Exposome, Molecular Pathways and One Health: The Invertebrate Caenorhabditis elegans
Abstract
:1. Introduction
2. C. elegans in the Environment
3. How Does C. elegans Encounter Pollutants?
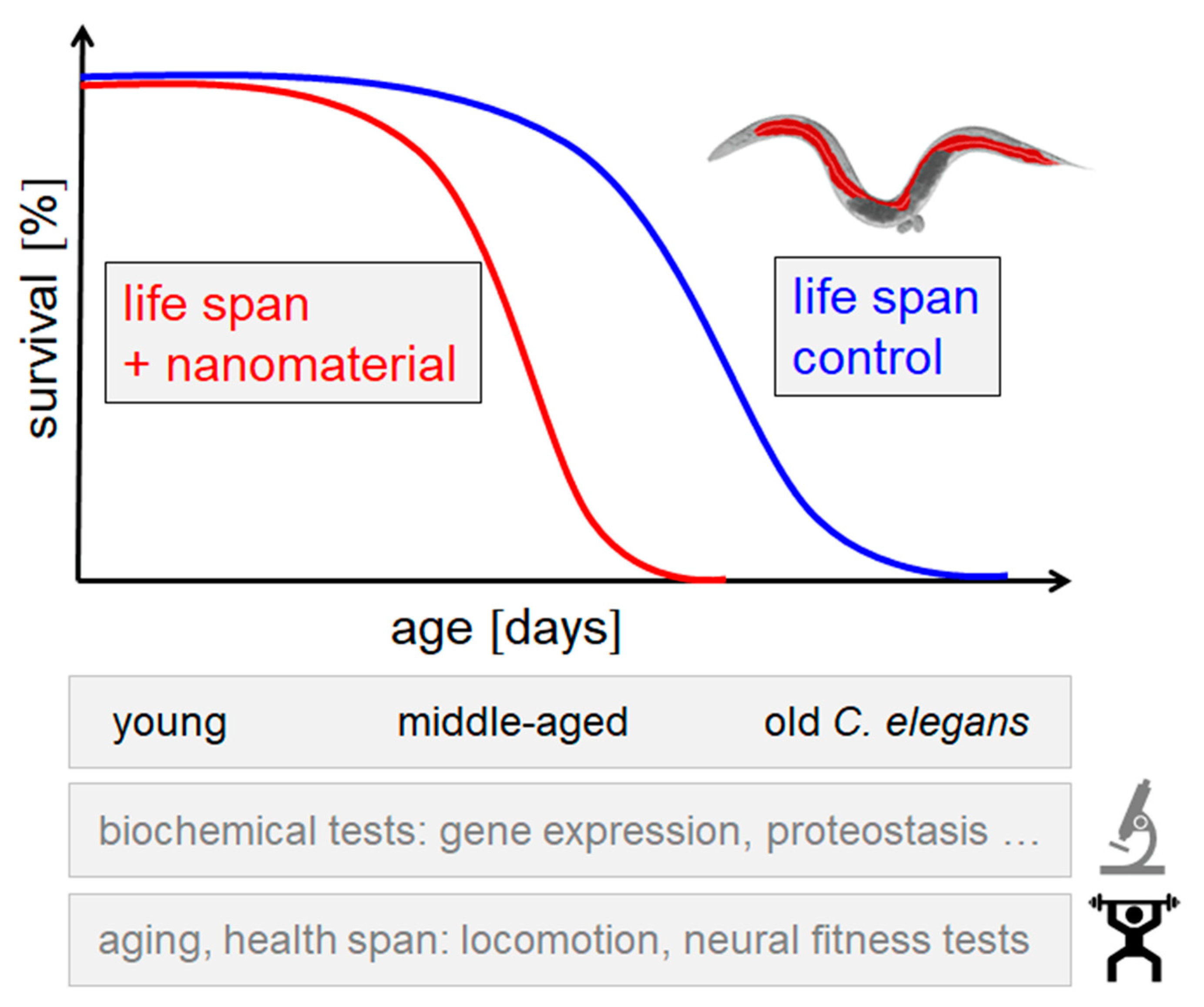
4. The Animal Model C. elegans
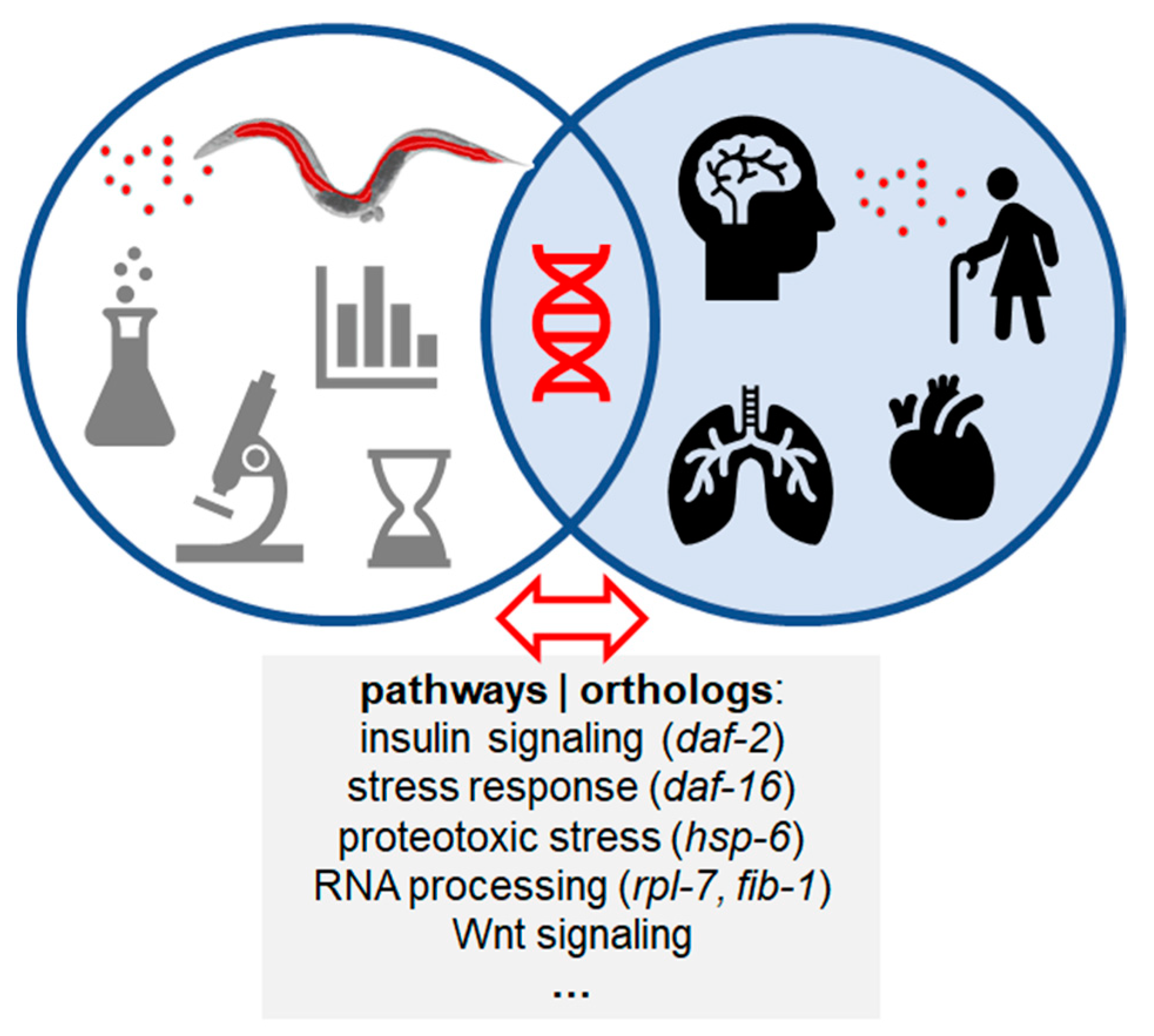
5. Contribution of C. elegans to the Exposome and One Health
6. From Worms to Men
7. Neurodegeneration and Neuronal Death in C. elegans PD Models
8. Investigation of Pollutant Effects in Wild C. elegans
9. Future Goals and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Hu, H.; Caravanos, J.; Cropper, M.L.; Hanrahan, D.; Sandilya, K.; Chiles, T.C.; Kumar, P.; Suk, W.A. Pollution and Global Health—An Agenda for Prevention. Environ. Health Perspect. 2018, 126, 084501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kalinina, A.; Sun, T.; Nowack, B. Probabilistic modeling of the flows and environmental risks of nano-silica. Sci. Total Environ. 2016, 545–546, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nowack, B. Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions. Environ. Pollut. 2018, 235, 589–601. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wick, P.; Nowack, B. Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 2021, 16, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Kiontke, K.; Sudhaus, W. Ecology of Caenorhabditis Species. In WormBook: The Online Review of C. elegans Biology. 2006. Available online: http://www.wormbook.org/chapters/www_ecolCaenorhabditis/ecolCaenorhabditis.html (accessed on 28 July 2022).
- Sommer, R.J.; Streit, A. Comparative genetics and genomics of nematodes: Genome structure, development, and lifestyle. Annu. Rev. Genet. 2011, 45, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.M.; Ashton, L.A.; Parr, C.L.; Eggleton, P. The impact of invertebrate decomposers on plants and soil. New Phytol. 2021, 231, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Frézal, L.; Félix, M.A. C. elegans outside the Petri dish. eLife 2015, 4, e05849. [Google Scholar] [CrossRef]
- Lamborg, C.H.; Hammerschmidt, C.R.; Bowman, K.L.; Swarr, G.J.; Munson, K.M.; Ohnemus, D.C.; Lam, P.J.; Heimbürger, L.E.; Rijkenberg, M.J.; Saito, M.A. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 2014, 512, 65–68. [Google Scholar] [CrossRef]
- Streets, D.G.; Horowitz, H.M.; Jacob, D.J.; Lu, Z.; Levin, L.; Ter Schure, A.F.H.; Sunderland, E.M. Total Mercury Released to the Environment by Human Activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef]
- Huang, J.H.; Shetaya, W.H.; Osterwalder, S. Determination of (Bio)-available mercury in soils: A review. Environ. Pollut. 2020, 263, 114323. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, C.S.; Murayama, M.; Hochella, M.F. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products. Environ. Sci. Technol. 2010, 44, 7509–7514. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, I.; Sun, T.Y.; Clark, J.R.; Dobson, P.J.; Hungerbuehler, K.; Owen, R.; Nowack, B.; Lead, J. Probabilistic modelling of prospective environmental concentrations of gold nanoparticles from medical applications as a basis for risk assessment. J. Nanobiotechnology 2015, 13, 93. [Google Scholar] [CrossRef]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- von Mikecz, A. Lifetime Eco-Nanotoxicology in an Adult Organism: Where and When Is the Invertebrate, C. elegans Vulnerable? Environ. Sci. Nano 2018, 5, 616–622. [Google Scholar] [CrossRef]
- Unrine, J.M.; Shoults-Wilson, W.A.; Zhurbich, O.; Bertsch, P.M.; Tsyusko, O.V. Trophic transfer of Au nanoparticles from soil along a simulated terrestrial food chain. Environ. Sci. Technol. 2012, 46, 9753–9760. [Google Scholar] [CrossRef]
- Koo, Y.; Wang, J.; Zhang, Q.; Zhu, H.; Chehab, E.W.; Colvin, V.L.; Alvarez, P.J.; Braam, J. Fluorescence reports intact quantum dot uptake into roots and translocation to leaves of Arabidopsis thaliana and subsequent ingestion by insect herbivores. Environ. Sci. Technol. 2015, 49, 626–632. [Google Scholar] [CrossRef]
- Pluskota, A.; Horzowski, E.; Bossinger, O.; von Mikecz, A. In Caenorhabditis elegans nanoparticle-bio-interactions become transparent: Silica-nanoparticles induce reproductive senescence. PLoS ONE 2009, 4, e6622. [Google Scholar] [CrossRef]
- Contreras, E.Q.; Cho, M.; Zhu, H.; Puppala, H.L.; Escalera, G.; Zhong, W.; Colvin, V.L. Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ. Sci. Technol. 2013, 47, 1148–1154. [Google Scholar] [CrossRef]
- Jung, S.K.; Qu, X.; Aleman-Meza, B.; Wang, T.; Riepe, C.; Liu, Z.; Li, Q.; Zhong, W. Multi-endpoint, high-throughput study of nanomaterial toxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2015, 49, 2477–2485. [Google Scholar] [CrossRef]
- Ratnasekhar, C.; Sonane, M.; Satish, A.; Mudiam, M.K. Metabolomics reveals the perturbations in the metabolome of Caenorhabditis elegans exposed to titanium dioxide nanoparticles. Nanotoxicology 2015, 9, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Gührs, K.H.; von Mikecz, A. Anti-amyloid compounds protect from silica nanoparticle-induced neurotoxicity in the nematode C. elegans. Nanotoxicology 2016, 10, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Piechulek, A.; von Mikecz, A. Life span-resolved nanotoxicology enables identification of age-associated neuromuscular vulnerabilities in the nematode Caenorhabditis elegans. Environ. Pollut. 2018, 233, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Nance, J.; Frøkjær-Jensen, C. The Caenorhabditis elegans Transgenic Toolbox. Genetics 2019, 212, 959–990. [Google Scholar] [CrossRef]
- Richardson, C.E.; Shen, K. Neurite Development and Repair in Worms and Flies. Annu. Rev. Neurosci. 2019, 42, 209–226. [Google Scholar] [CrossRef]
- Watanabe, S.; Liu, Q.; Davis, M.W.; Hollopeter, G.; Thomas, N.; Jorgensen, N.B.; Jorgensen, E.M. Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. eLife 2013, 2, e00723. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Watson, E.; MacNeil, L.T.; Ritter, A.D.; Yilmaz, L.S.; Rosebrock, A.P.; Caudy, A.A.; Walhout, A.J. Interspecies systems biology uncovers metabolites affecting C. elegan gene expression and life history traits. Cell 2014, 156, 759–770. [Google Scholar] [CrossRef]
- Solis, G.M.; Petrascheck, M. Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J. Vis. Exp. 2011, 49, 2496. [Google Scholar] [CrossRef]
- Scharf, A.; Piechulek, A.; von Mikecz, A. Effect of nanoparticles on the biochemical and behavioral aging phenotype of the nematode Caenorhabditis elegans. ACS Nano 2013, 7, 10695–10703. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.M.; Kasturi, P.; Zheng, M.; Pinkert, S.; Vecchi, G.; Ciryam, P.; Morimoto, R.I.; Dobson, C.M.; Vendruscolo, M.; Mann, M.; et al. Widespread Proteome Remodeling and Aggregation in Aging, C. elegans. Cell 2017, 168, 944. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Limke, A.; Guehrs, K.H.; von Mikecz, A. Pollutants corrupt resilience pathways of aging in the nematode C. elegans. iScience 2022, in press. [Google Scholar]
- Gao, P. The Exposome in the Era of One Health. Environ. Sci. Technol. 2021, 55, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Ijomone, O.M.; Iroegbu, J.D.; Aschner, M.; Bornhorst, J. Impact of environmental toxicants on p38- and ERK-MAPK signaling pathways in the central nervous system. Neurotoxicology 2021, 86, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Brignull, H.R.; Moore, F.E.; Tang, S.J.; Morimoto, R.I. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. J. Neurosci. 2006, 26, 7597–7606. [Google Scholar] [CrossRef]
- Lim, S.F.; Riehn, R.; Ryu, W.S.; Khanarian, N.; Tung, C.K.; Tank, D.; Austin, R.H. In vivo and scanning electron microscopy imaging of up-converting nanophosphors in Caenorhabditis elegans. Nano Lett. 2006, 6, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47–58. [Google Scholar] [CrossRef]
- Benner, J.; Daniel, H.; Spanier, B. A glutathione peroxidase, intracellular peptidases and the TOR complexes regulate peptide transporter PEPT-1 in C. elegans. PLoS ONE 2011, 6, e25624. [Google Scholar] [CrossRef]
- Piechulek, A.; Berwanger, L.C.; von Mikecz, A. Silica nanoparticles disrupt OPT-2/PEP-2-dependent trafficking of nutrient peptides in the intestinal epithelium. Nanotoxicology 2019, 13, 1133–1148. [Google Scholar] [CrossRef]
- Hering, I.; Le, D.T.; von Mikecz, A. How to keep up with the analysis of classic and emerging neurotoxins: Age-resolved fitness tests in the animal model Caenorhabditis elegans: A step-by-step protocol. EXCLI J. 2022, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Cornelius, T.; Morimoto, R.I. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 2008, 320, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Labella, S.; Korneeva, N.L.; Keiper, B.D.; Aamodt, E.J.; Zetka, M.; Rhoads, R.E. A C. elegans eIF4E-family member upregulates translation at elevated temperatures of mRNAs encoding MSH-5 and other meiotic crossover proteins. J. Cell Sci. 2010, 123, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Ujisawa, T.; Sonoda, S.; Kuhara, A. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat. Commun. 2014, 5, 4412. [Google Scholar] [CrossRef]
- Lee, S.J.; Kenyon, C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 2009, 19, 715–722. [Google Scholar] [CrossRef]
- C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- von Mikecz, A.; Schikowski, T. Effects of Airborne Nanoparticles on the Nervous System: Amyloid Protein Aggregation, Neurodegeneration and Neurodegenerative Diseases. Nanomater. 2020, 10, 1349. [Google Scholar] [CrossRef]
- Fatouros, C.; Pir, G.J.; Biernat, J.; Koushika, S.P.; Mandelkow, E.; Mandelkow, E.M.; Schmidt, E.; Baumeister, R. Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum. Mol. Genet. 2012, 21, 3587–3603. [Google Scholar] [CrossRef]
- Alexander, A.G.; Marfil, V.; Li, C. Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Front. Genet. 2014, 5, 279. [Google Scholar] [CrossRef]
- Volovik, Y.; Marques, F.C.; Cohen, E. The nematode Caenorhabditis elegans: A versatile model for the study of proteotoxicity and aging. Methods 2014, 68, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Gadea, A.G.; Pierce-Shimomura, J.T. Conserved role of dopamine in the modulation of behavior. Commun. Integr. Biol. 2012, 5, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Uversky, V.N.; Li, J.; Fink, A.L. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J. Biol. Chem. 2001, 276, 10737–10744. [Google Scholar] [CrossRef]
- Martinez, B.A.; Petersen, D.A.; Gaeta, A.L.; Stanley, S.P.; Caldwell, G.A.; Caldwell, K.A. Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. elegans Models of Parkinson’s Disease. J. Neurosci. 2017, 37, 11085–11100. [Google Scholar] [CrossRef]
- Vaux, D.L. CED-4—The third horseman of apoptosis. Cell 1997, 90, 389–390. [Google Scholar] [CrossRef]
- Waller, A.V. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. 1850, 140, 423–429. [Google Scholar] [CrossRef]
- Nichols, A.L.A.; Meelkop, E.; Linton, C.; Giordano-Santini, R.; Sullivan, R.K.; Donato, A.; Nolan, C.; Hall, D.H.; Xue, D.; Neumann, B.; et al. The Apoptotic Engulfment Machinery Regulates Axonal Degeneration in C. elegans Neurons. Cell Rep. 2016, 14, 1673–1683. [Google Scholar] [CrossRef]
- Merlini, E.; Coleman, M.P.; Loreto, A. Mitochondrial dysfunction as a trigger of programmed axon death. Trends Neurosci. 2022, 45, 53–63. [Google Scholar] [CrossRef]
- Rui, Y.; Tiwari, P.; Xie, Z.; Zheng, J.Q. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J. Neurosci. 2006, 26, 10480–10487. [Google Scholar] [CrossRef]
- van Duyn, N.; Nass, R. The putative multidrug resistance protein MRP-7 inhibits methylmercury-associated animal toxicity and dopaminergic neurodegeneration in Caenorhabditis elegans. J. Neurochem. 2014, 128, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Tsatsakis, A.; Santamaría, A.; Antunes Soare, F.A.; Tinkov, A.A.; Docea, A.O.; Skalny, A.; Bowman, A.B.; Aschner, M. Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology 2020, 77, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hobert, O. A map of terminal regulators of neuronal identity in Caenorhabditis elegans. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 474–498. [Google Scholar] [CrossRef] [PubMed]
- von Mikecz, A.; Scharf, A. Pollution—Bring the field into the lab. Nature 2022, 602, 386. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Moon, J.; An, Y.J. A highly efficient nonchemical method for isolating live nematodes (Caenorhabditis elegans) from soil during toxicity assays. Environ. Toxicol. Chem. 2015, 34, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Bensaddek, D.; Narayan, V.; Nicolas, A.; Murillo, A.B.; Gartner, A.; Kenyon, C.J.; Lamond, A.I. Micro-proteomics with iterative data analysis: Proteome analysis in C. elegans at the single worm level. Proteomics 2016, 16, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.; Peter, Q.; Seinstra, R.I.; Perni, M.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J.; Nollen, E.A.A. Assessing motor-related phenotypes of Caenorhabditis elegans with the wide field-of-view nematode tracking platform. Nat. Protoc. 2020, 15, 2071–2106. [Google Scholar] [CrossRef] [PubMed]
- Oeller, M.; Sormanni, P.; Vendruscolo, M. An open-source automated PEG precipitation assay to measure the relative solubility of proteins with low material requirement. Sci. Rep. 2021, 11, 21932. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Mikecz, A. Exposome, Molecular Pathways and One Health: The Invertebrate Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 9084. https://doi.org/10.3390/ijms23169084
von Mikecz A. Exposome, Molecular Pathways and One Health: The Invertebrate Caenorhabditis elegans. International Journal of Molecular Sciences. 2022; 23(16):9084. https://doi.org/10.3390/ijms23169084
Chicago/Turabian Stylevon Mikecz, Anna. 2022. "Exposome, Molecular Pathways and One Health: The Invertebrate Caenorhabditis elegans" International Journal of Molecular Sciences 23, no. 16: 9084. https://doi.org/10.3390/ijms23169084
APA Stylevon Mikecz, A. (2022). Exposome, Molecular Pathways and One Health: The Invertebrate Caenorhabditis elegans. International Journal of Molecular Sciences, 23(16), 9084. https://doi.org/10.3390/ijms23169084




