Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure Analysis
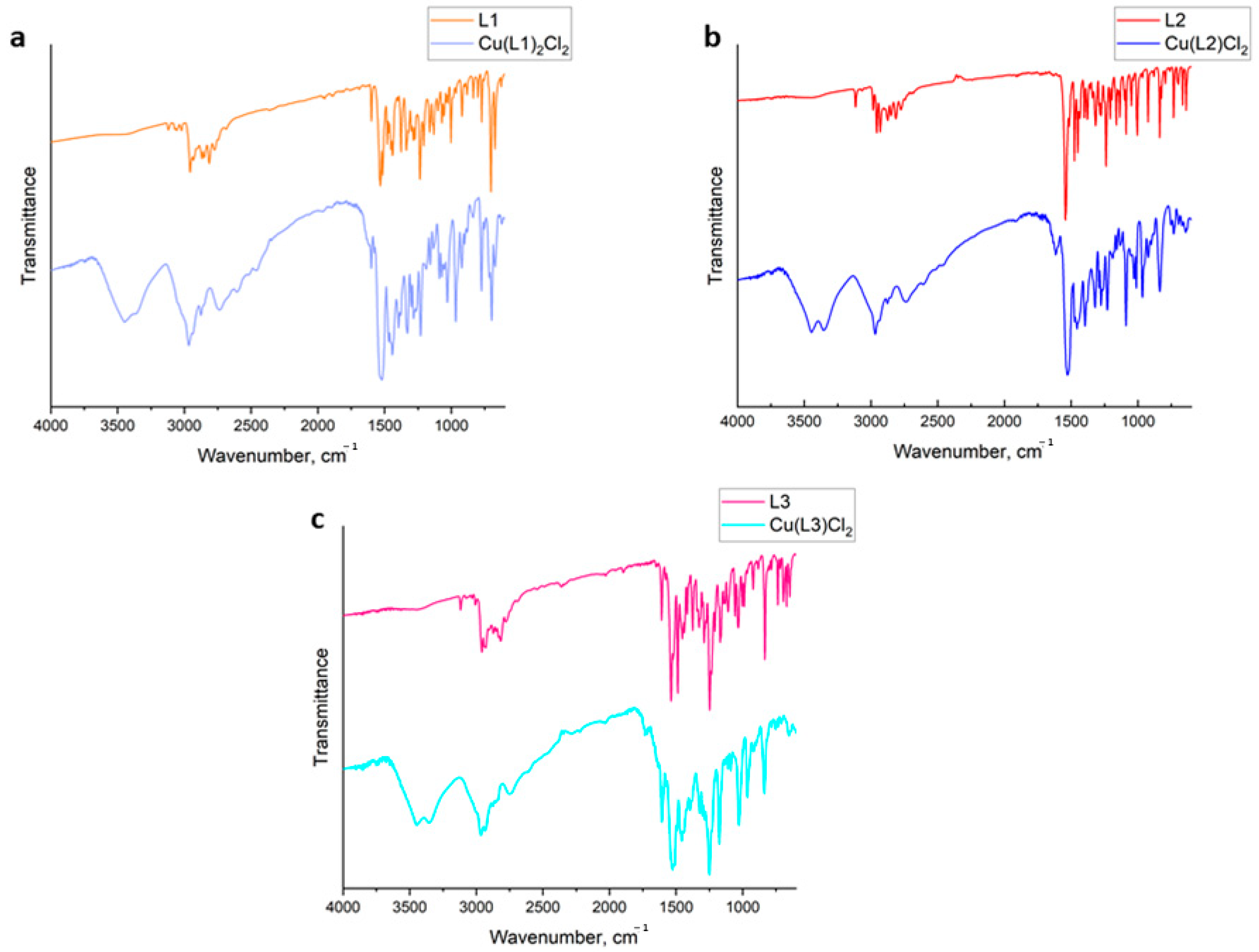
2.2. FTIR Spectra
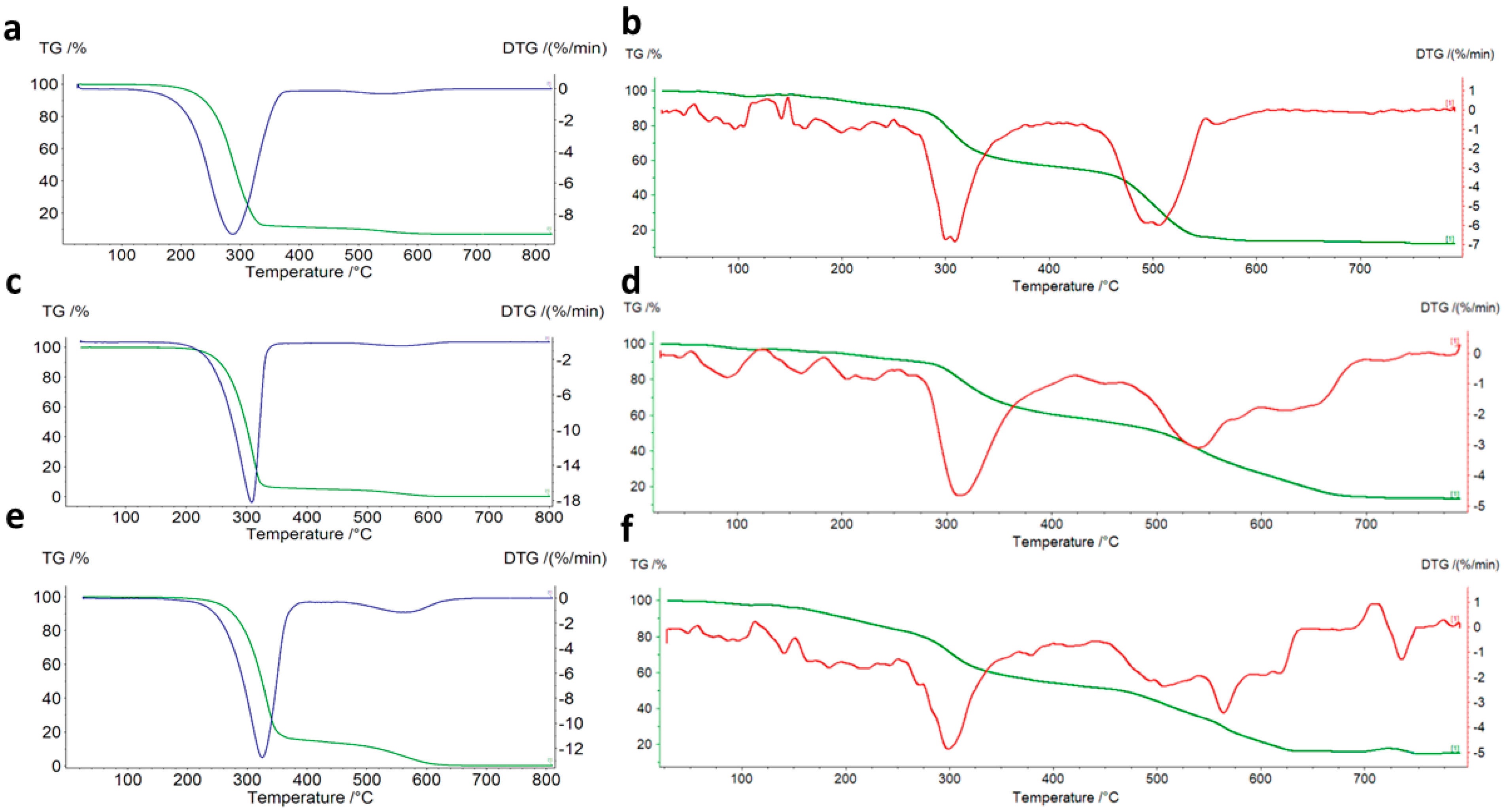
2.3. Thermogravimetric Study
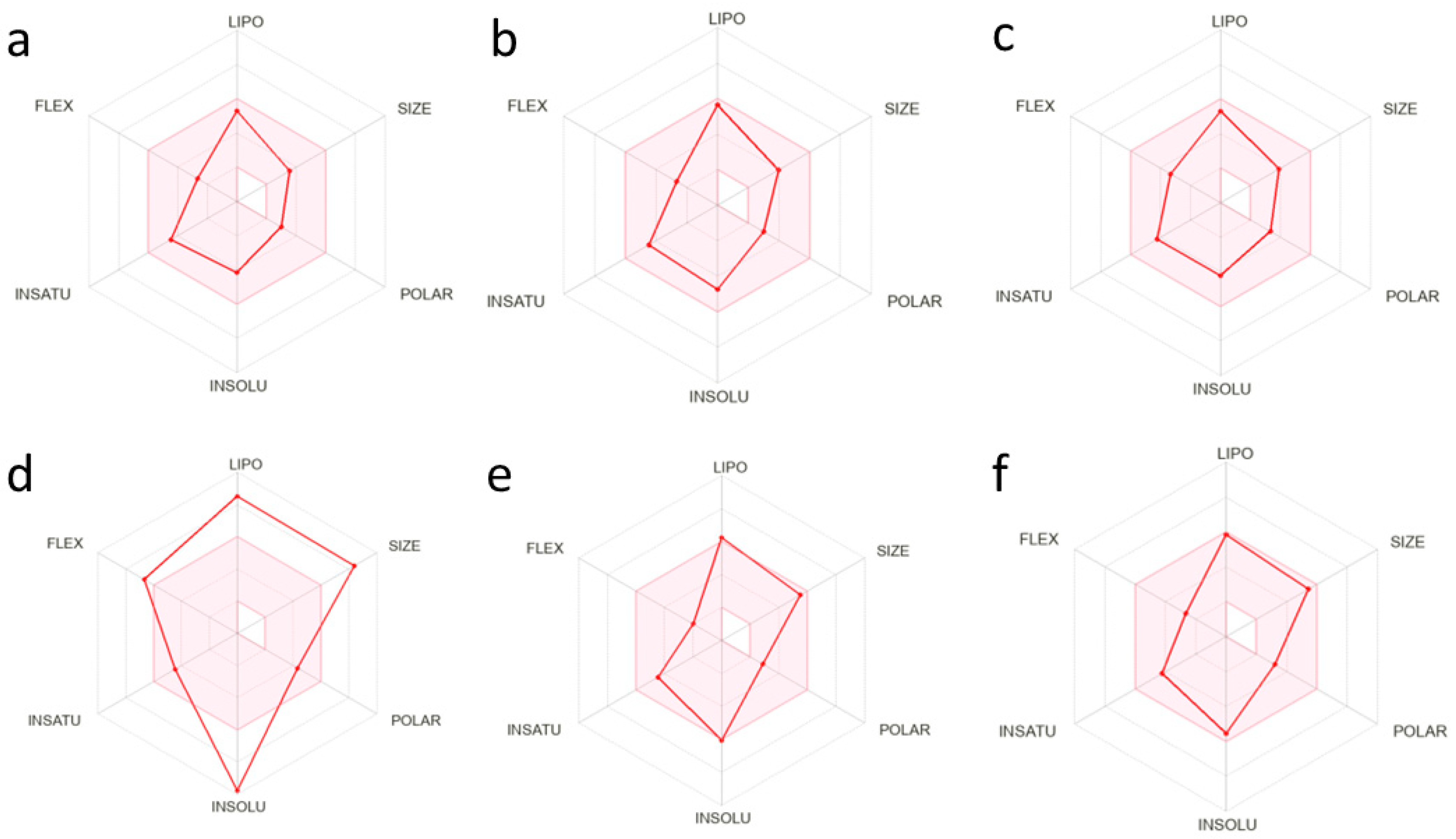
2.4. Biological Activity Predictions
2.5. ADME Analysis
2.6. Antimicrobial Activity
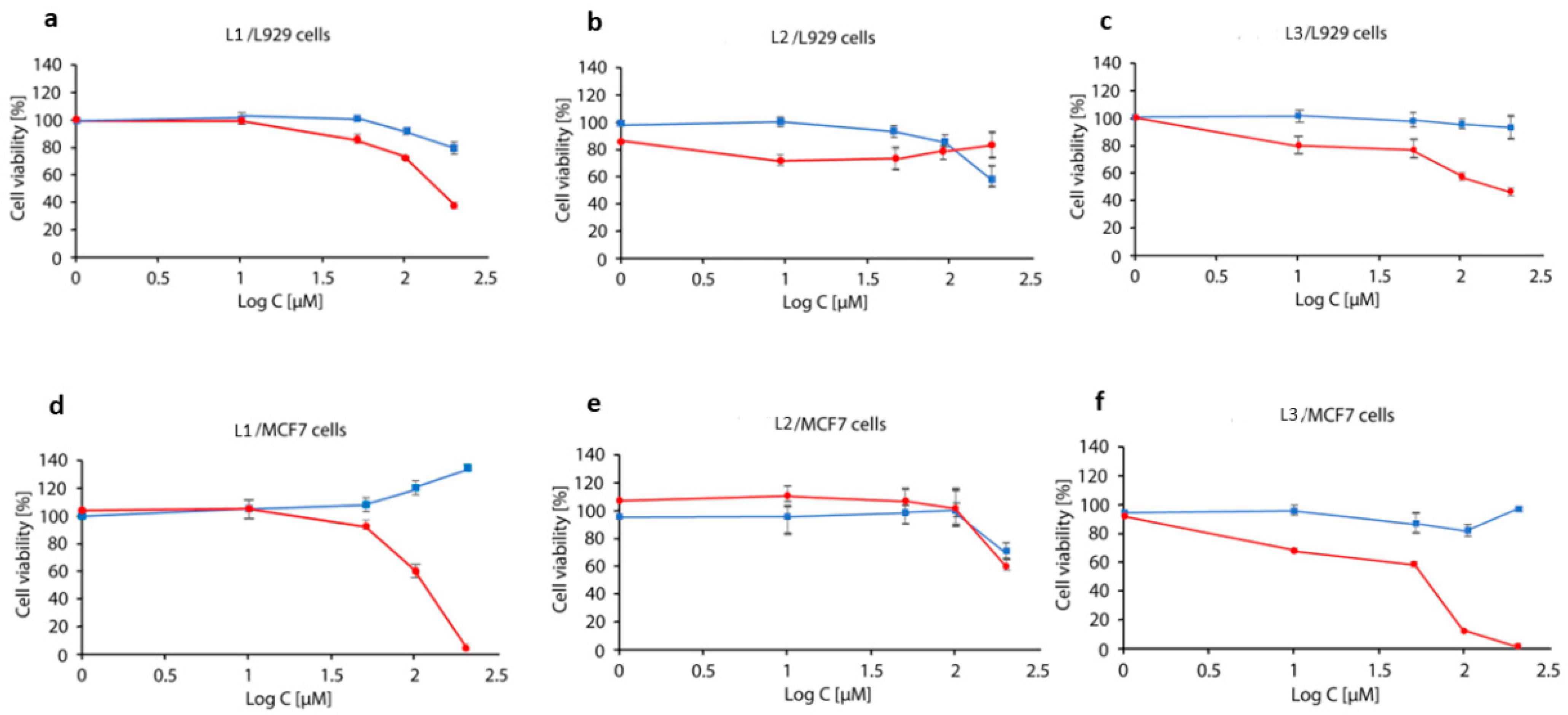
2.7. MTT Analysis
3. Materials and Methods
3.1. Chemistry
3.1.1. 1H, and 13C NMR
3.1.2. Flame Atomic Absorption Spectroscopy
3.1.3. Fourier-Transform Infrared Spectroscopy
3.1.4. Thermogravimetric Analysis
3.1.5. X-ray Measurement and Crystal Structure Determination
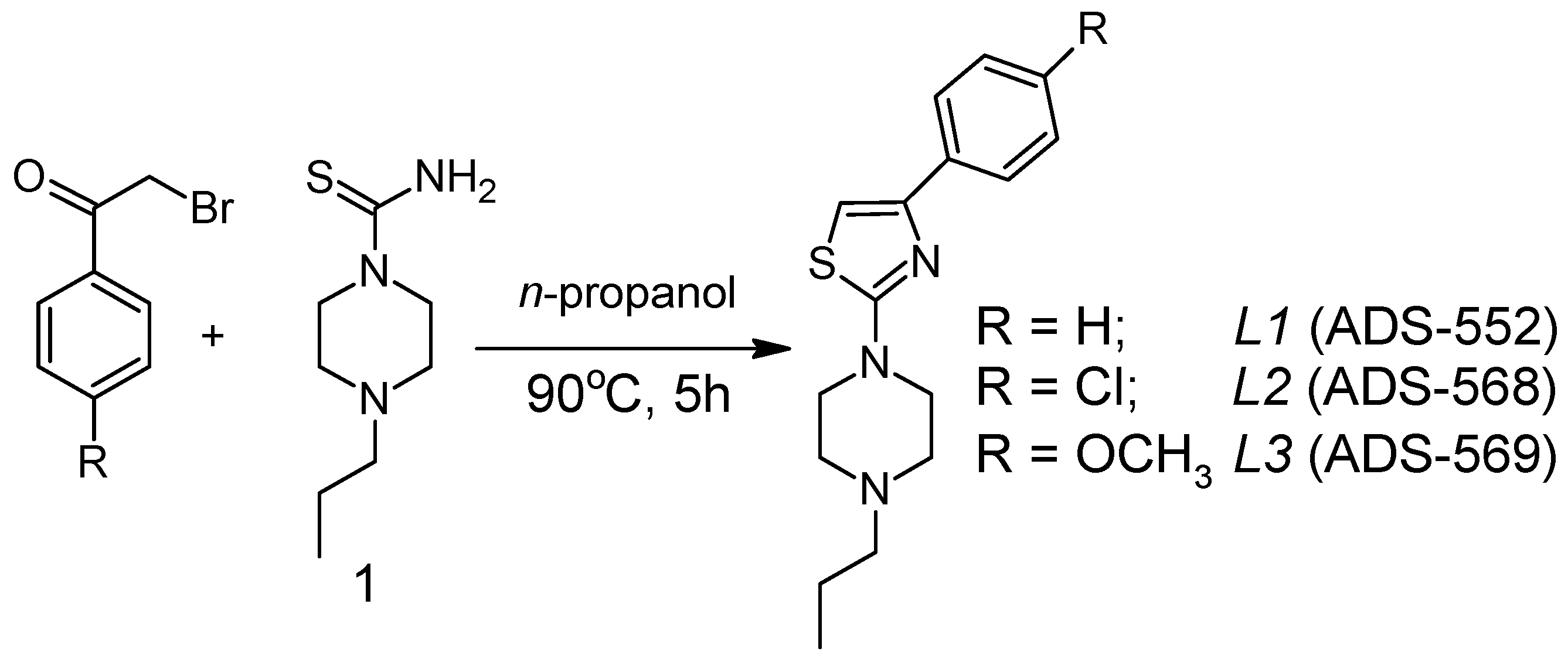
3.2. Synthesis of Organic Ligands (L1-L3)
3.3. Synthesis of Coordination Compounds
3.4. Biological Activity Predictions
3.5. ADME Analysis
3.6. Microbiology
3.7. Cell Viability Analysis by MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Hum. Fungal Pathog. Identif. 2017, 1508, 17–65. [Google Scholar] [CrossRef]
- Ravikant, K.T.; Gupte, S.; Kaur, M. A review on emerging fungal infections and their significance. J. Bacteriol. Mycol. 2015, 1, 39–41. [Google Scholar]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis, Characterization and Antimicrobial Activities of Some Thiazole Derivatives. Am. J. Org. Chem. 2012, 2, 69–73. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; El-Ghani, G.E.A.; Khalil, A.M. Synthesis and Antimicrobial Activities of Some New Thiazole and Pyrazole Derivatives Based on 4,5,6,7-Tetrahydrobenzothiophene Moiety. Eur. J. Med. Chem. 2010, 45, 1338–1345. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdallah, M.A.; Khedr, M.; Mahmoud, H.K. Synthesis, Antimicrobial Activity and Molecular Docking Study of Thiazole Derivatives. J. Heterocycl. Chem. 2017, 54, 2417–2425. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, P.K.; Verma, P.K.; Tomer, I.; Mathur, G.; Dhakad, P.K. Biological potential of thiazole derivatives of syn-thetic origin. J. Heterocycl. Chem. 2017, 54, 2103–2116. [Google Scholar]
- Edelsberg, J.; Weycker, D.; Barron, R.; Li, X.; Wu, H.; Oster, G.; Badre, S.; Langeberg, W.J.; Weber, D.J. Prevalence of antibiotic resistance in US hospitals. Diagn. Microbiol. Infect. Dis. 2014, 78, 255–262. [Google Scholar] [CrossRef]
- Bou-Antoun, S.; Davies, J.; Guy, R.; Johnson, A.P.; A Sheridan, E.; Hope, R. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Eurosurveillance 2016, 21, 30329. [Google Scholar] [CrossRef]
- Public Health England. Voluntary Surveillance of Staphylococcus aureus Bacteraemia in England, Wales and Northern Ireland: 2007–2014; Public Health England: London, UK, 2015; Volume 9. [Google Scholar]
- de Siqueira, L.R.P.; Gomes, P.A.T.D.M.; Ferreira, L.P.D.L.; Rêgo, M.J.B.D.M.; Leite, A.C.L. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019, 170, 237–260. [Google Scholar] [CrossRef]
- Desai, N.; Joshi, V.; Rajpara, K.; Vaghani, H.; Satodiya, H. Facile synthesis of novel fluorine containing pyrazole based thiazole derivatives and evaluation of antimicrobial activity. J. Fluor. Chem. 2012, 142, 67–78. [Google Scholar] [CrossRef]
- Chhabria, T.M.; Patel, S.; Modi, P.; Brahmkshatriya, S.P. Thiazole: A review on chemistry, synthesis and therapeutic importance of its derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [Google Scholar] [PubMed]
- Gomha, S.M.; Abdelaziz, M.R.; Abdel-Aziz, H.M.; Hassan, S.A. Green Synthesis and Molecular Docking of Thiazolyl-thiazole Derivatives as Potential Cytotoxic Agents. Mini Rev. Med. Chem. 2017, 17, 805–815. [Google Scholar] [CrossRef]
- Pinedo, H.M.; Schornagel, J.H. Platinum and Other Metal Coordination Compounds in Cancer Chemotherapy 2; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Mohareb, R.M.; Abdallah, A.E.M.; Ahmed, E.A. Synthesis and cytotoxicity evaluation of thiazole derivatives obtained from 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene- 3-carbonitrile. Acta Pharm. 2017, 67, 495–510. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, W.; Lu, W.; Xu, L.; Yang, S.; Cai, X.M.; Cao, F. High anticancer potency on tumor cells of dehydroabietylamine Schiff-base derivatives and a copper (II) complex. Eur. J. Med. Chem. 2018, 146, 451–459. [Google Scholar]
- Martinez-Bulit, P.; Garza-Ortiz, A.; Mijangos, E.; Barron-Sosa, L.; Sanchez-Bartez, F.; Gracia-Mora, I.; Barba-Behrens, N. 2, 6-Bis (2, 6-diethylphenyliminomethyl) pyridine coordination compounds with cobalt (II), nickel (II), copper (II), and zinc (II): Synthesis, spectroscopic characterization, X-ray study and in vitro cytotoxicity. J. Inorg. Biochem. 2015, 142, 1–7. [Google Scholar]
- Pellei, M.; Bagnarelli, L.; Luciani, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; De Franco, M.; Gandin, V.; Marzano, C.; et al. Synthesis and Cytotoxic Activity Evaluation of New Cu(I) Complexes of Bis(pyrazol-1-yl) Acetate Ligands Functionalized with an NMDA Receptor Antagonist. Int. J. Mol. Sci. 2020, 21, 2616. [Google Scholar] [CrossRef]
- Carlo, S.; Maura, P.; Valentina, G. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar]
- Gandin, V.; Trenti, A.; Porchia, M.; Tisato, F.; Giorgetti, M.; Zanusso, I.; Marzano, C. Homoleptic phosphino copper (I) com-plexes with in vitro and in vivo dual cytotoxic and anti-angiogenic activity. Metallomics 2015, 7, 1497–1507. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar]
- Rozenberg, M.; Shoham, G. FTIR spectra of solid poly-l-lysine in the stretching NH mode range. Biophys. Chem. 2007, 125, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Abu-Yamin, A.-A.; Abduh, M.S.; Saghir, S.A.M.; Al-Gabri, N. Synthesis, Characterization and Biological Activities of New Schiff Base Compound and Its Lanthanide Complexes. Pharmaceuticals 2022, 15, 454. [Google Scholar] [CrossRef]
- Zhou, S.; Shen, S.; Zhao, D.; Zhang, Z.; Yan, S. Evaporation and decomposition of eutectics of cupric chloride and sodium chloride. J. Therm. Anal. 2017, 129, 1445–1452. [Google Scholar] [CrossRef]
- Kowalska, M.; Fijałkowski, Ł.; Nowaczyk, A. The biological activity assessment of potential drugs acting on cardiovascular system using Lipinski and Veber Rules. J. Educ. Health Sport 2018, 8, 184–191. [Google Scholar]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol Microbial Metabolites Exhibit Gut and Blood–Brain Barrier Permeability and Protect Murine Microglia against LPS-Induced Inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef]
- Ansel, H.C.; Norred, W.P.; Roth, I.L. Antimicrobial Activity of Dimethyl Sulfoxide against Escherichia coli, Pseudomonas aeruginosa, and Bacillus megaterium. J. Pharm. Sci. 1969, 58, 836–839. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Frymarkiewicz, A.; Walczyński, K. Non-imidazole histamine H3 ligands, part IV: SAR of 1-[2-thiazol-5-yl-(2-aminoethyl)]-4-npropylpiperazine derivatives. Eur. J. Med. Chem. 2009, 44, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Guryn, R.; Staszewski, M.; Walczyński, K. Non-imidazole histamine H3 ligands: Part V. synthesis and preliminary pharmacological investigation of 1-[2-thiazol-4-yl-and 1-[2-thiazol-5-yl-(2-aminoethyl)]-4-n-propylpiperazine derivatives. Med. Chem. Res. 2013, 22, 3640–3652. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Kassel, D.B. Applications of high-throughput ADME in drug discovery. Curr. Opin. Chem. Biol. 2004, 8, 339–345. [Google Scholar] [CrossRef]
- Ekins, S.; Bugrim, A.; Brovold, L.; Kirillov, E.; Nikolsky, Y.; Rakhmatulin, E.; Nikolskaya, T. Algorithms for network analysis in systems-ADME/Tox using the MetaCore and MetaDrug platforms. Xenobiotica 2006, 36, 877–901. [Google Scholar] [CrossRef]
- Shou, W.Z. Current status and future directions of high-throughput ADME screening in drug discovery. J. Pharm. Anal. 2020, 10, 201–208. [Google Scholar] [CrossRef]
- Tetko, I.V.; Bruneau, P.; Mewes, H.W.; Rohrer, D.C.; Poda, G.I. Can we estimate the accuracy of ADME–Tox predictions? Drug Discov. Today 2006, 11, 700–707. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria that Grow Aerobically; Approved Standard—Ninth Edition 2012; Clinical and Laboratory Standards Institute (CLSI): Pennsylvania, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standards-Second Edition, in CLSI document M27-2A 2002, CLSI Pennsylvania, USA; Clinical and Laboratory Standards Institute (CLSI): Pennsylvania, PA, USA, 2002. [Google Scholar]
- Abu-Melha, S.; Edrees, M.M.; Salem, H.H.; Kheder, N.A.; Gomha, S.M.; Abdelaziz, M.R. Synthesis and Biological Evaluation of Some Novel Thiazole-Based Heterocycles as Potential Anticancer and Antimicrobial Agents. Molecules 2019, 24, 539. [Google Scholar] [CrossRef] [Green Version]
- Sujatha, K.; Vedula, R.R. Novel one-pot expeditious synthesis of 2,4-disubstituted thiazoles through a three-component reaction under solvent free conditions. Synth. Commun. 2018, 48, 302–308. [Google Scholar] [CrossRef]
- Kumar, S.; Aggarwal, R. Thiazole: A Privileged Motif in Marine Natural Products. Mini Rev. Org. Chem. 2019, 16, 26–34. [Google Scholar] [CrossRef]
- Nayak, S.; Gaonkar, S.L. A Review on Recent Synthetic Strategies and Pharmacological Importance of 1,3-Thiazole Derivatives. Mini. Rev. Med. Chem. 2019, 19, 215–238. [Google Scholar] [CrossRef] [PubMed]
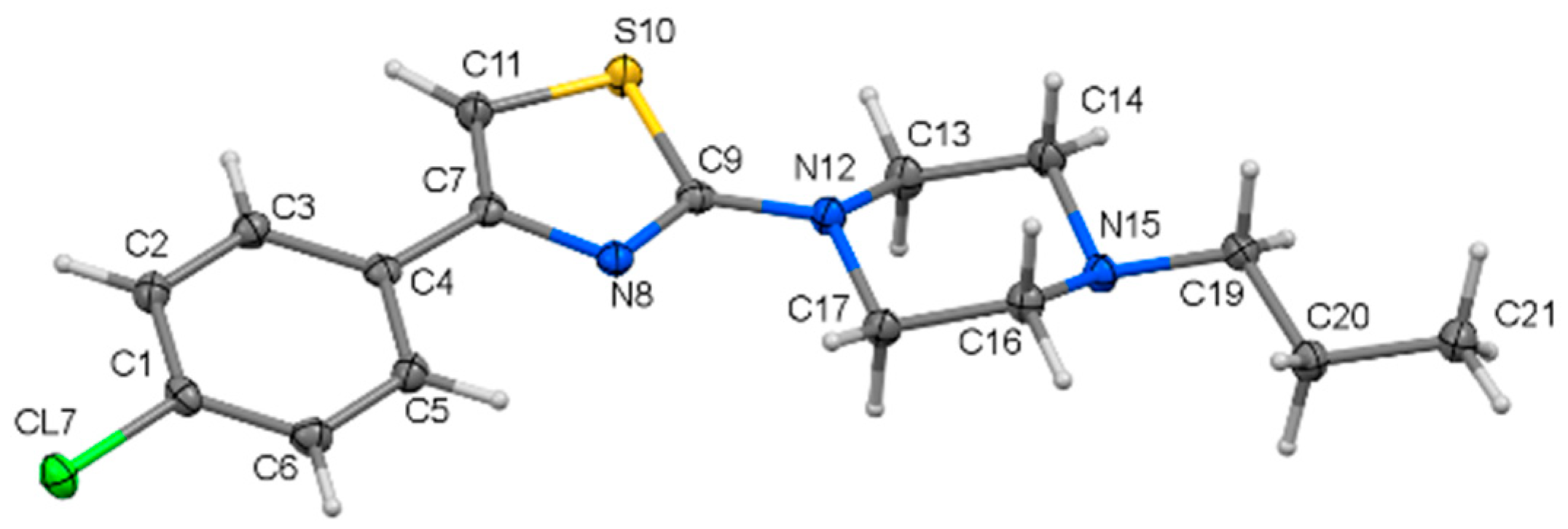
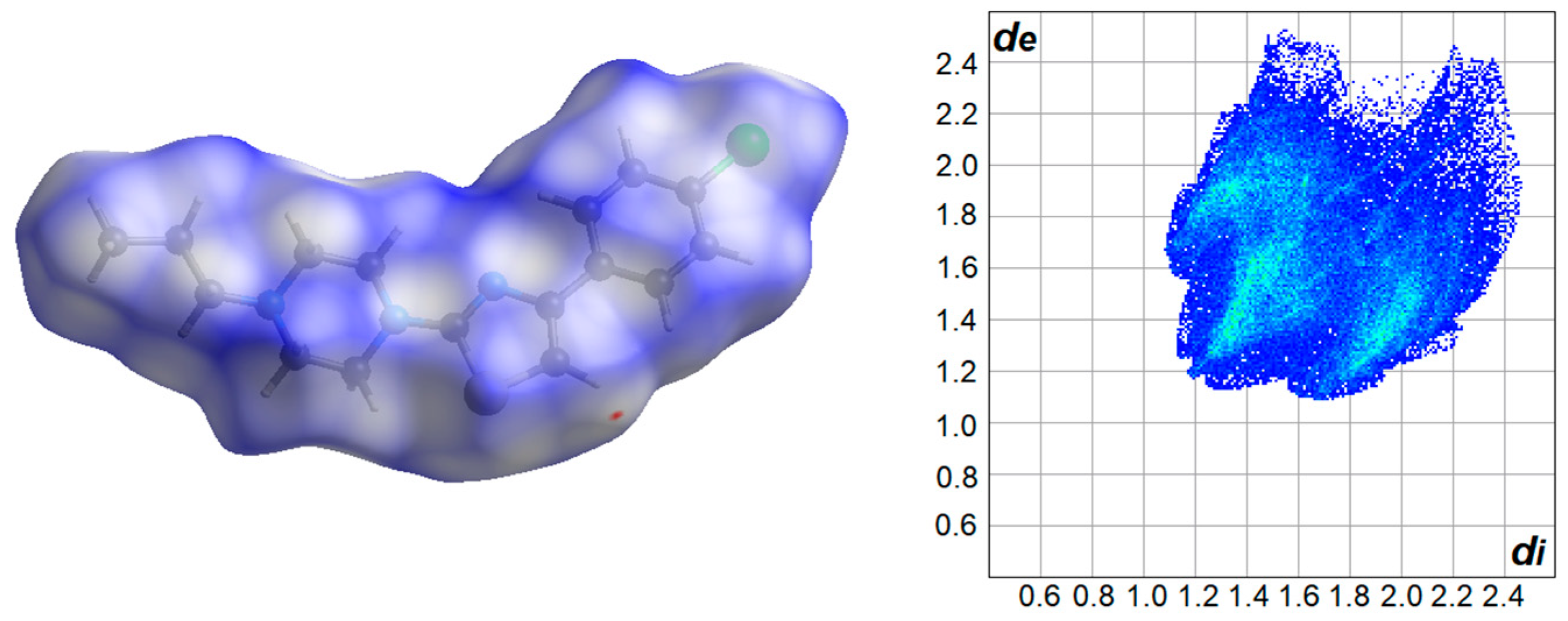

| i—j | dij (Å) | i—j—k | αijk (°) |
|---|---|---|---|
| C4—C7 | 1.4746(15) | C7—N8—C9 | 110.41(9) |
| C7—N8 | 1.3925(14) | N8—C9—S10 | 114.69(8) |
| N8—C9 | 1.3055(14) | C9—S10—C11 | 88.72(5) |
| C9—S10 | 1.7585(11) | S10—C11—C7 | 110.53(9) |
| S10—C11 | 1.7252(12) | C11—C7—N8 | 115.65(10) |
| C11—C7 | 1.3582(16) | N12—C13—C14 | 109.97(9) |
| C9—N12 | 1.3643(14) | C13—C14—N15 | 110.99(9) |
| N12—C13 | 1.4589(14) | C14—N15—C16 | 109.18(9) |
| C13—C14 | 1.5176(16) | N15—C16—C17 | 110.82(9) |
| C14—N15 | 1.4598(14) | C16—C17—N12 | 109.73(9) |
| N15—C16 | 1.4634(14) | C17—N12—C13 | 114.17(9) |
| C16—C17 | 1.5208(15) | ||
| C17—N12 | 1.4643(14) | ||
| N15—C19 | 1.4674(14) |
| H•••H | 49.6 | Cl•••N | 0.7 |
| H•••C | 21.1 | Cl•••S | 0.9 |
| H•••N | 5.4 | C•••C | 0.0 |
| H•••S | 7.0 | C•••N | 0.7 |
| H•••Cl | 13.2 | C•••S | 1.2 |
| Cl•••Cl | 0.0 | N•••S | 0.1 |
| Cl•••C | 0.0 | S•••S | 0.2 |
| Complex | Stages | Temperature Range, °C | Mass Loss | Final Solid Product of the Thermal Decomposition | |||
|---|---|---|---|---|---|---|---|
| I | II | III | Found | Calculated | |||
| L1 | 2 | 160–365 | 440–620 | - | 100.00 | 100.00 | - |
| L2 | 2 | 160–340 | 410–620 | - | 100.00 | 100.00 | - |
| L3 | 2 | 190–380 | 400–660 | - | 100.00 | 100.00 | - |
| Cu(L1)2Cl2 | 3 | 70–175 | 280–420 | 480–720 | 87.81 | 88.78 | CuO |
| Cu(L2)Cl2 | 3 | 50–250 | 305–415 | 500–750 | 86.74 | 82.57 | CuO |
| Cu(L3)Cl2 | 3 | 50–250 | 290–420 | 480–770 | 84.87 | 82.49 | CuO |
| Pa | Pi | Activity | |
|---|---|---|---|
| L1 | 0.767 | 0.003 | Anti-anorexic |
| 0.741 | 0.007 | Phosphatase inhibitor | |
| 0.634 | 0.012 | Neurodegenerative diseases treatment | |
| 0.598 | 0.007 | Transcription factor STAT inhibitor | |
| 0.621 | 0.058 | Glycosylphosphatidylinositol phospholipase D inhibitor | |
| 0.568 | 0.010 | Transcription factor STAT3 inhibitor | |
| 0.635 | 0.086 | Mucomembranous protector | |
| 0.543 | 0.020 | Anxiolytic | |
| L2 | 0.797 | 0.003 | Anti-anorexic |
| 0.740 | 0.007 | Phosphatase inhibitor | |
| 0.714 | 0.032 | Glycosylphosphatidylinositol phospholipase D inhibitor | |
| 0.590 | 0.007 | Transcription factor STAT inhibitor | |
| 0.596 | 0.015 | Insulin promoter | |
| 0.669 | 0.091 | Phobic disorders treatment | |
| 0.594 | 0.017 | Neurodegenerative diseases treatment | |
| 0.586 | 0.021 | Cholesterol antagonist | |
| L3 | 0.705 | 0.012 | Phosphatase inhibitor |
| 0.694 | 0.004 | Anti-anorexic | |
| 0.607 | 0.006 | Transcription factor STAT inhibitor | |
| 0.592 | 0.008 | Transcription factor STAT3 inhibitor | |
| 0.569 | 0.019 | Neurodegenerative diseases treatment | |
| 0.612 | 0.067 | Antineurotic | |
| 0.528 | 0.019 | Anti-ulcerative | |
| 0.526 | 0.032 | Cholesterol antagonist |
| Name | Toxicity Class | Predicted LD50 |
|---|---|---|
| L1 | 4 | 1000 mg/kg |
| L2 | 4 | 1000 mg/kg |
| L3 | 4 | 1000 mg/kg |
| Cu(L1)2Cl2 | 4 | 540 mg/kg |
| Cu(L2)Cl2 | 4 | 540 mg/kg |
| Cu(L3)Cl2 | 4 | 540 mg/kg |
| Complex | S. aureus | E. coli | C. glabrata |
|---|---|---|---|
| L1 | >1000 | 128 | 32 |
| L2 | >1000 | >1000 | 128 |
| L3 | >1000 | >1000 | 64 |
| Cu(L1)2Cl2 | >1000 | >1000 | >1000 |
| Cu(L2)Cl2 | >1000 | >1000 | 64 |
| Cu(L3)Cl2 | >1000 | >1000 | 64 |
| Nystatin | - | - | 4 |
| Compound | L2 |
|---|---|
| Empirical formula | C16H20ClN3S |
| Formula weight | 321.86 |
| Temperature (K) | 100(1) |
| Crystal system | monoclinic |
| Space group | P21/c |
| a (Å) | 11.62470(10) |
| b (Å) | 17.19590(10) |
| c (Å) | 8.25110(10) |
| α (°) | 90 |
| β (°) | 109.8670(10) |
| γ (°) | 90 |
| Volume (Å3) | 1551.21(3) |
| Z | 4 |
| Calculated density (g/cm3) | 1.378 |
| Absorption coefficient (mm−1) | 3.399 |
| F(000) | 680.0 |
| Crystal size (mm) | 0.089 × 0.054 × 0.028 |
| Radiation | Cu Kα (λ = 1.54184) |
| θ Range for data collection (°) | 8.088 to 157.678 |
| Index ranges | −14 ≤ h ≤ 13, −21 ≤ k ≤ 21, −10 ≤ l ≤ 9 |
| Reflections collected/independent | 30763/3280 |
| Rint | 0.0285 |
| Completeness (%) | 100.0 |
| Data/restraints/parameters | 3280/0/191 |
| Goodness-of-fit on F2 | 1.048 |
| Final R indices [I > 2σ(I)] | R1 = 0.0241, wR2 = 0.0610 |
| Final R indexes [all data] | R1 = 0.0256, wR2 = 0.0618 |
| Largest diff. peak/hole (e•Å−3) | 0.31/−0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivovarova, E.; Climova, A.; Świątkowski, M.; Staszewski, M.; Walczyński, K.; Dzięgielewski, M.; Bauer, M.; Kamysz, W.; Krześlak, A.; Jóźwiak, P.; et al. Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents. Int. J. Mol. Sci. 2022, 23, 9844. https://doi.org/10.3390/ijms23179844
Pivovarova E, Climova A, Świątkowski M, Staszewski M, Walczyński K, Dzięgielewski M, Bauer M, Kamysz W, Krześlak A, Jóźwiak P, et al. Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents. International Journal of Molecular Sciences. 2022; 23(17):9844. https://doi.org/10.3390/ijms23179844
Chicago/Turabian StylePivovarova, Ekaterina, Alina Climova, Marcin Świątkowski, Marek Staszewski, Krzysztof Walczyński, Marek Dzięgielewski, Marta Bauer, Wojciech Kamysz, Anna Krześlak, Paweł Jóźwiak, and et al. 2022. "Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents" International Journal of Molecular Sciences 23, no. 17: 9844. https://doi.org/10.3390/ijms23179844
APA StylePivovarova, E., Climova, A., Świątkowski, M., Staszewski, M., Walczyński, K., Dzięgielewski, M., Bauer, M., Kamysz, W., Krześlak, A., Jóźwiak, P., & Czylkowska, A. (2022). Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents. International Journal of Molecular Sciences, 23(17), 9844. https://doi.org/10.3390/ijms23179844











