Gene Expression Profiling of Mono- and Co-Culture Models of the Respiratory Tract Exposed to Crystalline Quartz under Submerged and Air-Liquid Interface Conditions
Abstract
:1. Introduction
2. Results
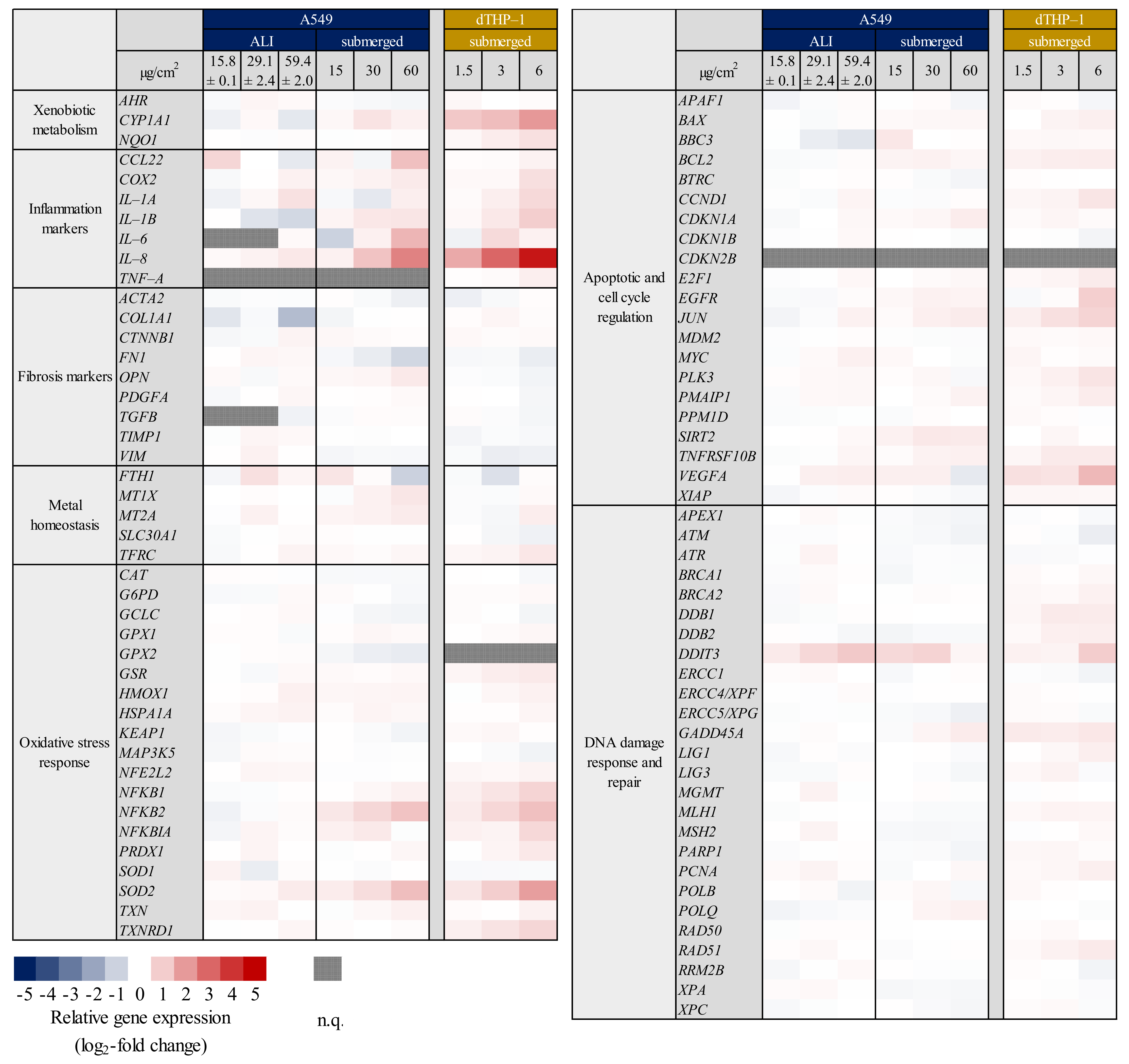
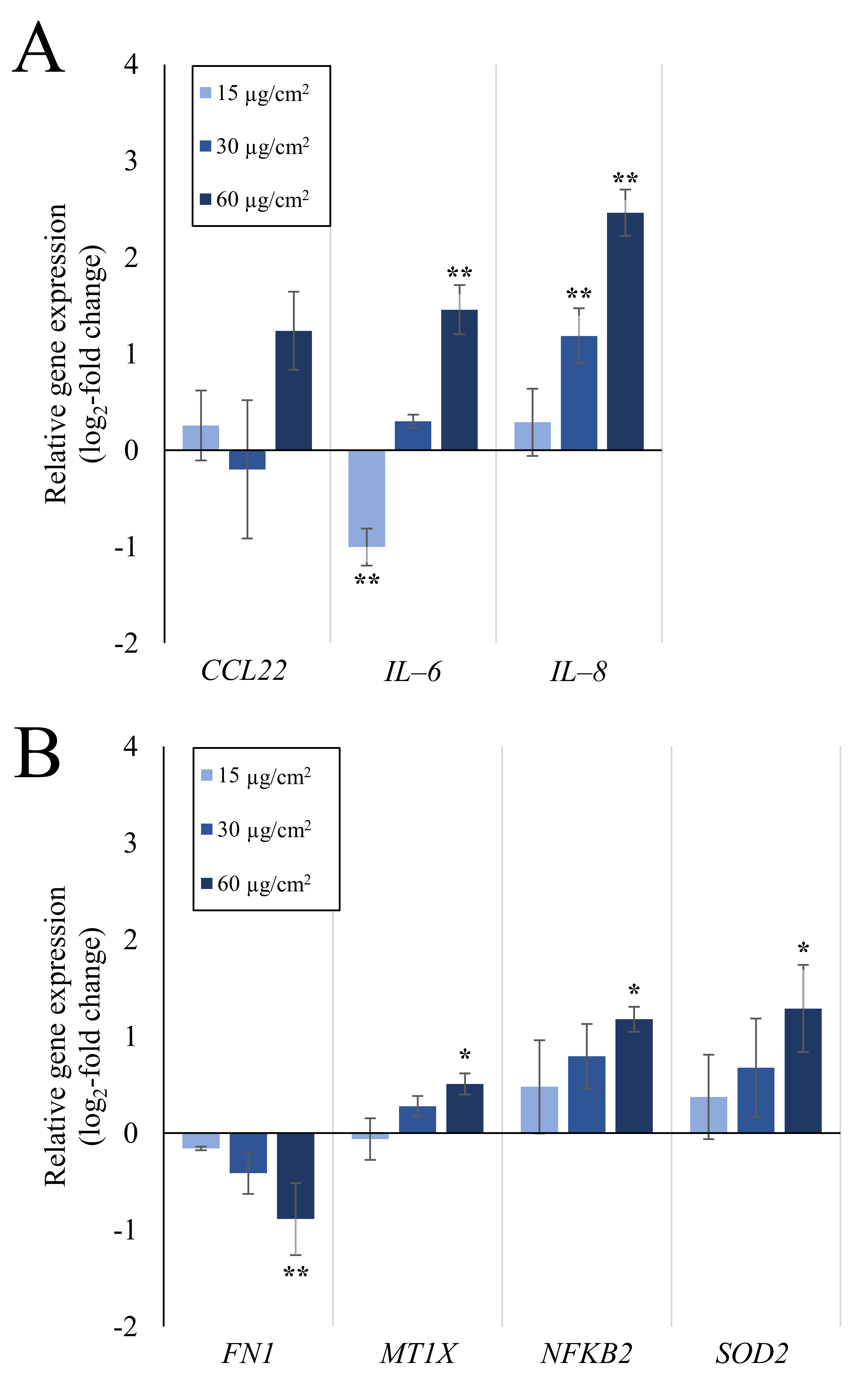
2.1. Inflammation- and Oxidative Stress-Related Gene Expression Is Upregulated in A549 and dTHP-1 Mono-Cultures upon Exposure to Quartz
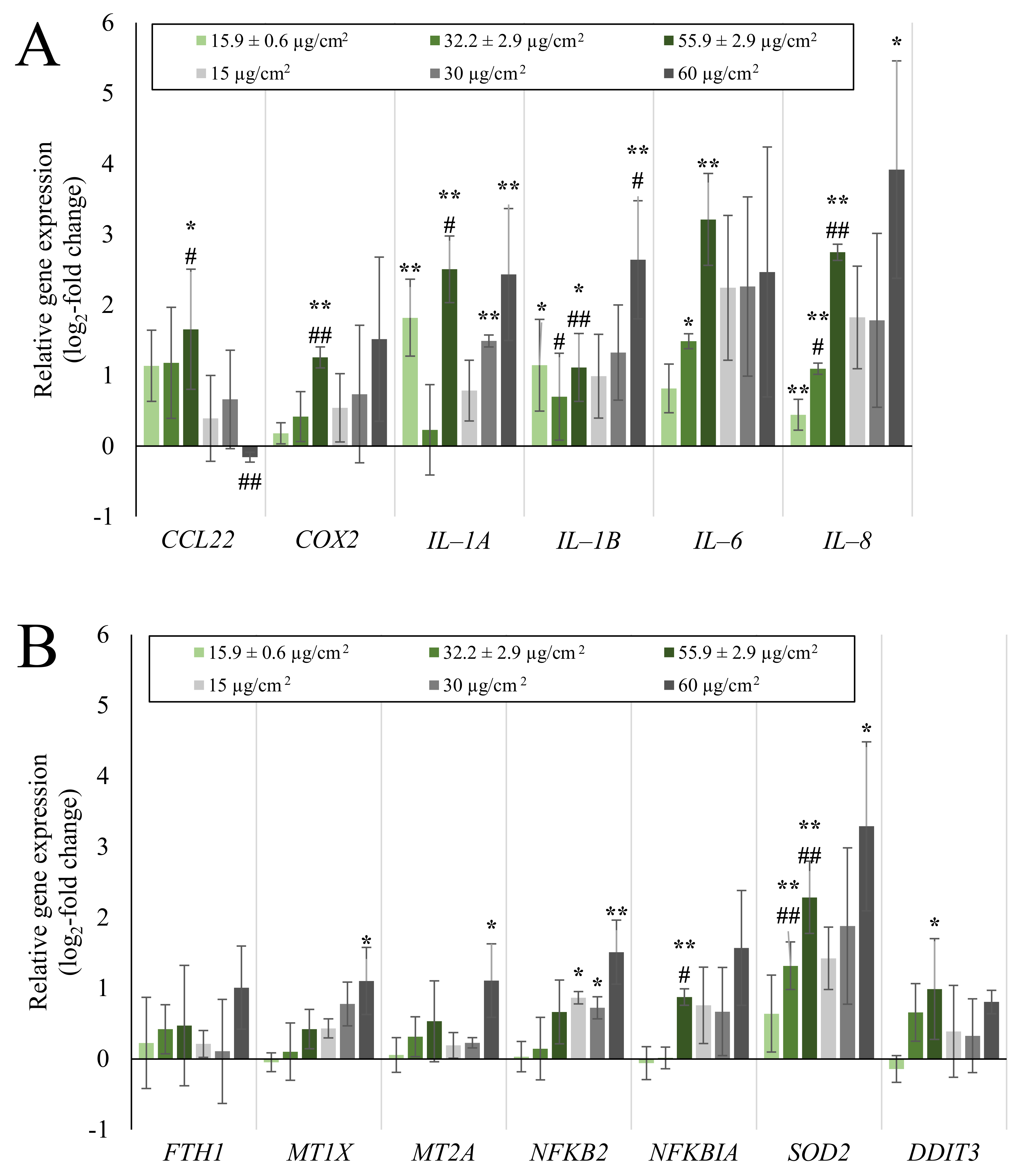
2.2. Inflammatory Gene Induction Is More Pronounced in A549/dTHP-1 Co-Cultures Compared to Mono-Cultures in Response to Quartz
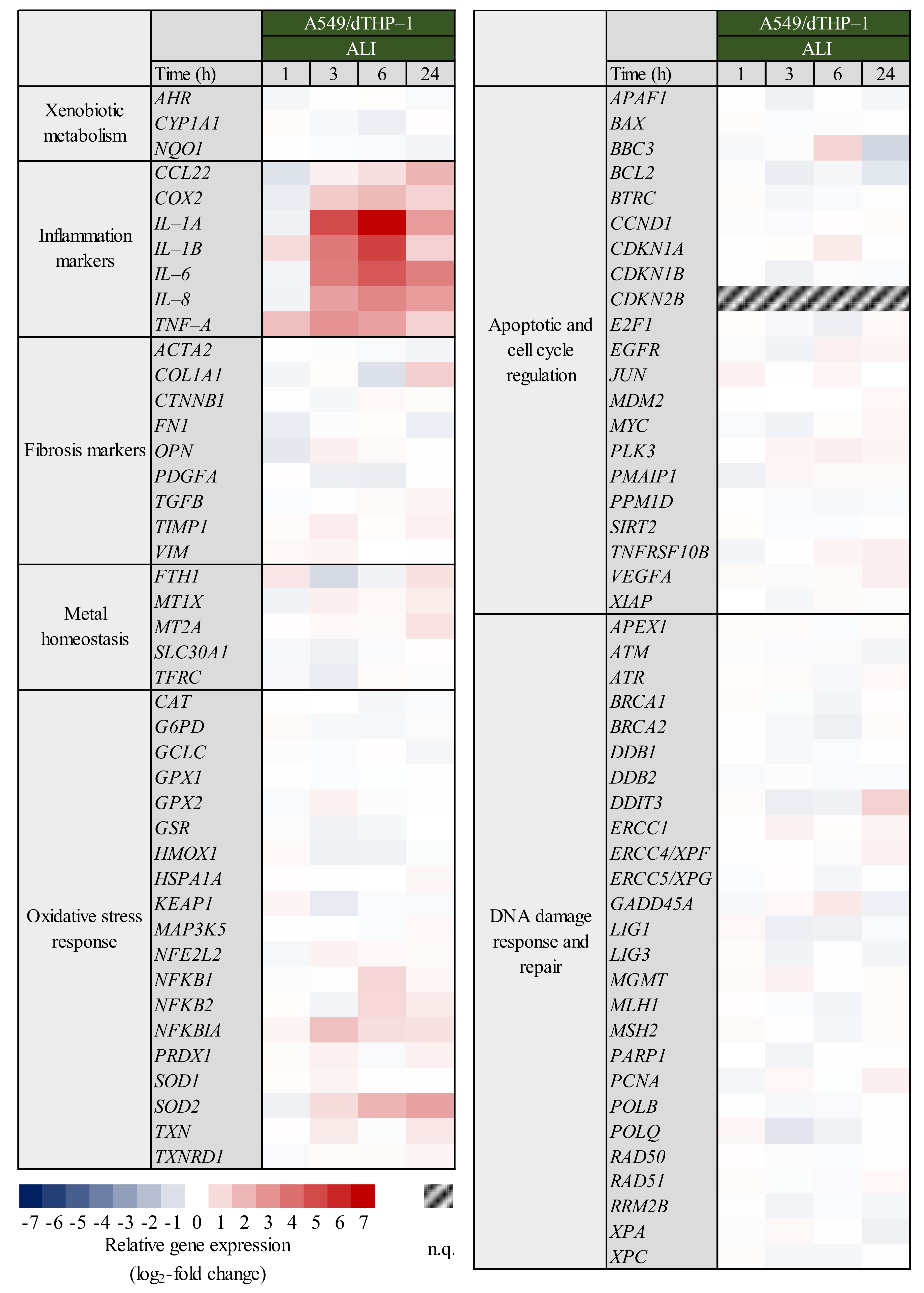
2.3. Absence of DNA Strand Breaks after ALI Exposure to Quartz
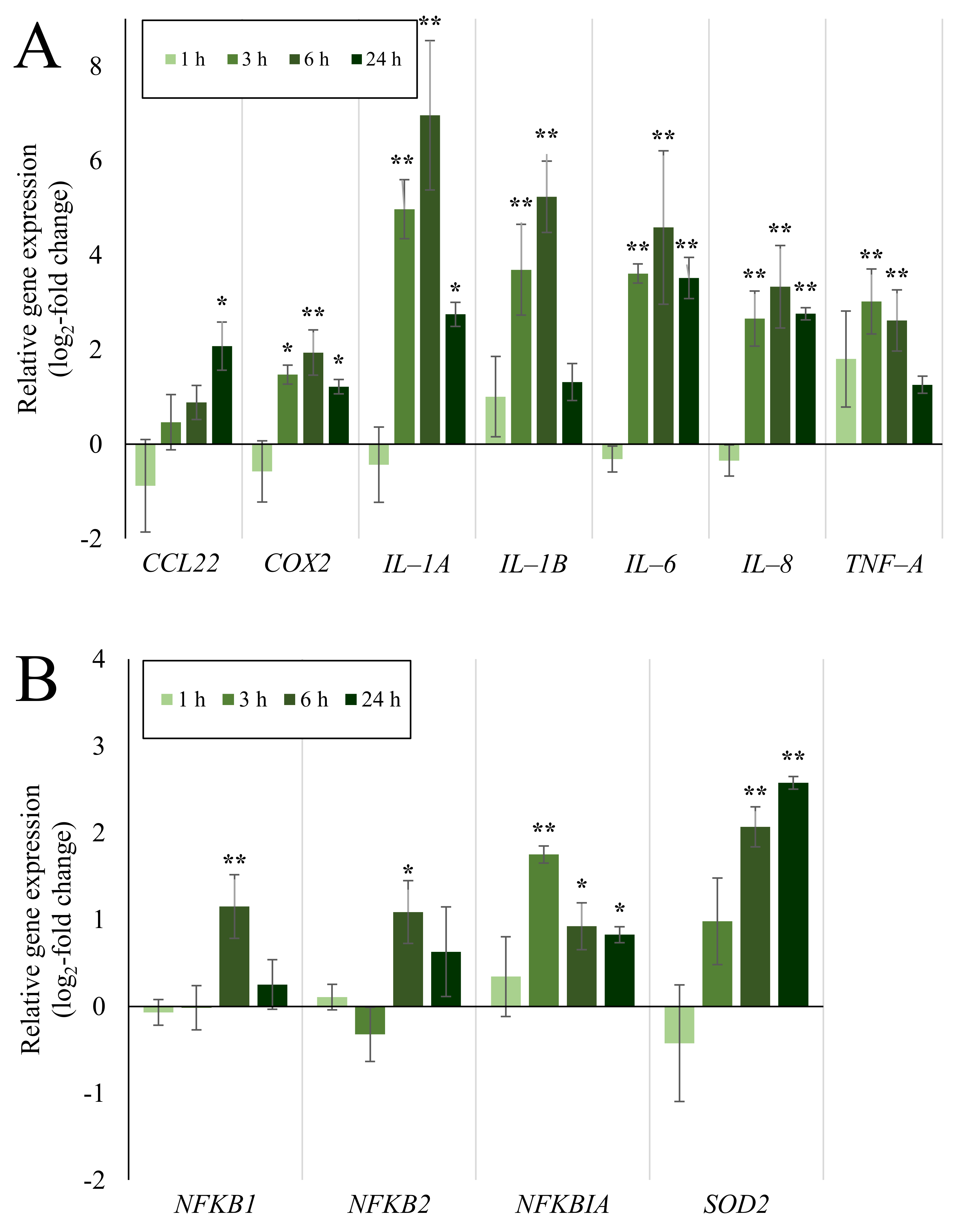
2.4. Expression of Inflammatory and Oxidative Stress Response Genes Induced by Quartz Is Highly Time-Dependent
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Particle Preparation and Characterization
4.3. Cell Culture
4.4. Particle Exposure
4.5. Gene Expression Analysis via High-Thoughout RT-qPCR
4.6. Evaluation of DNA Strand Breaks by Alkaline Unwinding
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Air-liquid interface |
| AU | Alkaline unwinding |
| BSA | Bovine serum albumin |
| CCL22 | Gene coding for C-C motif chemokine 22 |
| COX2 | Gene coding for cyclooxygenase 2 |
| DDIT3 | Gene coding for DNA damage inducible protein |
| DLS | Dynamic light scattering |
| dTHP-1 | differentiated THP-1 cells |
| FBS | Fetal bovine serum |
| HT RT qPCR | High-throughput RT qPCR |
| IL-1A/1B/6/8 | Genes coding for interleukin-1α/1β/6/8 |
| JUN | Gene coding for jun proto-oncogene |
| MT1X/2A | Genes coding for metallotionein 1X/2A |
| NF-κB | Nuclear factor κB |
| NLRP3 | NOD-, LRR- and pyrin domain containing protein 3 |
| PMA | Phorbol 12-myristate 13-acetate |
| QCM | Quartz crystal microbalance |
| ROS | Reactive oxygen species |
| SOD2 | Gene coding for superoxide dismutase 2 |
| TEM | transmission electron microscopy |
| TNF-A | Gene coding for tumor necrosis factor α |
| TNFRSF10B | Gene coding for tumor necrosis factor receptor superfamily, member 10b/death receptor 5 |
| VEGFA | Gene coding for vasular endothelial growth factor A |
References
- Greim, H. Siliciumdioxid, kristallin: Quarz-, Cristobalit-, Tridymitstaub (Alveolengängiger Anteil) [MAK Value Documentation in German language]. In The MAK-Collection for Occupational Health and Safety; Wiley-VCH: Weinheim, Germany, 1999; pp. 1–66. [Google Scholar]
- International Agency for Research on Cancer. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Silica, Some Silicates, Coal Dust and Para-Aramid Fibrils. Lyon, 15–22 October 1996. IARC Monogr. Identif. Carcinog. Hazards Hum. 1997, 68, 1–475. [Google Scholar]
- Calvert, G.; Rice, F.; Boiano, J.; Sheehy, J.; Sanderson, W. Occupational silica exposure and risk of various diseases: An analysis using death certificates from 27 states of the United States. Occup. Environ. Med. 2003, 60, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Pavan, C.; Fubini, B. Unveiling the variability of “quartz hazard” in light of recent toxicological findings. Chem. Res. Toxicol. 2017, 30, 469–485. [Google Scholar] [CrossRef]
- Upadhyay, S.; Palmberg, L. Air-liquid interface: Relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B. Air–liquid Interface in vitro models for respiratory toxicology research: Consensus workshop and recommendations. Appl. Vitr. Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Paur, H.; Mülhopt, S.; Weiss, C.; Diabaté, S. In vitro exposure systems and bioassays for the assessment of toxicity of nanoparticles to the human lung. J. Für Verbrauch. Und Lebensm. 2008, 3, 319–329. [Google Scholar]
- Barosova, H.; Karakocak, B.B.; Septiadi, D.; Petri-Fink, A.; Stone, V.; Rothen-Rutishauser, B. An In Vitro Lung System to Assess the Proinflammatory Hazard of Carbon Nanotube Aerosols. Int. J. Mol. Sci. 2020, 21, 5335. [Google Scholar] [CrossRef]
- Öhlinger, K.; Kolesnik, T.; Meindl, C.; Gallé, B.; Absenger-Novak, M.; Kolb-Lenz, D.; Fröhlich, E. Air-liquid interface culture changes surface properties of A549 cells. Toxicol. Vitr. 2019, 60, 369–382. [Google Scholar] [CrossRef]
- Skuland, T.; Låg, M.; Gutleb, A.C.; Brinchmann, B.C.; Serchi, T.; Øvrevik, J.; Holme, J.A.; Refsnes, M. Pro-inflammatory effects of crystalline- and nano-sized non-crystalline silica particles in a 3D alveolar model. Part. Fibre Toxicol. 2020, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Herseth, J.I.; Refsnes, M.; Låg, M.; Schwarze, P.E. Role of IL-1β and COX2 in silica-induced IL-6 release and loss of pneumocytes in co-cultures. Toxicol. Vitr. 2009, 23, 1342–1353. [Google Scholar] [CrossRef]
- Kanj, R.S.; Kang, J.; Castranova, V. Interaction between primary alveolar macrophages and primary alveolar type II cells under basal conditions and after lipopolysaccharide or quartz exposure. J. Toxicol. Environ. Health A 2006, 69, 1097–1116. [Google Scholar]
- Hindman, B.; Ma, Q. Carbon nanotubes and crystalline silica stimulate robust ROS production, inflammasome activation, and IL-1β secretion in macrophages to induce myofibroblast transformation. Arch. Toxicol. 2019, 93, 887–907. [Google Scholar] [CrossRef]
- van Berlo, D.; Knaapen, A.M.; van Schooten, F.-J.; Schins, R.P.; Albrecht, C. NF-κB dependent and independent mechanisms of quartz-induced proinflammatory activation of lung epithelial cells. Part. Fibre Toxicol. 2010, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Pozzolini, M.; Vergani, L.; Ragazzoni, M.; Delpiano, L.; Grasselli, E.; Voci, A.; Giovine, M.; Scarfì, S. Different reactivity of primary fibroblasts and endothelial cells towards crystalline silica: A surface radical matter. Toxicology 2016, 361, 12–23. [Google Scholar] [CrossRef]
- Schins, R.P.F.; Duffin, R.; Höhr, D.; Knaapen, A.M.; Shi, T.; Weishaupt, C.; Stone, V.; Donaldson, K.; Borm, P.J.A. Surface Modification of Quartz Inhibits Toxicity, Particle Uptake, and Oxidative DNA Damage in Human Lung Epithelial Cells. Chem. Res. Toxicol. 2002, 15, 1166–1173. [Google Scholar] [CrossRef]
- Schins, R.P.F.; Knaapen, A.M.; Cakmak, G.D.; Shi, T.; Weishaupt, C.; Borm, P.J.A. Oxidant-induced DNA damage by quartz in alveolar epithelial cells. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2002, 517, 77–86. [Google Scholar] [CrossRef]
- Li, H.; Van Berlo, D.; Shi, T.; Speit, G.; Knaapen, A.M.; Borm, P.J.; Albrecht, C.; Schins, R.P. Curcumin protects against cytotoxic and inflammatory effects of quartz particles but causes oxidative DNA damage in a rat lung epithelial cell line. Toxicol. Appl. Pharmacol. 2008, 227, 115–124. [Google Scholar] [CrossRef]
- Antognelli, C.; Gambelunghe, A.; Del Buono, C.; Murgia, N.; Talesa, V.N.; Muzi, G. Crystalline silica Min-U-Sil 5 induces oxidative stress in human bronchial epithelial cells BEAS-2B by reducing the efficiency of antiglycation and antioxidant enzymatic defenses. Chem.-Biol. Interact. 2009, 182, 13–21. [Google Scholar] [CrossRef]
- Albrecht, C.; Schins, R.P.; Hohr, D.; Becker, A.; Shi, T.; Knaapen, A.M.; Borm, P.J. Inflammatory time course after quartz instillation: Role of tumor necrosis factor-α and particle surface. Am. J. Respir. Cell Mol. Biol. 2004, 31, 292–301. [Google Scholar] [CrossRef]
- Duffin, R.; Gilmour, P.S.; Schins, R.P.; Clouter, A.; Guy, K.; Brown, D.M.; MacNee, W.; Borm, P.J.; Donaldson, K.; Stone, V. Aluminium lactate treatment of DQ12 quartz inhibits its ability to cause inflammation, chemokine expression, and nuclear factor-κB activation. Toxicol. Appl. Pharmacol. 2001, 176, 10–17. [Google Scholar] [CrossRef]
- Wu, R.; Högberg, J.; Adner, M.; Ramos-Ramírez, P.; Stenius, U.; Zheng, H. Crystalline silica particles cause rapid NLRP3-dependent mitochondrial depolarization and DNA damage in airway epithelial cells. Part. Fibre Toxicol. 2020, 17, 39. [Google Scholar] [CrossRef]
- Peeters, P.M.; Eurlings, I.M.; Perkins, T.N.; Wouters, E.F.; Schins, R.P.; Borm, P.J.; Drommer, W.; Reynaert, N.L.; Albrecht, C. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part. Fibre Toxicol. 2014, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Peeters, P.M.; Perkins, T.N.; Wouters, E.F.; Mossman, B.T.; Reynaert, N.L. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Part. Fibre Toxicol. 2013, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hindman, B.; Ma, Q. Carbon nanotubes and crystalline silica induce matrix remodeling and contraction by stimulating myofibroblast transformation in a three-dimensional culture of human pulmonary fibroblasts: Role of dimension and rigidity. Arch. Toxicol. 2018, 92, 3291–3305. [Google Scholar] [CrossRef]
- Friesen, A.; Fritsch-Decker, S.; Hufnagel, M.; Mülhopt, S.; Stapf, D.; Hartwig, A.; Weiss, C. Comparing a-Quartz-Induced Cytotoxicity and Interleukin-8 Release in Pulmonary Mono- and Co-Cultures Exposed under Submerged and Air-Liquid Interface Conditions. Int. J. Mol. Sci. 2022, 23, 6412. [Google Scholar] [CrossRef]
- Fischer, B.M.; Neumann, D.; Piberger, A.L.; Risnes, S.F.; Köberle, B.; Hartwig, A. Use of high-throughput RT-qPCR to assess modulations of gene expression profiles related to genomic stability and interactions by cadmium. Arch. Toxicol. 2016, 90, 2745–2761. [Google Scholar] [CrossRef] [Green Version]
- Freyria, F.S.; Bonelli, B.; Tomatis, M.; Ghiazza, M.; Gazzano, E.; Ghigo, D.; Garrone, E.; Fubini, B. Hematite nanoparticles larger than 90 nm show no sign of toxicity in terms of lactate dehydrogenase release, nitric oxide generation, apoptosis, and comet assay in murine alveolar macrophages and human lung epithelial cells. Chem. Res. Toxicol. 2012, 25, 850–861. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 7, 1–20. [Google Scholar]
- Tripathi, P.; Aggarwal, A. NF-kB transcription factor: A key player in the generation of immune response. Curr. Sci. 2006, 90, 519–531. [Google Scholar]
- Hartwig, A.; Dally, H.; Schlepegrell, R. Sensitive analysis of oxidative DNA damage in mammalian cells: Use of the bacterial Fpg protein in combination with alkaline unwinding. Toxicol. Lett. 1996, 88, 85–90. [Google Scholar] [CrossRef]
- Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.-E.; Biola-Clier, M.; Ambrose, S. Air–liquid interface exposure of lung epithelial cells to low doses of nanoparticles to assess pulmonary adverse effects. Nanomaterials 2021, 11, 65. [Google Scholar] [CrossRef]
- Murugadoss, S.; Mülhopt, S.; Diabaté, S.; Ghosh, M.; Paur, H.-R.; Stapf, D.; Weiss, C.; Hoet, P.H. Agglomeration State of Titanium-Dioxide (TiO2) Nanomaterials Influences the Dose Deposition and Cytotoxic Responses in Human Bronchial Epithelial Cells at the Air-Liquid Interface. Nanomaterials 2021, 11, 3226. [Google Scholar] [CrossRef]
- Blank, F.; Rothen-Rutishauser, B.M.; Schurch, S.; Gehr, P. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J. Aerosol Med. 2006, 19, 392–405. [Google Scholar] [CrossRef]
- Hufnagel, M.; Neuberger, R.; Wall, J.; Link, M.; Friesen, A.; Hartwig, A. Impact of Differentiated Macrophage-Like Cells on the Transcriptional Toxicity Profile of CuO Nanoparticles in Co-Cultured Lung Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5044. [Google Scholar] [CrossRef]
- Hufnagel, M.; Schoch, S.; Wall, J.; Strauch, B.M.; Hartwig, A. Toxicity and Gene Expression Profiling of Copper-and Titanium-Based Nanoparticles Using Air–Liquid Interface Exposure. Chem. Res. Toxicol. 2020, 33, 1237–1249. [Google Scholar] [CrossRef]
- Strauch, B.M.; Hubele, W.; Hartwig, A. Impact of endocytosis and lysosomal acidification on the toxicity of copper oxide nano-and microsized particles: Uptake and gene expression related to oxidative stress and the DNA damage response. Nanomaterials 2020, 10, 679. [Google Scholar] [CrossRef] [Green Version]
- Strauch, B.M.; Niemand, R.K.; Winkelbeiner, N.L.; Hartwig, A. Comparison between micro-and nanosized copper oxide and water soluble copper chloride: Interrelationship between intracellular copper concentrations, oxidative stress and DNA damage response in human lung cells. Part. Fibre Toxicol. 2017, 14, 1–17. [Google Scholar] [CrossRef]
- Jensen, K.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media-Standard Operation Procedure (SOP) and Background Documentation. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 7 June 2022).
- Docter, D.; Bantz, C.; Westmeier, D.; Galla, H.J.; Wang, Q.; Kirkpatrick, J.C.; Nielsen, P.; Maskos, M.; Stauber, R.H. The protein corona protects against size-and dose-dependent toxicity of amorphous silica nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 1380–1392. [Google Scholar] [CrossRef] [Green Version]
- Leibe, R.; Hsiao, I.-L.; Fritsch-Decker, S.; Kielmeier, U.; Wagbo, A.M.; Voss, B.; Schmidt, A.; Hessman, S.D.; Duschl, A.; Oostingh, G.J. The protein corona suppresses the cytotoxic and pro-inflammatory response in lung epithelial cells and macrophages upon exposure to nanosilica. Arch. Toxicol. 2019, 93, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Hurst, G.B.; Wang, W.; Foster, C.M.; Nallathamby, P.D.; Retterer, S.T. Dynamic development of the protein corona on silica nanoparticles: Composition and role in toxicity. Nanoscale 2013, 5, 6372–6380. [Google Scholar] [CrossRef] [PubMed]
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.-R. Silica nanoparticles are less toxic to human lung cells when deposited at the air–liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panas, A.; Marquardt, C.; Nalcaci, O.; Bockhorn, H.; Baumann, W.; Paur, H.-R.; Mülhopt, S.; Diabaté, S.; Weiss, C. Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology 2012, 7, 259–273. [Google Scholar] [CrossRef] [PubMed]
- te Velde, A.A.; Huijbens, R.J.; Heije, K.; de Vries, J.E.; Figdor, C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood 1990, 76, 1392–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornace, A., Jr.; Nebert, D.; Hollander, M.; Luethy, J.; Papathanasiou, M.; Fargnoli, J.; Holbrook, N. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol. 1989, 9, 4196–4203. [Google Scholar]
- Jauhiainen, A.; Thomsen, C.; Strömbom, L.; Grundevik, P.; Andersson, C.; Danielsson, A.; Andersson, M.K.; Nerman, O.; Rörkvist, L.; Ståhlberg, A. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153. PLoS ONE 2012, 7, e33208. [Google Scholar]
- Fanizza, C.; Ursini, C.L.; Paba, E.; Ciervo, A.; Di Francesco, A.; Maiello, R.; De Simone, P.; Cavallo, D. Cytotoxicity and DNA-damage in human lung epithelial cells exposed to respirable α-quartz. Toxicol. Vitr. 2007, 21, 586–594. [Google Scholar] [CrossRef]
- Hetland, R.B.; Schwarze, P.E.; Johansen, B.V.; Myran, T.; Uthus, N.; Refsnes, M. Silica-induced cytokine release from A549 cells: Importance of surface area versus size. Hum. Exp. Toxicol. 2001, 20, 46–55. [Google Scholar] [CrossRef]
- Williams, L.; Zosky, G. The inflammatory effect of iron oxide and silica particles on lung epithelial cells. Lung 2019, 197, 199–207. [Google Scholar] [CrossRef]
- Perkins, T.N.; Shukla, A.; Peeters, P.M.; Steinbacher, J.L.; Landry, C.C.; Lathrop, S.A.; Steele, C.; Reynaert, N.L.; Wouters, E.F.; Mossman, B.T. Differences in gene expression and cytokine production by crystalline vs. amorphous silica in human lung epithelial cells. Part. Fibre Toxicol. 2012, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Castranova, V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: Role of reactive oxygen/nitrogen species. Free Radical Biol. Med. 2004, 37, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, R.; Monshouwer, M. Signal transduction in inflammatory processes, current and future therapeutic targets: A mini review. Vet. Q. 2000, 22, 11–16. [Google Scholar] [CrossRef]
- Tracey, M.; Kevin, J.; Cerami, P.D. Anthony, Tumor necrosis factor: A pleiotropic cytokine and therapuetic target. Annu. Rev. Med. 1994, 45, 491–503. [Google Scholar] [CrossRef]
- Chen, C.-C.; Sun, Y.-T.; Chen, J.-J.; Chiu, K.-T. TNF-α-induced cyclooxygenase-2 expression in human lung epithelial cells: Involvement of the phospholipase C-γ2, protein kinase C-α, tyrosine kinase, NF-κB-inducing kinase, and I-κB kinase 1/2 pathway. J. Immunol. 2000, 165, 2719–2728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef] [Green Version]
- Fritsch-Decker, S.; Marquardt, C.; Stoeger, T.; Diabaté, S.; Weiss, C. Revisiting the stress paradigm for silica nanoparticles: Decoupling of the anti-oxidative defense, pro-inflammatory response and cytotoxicity. Arch. Toxicol. 2018, 92, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre Toxicol. 2015, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Xu, R.; Zhang, H.; Peng, Z.; Feng, M.; Yu, B.; Wang, Y.; Shi, T.; Zhou, Y.; Liu, Y. Exogenous Clara cell protein 16 attenuates silica particles-induced inflammation in THP-1 macrophages by down-regulating NF-κB and caspase-1 activation. J. Toxicol. Sci. 2020, 45, 651–660. [Google Scholar] [CrossRef]
- Premshekharan, G.; Nguyen, K.; Zhang, H.; Forman, H.J.; Leppert, V.J. Low dose inflammatory potential of silica particles in human-derived THP-1 macrophage cell culture studies–Mechanism and effects of particle size and iron. Chem.-Biol. Interact. 2017, 272, 160–171. [Google Scholar] [CrossRef]
- Godiska, R.; Chantry, D.; Raport, C.J.; Sozzani, S.; Allavena, P.; Leviten, D.; Mantovani, A.; Gray, P.W. Human macrophage–derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1997, 185, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Vulcano, M.; Albanesi, C.; Stoppacciaro, A.; Bagnati, R.; D’Amico, G.; Struyf, S.; Transidico, P.; Bonecchi, R.; Del Prete, A.; Allavena, P. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur. J. Immunol. 2001, 31, 812–822. [Google Scholar] [CrossRef]
- Kumari, M.R.; Hiramatsu, M.; Ebadi, M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic. Res. 1998, 29, 93–101. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihantola, T.; Di Bucchianico, S.; Happo, M.; Ihalainen, M.; Uski, O.; Bauer, S.; Kuuspalo, K.; Sippula, O.; Tissari, J.; Oeder, S. Influence of wood species on toxicity of log-wood stove combustion aerosols: A parallel animal and air-liquid interface cell exposure study on spruce and pine smoke. Part. Fibre Toxicol. 2020, 17, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kanashova, T.; Sippula, O.; Oeder, S.; Streibel, T.; Passig, J.; Czech, H.; Kaoma, T.; Sapcariu, S.C.; Dilger, M.; Paur, H.-R. Emissions from a modern log wood masonry heater and wood pellet boiler: Composition and biological impact on air-liquid interface exposed human lung cancer cells. J. Mol. Clin. Med. 2018, 1, 23. [Google Scholar]
- Sapcariu, S.C.; Kanashova, T.; Dilger, M.; Diabaté, S.; Oeder, S.; Passig, J.; Radischat, C.; Buters, J.; Sippula, O.; Streibel, T. Metabolic profiling as well as stable isotope assisted metabolic and proteomic analysis of RAW 264.7 macrophages exposed to ship engine aerosol emissions: Different effects of heavy fuel oil and refined diesel fuel. PLoS ONE 2016, 11, e0157964. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Kobayashi, Y.; Goto, Y.; Okumura, H.; Nakae, S.; Konno, T.; Tada, K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982, 42, 1530–1536. [Google Scholar]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friesen, A.; Fritsch-Decker, S.; Hufnagel, M.; Mülhopt, S.; Stapf, D.; Weiss, C.; Hartwig, A. Gene Expression Profiling of Mono- and Co-Culture Models of the Respiratory Tract Exposed to Crystalline Quartz under Submerged and Air-Liquid Interface Conditions. Int. J. Mol. Sci. 2022, 23, 7773. https://doi.org/10.3390/ijms23147773
Friesen A, Fritsch-Decker S, Hufnagel M, Mülhopt S, Stapf D, Weiss C, Hartwig A. Gene Expression Profiling of Mono- and Co-Culture Models of the Respiratory Tract Exposed to Crystalline Quartz under Submerged and Air-Liquid Interface Conditions. International Journal of Molecular Sciences. 2022; 23(14):7773. https://doi.org/10.3390/ijms23147773
Chicago/Turabian StyleFriesen, Alexandra, Susanne Fritsch-Decker, Matthias Hufnagel, Sonja Mülhopt, Dieter Stapf, Carsten Weiss, and Andrea Hartwig. 2022. "Gene Expression Profiling of Mono- and Co-Culture Models of the Respiratory Tract Exposed to Crystalline Quartz under Submerged and Air-Liquid Interface Conditions" International Journal of Molecular Sciences 23, no. 14: 7773. https://doi.org/10.3390/ijms23147773
APA StyleFriesen, A., Fritsch-Decker, S., Hufnagel, M., Mülhopt, S., Stapf, D., Weiss, C., & Hartwig, A. (2022). Gene Expression Profiling of Mono- and Co-Culture Models of the Respiratory Tract Exposed to Crystalline Quartz under Submerged and Air-Liquid Interface Conditions. International Journal of Molecular Sciences, 23(14), 7773. https://doi.org/10.3390/ijms23147773





