Hair Loss and Telogen Effluvium Related to COVID-19: The Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies
Abstract
:1. Introduction
2. SARS-CoV-2 Related COVID-19, and Hair Loss
2.1. Study Overview
2.2. Historical Context
2.3. Clinical Studies Analyses Reporting Hair Loss and Telogen Effluvium Increasing in COVID-19 Patients
2.4. The Role of Systemic Inflammation, Oxidative Stress, and Ischemia in COVID-19 Related Hair Loss
2.5. The Correlation between Inflammation-Related Covid-19 Related and Telogen Effluvium
3. The COVID-19 Pandemic Provoked Stress-Induced Hair Loss
4. Regenerative Therapies in COVID-19 Related Hair Loss
5. Clinical Studies on PRP, HFSCs, and/or AD-MSCs Effects in AGA and Correlations between AGA and SARS-CoV-2
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HL | Hair Loss |
| MPHL | Male Pattern Hair Loss |
| FPHL | Female Pattern Hair Loss |
| AA | Alopecia Areata |
| AGA | Androgenetic Alopecia |
| COVID-19 | Coronavirus Disease 2019 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| ROS | Reactive Oxygen Species |
| IL-1 | Interleukin 1 |
| TNF-α | Tumor Necrosis Factor α |
| PHL | Pattern Hair Loss |
| IFN-γ | Interferon γ |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| PRP | Platelet-Rich Plasma |
| HFSCs | Human Follicle Stem Cells |
| LLLT | Low-Level Led Therapy |
| VEGF | Vascular Endothelial Growth Factor |
| GFs | Growth Factors |
| US | United States |
| FDA | Federal Drug Administration |
| WHO | World Health Organization |
| TE | Telogen Effluvium |
| Fibroblast | Growth Factor-7 FGF-7 |
| MSC | Mesenchymal Stem Cells |
| AD-MSCs | Adipose-Derived Mesenchymal Stem Cells |
| HGF | Hepatocyte growth factor |
| DPC | Dermal Papilla Cell |
| ECs | Epithelial Cells |
| EGF | Epidermal Growth Factor (EGF) |
| FGF-b | fibroblast growth factor-beta |
| IL-6 | Interleukin-6 |
| TGF-β | transforming growth factor-beta |
| IGF-1 | Insulin-like growth factor-1 |
| HF | Human Follicles |
| BMP | Bone Morphogenetic Protein |
| BMPR1a | Bone Morphogenetic Protein Receptor Type 1A |
| M-CSF | Macrophage Colony-Stimulating |
| PDGF | platelet-derived growth factor |
| PGE2 | Prostaglandin E2 |
| PGF2α | ProstaglandinF2α |
| ARs | Androgen Receptors |
| TMPRSS2 | Transmembrane Protease Serine 2 |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| usCRP | ultrasensitive C-reactive protein |
| LDH | Lactate dehydrogenase |
| ESR | Erythrocyte sedimentation rate |
| DPCs | Dermal papilla cells |
References
- Gentile, P.; Garcovich, S. The Effectiveness of Low-Level Light/Laser Therapy on Hair Loss. Facial Plast. Surg. Aesthet. Med. 2021. [CrossRef] [PubMed]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Dionisi, L.; Pizzicannella, J.; Kothari, A.; De Fazio, D.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Autologous Non-Activated Platelet-Rich Plasma (A-PRP) and Activated Platelet-Rich Plasma (AA-PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation. Int. J. Mol. Sci. 2020, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Insalaco, C.; Cervelli, V.; Piva, T. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int. J. Mol. Sci. 2017, 18, 408. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef]
- Jaworsky, C.; Gilliam, A.C. Immunopathology of the human hair follicle. Dermatol. Clin. 1999, 17, 561–568. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Garza, L.A.; Yang, C.-C.; Zhao, T.; Blatt, H.B.; Lee, M.; He, H.; Stanton, D.C.; Carrasco, L.; Spiegel, J.H.; Tobias, J.W.; et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Investig. 2011, 121, 613–622. [Google Scholar] [CrossRef]
- Seirafianpour, F.; Sodagar, S.; Pour Mohammad, A.; Panahi, P.; Mozafarpoor, S.; Almasi, S.; Goodarzi, A. Cutaneous manifestations and considerations in COVID-19 pandemic: A systematic review. Dermatol. Ther. 2020, 33, e13986. [Google Scholar] [CrossRef]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef]
- Kutlu, Ö. Analysis of dermatologic conditions in Turkey and Italy by using Google Trends analysis in the era of the COVID-19 pandemic. Dermatol. Ther. 2020, 33, e13949. [Google Scholar] [CrossRef]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Vaño-Galván, S.; Wambier, C.G.; McCoy, J.; Gomez-Zubiaur, A.; Moreno-Arrones, O.M.; Shapiro, J.; Sinclair, R.D.; Gold, M.H.; Kovacevic, M.; et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain—A potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020, 19, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Gomez-Zubiaur, A.; Herrera, S.; Hermosa-Gelbard, Á.; Moreno-Arrones, O.M.; Jiménez-Gómez, N.; Gonzalez-Cantero, A.; Fonda-Pascual, P.; et al. Androgenetic alopecia present in most patients hospitalized with COVID-19: The “Gabrin sign”. J. Am. Acad. Dermatol. 2020, 83, 680–682. [Google Scholar] [CrossRef]
- Wambier, C.G.; Vaño-Galván, S.; McCoy, J.; Pai, S.; Dhurat, R.; Goren, A. Androgenetic alopecia in COVID-19: Compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. J. Am. Acad. Dermatol. 2020, 83, e453–e454. [Google Scholar] [CrossRef]
- Wambier, C.G. Reply to “Comment on androgenetic alopecia present in the majority of patients hospitalized with COVID-19”. J. Am. Acad. Dermatol. 2020, 84, E53–E54. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-center longitudinal study. Clin. Microbiol. Infect. 2020, 27, 89–95. [Google Scholar] [CrossRef]
- Olds, H.; Liu, J.; Luk, K.; Lim, H.W.; Ozog, D.; Rambhatla, P.V. Telogen effluvium associated with COVID-19 infection. Dermatol. Ther. 2021, 34, e14761. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Jabbar, R.I. COVID-19 infection is a major cause of acute telogen effluvium. Ir. J. Med. Sci. 2021, 191, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Lobato-Berezo, A.; Gomez-Zubiaur, A.; Arias-Santiago, S.; Saceda-Corralo, D.; Bernardez-Guerra, C.; Grimalt, R.; Fernandez-Crehuet, P.; Ferrando, J.; Gil, R.; et al. SARS-CoV-2-induced telogen effluvium: A multicentric study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e181–e183. [Google Scholar] [CrossRef] [PubMed]
- Shome, D.; Kapoor, R.; Surana, M.; Vadera, S.; Shah, R. Efficacy of QR678 Neo® hair growth factor formulation for the treatment of hair loss in Covid-19- induced persistent Telogen Effluvium-A prospective, clinical, single-blind study. J. Cosmet. Dermatol. 2022, 21, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Agarwala, P.; Iqbal, K.; Omar, H.M.S.; Jangid, G.; Patel, V.; Rathore, S.S.; Kumari, C.; Velasquez-Botero, F.; López, G.A.B.; et al. A systematic review of acute telogen effluvium, a harrowing post-COVID-19 manifestation. J. Med. Virol. 2022, 94, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Seyfi, S.; Alijanpour, R.; Aryanian, Z.; Ezoji, K.; Mahmoudi, M. Prevalence of telogen effluvium hair loss in COVID-19 patients and its relationship with disease severity. J. Med. Life 2022, 15, 631–634. [Google Scholar] [PubMed]
- Monari, P.; Gualdi, G.; Bettoni, G.; Costa, R.; Ragni, G.; Zani, F.; Bianchi, G.; Casella, S.; Casella, E.; Crippa, M.; et al. Post-SARS-CoV-2 Acute Telogen Effluvium: An Expected Complication. J. Clin. Med. 2022, 11, 1234. [Google Scholar] [CrossRef]
- Kircik, L.H. A new look at the pathogenesis of hair loss. J. Drugs Dermatol. 2017, 16, s133–s134. [Google Scholar]
- Magro, C.M.; Rossi, A.; Poe, J.; Manhas-Bhutani, S.; Sadick, N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. J. Drugs Dermatol. 2011, 10, 1404–1411. [Google Scholar]
- Sadick, N.S.; Callender, V.D.; Kircik, L.H.; Kogan, S. New insight into the pathophysiology of hair loss trigger a paradigm shift in the treatment approach. J. Drug. Derm. 2017, 16, s135–s140. [Google Scholar]
- Jaworsky, C.; Kligman, A.M.; Murphy, G.F. Characterization of inflammatory infiltrates in male pattern alopecia: Implications for pathogenesis. Br. J. Dermatol. 1992, 127, 239–246. [Google Scholar] [CrossRef]
- Mahé, Y.F.; Michelet, J.F.; Billoni, N.; Jarrousse, F.; Buan, B.; Commo, S.; Saint-Leger, D.; Bernard, B.A. Androgenetic alopecia and microinflammation. Int. J. Dermatol. 2000, 39, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Prie, B.E.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative stress in androgenetic alopecia. L. Med. Life 2016, 9, 79–83. [Google Scholar]
- Kato, H.; Kinoshita, K.; Saito, N.; Kanayama, K.; Mori, M.; Asahi, N.; Sunaga, A.; Yoshizato, K.; Itami, S.; Yoshimura, K. The Effects of Ischemia and Hyperoxygenation on Hair Growth and Cycle. Organogenesis 2020, 16, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Matsudo, K.; Fujita, T. Management of hair loss after severe acute respiratory syndromecoronavirus 2 infection: Insight into the pathophysiology with implication for better management. J. Dermatol. 2022. early view. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saathoff, M.; Bettermann, A.; Takigawa, M.; Paus, R. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br. J. Dermatol. 2005, 152, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Tufan, A.; Avanoglu Güler, A.; Matucci-Cerinic, M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020, 50, 620–632. [Google Scholar] [CrossRef]
- World Health Organisation. Mental Health And COVID-19. 2020. Available online: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/technical-guidance/mental-health-and-covid-19 (accessed on 5 October 2020).
- Asghar, F.; Shamim, N.; Farooque, U.; Sheikh, H.; Aqeel, R. Telogen Effluvium: A Review of the Literature. Cureus 2020, 12, e8320. [Google Scholar] [CrossRef]
- Arck, P.C.; Handjiski, B.; Peters, E.M.; Peter, A.S.; Hagen, E.; Fischer, A.; Klapp, B.F.; Paus, R. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am. J. Pathol. 2003, 62, 803–814. [Google Scholar] [CrossRef]
- Franke, H.A. Toxic Stress: Effects, Prevention and Treatment. Children 2014, 1, 390–402. [Google Scholar] [CrossRef]
- Headington, J.T. Telogen effluvium. New concepts and review. Arch. Dermatol. 1993, 129, 356–363. [Google Scholar] [CrossRef]
- Thom, E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J. Drugs Dermatol. 2016, 15, 1001–1004. [Google Scholar] [PubMed]
- Zhang, B.; Ma, S.; Rachmin, I.; He, M.; Baral, P.; Choi, S.; Gonçalves, W.A.; Shwartz, Y.; Fast, E.M.; Su, Y.; et al. Hyperactivation of sympathetic nerves drive depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, D. Stress-Induced Hair Loss in the Age of COVID-19. Miami Health News—University of Miami Hospitals and Clinics. August 2020. Available online: https://news.umiamihealth.org/en/stress-induced-hair-loss-in-the-age-of-covid-19 (accessed on 9 October 2020).
- Turkmen, D.; Altunisik, N.; Sener, S.; Colak, C. Evaluation of the effects of COVID-19 pandemic on hair diseases through a web-based questionnaire. Dermatol. Ther. 2020, 33, e13923. [Google Scholar] [CrossRef] [PubMed]
- Sweet, J. COVID-19 Survivors Are Losing Their Hair—Here’s Why. Healthline. August 2020. Available online: https://www.healthline.com/health-news/covid-19-survivors-are-losing-their-hair-heres-why (accessed on 12 October 2020).
- Pawlowski, A. I Was Freaking out: COVID-19 Survivors Say the Hair Loss is a Lingering Problem. August 2020. Available online: https://www.today.com/health/covid-19-hair-loss-blamed-telogen-effluvium-treatment-regrowth-t188361 (accessed on 12 October 2020).
- Gentile, P. Autologous Cellular Method Using Micrografts of Human Adipose Tissue-Derived Follicle Stem Cells in Androgenic Alopecia. Int. J. Mol. Sci. 2019, 20, 3446. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; De Angelis, B.; De Sio, C.; De Fazio, D.; Ceccarelli, G.; Trivisonno, A.; Orlandi, A.; Cervelli, V.; et al. Platelet-Rich Plasma and Micrografts Enriched with Autologous Human Follicle Mesenchymal Stem Cells Improve Hair Re-Growth in Androgenetic Alopecia. Biomolecular Pathway Analysis and Clinical Evaluation. Biomedicines 2019, 7, 27. [Google Scholar]
- Gentile, P.; Garcovich, S. Systematic review: The platelet-rich plasma use in female androgenetic alopecia as effective autologous treatment of regenerative plastic surgery. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 850–859. [Google Scholar] [CrossRef]
- Talei, B.; Shauly, O.; Gould, D. Platelet Rich Plasma Hybridized Adipose Transplant (PHAT) for the Treatment of Hair Loss: A Case Series. Aesthetic Plast. Surg. 2021, 45, 2760–2767. [Google Scholar] [CrossRef]
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 659–660. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Wang, Z.; Hare, J.M.; Bu, G.; Mallea, J.M.; Pascual, J.M.; Caplan, A.I.; Kurtzberg, J.; Zubair, A.C.; Kubrova, E.; et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl. Med. 2020, 9, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Sterodimas, A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin. Biol. Ther. 2020, 20, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Sterodimas, A. Adipose Stem Cells (ASCs) and Stromal Vascular Fraction (SVF) as a Potential Therapy in Combating (COVID-19)-Disease. Aging Dis. 2020, 11, 465–469. [Google Scholar] [CrossRef]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Calabrese, C.; Garcovich, S. Research progress on Mesenchymal Stem Cells (MSCs), Adipose-Derived Mesenchymal Stem Cells (AD-MSCs), Drugs, and Vaccines in Inhibiting COVID-19 Disease. Aging Dis. 2020, 11, 1191–1201. [Google Scholar] [CrossRef]
- Gentile, P. SARS-CoV-2: The “Uncensored” Truth about Its Origin and Adipose-Derived Mesenchymal Stem Cells as New Potential Immune-Modulatory Weapon. Aging Dis. 2021, 12, 330–344. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Mechanical and Controlled PRP Injections in Patients Affected by Androgenetic Alopecia. J. Vis. Exp. 2018, 131, 56406. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Stem cells from human hair follicles: First mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017, 4, 58. [Google Scholar] [CrossRef]
- Rathnayake, D.; Sinclair, R. Male androgenetic alopecia. Expert Opin. Pharmacother. 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Randall, V.A.; Hibberts, N.A.; Thornton, M.J.; Hamada, K.; Merrick, A.E.; Kato, S.; Jenner, T.J.; De Oliveira, I.; Messenger, A.G. The hair follicle: A paradoxical androgen target organ. Horm. Res. 2000, 54, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, L.; Kirchhoff, F.; Münch, J. The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; McCoy, J.; Gustavo Wambier, C.; Goren, A. Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial—Biochemical). Cureus 2021, 13, e13047. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yu, A.; Luo, Y.; Tian, T.; Dong, Y.; Zong, H.; Chen, H.; Gao, X.; Xu, X.; Li, Y. Genomewide differential expression profiling of long non-coding RNAs in androgenetic alopecia in a Chinese male population. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Lin, E.; Zhang, H.; Liu, Y.; Cao, G.; Fu, C.; Chen, L.; Zeng, Y.; Cai, B.; Yuan, Y.; et al. LncRNA H19 Overexpression Activates Wnt Signaling to Maintain the Hair Follicle Regeneration Potential of Dermal Papilla Cells. Front. Genet. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Ma, T.; Liu, Q. Vascular Endothelial Growth Factor Protects CD200-Rich and CD34-Positive Hair Follicle Stem Cells Against Androgen-Induced Apoptosis Through the Phosphoinositide 3-Kinase/Akt Pathway in Patients With Androgenic Alopecia. Dermatol. Surg. 2020, 46, 358–368. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Cervelli, V.; Orlandi, A.; Garcovich, S. Autologous Micrografts from Scalp Tissue: Trichoscopic and Long-Term Clinical Evaluation in Male and Female Androgenetic Alopecia. Biomed. Res. Int. 2020, 2020, 7397162. [Google Scholar] [CrossRef]
- Huang, Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J. Cell. Mol. Med. 2018, 22, 5768–5775. [Google Scholar] [CrossRef]
- Wang, E.C.E.; Higgins, C.A. Immune cell regulation of the hair cycle. Exp. Dermatol. 2020, 29, 322–333. [Google Scholar] [CrossRef]
- Yang, L.; Peng, R. Unveiling hair follicle stem cells. Stem Cell Rev. Rep. 2010, 6, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Liu, C.; Li, L.; Lan, M.; Yu, Y.; Gu, L.; Su, Y.; Zhang, K.; Zhang, Y.; Wang, T.; et al. miR-29a/b1 Inhibits Hair Follicle Stem Cell Lineage Progression by Spatiotemporally Suppressing WNT and BMP Signaling. Cell Rep. 2019, 29, 2489–2504.e2484. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Daszczuk, P.; Mazurek, P.; Pieczonka, T.D.; Olczak, A.; Boryń, Ł.M.; Kobielak, K. An Intrinsic Oscillation of Gene Networks Inside Hair Follicle Stem Cells: An Additional Layer That Can Modulate Hair Stem Cell Activities. Front. Cell Dev. Biol. 2020, 8, 595178. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Hawkshaw, N.J.; Hardman, J.A.; Alam, M.; Jimenez, F.; Paus, R. Deciphering the molecular morphology of the human hair cycle: Wnt signaling during the telogen-anagen transformation. Br. J. Dermatol. 2020, 182, 1184–1193. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell. Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef]
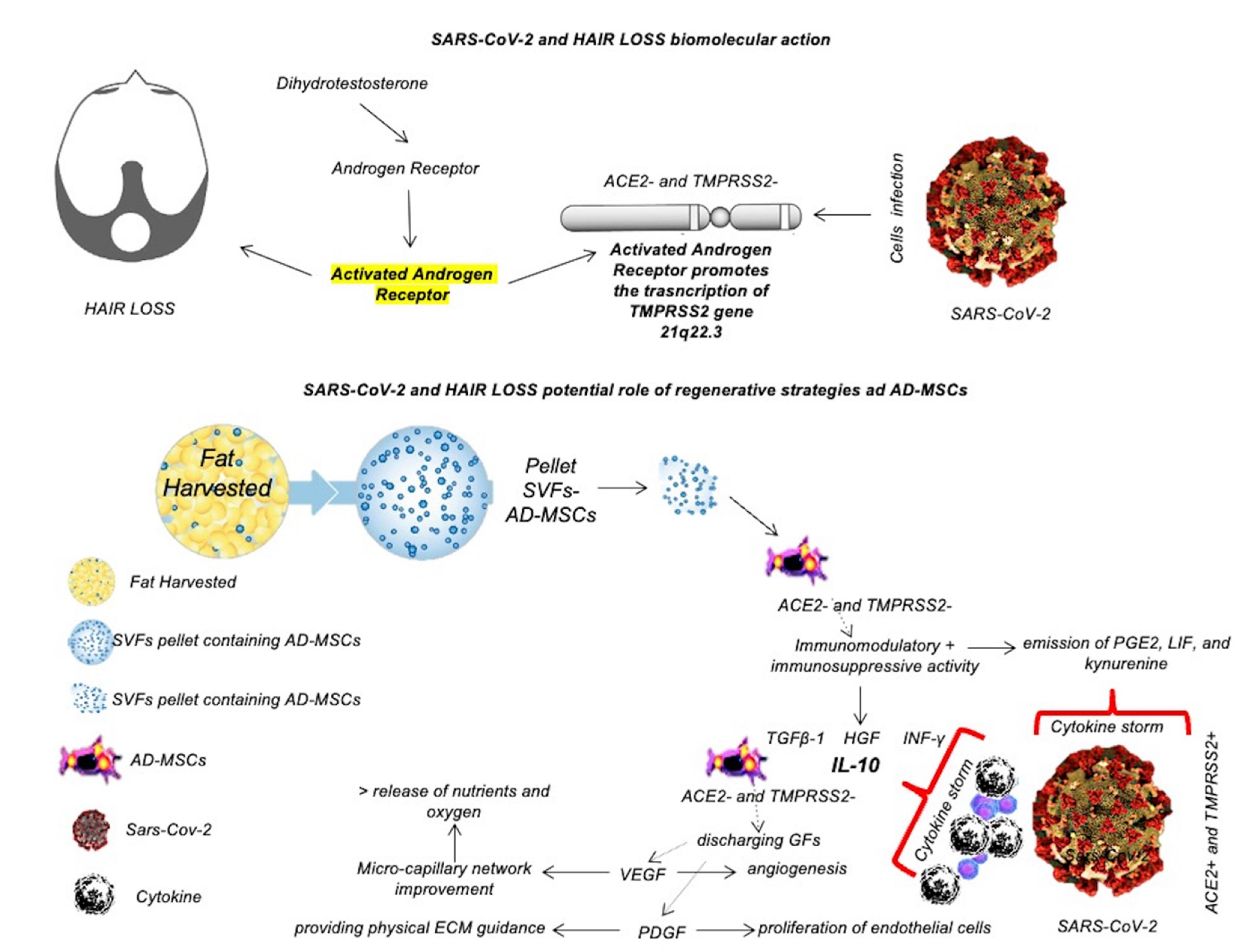
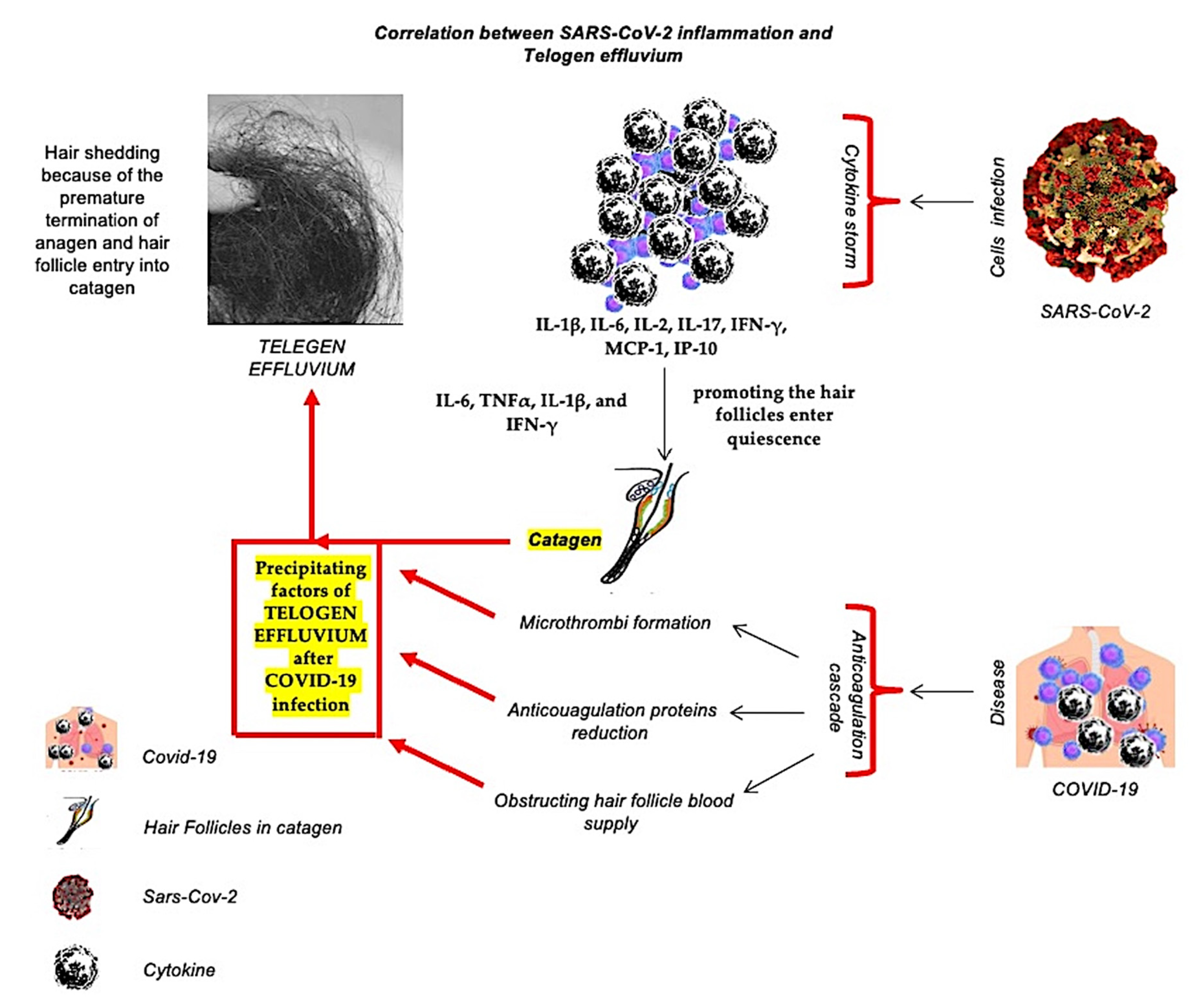
| Clinical Studies | Characteristics | Results | Year | References |
|---|---|---|---|---|
| Goren, A.; et al. | A descriptive study on 41 Caucasian males admitted to hospitals in Spain with a diagnosis of bilateral SARS-CoV-2 pneumonia (mean age = 58 years). | 71% of the subjects were diagnosed with significant MPHL of which 39% had a severe involvement. | 2020 | [15] |
| Wambier, C.G.; et al. | Multicenter study with a follow-up on 175 patients (males and females) hospitalized with COVID-19 | 79% (95% CI: 70–85%) of men and 42% (95% CI: 29–55%) of women had significant PHL. These values are in sharp contrast with the expected prevalence rates among age- and race-matched populations. The prevalence of MPHL in a similar white population is expected to be at 31–53%, and that of FPHL to be at a maximum of 38%. | 2020 | [16] |
| Wambier, C.G. | Reply to “Comment on androgenetic alopecia present in the majority of patients hospitalized with COVID-19” | The data available up to date points to a considerably higher prevalence and severity of PHL among hospitalized COVID-19 patients. | 2020 | [18] |
| Wambier, C.G.; et al. | Comparing study on androgenetic alopecia in COVID-19 on age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. | Hypoxemia leading to skin ischemia is another potential pathogenetic factor that connects lung damage secondary to SARS-CoV-2 infection with hair growth impairment. | 2020 | [17] |
| Xiong, Q.; et al. | A single-center longitudinal study on 538 COVID-19 survivors and 184 controls in Wuhan, China: | Almost half of the female participants started experiencing hair loss after being infected by SARS-CoV-2 compared to no case in the control group. 27% of affected cases experienced alopecia during their hospitalization while 73% first recognized it after being discharged. 3 to 4 months after discharge, alopecia was among the most prevalent complaints in convalescent COVID-19 patients, reported more commonly by women. Patients with higher stages of HL had worse clinical outcomes (use of ventilators and deaths). Some authors proposed the eponym ‘Gabrin sign’ to refer to the phenomenon of severe baldness in COVID-19 patients with a higher risk of unfavorable outcomes. | 2020 | [19] |
| Clinical Studies | Characteristics | Results | Year | References |
|---|---|---|---|---|
| Olds, H.; et al. | Case series of 10 patients with telogen effluvium attributed to COVID-19 infection | The mean age was 48.5 years old and 90% were female. Six of the patients were Black, one was middle eastern, and three were White. On average, the hair shedding began 50 days after the first symptom of COVID-19 infection. About 80% of these patients were treated with antibiotics, systemic corticosteroids, and/or hydroxychloroquine for their COVID-19 infection, and 70% were hospitalized. The presentations of these patients suggested that COVID-19 infection may be a significant trigger of TE. | 2021 | [20] |
| Sharquie, K.E.; et al. | Observational cross-sectional study that had been conducted during the period from September 2020 to March 2021 years on 39 patients with post COVID-19 hair loss confirmed by polymerase chain reaction (PCR) or antibody testing | 39 patients were evaluated; their ages ranged from 22 to 67 years with a mean and SD of 41.3 ± 11.6 years with 36 (92.3%) females and 3 (7.69%) males. All patients with a diagnosis of acute telogen effluvium were enrolled in this study and had a laboratory-confirmed diagnosis of prior SARS-CoV-2 infection; 15 (38.46%) patients reported mild symptoms, 24 (61.53%) patients presented with moderate disease, and no patient required hospitalization. They all experienced excessive hair loss within two to three months after infection. Pull tests were strongly positive (>10–50% with a mean of 35% of pulled hair away from scalp). | 2021 | [21] |
| Moreno-Arrones, O.M. et al. | Prospective Multicentric study which enrolled 214 patients from March to August 2020 with acute telogen effluvium (ATE) that had a prior SARS-CoV-2 infection confirmed either by serological tests [e.g., detection of serum antibodies against the virus via enzyme-linked immunosorbent assays (ELISAs)] or by detection of viral RNA using real-time reverse transcription polymerase chain reaction (RT-PCR). | Mean number of days since SARS-CoV-2 diagnosis and significant hair shedding was 57.1 days (SD of 18.3). Regarding the severity of hair shedding, which was evaluated by the Sinclair Shedding Scale, 3 (4.7%) of the patients (9) had a hair shedding score of 1, 10.5% (20) of 2, 12.6 (24) of 3, 20.4% (39) of 4, 22% (42) of 5 and 29.8% (57) of 6. In 72.8% of the cases (139 patients), the ATE was active four weeks after the diagnosis. History of fever was associated (p = 0.04) with an increased hair shedding (Sinclair score of 5 or 6). The use of heparinoids was not associated with severity. | 2021 | [22] |
| Shome et al. | Case series study which enrolled 20 patients (all women) presenting with persistent TE starting a few weeks after recovery from Covid-19 infection, and continuing beyond six months | Management of Covid-19-induced persistent Telogen Effluvium has been unclear and futile so far. Intra-dermal administration of QR678 Neo® hair growth factor formulation in the scalp reduced hair fall, improved hair regrowth, and increased hair density. | 2022 | [23] |
| Hussain et al. | A systematic review involving 465 patients diagnosed with acute TE. | The mean age was 44 years, and 67.5% were women. The most common trichoscopy findings were a decrease in hair density and empty follicles. The average duration from the onset of COVID-19 symptoms to the appearance of acute TE was 74 days, earlier than classic acute TE. | 2022 | [24] |
| Seyfi S. et al. | This observational cross-sectional study included 198 patients, confirming that TE is one of the consequences of the COVID-19 pandemic. | The study affirmed that COVID-19, via medication and stress, triggers TE. | 2022 | [25] |
| Monari et al. | A cross-sectional study which enrolled 96 patients with a diagnosis of SARS-CoV-2 pneumonia, assessed TE in 31.3% of patients, with a significant difference in sex (females 73%, males 26.7%). | The average time detected from the onset of the first symptoms to TE was 68.43 days. There were no significant associations between TE and COVID-19-related features (length of hospitalization, virologic positivity, fever’ duration), treatment characteristics, or laboratory findings. Post-infection acute TE occurs in a significant number of COVID-19 patients. | 2022 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, P. Hair Loss and Telogen Effluvium Related to COVID-19: The Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies. Int. J. Mol. Sci. 2022, 23, 9116. https://doi.org/10.3390/ijms23169116
Gentile P. Hair Loss and Telogen Effluvium Related to COVID-19: The Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies. International Journal of Molecular Sciences. 2022; 23(16):9116. https://doi.org/10.3390/ijms23169116
Chicago/Turabian StyleGentile, Pietro. 2022. "Hair Loss and Telogen Effluvium Related to COVID-19: The Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies" International Journal of Molecular Sciences 23, no. 16: 9116. https://doi.org/10.3390/ijms23169116
APA StyleGentile, P. (2022). Hair Loss and Telogen Effluvium Related to COVID-19: The Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies. International Journal of Molecular Sciences, 23(16), 9116. https://doi.org/10.3390/ijms23169116






