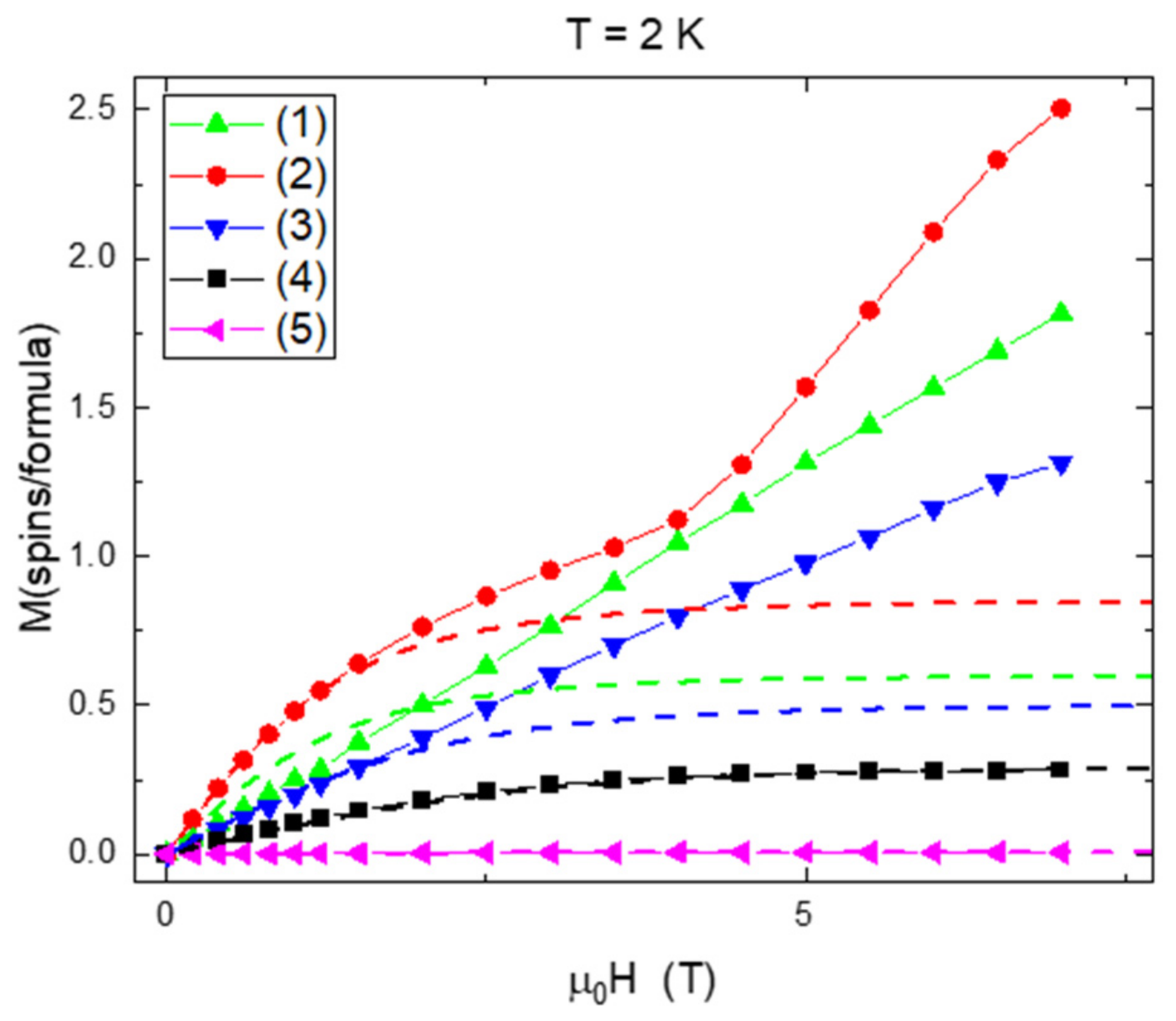
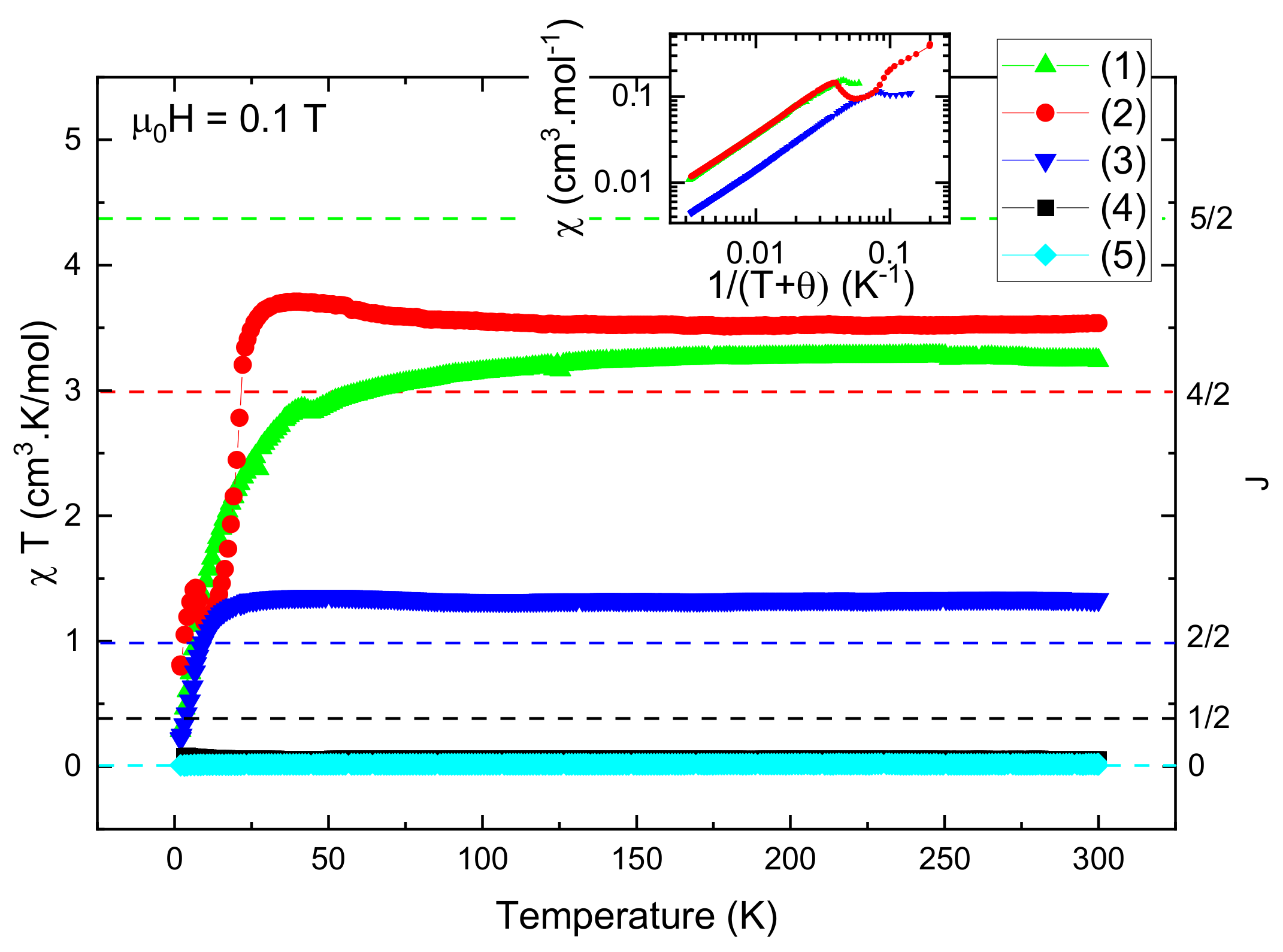
2.3. Magnetic Study
2.4. EPR Spectra
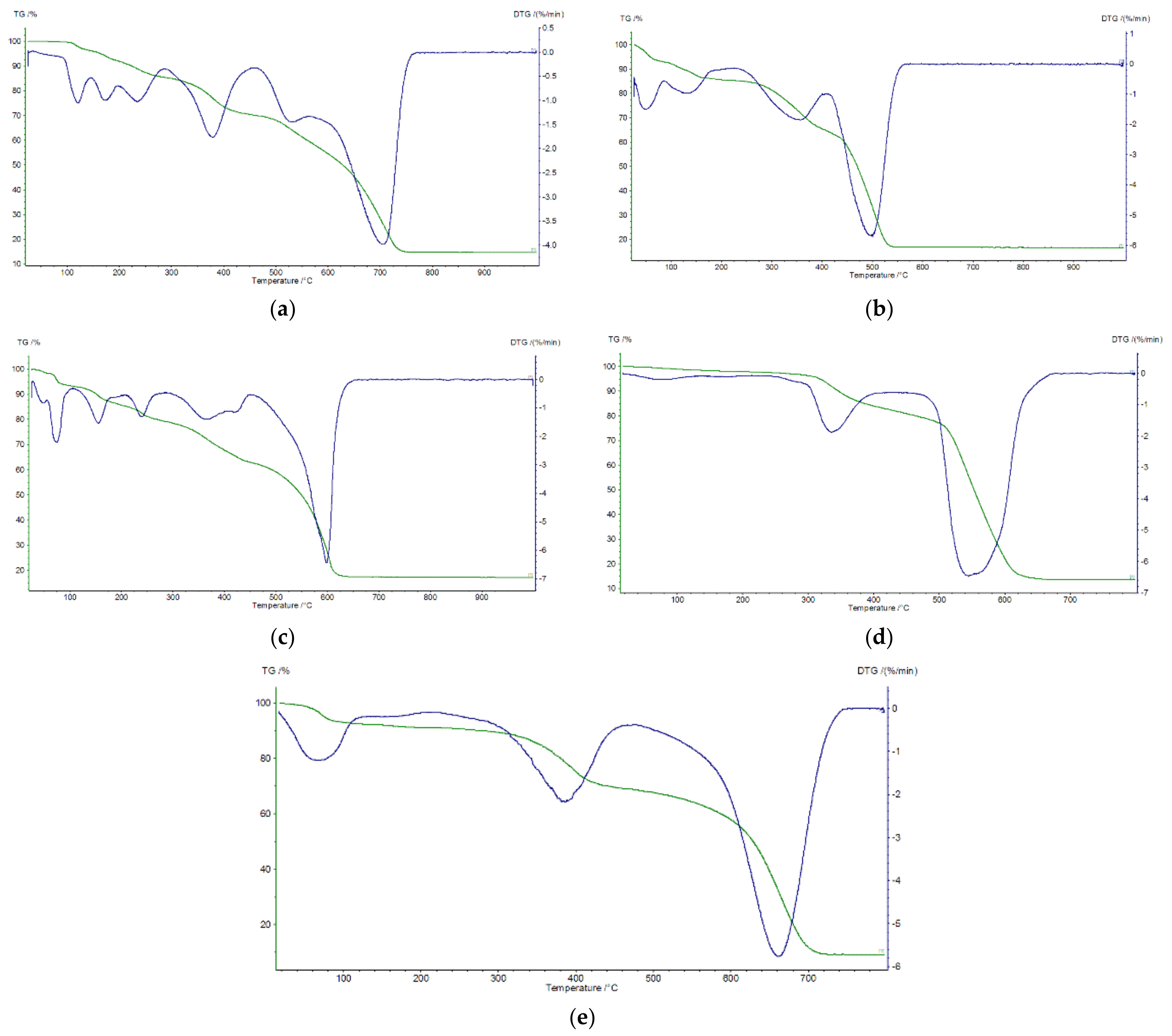
2.5. Thermal Decomposition of Complexes
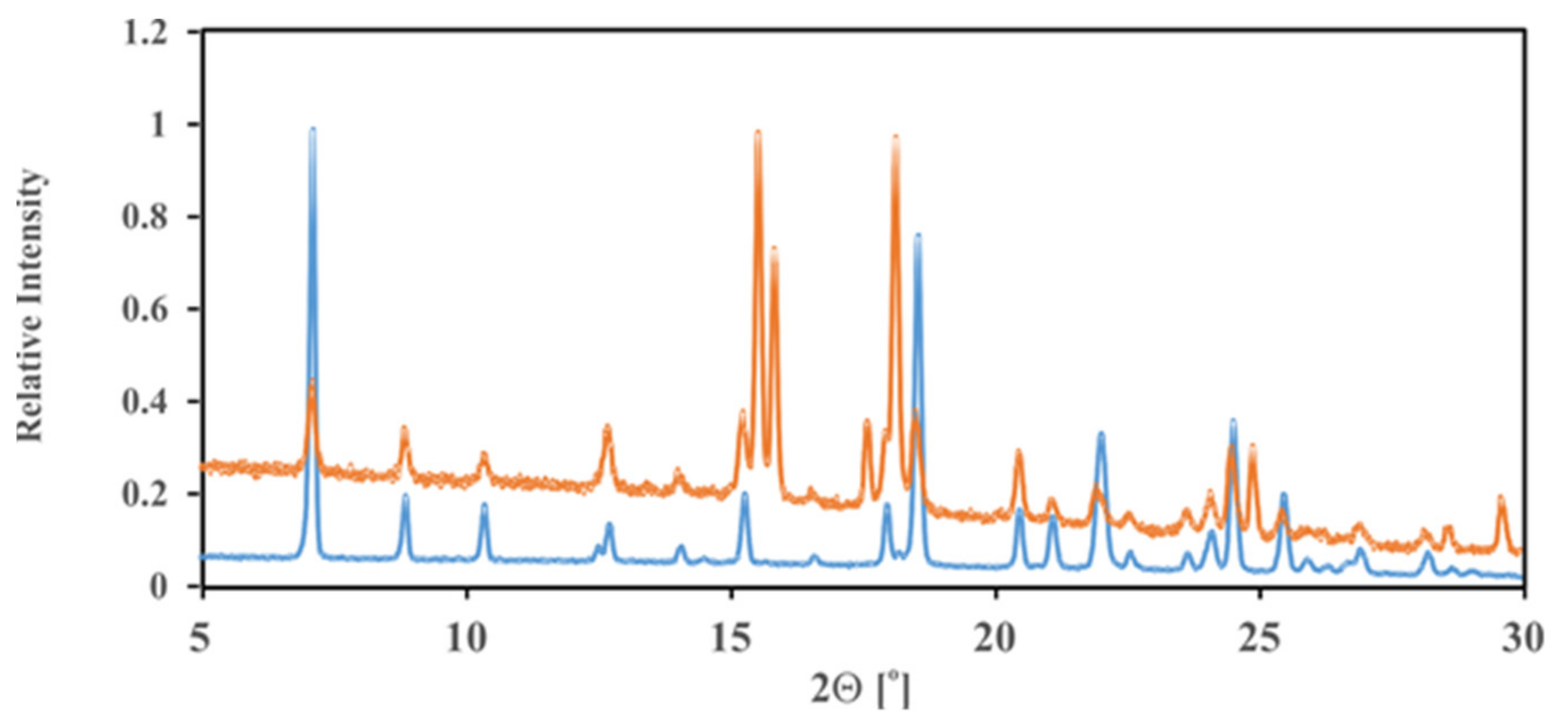
2.6. PXRD Analysis for Complexes (1), (2) and (3)
2.7. Cell Line Cytotoxicity Predictor: In Silico Prediction of Cytotoxicity for Tumour
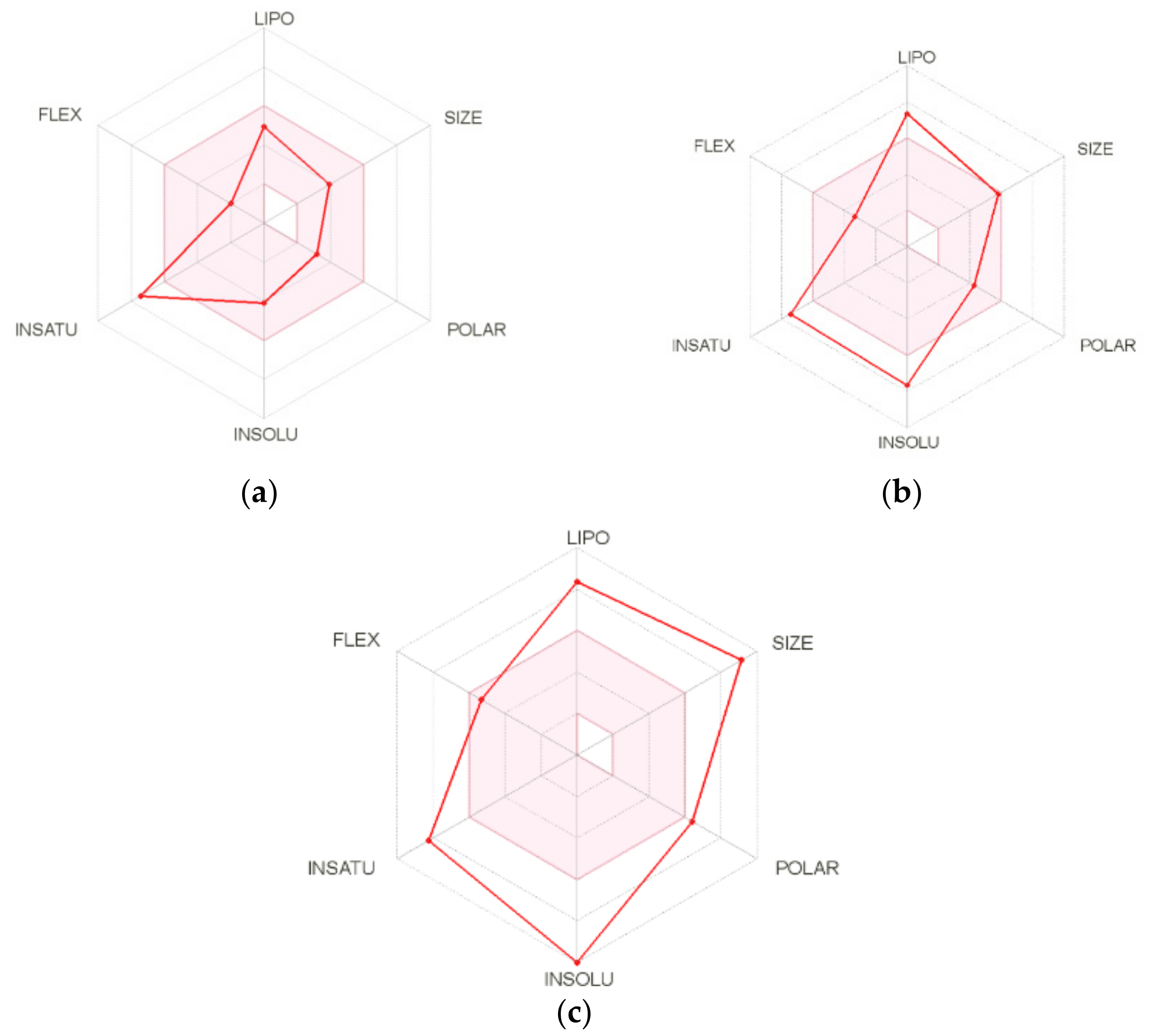
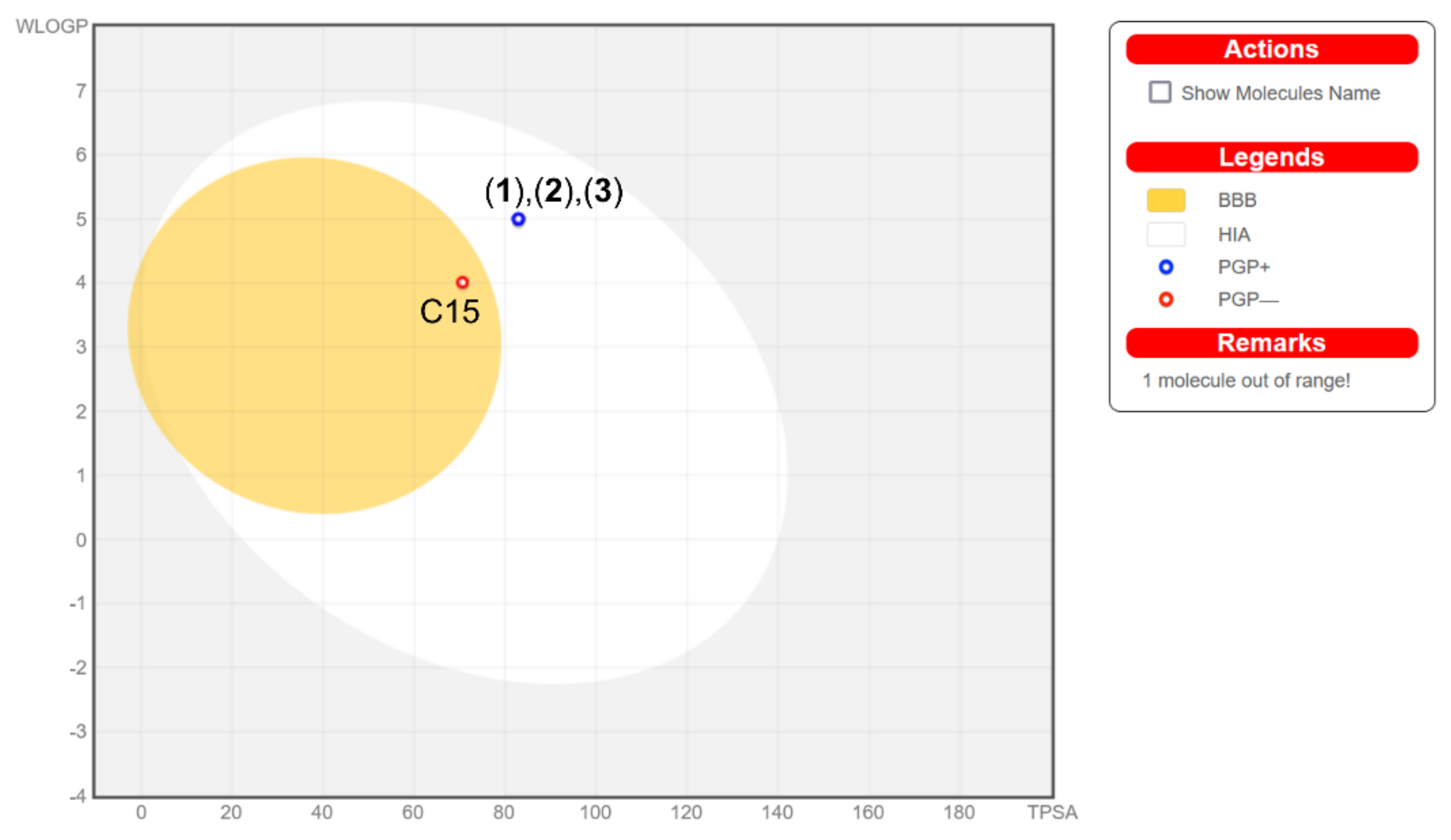
2.8. ADMET Analysis
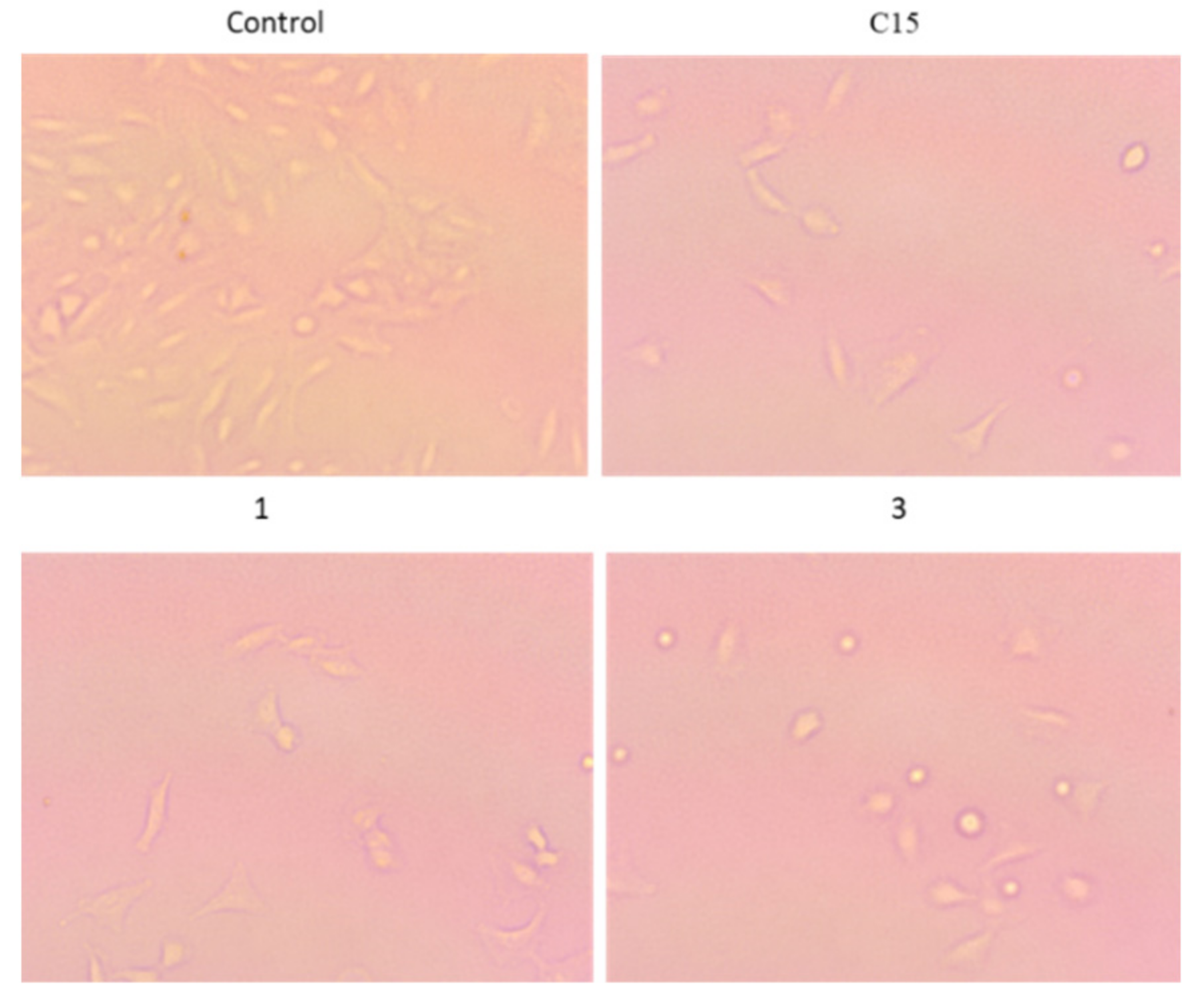
2.9. In Vitro Cytotoxicity of Compounds against A549 and HT29 Cancer Cells
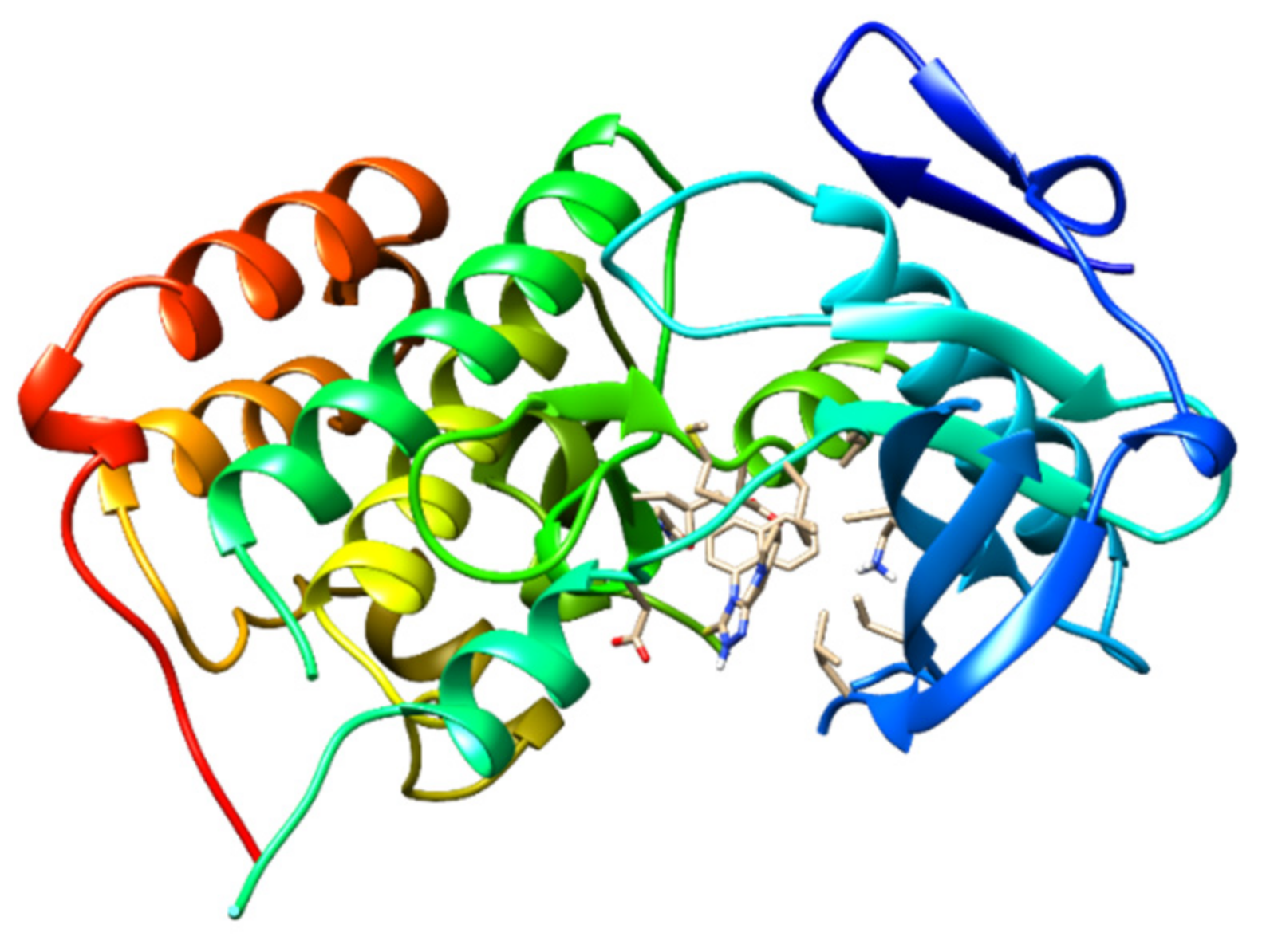
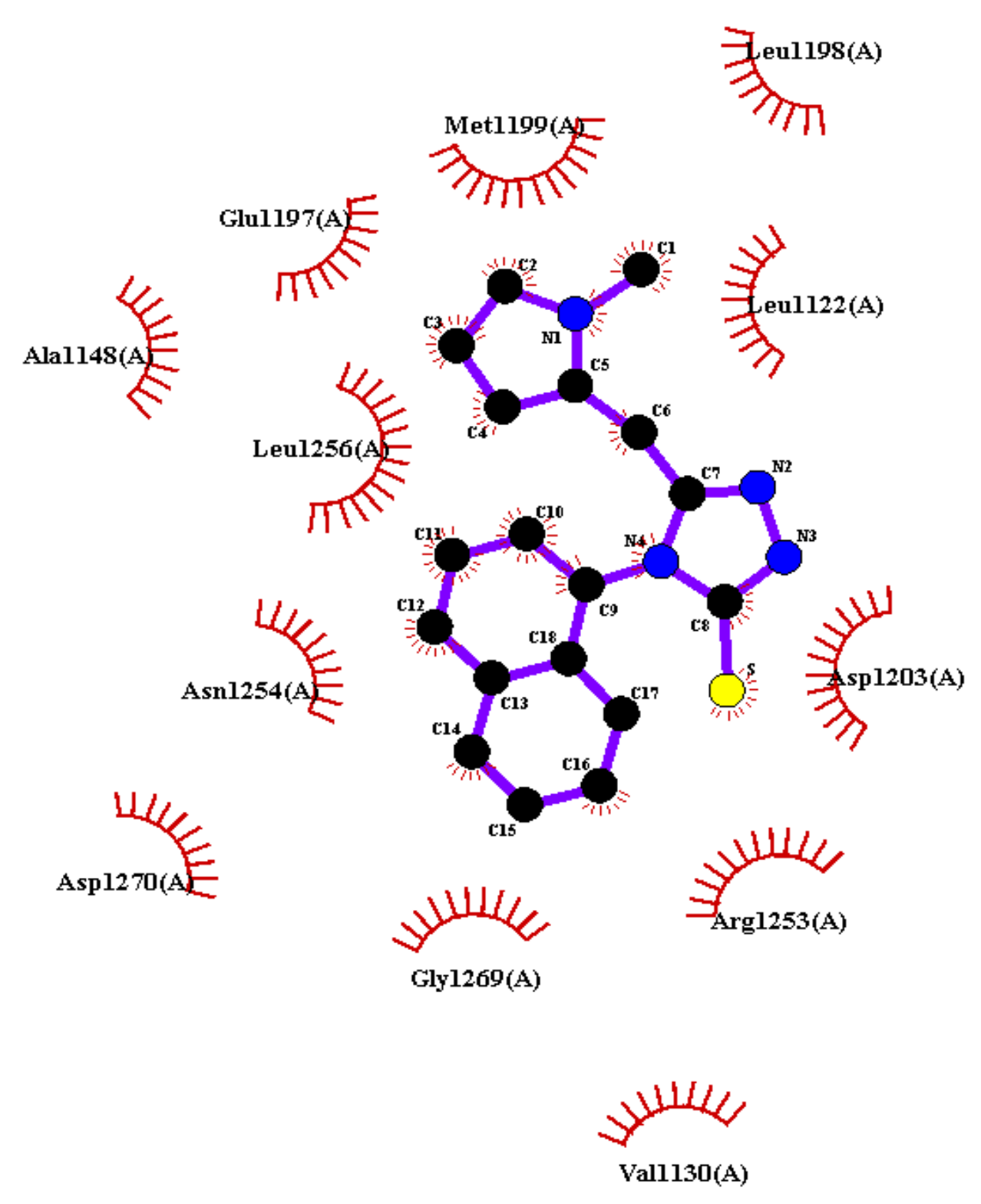
2.10. Molecular Docking
3. Materials and Methods
3.1. Synthesis of 2-(1-Methylpyrrol-2-yl)Acetohydrazide (A)
3.2. Synthesis of 1-(1-Methylpyrrol-2-yl)Acetyl-4-(1-Naphtyl) Thiosemicarbazide (B)
3.3. Synthesis of 5-((1-Methyl-Pyrrol-2-yl)Methyl)-4-(Naphthalen-1-yl)-1,2,4-Triazoline-3-Thione (C15)
3.4. Synthesis of the Metal(II) Complexes Containing Heterocyclic 4,5-Disbustituted 1,2,4-Triazole-3-Thione (1-5)
3.5. Chemistry
3.6. Inductively Coupled Plasma Analysis
3.7. Electron Paramagnetic Resonance Measurement
3.8. PXRD Analysis
3.9. ADMET Analysis
3.10. Cell Culture
3.11. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Hambley, T.W. Is anticancer drug development heading in the right direction? Cancer Res. 2009, 69, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S.; Thurston, D.E. Chemical approaches to the discovery and development of cancer therapies. Nat. Rev. Cancer 2005, 5, 285–296. [Google Scholar] [CrossRef]
- Orvig, C.; Abrams, M.J. Medicinal inorganic chemistry: Introduction. Chem. Rev. 1999, 99, 2201–2204. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Kaya, G.; Yekeler, H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J. Gastroenterol. 2007, 13, 612–618. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C. Boon and bane of metal ions in medicine. Science 2003, 300, 936–939. [Google Scholar] [CrossRef]
- Pitucha, M.; Korga-Plewko, A.; Czylkowska, A.; Rogalewicz, B.; Drozd, M.; Iwan, M.; Kubik, J.; Humeniuk, E.; Adamczuk, G.; Karczmarzyk, Z.; et al. Influence of complexation of thiosemicarbazone derivatives with Cu (II) ions on their antitumor activity against melanoma cells. Int. J. Mol. Sci. 2021, 22, 3104. [Google Scholar] [CrossRef]
- Raducka, A.; Czylkowska, A.; Gobis, K.; Czarnecka, K.; Szymański, P.; Świątkowski, M. Characterization of Metal-Bound Benzimidazole Derivatives, Effects on Tumor Cells of Lung Cancer. Materials 2021, 14, 2958. [Google Scholar] [CrossRef] [PubMed]
- Raducka, A.; Świątkowski, M.; Korona-Głowniak, I.; Kaproń, B.; Plech, T.; Szczesio, M.; Gobis, K.; Szynkowska-Jóźwik MI Czylkowska, A. Zinc Coordination Compounds with Benzimidazole Derivatives: Synthesis, Structure, Antimicrobial Activity and Potential Anticancer Application. Int. J. Mol. Sci. 2022, 23, 6595. [Google Scholar] [CrossRef] [PubMed]
- Murugesh, N.; Haribabu, J.; Arumugam, K.; Balachandran, C.; Swaathy, R.; Aoki, S.; Sreekanth, A.; Karvembu, R.; Vedachalam, S. NHC-catalyzed green synthesis of functionalized chromones: DFT mechanistic insights and: In vitro activities in cancer cells. New J. Chem. 2019, 43, 13509–13525. [Google Scholar] [CrossRef]
- Subhashree, G.R.; Haribabu, J.; Saranya, S.; Yuvaraj, P.; Anantha Krishnan, D.; Karvembu, R.; Gayathri, D. In vitro antioxidant, antiinflammatory and in silico molecular docking studies of thiosemicarbazones. J. Mol. Struct. 2017, 1145, 160–169. [Google Scholar] [CrossRef]
- Kalaiyarasi, A.; Haribabu, J.; Gayathri, D.; Gomathi, K.; Bhuvanesh, N.S.P.; Karvembu, R.; Biju, V.M. Chemosensing, molecular docking and antioxidant studies of 8-aminoquinoline appended acylthiourea derivatives. J. Mol. Struct. 2019, 1185, 450–460. [Google Scholar] [CrossRef]
- Kumar, P.; Udupi, R.; Dubey, P. Synthesis and biological study of substituted 1,3,4-oxadiazoles and 1,2,4-triazoles. Int. J. Pharm. Tech Res. 2009, 4, 1654–1662. [Google Scholar]
- Kaur, R.; Ranjan Dwivedi, A.; Kumar, B.; Kumar, V. Recent developments on 1, 2, 4-triazole nucleus in anticancer compounds: A review. Anticancer Agents Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef]
- Ivolgina, V.A.; Chernov’yants, M.S.; Popov, L.D.; Suslonov, V.V.; Avtushenko, N.A.; Luanguzov, N.V. Structural study and thermal behavior of novel interaction product of 4-amino-5-(furan-2-yl)-4H-1, 2, 4-triazole-3-thione with molecular iodine. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 421–428. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1, 2, 4-triazoles. Eur J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Collin, X.; Sauleau, A.; Coulon, J. 1, 2, 4-Triazolo mercapto and aminonitriles as potent antifungal agents. Bioorganic Med. Chem. Lett. 2003, 13, 2601–2605. [Google Scholar] [CrossRef]
- Pibiri, I.; Buscemi, S. A recent portrait of bioactive triazoles. Curr. Bioact. Compd. 2010, 6, 208–242. [Google Scholar] [CrossRef]
- Hemdan, M.M.; El-Sayed, A.A. Synthesis of Some New Heterocycles Derived from Novel 2-(1,3-Dioxisoindolin-2-yl)Benzoyl Isothiocyanate. J. Heterocycl. Chem. 2015, 53, 487–492. [Google Scholar] [CrossRef]
- Hemdan, M.M.; Fahmy, A.F.; Ali, N.F.; Hegazi, E.; Abd-Elhaleem, A. Synthesis of some new heterocycles derived from phenylacetyl isothiocyanate. Chin. J. Chem. 2008, 26, 388–391. [Google Scholar] [CrossRef]
- Hemdan, M.M.; Elshahawi, M.M. ChemInform Abstract: Synthesis of 1,2,4-Triazoles, Imidazoles, Pyrimidines, Quinazolines, 1,3,5-Triazines, and 1,3-Thiazines from 3-Oxo-5,6-diphenyl-2,3-dihydropyridazine-4-carbonyl Isothiocyanate (I). J. Chem. Res. 2009, 2, 75–77. [Google Scholar] [CrossRef]
- Hemdan, M.M.; El-Sayed, A.A.-E.; Hemdan, M.M.; El-Sayed, A.A.-E. Use of Phthalimidoacetyl isothiocyanate as a scaffold in the synthesis of target heterocyclic systems, and their antimicrobial assessment. Chem. Pharm. Bull. 2016, 64, 483–489. [Google Scholar] [CrossRef]
- Hemdan, M.M. Addition–cyclisation of 3-(2-thienyl)acryloyl isothiocyanate with hydrazine derivatives as a source of triazoles and thiadiazoles. J. Chem. Res. 2009, 8, 489–491. [Google Scholar] [CrossRef]
- Hemdan, M.M. Synthesis and antimicrobial activities of some heterocyclic systems from 2-furoyl isothiocyanate. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 620–627. [Google Scholar] [CrossRef]
- Hemdan, M.M.; Fahmy, A.F.M.; Aly, N.F.; Hegazi, I.A.; El-Sayed, A.A. Utility of phthalimidoacyl isothiocyanate in synthesis of quinazolines, benzoxazoles, benzimidazoles, 1, 2, 4-triazoles, and oxatriazepines. Phosphorus Sulfur Silicon Relat Elem. 2012, 187, 181–189. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Sachs, G.; Shin, J.M.; Howden, C.W. Review article: The clinical pharmacology of proton pump inhibitors. Aliment. Pharmacol. Ther. 2006, 23, 2–8. [Google Scholar] [CrossRef]
- Tkach, V.V.; Kushnir, M.V.; de Oliveira Sílvio, C.; Shevchenko, I.M.; Odyntsova, V.M.; Omelyanchik, V.M.; Luganska, O.V.; Kopiika, V.V.; Kormosh, Z.O.; Ivanushko, Y.G.; et al. Theoretical Description for Anti-COVID-19 Drug Molnupiravir Electrochemical Determination over the Poly-((1,2,4-triazole)-co-(squaraine dye)) Composite with Cobalt (III) Oxyhydroxide. Biointerface Res. Appl. Chem. 2022, 13, 74. [Google Scholar]
- El Ashry, E.S.H.; Farahat Mohamed, M.K.; Awad Laila, F.; Mahmoud, B.; Hoda, Y.; Badawy Mohamed, E.I.; Abd Al Moaty Mohamed, N.J. Synthesis, antibacterial, antioxidant, and molecular docking studies of 6-methylpyrimidin-4(3H)-one and oxo-1,2,4-triazolo [4,3-a]pyrimidine derivatives. Mol. Struct. 2022, 1259, 132733. [Google Scholar] [CrossRef]
- Alsaad, H.; Kubba, A.; Lubna, H.; Tahtamouni, A.; Hamzah, H. Synthesis, docking study, and structure activity relationship of novel anti-tumor 1, 2, 4 triazole derivatives incorporating 2-(2, 3-dimethyl aminobenzoic acid) moiety. Pharmacia 2022, 69, 415–428. [Google Scholar] [CrossRef]
- Demirbas, A.; Sahin, D.; Demirbas, N.; Karaoglu, S.A. Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Singh, V.; Pandey, D.D.; Maurya, R.K. Synthesis, characterization and antimicrobial Evaluation of some 1, 2, 4-triazole derivatives. Int. J. Pharm. Pharm. Sci. 2014, 6, 213–216. [Google Scholar]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1, 2, 4-Triazoles as Antifungal Agents. BioMed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansury, S.; Aboktifa, M.A.; Jassim, A.M.; Balakit, A.A.; Alkazazz, F.F. Evaluation the Antioxidant Enzymes Activity in Adults Male Rats Treated with Some New 3-mercapto1, 2, 4-triazole Derivatives. Res. J. Pharm. Technol. 2022, 15, 224–228. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J. The Antibacterial Activity of 1, 2, 3-triazole-and 1, 2, 4-Triazole-containing Hybrids against Staphylococcus aureus: An Updated Review (2020-Present). Curr. Top. Med. Chem. 2022, 22, 41–63. [Google Scholar] [CrossRef]
- Hou, Y.P.; Sun, J.; Pang, Z.H.; Lv, P.C.; Li, D.D.; Yan, L.; Zhang, H.J.; Zheng, E.X.; Zhao, J.; Zhu, H.L. Synthesis and antitumor activity of 1,2,4-triazoles having 1,4-benzodioxan fragment as a novel class of potent methionine aminopeptidase type II inhibitors. Bioorganic Med. Chem. 2011, 19, 5948–5954. [Google Scholar] [CrossRef]
- Lin, R.; Connolly, P.J.; Huang, S.; Wetter, S.K.; Lu, Y.; Murray, W.V.; Emanuel, S.L.; Gruninger, R.H.; Fuentes-Pesquera, A.R.; Rugg, C.A.; et al. 1-Acyl-1H-[1,2,4]triazole-3,5-diamine analogues as novel and potent anticancer cyclin-dependent kinase inhibitors: Synthesis and evaluation of biological activities. J. Med. Chem. 2005, 48, 4208–4211. [Google Scholar] [CrossRef]
- Saad, H.A.; Osman, N.A.; Moustafa, A.H. Synthesis and analgesic activity of some new pyrazoles and triazoles bearing a 6,8-dibromo-2-methylquinazoline moiety. Molecules 2011, 16, 10187–10201. [Google Scholar] [CrossRef] [PubMed]
- Kumidha, J.T.D.; Leonard, M.; Muthumani, N.; Chidhambaranathan, T.K. Synthesis and evaluation of some 1, 2, 4-triazole derivatives as anticonvulsant, anti-inflammatory and antimicrobial agents. Asian J. Pharm. Clin. 2013, 6, 5. [Google Scholar]
- Shashikala, M.D.K.; Ramaiah, G.K.; Vanita, K.; Veena, V.P.V. Synthesis and analgesic activity of triazolothiadiazoles and triazolothiadiazines encompassing 3–nitronaphtho[2,1-b]furan. Chem. Pharm. Res. 2011, 3, 445. [Google Scholar]
- Murty, M.S.R.; Ram, K.R.; Rao, B.R.; Rao, R.V.; Katiki, M.R.; Rao, J.V.; Pamanji, R.; Velatooru, L.R. Synthesis, characterization, and anticancer studies of S and N alkyl piperazine-substituted positional isomers of 1,2,4-triazole derivatives. Med. Chem. Res. 2014, 23, 1661–1671. [Google Scholar] [CrossRef]
- Cao, X.; Wang, W.; Wang, S.; Bao, L. Asymmetric synthesis of novel triazole derivatives and their in vitro antiviral activity and mechanism of action. Eur. J. Med. Chem. 2017, 139, 718–725. [Google Scholar] [CrossRef]
- Singh, R.; Kashaw, S.K.; Mishra, V.K.; Mishra, M.; Rajoriya, V.; Kashaw, V. Design and synthesis of new bioactive 1,2,4-triazoles, potential antitubercular and antimicrobial agents. Indian J. Pharm. Sci. 2018, 80, 36–45. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Keivanloo, A.; Fakharian, M.; Sepehri, S. 1,2,3-Triazoles based 3-substituted 2-thioquinoxalines: Synthesis, anti-bacterial activities, and molecular docking studies. J. Mol. Struct. 2020, 1202, 127262. [Google Scholar] [CrossRef]
- Haegler, P.; Joerin, L.; Krähenbühl, S.; Bouitbir, J. Hepatocellular toxicity of imidazole and triazole antimycotic agents. Toxicol. Sci. 2017, 157, 183–195. [Google Scholar] [CrossRef]
- Foroumadi, A.; Mansouri, S.; Kiani, Z.; Rahmani, A. Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl] piperazinyl quinolones. Eur. J. Med. Chem. 2003, 38, 851–854. [Google Scholar] [CrossRef]
- Zmejkovski, B.B.; Savić, A.; Poljarević, J.; Pantelić, N.; Aranđelović, S.; Radulović, S.; Sabo, T.J. Synthesis, characterization and in vitro antitumor activity of new palladium (II) complexes with (S, S)-R2edda-type esters. Polyhedron 2014, 80, 106–111. [Google Scholar] [CrossRef]
- Aguilà, D.; Escribano, E.; Speed, S.; Talancón, D.; Yermán, L.; Alvarez, S. Calibrating the coordination chemistry tool chest: Metrics of bi-and tridentate ligands. Dalton Trans. 2009, 33, 6610. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A.L.; Bosnich, B. Principles of mononucleating and binucleating ligand design. Chem. Rev. 2004, 104, 349–384. [Google Scholar] [CrossRef] [PubMed]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Ping Dou, Q. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, P.; Korga-Plewko, A.; Karczmarzyk, Z.; Hawrył, A.; Wysocki, W.; Człapski, M.; Iwan, M.; Ostrowska-Leśko, M.; Fornal, E.; Pitucha, M. Potential Anticancer Agents against Melanoma Cells Based on an As-Synthesized Thiosemicarbazide Derivative. Biomolecules 2022, 12, 151. [Google Scholar] [CrossRef]
- Pitucha, M.; Korga-Plewko, A.; Kozyra, P.; Iwan, M.; Kaczor, A.A. 2, 4-Dichlorophenoxyacetic Thiosemicarbazides as a new class of compounds against stomach cancer potentially intercalating with DNA. Biomolecules 2020, 10, 296. [Google Scholar] [CrossRef]
- Bharty, M.K.; Bharti, A.; Chaurasia, R.; Chaudhari, U.K.; Kushawaha, S.K.; Sonkar, P.K.; Ganesan, V.; Butcher, J.R. Synthesis and characterization of Mn (II) complexes of 4-phenyl (phenyl-acetyl)-3-thiosemicarbazide, 4-amino-5-phenyl-1, 2, 4-triazole-3-thiolate, and their application towards electrochemical oxygen reduction reaction. Polyhedron 2019, 173, 114125. [Google Scholar] [CrossRef]
- Paqhaleh, D.M.S.; Aminjanov, A.A.; Amani, V.J. Discrete and polymeric lead (II) complexes containing 4-methyl-1, 2, 4-triazole-3-thiol ligand: X-ray studies, spectroscopic characterization, and thermal analyses. Inorg. Organomet. Polym. 2014, 24, 340–346. [Google Scholar] [CrossRef]
- Kahn, O. Introduction to Molecular Magnetism; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2015; p. 396. [Google Scholar]
- Sivasankar Reddy, M.; Prathima, B.; Saraswathi, M.; Babu, S.; Sarala, Y.; Varada Reddy, A.J. Synthesis, spectral aspects and biological activities of 5-hydroxy-2- nitrobenzaldehydethiosemicarbazone and their Mn(II), Co(II) and Ni(II) complexes. Appl. Pharm. Sci. 2016, 6, 90–96. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Vlček, A.J. Electronic structure of Bis(o-iminobenzo-semiquinonato)metal complexes (Cu, Ni, Pd): The art of establishing physical oxidation states in transition-metal complexes containing radical ligands. Chemtracts 2001, 14, 683–689. [Google Scholar]
- Rahman, K.N.A.; Haribabu, J.; Balachandran, C.; Bhuvanesh, N.S.P.; Karvembu, R.; Sreekanth, A. Copper, nickel and zinc complexes of 3-acetyl coumarin thiosemicarbazone: Synthesis, characterization and in vitro evaluation of cy-totoxicity and DNA/protein binding properties. Polyhedron 2017, 135, 26–35. [Google Scholar] [CrossRef]
- Haribabu, J.; Alajrawy, O.I.; Jeyalakshmi, K.; Balachandran, C.; Krishnan, D.A.; Bhuvanesh, N.; Aoki, S.; Natarajan, K.; Karvembu, R. N-substitution in isatin thiosemicarbazones decides nuclearity of Cu(II) complexes—Spectroscopic, molecular docking and cytotoxic studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118963. [Google Scholar] [CrossRef]
- Yvonne, C.; Martin, A. Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D.J. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Haribabu, J.; Srividya, S.; Mahendiran, D.; Gayathri, D.; Venkatramu, V.; Bhuvanesh, N.; Karvembu, R. Synthesis of palladium (II) complexes via Michael addition: Antiproliferative effects through ROS-mediated mitochondrial apoptosis and docking with SARS-CoV-2. Inorg. Chem. 2020, 59, 17109–17122. [Google Scholar] [CrossRef]
- Haribabu, J.; Tamura, Y.; Yokoi, K.; Balachandran, C.; Umezawa, M.; Tsuchiya, K.; Yamada, Y.; Karvembu, R. Synthesis and Anticancer Properties of Bis- and Mono(cationic peptide) Hybrids of Cyclometalated Iridium(III) Complexes: Effect of the Number of Peptide Units on Anticancer Activity. Eur. J. Inorg. Chem. 2021, 2021, 1709–1814. [Google Scholar] [CrossRef]
- Kaplan, A.; Akalin, C.G.; Kutlu, M.P. Titanyum Dioksitin A549 Hücreleri Üzerindeki Apoptotik Etkileri. Tumor Biol. 2017, 1–12. [Google Scholar]
- Lovejoy, K.A.; Serova, M.; Bieche, I.; Emami, S.; D’Incalci, M.; Broggini, M.; Erba, E.; Gespach, C.; Cvitkovic, E.; Faivre, S.; et al. Spectrum of Cellular Responses to Pyriplatin, a Monofunctional Cationic Antineoplastic Platinum(II) Compound, in Human Cancer Cells. Mol. Cancer Ter. 2011, 10, 1709–1719. [Google Scholar]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R.J. Development and validation of a genetic algorithm for flexible docking. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Jeya, R.A.; Balachandran, C.; Stalin, A.; Saravanan, R.; Loganathan, K.; Chennakesava, R.K.; Suresh, A.; Hiteshkumar, B.N. Synthesis of novel β-amino alcohols from phenylacetylcarbinol: Cytotoxicity activity against A549 cells and molecular docking. Res. Chem. Intermed. 2018, 44, 535–552. [Google Scholar]
- Pitucha, M.; Wujec, M.D. Synthesis of new derivatives of 3- (1-methylpyrrole-2-ylmethyl) -4-substituted-1,2,4-triazolin-5-thione. Ann. UMCS Lublin. 2004, 59, 144–153. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Kłosiński, K.; Girek, M.; Czarnecka, K.; Pasieka, Z.; Skibiński, R.; Szymański, P. Biological assessment of new tetrahydroacridine derivatives with fluorobenzoic moiety in vitro on A549 and HT-29 cell lines and in vivo on animal model. Hum. Cell 2020, 33, 859–867. [Google Scholar] [CrossRef]
- Girek, M.; Kłosiński, K.; Grobelski, B.; Pizzimenti, S.; Cucci, M.A.; Daga, M.; Barrera, G.; Pasieka, Z.; Czarnecka, K.; Szymański, P. Novel tetrahydroacridine derivatives with iodobenzoic moieties induce G0/G1 cell cycle arrest and apoptosis in A549 non-small lung cancer and HT-29 colorectal cancer cells. Mol. Cell. Biochem. 2019, 460, 123–150. [Google Scholar] [CrossRef] [PubMed]


| Complex | g-Factor | Profile |
|---|---|---|
| (1) | giso = 2.021 | Gauss |
| (2) | giso = 2.016 | Gauss |
| (3) | giso = 2.26 | Gauss |
| (4) | [g⊥, g||] = [2.05, 2.24] | Gauss |
| (5) | giso = 2.003 | Lorentz |
| Pa | Pi | Cell-Line Name | Tissue/Organ |
|---|---|---|---|
| 0.526 | 0.004 | Pancreatic carcinoma | Pancreas |
| 0.518 | 0.018 | Renal carcinoma | Kidney |
| 0.365 | 0.007 | Urothelial bladder carcinoma | Urinary tract |
| 0.362 | 0.061 | Colon carcinoma | Colon |
| 0.295 | 0.084 | Leukemic T-cells | Blood |
| 0.289 | 0.088 | Adult immunoblastic lymphoma | Haematopoietic and lymphoid tissue |
| 0.188 | 0.004 | Bladder carcinoma | Urinary tract |
| 0.297 | 0.125 | Glioblastoma | Brain |
| 0.282 | 0.139 | Melanoma | Skin |
| 0.250 | 0.156 | Non-small cell lung carcinoma | Lung |
| 0.238 | 0.146 | Non-small cell lung carcinoma | Lung |
| 0.229 | 0.153 | Colon adenocarcinoma | Colon |
| Compound | IC50 against A549 (µM) | IC50 against HT29 (µM) |
|---|---|---|
| C15 | 1092.35 ± 124.84 | 1058.02 ± 87.39 |
| (1) | 794.37 ± 83.62 | 654.31 ± 25.09 |
| (2) | no activity | no active |
| (3) | no activity | 1064.05 ± 43.56 |
| (4) | no activity | no activity |
| (5) | no activity | no activity |
| Etoposide | 451.47 ± 18.27 | 654.03 ± 39.51 |
| 5-fluorouracil | >1800 | 1626.85 ± 49.26 |
| Cisplatin | 31.25 ± 1.76 [74] | 11.9 ± 4.1 [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czylkowska, A.; Lanka, S.; Szczesio, M.; Czarnecka, K.; Szymański, P.; Pitucha, M.; Drabińska, A.; Camargo, B.C.; Szczytko, J. New Derivatives of 5-((1-Methyl-Pyrrol-2-yl) Methyl)-4-(Naphthalen-1-yl)-1,2,4-Triazoline-3-Thione and Its Coordination Compounds with Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 9162. https://doi.org/10.3390/ijms23169162
Czylkowska A, Lanka S, Szczesio M, Czarnecka K, Szymański P, Pitucha M, Drabińska A, Camargo BC, Szczytko J. New Derivatives of 5-((1-Methyl-Pyrrol-2-yl) Methyl)-4-(Naphthalen-1-yl)-1,2,4-Triazoline-3-Thione and Its Coordination Compounds with Anticancer Activity. International Journal of Molecular Sciences. 2022; 23(16):9162. https://doi.org/10.3390/ijms23169162
Chicago/Turabian StyleCzylkowska, Agnieszka, Suneel Lanka, Małgorzata Szczesio, Kamila Czarnecka, Paweł Szymański, Monika Pitucha, Aneta Drabińska, Bruno Cury Camargo, and Jacek Szczytko. 2022. "New Derivatives of 5-((1-Methyl-Pyrrol-2-yl) Methyl)-4-(Naphthalen-1-yl)-1,2,4-Triazoline-3-Thione and Its Coordination Compounds with Anticancer Activity" International Journal of Molecular Sciences 23, no. 16: 9162. https://doi.org/10.3390/ijms23169162
APA StyleCzylkowska, A., Lanka, S., Szczesio, M., Czarnecka, K., Szymański, P., Pitucha, M., Drabińska, A., Camargo, B. C., & Szczytko, J. (2022). New Derivatives of 5-((1-Methyl-Pyrrol-2-yl) Methyl)-4-(Naphthalen-1-yl)-1,2,4-Triazoline-3-Thione and Its Coordination Compounds with Anticancer Activity. International Journal of Molecular Sciences, 23(16), 9162. https://doi.org/10.3390/ijms23169162







