Abstract
Mesenchymal stem/stromal cells (MSCs) are widely used in disease models in order to control several phases in the response to injuries, immune reaction, wound healing, and regeneration. MSCs can act upon both the innate and adaptive immune systems and target a broad number of functions, such as the secretion of cytokines, proteolytic enzymes, angiogenic factors, and the regulating of cell proliferation and survival. The role of MSCs in coagulation has been less studied. This review evaluates the properties and main functions of MSCs in coagulation. MSCs can regulate coagulation in a wide range of pathways. MSCs express and release tissue factors (TF), one of the key regulators of the extrinsic coagulation pathways; MSCs can trigger platelet production and contribute to platelet activation. Altogether, MSCs seem to have a pro-thrombotic role and their superior characterization prior to their administration is necessary in order to prevent adverse coagulation events.
1. Introduction
Mesenchymal stem/stromal cells (MSCs) are multipotent cells that can differentiate into several mesenchymal tissue lineages such as osteoblasts, chondrocytes, and adipocytes, but not hematopoietic stem cells [1,2,3,4,5]. As claimed by the International Society for Cell & Gene Therapy (ISCT), the abbreviation “MSC” refers to “stromal” and not to “stem”, unless stemness is proven. MSCs are resident in many tissues but can mostly be obtained from bone marrow, adipose tissue, and the umbilical cord, and the source will modify their characteristics [6]. MSCs can act upon both the innate and adaptive arms of the immune system and target a broad number of functions, such as the secretion of cytokines, proteolytic enzymes, and angiogenic factors, as well as regulating cell proliferation and survival [7]. Immunomodulatory, anti-inflammatory, anti-fibrotic, and anti-microbial effects have been linked to MSCs in many published pre-clinical studies. MSCs are fairly unique cells from an immunological standpoint and their properties enable the MSCs to escape recognition by the immune system, thereby modulating/mediating the T-cell, B-cell, NK, dendritic cell (DC), and macrophage functions [8,9,10,11].
MSCs have been used in disease models in order to control several phases in the response to injuries, immune reaction, wound healing, and regeneration. [12]. Furthermore, MSCs have been used to treat cardiac and pulmonary diseases, vascular and metabolic pathologies, and neurological disorders. MSCs exert their functions through several mechanisms such as cell to cell contact and cause a paracrine-derived effect by secreting the factors or the exosomes/microvesicles (EVs) into the microenvironment.
The role of MSCs in coagulation has been less studied, but in recent months, it has peaked the interest of many and a number of articles have been published, largely due to the importance of the clinical implications [5,13,14]. The sources and production processes will lead to different MSC-therapies that display varying levels of the procoagulant tissue factor (TFs) [6]. The TF is the major determinant of the cell product hemocompatibility and may negatively trigger an inflammatory systemic response under certain conditions. This review describes the properties and main functions of MSCs in coagulation. Coagulation and inflammation pathways have many nodes in common, and we examine how MSCs affect coagulation directly or indirectly.
2. Coagulation Factors and Inflammatory Linked Pathways
Coagulation and inflammation are highly cohesive systems; both are precisely regulated biological systems with extensive cross-talk that optimizes the organism’s response to any damage. An increased inflammation can trigger coagulation and that, sequentially, can augment inflammation [15]. The failure of the anticoagulant mechanisms to control the clotting procedure would increase the inflammatory process. In addition, the inflammatory mechanisms alter the hemostatic balance to promote the activation of coagulation, and it could produce intravascular coagulation or thrombosis.
It has been described that the inflammatory mediators can elevate the platelet count and reactivity, downregulate the natural anticoagulant mechanisms, initiate and propagate the coagulation response, and impair fibrinolysis. Correspondingly, clotting can increase the inflammatory response by releasing mediators from the platelets and activating cells that intensify the inflammatory responses.
The molecular and cellular nexuses between coagulation and inflammation are slowly being described, and here we summarized the most well-known and demonstrated interactions. The dysregulation of one or both systems impacts the entire balance, resulting in a pathological state with excessive inflammation or thrombosis.
2.1. Tissue Factor
The tissue factor (TF), also called CD142, is one of the central nexuses between coagulation and inflammation. The TF is a type I integral transmembrane glycoprotein expressed by various cells. The TF is typically detected at high levels in the brain, skin, lungs, and placenta as well as in monocytes [16]. The TF activates the extrinsic blood coagulation cascade [6,17]. When a vascular injury occurs, the subendothelial TF is exposed to the blood flow and binds the plasma factor VIIa. The TF is the factor VII receptor and forms the complex TF-VIIa which initiates the coagulation by activating the zymogens factor X and IX to their respective serine proteases, factor IXa and factor Xa. Factor IXa and Xa form complex enzymes with their nonenzymatic co-factors (factor VIIIa and factor Va) on the surface of the membranes containing acidic phospholipids. Altogether, this leads to the thrombin generation from the zymogen prothrombin (Figure 1). Subsequently, thrombin activates fibrinogen in order to form fibrin, which leads to the clot formation [17,18]. Moreover, thrombin can stimulate its own production. The complex formed by the TF and factor VIIa (TF-VIIa) is essential for normal hemostasis [16,19,20,21]. Habitually, cells in contact with blood do not express a physiologically active TF and the exposure of the TF to blood is the critical cause for most of its functions [22]. The TF is strategically located in cells underneath the endothelium of the vasculature, which allows for its rapid recruitment in response to chemical or physical damage. The inflammatory cytokines (TNFα and IL-1β), infectious agents, and other injuries can upregulate the TF expression, and it will become exposed to the circulation.
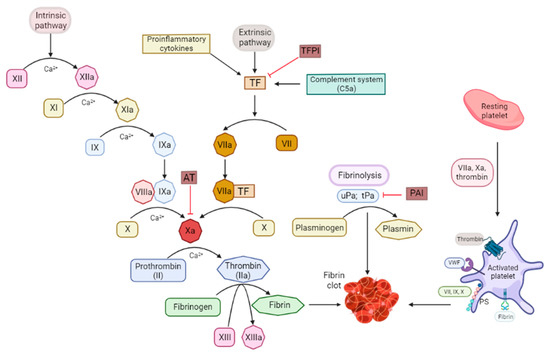
Figure 1.
Schema of the intrinsic and extrinsic coagulation pathways and the fibrinolysis and platelet-coagulation interaction nodes. PAI: plasminogen activator inhibitor; PS: phosphatidylserine; TF: tissue factor; TFPI: tissue factor pathway inhibitor; tPA: tissue plasminogen activator; uPA: urokinase type-plasminogen activator; VWF: von Willebrand factor.
As an additional regulatory mechanism, the TF is produced in an inactive form that becomes activated following several poorly understood mechanisms: the dimer formation, lipid raft, disulfide bonds, or phosphatidylserine exposure. Phosphatidylserine is a glycerophospholipid that participates in the activation of several key signaling pathways, such as the coagulation pathway. Still, it is not clear how the TF activation works, but there may be more than one mechanism acting simultaneously. For example, the exposure of phosphatidylserine to the cell surface seems to be able to trigger the formation of activated factors Va, IXa, VIIIa, and Xa, which will contribute to the correct orientation of the TF-VII complex [17,23]. However, the contribution to coagulation by the TF and phosphatidylserine is different; the TF appears to be the physiological activator, and phosphatidylserine is the driving force behind the propagation of coagulation.
The activation of factors VIIa, Xa, and thrombin activates the platelets and induces the protease-activated receptor (PAR) -mediated signaling, which leads to the release of pro-inflammatory cytokines and upregulates the expression of the leukocyte and vascular adhesion molecules (ICAM-1 and VCAM-1).
The TF may cause the activation of damage-associated molecular patterns (DAMPs) that are generated during infection, inflammation, or stress. Consequently, it triggers the activation of toll-like receptors (TLRs) mostly upon immune cells and the complement system, which induces the release of pro-inflammatory cytokines and chemokines, the upregulation of leukocyte adhesion molecules, and the expression of the TF.
2.2. Thrombin and PARs
Thrombin cleaves fibrinogen and generates a fibrin clot (Figure 1). Thrombin has a positive feedback loop through a pro-cofactor activation that increases its own production. Moreover, it can activate other cell types via the cleavage of the PARs. The PARs are primarily expressed in platelets and endothelial, immune, and epithelial cells. The PAR activation triggers the production of the monocyte chemoattractant protein-1, TNFα, IL-1β, and IL-6.
The PAR signaling in the endothelial cells causes their activation with an increase in the P- and E-selectin expression, as well as the increase in MCP1/CCL2, IL8, and the plasminogen activator inhibitor 1 (PAI-1). Altogether, this triggers the leukocyte and platelet recruitment and the adhesion to the endothelium, thereby increasing the risk of thrombosis. Overall, this process is a powerful positive feedback loop that intensifies the inflammation and pro-coagulant processes.
2.3. Protein C–Thrombomodulin–Endothelial Cell Protein C Receptor
Protein C (PC) is a precursor for the activated protein C (APC), which is a serine protease necessary to prevent the dysregulated activation of the coagulation and inflammation. The APC generation depends on the cleavage by thrombin and is catalyzed by thrombomodulin (TM) and the endothelial protein C receptor (EPCR). It has been described that the APC is able to suppress the nuclear translocation of NF-κB. Therefore, the APC reduces the release of the pro-inflammatory cytokines, including TNFα, IL-1β, IL-6, IL-8, and the macrophage inflammatory protein-1α by the monocytes/macrophages, it diminishes the TF, and inhibits the neutrophil activation and chemotaxis, thereby reducing inflammation.
2.4. Toll-Like Receptors
Toll-Like-Receptors (TLRs) are expressed in a broad number of cells such as the endothelial cells, platelets, macrophages, and dendritic cells and these play a major role in regulating the innate immune system and have the capacity to trigger several inflammatory pathways [24]. The TLRs may contribute to thrombosis, but the mechanisms are not fully understood. Several pre-clinical models of diabetes, hypercholesterolemia, and endotoxemia have shown that TLR2 and TLR4 mediate the TF expression and the platelet activation, thereby increasing the coagulation response [25,26,27,28]. Fibrinogen may act as a ligand for the TLR-mediated signaling. The signaling through TLR9 in human coronary artery endothelial cells and pre-clinical mouse models are involved in initiating the TF expression [29].
2.5. Complement System
The complement system is essential for the innate immune system response. The complement system is activated via the classical, lectin, or alternative pathways [30,31]. These three pathways converge at the C3 complement node, which is a pivotal protein. In addition, the complement system contributes to the inflammation and thrombosis [32,33]. Mainly, the complement enzymes comprise a single serine protease with a high substrate specificity. However, it has been shown that the complement and coagulation pathways share some serine-proteases that may be shared by activating at the same time factor (F) XIIa, which is able to activate C1q [34].
C5a was generated in the absence of C3, with thrombin acting as a potent C5 convertase [35]. The complement system also can amplify the coagulation through the C5a-mediated induction of the expression of the TF in neutrophils, endothelial cells, monocytes [36,37], plasminogen-activator inhibitor 1 (PAI-1), and on the von Willebrand factor (VWF) secretion from the endothelial cells.
2.6. Plasminogen System and Fibrinolysis
The fibrinolytic system is responsible for the resolution of blood clots; nonetheless, this system can also trigger the inflammatory processes involved in thrombosis [38]. The plasminogen system leads to fibrinolysis by progressively degrading fibrin which initiates several immune processes that help wound healing and angiogenesis [39]. The fibrinolytic system is activated directly upon the fibrin formation.
Thrombin produced during hemostasis or thrombosis is able to activate the endothelium and produce and secrete the plasminogen activators and the urokinase type-plasminogen activator (uPA). The plasminogen activators and the uPA cleave the plasminogen in order to generate plasmin, which is the principal fibrinolysis enzyme. Fibrin facilitates the interaction between the tissue plasminogen activator (tPA) and the plasminogen, thereby acting as a cofactor for the tPA-mediated plasminogen activation. Moreover, the cellular and fibrin-based plasmin generation can activate C3 and C5 of the complement system, thereby mediating the inflammation [40].
2.7. Platelets and Clot Formation
The platelet and coagulation activations are interconnected processes. Platelets are one of the key players in clot formation [41]. Collagen and thrombin via the TF initiate the arterial thrombus formation. At the initial stages of the process, the coagulation factors bind to the membrane receptors on resting platelets, which initiate the platelet adhesion and thrombus formation. When platelets become active, they express more binding sites a with high affinity to the activated coagulation factors. The activated platelets cluster together, they release their content, and form a clot. The released content, mainly thrombin, can activate other platelets and interact with the pro-coagulation factors that respond in a cascade, thereby converting fibrinogen, a blood-soluble protein, into fibrin that is the end coagulation product and that lays down into the clot and strengthens it.
3. Mesenchymal Stromal Cells (MSCs) Modulate Coagulation
The MSCs can regulate the coagulation in a wide range of pathways. Here, we will review the mechanisms that have been fully confirmed and summarize other non-well-understood possible pathways.
3.1. TF-Mediated Pro-Coagulant Activity of the MSCs
Growing evidence is emerging concerning the pro-coagulant effect of MSCs through the release of the tissue factor (TF) [5,6,13,14,42] which could increase the risk of clot formation. MSCs express the surface tissue factor (TF) and can also release it in the microenvironment. Several inflammatory mediators can trigger the expression of the TF by the MSCs [43]. Moll et al. first described that the increased passaging of MSCs during culture leading to a remarkable increase in the expression of the TF on the cell surface of the MSCs [44]. This was also independently verified by Tatsumi and Liao in subsequent studies that further discussed the tissue sources from which the cells were derived [45,46]. Recently, Moll et al. summarized the occurrence of the adverse thrombotic reactions upon the infusion of the TF expressing MSCs in patients [5]. The intrinsic blood-activating properties of MSCs, depending on their source, have been studied by several authors who have discussed how to control the activation of the clotting system [47,48].
As mentioned in the previous chapter, the TF triggers the extrinsic blood coagulation cascade by forming the complex TF:VIIa, followed by the activation of factors X and IX, thereby leading to the thrombin formation from the zymogen prothrombin [6,17]. The cultured MSCs, once they are exposed to blood, generate an immune response called the instant mediated blood mediated inflammatory response (IBMIR) that complements the coagulation activation together with the activation of the leukocyte and platelet populations; in 2012, Moll et al. reported for first time about the IBMIR, in relation to clinical MSC products [44,49]. This response was initiated by the increased formation of thrombin and clotting factors, such as activated factors VIIa, XIa, and XIIa. A clear dose-dependent correlation has been described between the MSC-associated TF with a clot formation; a higher TF density measured by flow cytometry was associated with a shorter clotting time and a greater thrombin production [23]. Different MSC tissue sources display highly variable expressions of the TF. For example, it has been shown that isolated umbilical cord (UC), bone marrow (BM), and adipose tissue (AT) derived MSCs express the TF in cell culture conditions. However, the BM-MSC expresses lower levels of the TF than the AT-MSC or the UC-MSC [48,50]; the BM-MSC showed a much lower pro-coagulant activity in vitro; meanwhile, the AT-MSC exhibited a high pro-coagulant activity.
In vivo, the administration of the low TF-expressing BM-MSCs in rats did not show any intravascular clot formation; meanwhile, the administration of the TF-expressing UC-MSC triggered an intravascular thromboembolism in the lungs, liver, and spleen [48]. Likewise, it has been reported that the TF-expressing BM-MSC intravenous administration in mice spreads the micro-thrombi in the heart, kidney, spleen, and liver [46]; that effect could be prevented by the administration of heparin (400 U/kg). Consequently, as the AT-MSCs seems more consistently pro-coagulant than the BM-MSCs, they are presenting a potential safety concern for the systemic administration in coagulopathic patients.
While a considerable number of in vitro and pre-clinical studies have confirmed that MSCs exert a pro-coagulant effect after blood contact, it is clear that in clinical studies, the adverse events related to thrombosis exist, however they are underreported and these limited studies report these thrombotic events in humans associated with an MSC administration [51]; here we summarize them. The intravenous administration of MSCs in 44 patients, who underwent a hematopoietic stem cell transplantation and who suffered from a weak IBMIR, the response was highly variable depending on the donor, the number of administered cells, and their cellular passage [44]. Moreover, the cell cryopreservation and freeze-thawing effects should also be considered; the IBMIR was slightly augmented in the thawed cells, likely due to the release of the intracellular TF that may increase clotting and trigger a complement activation [52,53,54]. Moll et al. found that the patients exhibited an increased formation of the blood activation markers but no formation of the hyperfibrinolysis marker D-dimer [44]. In Phase 1b/2a of the clinical study into Crohn’s disease, Melmed et al. reported two patients suffering from venous thrombosis after the infusion of a placenta isolated high-TF-expressing MSC. They associated the adverse effect with the TF expression as a cause [6,14,44,55]. A man who received multiple systemic AT-MSC infusions for a herniated cervical disc, presented with a bilateral pulmonary embolus one month after receiving the last one [56]. Another 73-year-old man enrolled in a clinical trial died from a pulmonary embolism after receiving an infusion of AT-MSCs [57]. However, both studies do not describe the cell source, the manufacturing process, or the internal control of the administered cells. Numerous clinical trials using MSC therapies are in progress, and the TF expression may arise as a safety principle. Furthermore, many patients who may benefit from MSC therapy are likely to be in a hypercoagulable state or at a high risk of a thrombotic event due to their primary pathology. Lalu et al. reported, in their systematic review and meta-analysis, that MSC therapy appears to be safe based on the current clinical trials, nevertheless they mention that further larger scale controlled clinical trials are required in order to further define the safety profile of the MSCs [58]. Therefore, monitoring the pro-thrombotic effects of the MSCs during manufacture in order to ensure that they show the desired characteristics prior to patient administration should be part of the evaluated criteria in clinical trials. Moll et al. highlight, in several of their most recent publications, the clear need for new and improved clinical guidelines regarding the usage of MSC-derived therapeutics [5,6,13].
The TF is not the unique pro-coagulation factor expressed by MSCs; MSCs also express phosphatidylserine, which, after moving from the cytoplasm to the cell surface (membrane), can trigger the thrombin-activating complex and thereby activate coagulation [59,60].
Moreover, an preliminary in vitro study that confirmed that cultured fibrin-embedded MSCs express the plasminogen activators uPA, tPA, and PAI [61], thereby suggesting that MSCs are also involved in the fibrinolytic cascade. In vivo, the autologous AT-MSCs administration induced peripheral micro thrombosis in 30 diabetic patients (type 1 and 2) with limb ischemia [62]. Furthermore, the AT-MSCs derived from the type 2 diabetic patients exhibited a decreased serum-independent fibrinolytic activity. Later studies demonstrated that the AT-MSCs isolated from the diabetic patients released greater amounts of PAI-1, less tPA, and formed fewer D-dimers compared with the MSCs isolated from healthy patients. In addition, the diabetic AT-MSCs presented an upregulation of the expression of the TF and displayed an altered signaling of platelet-derived growth factor (PDGF) [63]. Consequently, MSCs seem to play a dual role by provoking the IBMIR explained by the TF secretion and by producing fibrinolytic enzymes that can activate the fibrinolytic cascade.
3.2. Effect of MSCs in Platelet Formation
MSCs can play a role in coagulation by acting directly in the platelet production and activation. Kim et al. have shown that the co-administration of MSCs together with a bone marrow transplantation (BMT) can trigger a platelet production, thereby enhancing coagulation. These results suggest that MSCs can be therapeutic in preventing thrombocytopenia after the BMT by promoting megakaryopoiesis and platelet differentiation [64].
In agreement, Liao et al. described that the BM-MSC infusion decreased platelet numbers in blood in a concentration-dependent manner [46]. The infusion of more than 1 × 106 MSCs prolonged the prothrombin time (PT), activated the partial thromboplastin time (APTT), and decreased fibrinogen and factor VIII concentrations. The increased numbers of the MSC administration were associated with acute adverse events, including an IBMIR [44] and microvascular embolisms [65,66,67]. The infusion of a small dose of MSCs affected the PT but not the APTT, thereby suggesting that the main activation of coagulation occurred through the TF and the extrinsic coagulation pathway. The administration of a TFPI was able to prevent an MSC-induced coagulation. The pre-injection of the heparin also prevented the MSC-induced coagulation reaction, which prevented the circulatory and respiratory failure produced for MSCs in higher doses.
3.3. MSC Released Soluble Factors Affecting Coagulation
The MSC-conditioned medium was tested for platelet activation in order to demonstrate whether they released any soluble factor that could trigger clot formation and enhance coagulation. The MSC-conditioned medium did not affect the platelet activation, in neither the resting platelets nor in the stimulated ones. The conditioned medium obtained from the MSC culturing slightly suppressed the expression of CD62p and Pac-1, which are two platelet activation markers.
Moreover, the extravesicles (EVs) secreted by MSCs were also analyzed. The TF was not detected in the EVs, which supports that the MSC-induced coagulation may be TF-mediated, but the TF is not a relevant factor in the pro-coagulant effect of the EVs. Previously, it has been shown that the monocyte and platelet-secreted EVs trigger a thrombin generation and propagation of the coagulation through the exposure of phosphatidylserine, and the TF was only detected in pathological conditions [68]. It has been described that the MSC-derived EVs can expose phosphatidylserine onto their surface [69].Phosphatidylserine offers a binding, the catalytic point that leads to the formation of factors Va, VIIIa, Ixa, X, and Xa and to the activation of the TF.
We may speculate that the coagulation activity of the EVs derived from MSCs is associated with the activation of the coagulation cascade by phosphatidylserine rather than the TF [59,70]. Silachev et al. revealed that only a part of the MSC and EV populations (3–4%) were annexin V-positive and presented phosphatidylserine on their surface; the authors confirmed the phosphatidylserine involvement in the pro-coagulant effects caused by MSCs and their EVs [60].
4. Conclusions
The MSC-associated coagulation effects have been described and studied; however, a lot of research is still needed in order to better determine which cell source for obtaining MSCs is the best in order to avoid a pro-thrombotic response due to their infusion. In addition, in order to characterize the MSC expression markers such as the TF, we need to control the cell dose, the culture passage, and the administration route, which may help to reduce and avoid adverse coagulation events triggered by MSC therapies.
Funding
This study was funded by Instituto de Salud Carlos III through the Miguel Servet, contract “CP20/00133 (ISCIII-AES-2019/002067) “and co-funded by the European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”.
Conflicts of Interest
The author declares no conflict of interest.
References
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning in Vitro and Retransplantation in Vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem versus Stromal Cells: International Society for Cell and Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Olson, S.D.; Nolta, J.A. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Transl. Med. 2022, 11, 2–13. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringdén, O.; Volk, H.-D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Jiang, X.-X.; Zhang, Y.; Liu, B.; Zhang, S.-X.; Wu, Y.; Yu, X.-D.; Mao, N. Human Mesenchymal Stem Cells Inhibit Differentiation and Function of Monocyte-Derived Dendritic Cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef]
- Ramasamy, R.; Fazekasova, H.; Lam, E.W.-F.; Soeiro, I.; Lombardi, G.; Dazzi, F. Mesenchymal Stem Cells Inhibit Dendritic Cell Differentiation and Function by Preventing Entry into the Cell Cycle. Transplantation 2007, 83, 71–76. [Google Scholar] [CrossRef]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human Mesenchymal Stem Cells Alter Antigen-Presenting Cell Maturation and Induce T-Cell Unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Moll, G.; Drzeniek, N.; Kamhieh-Milz, J.; Geissler, S.; Volk, H.-D.; Reinke, P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front. Immunol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Ringdén, O.; Moll, G.; Gustafsson, B.; Sadeghi, B. Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-Versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome. Front. Immunol. 2022, 13, 839844. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.A.; Morrissey, J.H.; Edgington, T.S. Selective Cellular Expression of Tissue Factor in Human Tissues. Implications for Disorders of Hemostasis and Thrombosis. Am. J. Pathol. 1989, 134, 1087–1097. [Google Scholar]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue Factor as a Link between Inflammation and Coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef]
- Catar, R.; Moll, G.; Hosp, I.; Simon, M.; Luecht, C.; Zhao, H.; Wu, D.; Chen, L.; Kamhieh-Milz, J.; Korybalska, K.; et al. Transcriptional Regulation of Thrombin-Induced Endothelial VEGF Induction and Proangiogenic Response. Cells 2021, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.H.; Butenas, S.; Mann, K.G. The Evaluation of Complex-Dependent Alterations in Human Factor VIIa. J. Biol. Chem. 1992, 267, 4834–4843. [Google Scholar] [CrossRef]
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arter. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef]
- Rao, L.V.M.; Pendurthi, U.R. Regulation of Tissue Factor Coagulant Activity on Cell Surfaces. J. Thromb. Haemost. 2012, 10, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Butenas, S.; Mann, K.G. Active Tissue Factor in Blood? Nat. Med. 2004, 10, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- George, M.J.; Prabhakara, K.; Toledano-Furman, N.E.; Wang, Y.-W.; Gill, B.S.; Wade, C.E.; Olson, S.D.; Cox, C.S. Clinical Cellular Therapeutics Accelerate Clot Formation. Stem Cells Transl. Med. 2018, 7, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Sabroe, I.; Parker, L.C.; Dower, S.K.; Whyte, M.K.B. The Role of TLR Activation in Inflammation. J. Pathol. 2008, 214, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Laplante, P.; Fuentes, R.; Salem, D.; Subang, R.; Gillis, M.-A.; Hachem, A.; Farhat, N.; Qureshi, S.T.; Fletcher, C.A.; Roubey, R.A.S.; et al. Antiphospholipid Antibody-Mediated Effects in an Arterial Model of Thrombosis Are Dependent on Toll-like Receptor 4. Lupus 2016, 25, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.J.; Aghakasiri, N.; Rumbaut, R.E. Platelet-Derived Toll-like Receptor 4 (Tlr-4) Is Sufficient to Promote Microvascular Thrombosis in Endotoxemia. PLoS ONE 2012, 7, e41254. [Google Scholar] [CrossRef]
- Ren, M.; Li, R.; Luo, M.; Chen, N.; Deng, X.; Yan, K.; Zeng, M.; Wu, J. Endothelial Cells but Not Platelets Are the Major Source of Toll-like Receptor 4 in the Arterial Thrombosis and Tissue Factor Expression in Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R901–R907. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Liu, J.; Lv, B.; Chen, F. Extracellular Histones Induce Tissue Factor Expression in Vascular Endothelial Cells via TLR and Activation of NF-ΚB and AP-1. Thromb. Res. 2016, 137, 211–218. [Google Scholar] [CrossRef]
- El Kebir, D.; Damlaj, A.; Makhezer, N.; Filep, J.G. Toll-like Receptor 9 Signaling Regulates Tissue Factor and Tissue Factor Pathway Inhibitor Expression in Human Endothelial Cells and Coagulation in Mice. Crit. Care Med. 2015, 43, e179–e189. [Google Scholar] [CrossRef]
- Fujita, T. Evolution of the Lectin-Complement Pathway and Its Role in Innate Immunity. Nat. Rev. Immunol. 2002, 2, 346–353. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, T. The Lectin Pathway. Res. Immunol. 1996, 147, 115–118. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Ricklin, D.; Ward, P.A.; Lambris, J.D. Interactions between Coagulation and Complement—Their Role in Inflammation. Semin. Immunopathol. 2012, 34, 151–165. [Google Scholar] [CrossRef]
- Afshar-Kharghan, V. Complement and Clot. Blood 2017, 129, 2214–2215. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.E.; Vaporciyan, A.A.; Bonish, B.K.; Jones, M.L.; Johnson, K.J.; Glovsky, M.M.; Eddy, S.M.; Ward, P.A. C5a-Induced Expression of P-Selectin in Endothelial Cells. J. Clin. Invest. 1994, 94, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S.; Rittirsch, D.; Neff, T.A.; McGuire, S.R.; Lambris, J.D.; Warner, R.L.; Flierl, M.A.; Hoesel, L.M.; et al. Generation of C5a in the Absence of C3: A New Complement Activation Pathway. Nat. Med. 2006, 12, 682–687. [Google Scholar] [CrossRef]
- Kastl, S.P.; Speidl, W.S.; Kaun, C.; Rega, G.; Assadian, A.; Weiss, T.W.; Valent, P.; Hagmueller, G.W.; Maurer, G.; Huber, K.; et al. The Complement Component C5a Induces the Expression of Plasminogen Activator Inhibitor-1 in Human Macrophages via NF-KappaB Activation. J. Thromb. Haemost. 2006, 4, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Spath, B.; Fischer, C.; Stolz, M.; Ayuk, F.A.; Kröger, N.; Bokemeyer, C.; Ruf, W. Rapid Activation of Monocyte Tissue Factor by Antithymocyte Globulin Is Dependent on Complement and Protein Disulfide Isomerase. Blood 2013, 121, 2324–2335. [Google Scholar] [CrossRef]
- Collen, D. The Plasminogen (Fibrinolytic) System. Thromb. Haemost. 1999, 82, 259–270. [Google Scholar] [CrossRef]
- Pepper, M.S. Extracellular Proteolysis and Angiogenesis. Thromb. Haemost. 2001, 86, 346–355. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular Intercommunication between the Complement and Coagulation Systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating Platelet and Coagulation Activation in Fibrin Clot Formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Procoagulant Activity of Human Mesenchymal Stem Cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Rasmusson-Duprez, I.; von Bahr, L.; Connolly-Andersen, A.-M.; Elgue, G.; Funke, L.; Hamad, O.A.; Lönnies, H.; Magnusson, P.U.; Sanchez, J.; et al. Are Therapeutic Human Mesenchymal Stromal Cells Compatible with Human Blood? Stem Cells 2012, 30, 1565–1574. [Google Scholar] [CrossRef]
- Tatsumi, K.; Ohashi, K.; Matsubara, Y.; Kohori, A.; Ohno, T.; Kakidachi, H.; Horii, A.; Kanegae, K.; Utoh, R.; Iwata, T.; et al. Tissue Factor Triggers Procoagulation in Transplanted Mesenchymal Stem Cells Leading to Thromboembolism. Biochem. Biophys. Res. Commun. 2013, 431, 203–209. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin Improves BMSC Cell Therapy: Anticoagulant Treatment by Heparin Improves the Safety and Therapeutic Effect of Bone Marrow-Derived Mesenchymal Stem Cell Cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef]
- Moll, G.; Ignatowicz, L.; Catar, R.; Luecht, C.; Sadeghi, B.; Hamad, O.; Jungebluth, P.; Dragun, D.; Schmidtchen, A.; Ringdén, O. Different Procoagulant Activity of Therapeutic Mesenchymal Stromal Cells Derived from Bone Marrow and Placental Decidua. Stem Cells Dev. 2015, 24, 2269–2279. [Google Scholar] [CrossRef]
- Oeller, M.; Laner-Plamberger, S.; Hochmann, S.; Ketterl, N.; Feichtner, M.; Brachtl, G.; Hochreiter, A.; Scharler, C.; Bieler, L.; Romanelli, P.; et al. Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells. Theranostics 2018, 8, 1421–1434. [Google Scholar] [CrossRef]
- Moll, G.; Jitschin, R.; von Bahr, L.; Rasmusson-Duprez, I.; Sundberg, B.; Lönnies, L.; Elgue, G.; Nilsson-Ekdahl, K.; Mougiakakos, D.; Lambris, J.D.; et al. Mesenchymal Stromal Cells Engage Complement and Complement Receptor Bearing Innate Effector Cells to Modulate Immune Responses. PLoS ONE 2011, 6, e21703. [Google Scholar] [CrossRef]
- Wu, X.; Darlington, D.N.; Christy, B.A.; Liu, B.; Keesee, J.D.; Salgado, C.L.; Bynum, J.A.; Cap, A.P. Intravenous Administration of Mesenchymal Stromal Cells Leads to a Dose-Dependent Coagulopathy and Is Unable to Attenuate Acute Traumatic Coagulopathy in Rats. J. Trauma Acute Care Surg. 2022, 92, 542–552. [Google Scholar] [CrossRef]
- Bauer, G.; Elsallab, M.; Abou-El-Enein, M. Concise Review: A Comprehensive Analysis of Reported Adverse Events in Patients Receiving Unproven Stem Cell-Based Interventions. Stem Cells Transl. Med. 2018, 7, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Alm, J.J.; Davies, L.C.; von Bahr, L.; Heldring, N.; Stenbeck-Funke, L.; Hamad, O.A.; Hinsch, R.; Ignatowicz, L.; Locke, M.; et al. Do Cryopreserved Mesenchymal Stromal Cells Display Impaired Immunomodulatory and Therapeutic Properties? Stem Cells 2014, 32, 2430–2442. [Google Scholar] [CrossRef]
- Cottle, C.; Porter, A.P.; Lipat, A.; Turner-Lyles, C.; Nguyen, J.; Moll, G.; Chinnadurai, R. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Curr. Stem Cell Rep. 2022, 8, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Geißler, S.; Catar, R.; Ignatowicz, L.; Hoogduijn, M.J.; Strunk, D.; Bieback, K.; Ringdén, O. Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? Adv. Exp. Med. Biol. 2016, 951, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.Y.; Pandak, W.M.; Casey, K.; Abraham, B.; Valentine, J.; Schwartz, D.; Awais, D.; Bassan, I.; Lichtiger, S.; Sands, B.; et al. Human Placenta-Derived Cells (PDA-001) for the Treatment of Moderate-to-Severe Crohn’s Disease: A Phase 1b/2a Study. Inflamm. Bowel Dis. 2015, 21, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial Occurrence of Pulmonary Embolism after Intravenous, Adipose Tissue-Derived Stem Cell Therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Korean Deaths Spark Inquiry. Nature 2010, 468, 485–486. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Canadian Critical Care Trials Group Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Spronk, H.M.H.; ten Cate, H.; van der Meijden, P.E.J. Differential Roles of Tissue Factor and Phosphatidylserine in Activation of Coagulation. Thromb. Res. 2014, 133, S54–S56. [Google Scholar] [CrossRef]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef]
- Neuss, S.; Schneider, R.K.M.; Tietze, L.; Knüchel, R.; Jahnen-Dechent, W. Secretion of Fibrinolytic Enzymes Facilitates Human Mesenchymal Stem Cell Invasion into Fibrin Clots. Cells Tissues Organs 2010, 191, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Acosta, L.; Hmadcha, A.; Escacena, N.; Pérez-Camacho, I.; de la Cuesta, A.; Ruiz-Salmeron, R.; Gauthier, B.R.; Soria, B. Adipose Mesenchymal Stromal Cells Isolated from Type 2 Diabetic Patients Display Reduced Fibrinolytic Activity. Diabetes 2013, 62, 4266–4269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capilla-González, V.; López-Beas, J.; Escacena, N.; Aguilera, Y.; de la Cuesta, A.; Ruiz-Salmerón, R.; Martín, F.; Hmadcha, A.; Soria, B. PDGF Restores the Defective Phenotype of Adipose-Derived Mesenchymal Stromal Cells from Diabetic Patients. Mol. Ther. 2018, 26, 2696–2709. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Lee, H.-J.; Cho, K.-A.; Kim, J.; Park, J.-W.; Woo, S.-Y.; Ryu, K.-H. Promotion of Platelet Production by Co-Transplantation of Mesenchymal Stem Cells in Bone Marrow Transplantation. Tissue Eng. Regen. Med. 2022, 19, 131–139. [Google Scholar] [CrossRef]
- Gleeson, B.M.; Martin, K.; Ali, M.T.; Kumar, A.H.S.; Pillai, M.G.-K.; Kumar, S.P.G.; O’Sullivan, J.F.; Whelan, D.; Stocca, A.; Khider, W.; et al. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells 2015, 33, 2726–2737. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of Culture-Expanded Mesenchymal Stem Cells in the Microvasculature: In Vivo Observations of Cell Kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary Passage Is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef]
- Lai, R.C.; Lim, S.K. Membrane Lipids Define Small Extracellular Vesicle Subtypes Secreted by Mesenchymal Stromal Cells. J. Lipid Res. 2019, 60, 318–322. [Google Scholar] [CrossRef]
- van der Poll, T.; Herwald, H. The Coagulation System and Its Function in Early Immune Defense. Thromb. Haemost. 2014, 112, 640–648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).