Lamin-A/C Is Modulated by the Involvement of Histamine-Mediated Calcium/Calmodulin-Dependent Kinase II in Lung Cancer Cells
Abstract
1. Introduction
2. Results
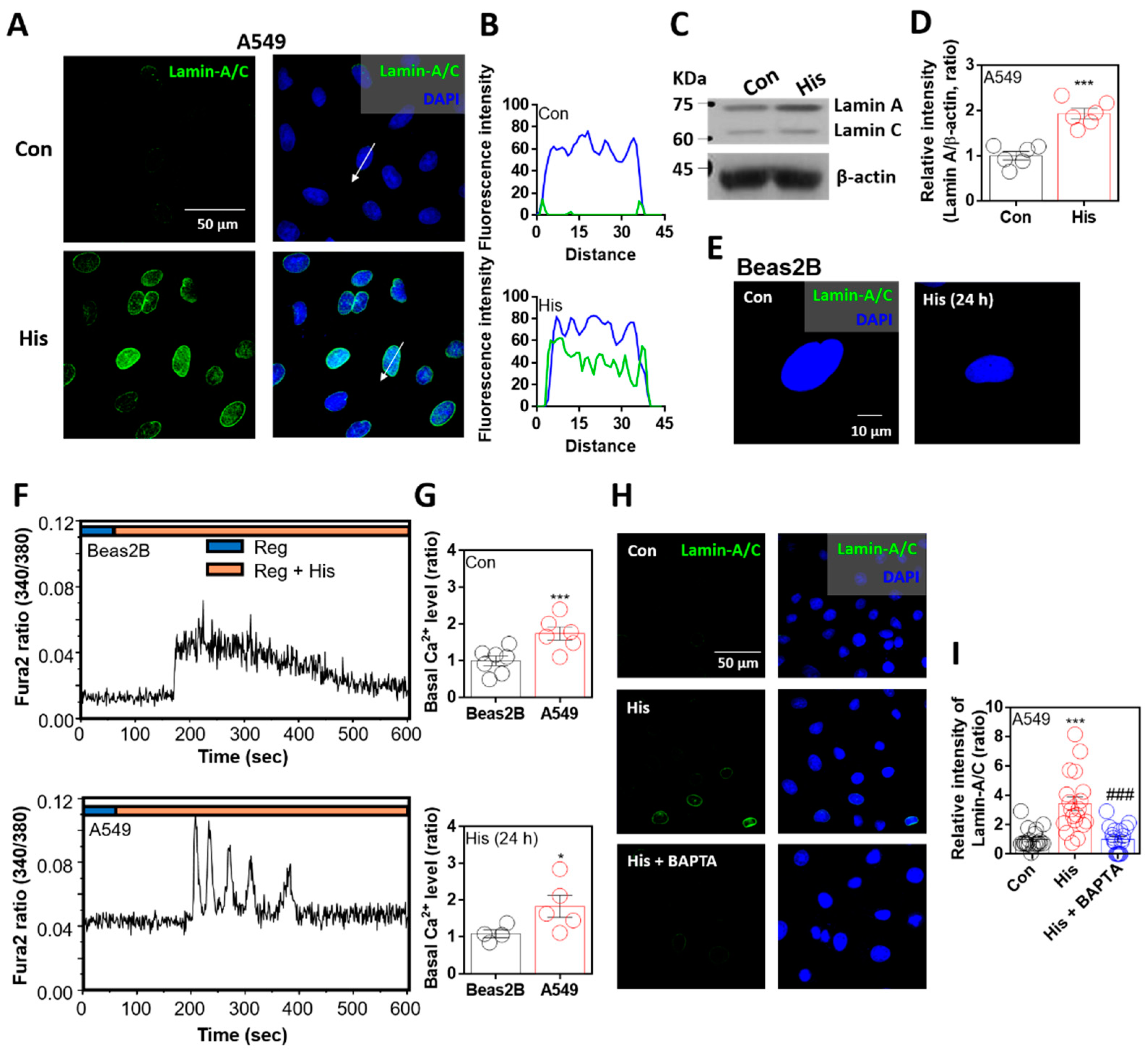
2.1. Lamin-A/C Expression Was Attenuated by Intracellular Calcium Chelation
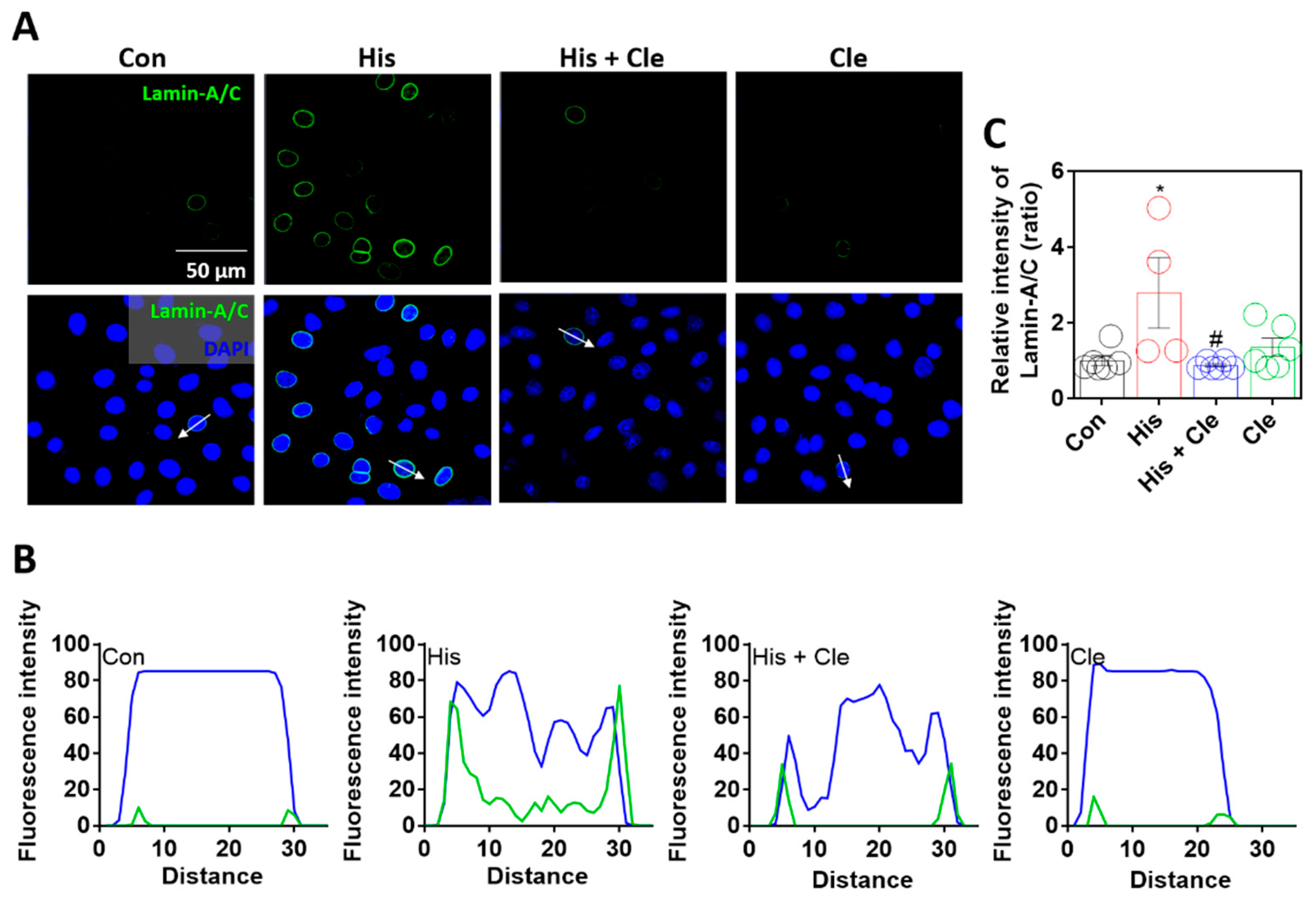
2.2. Lamin-A/C Expression Was Dependent on Histamine-Mediated Signaling
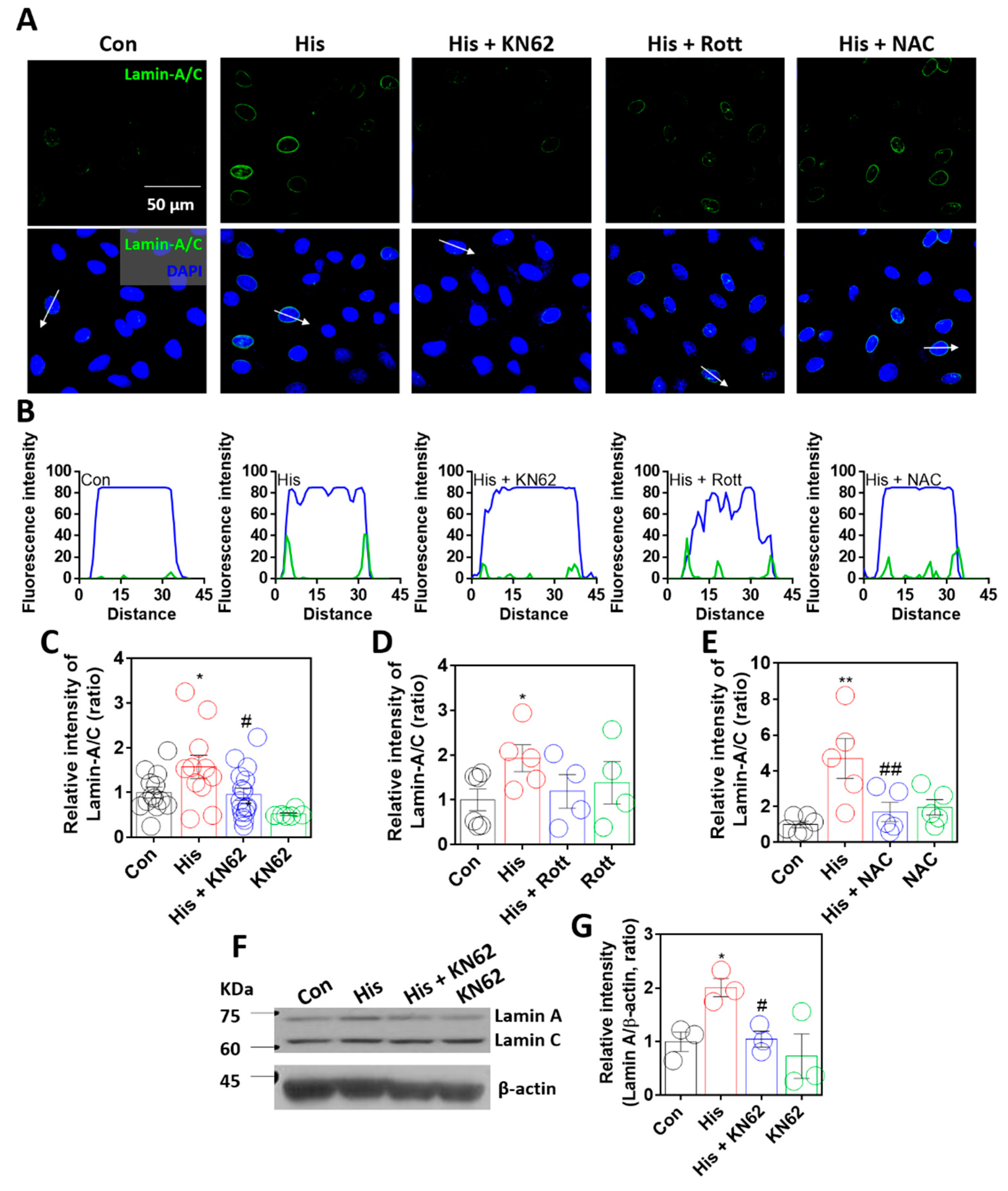
2.3. Lamin-A/C Expression Was Dependent on Calcium/Calmodulin-Dependent Kinase II
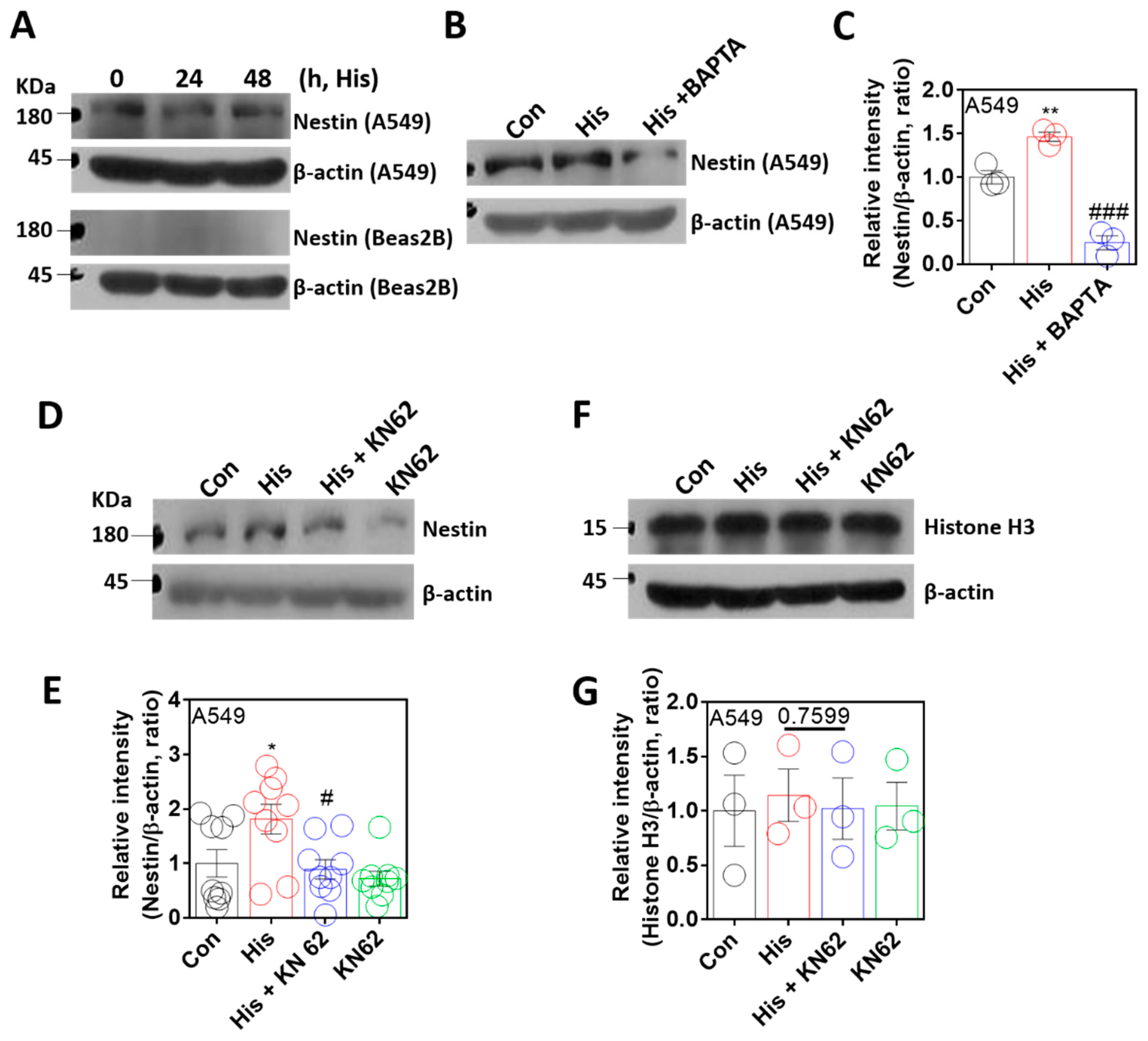
2.4. Nuclear Protein Nestin Expression Was Modulated by Ca/CaMKII
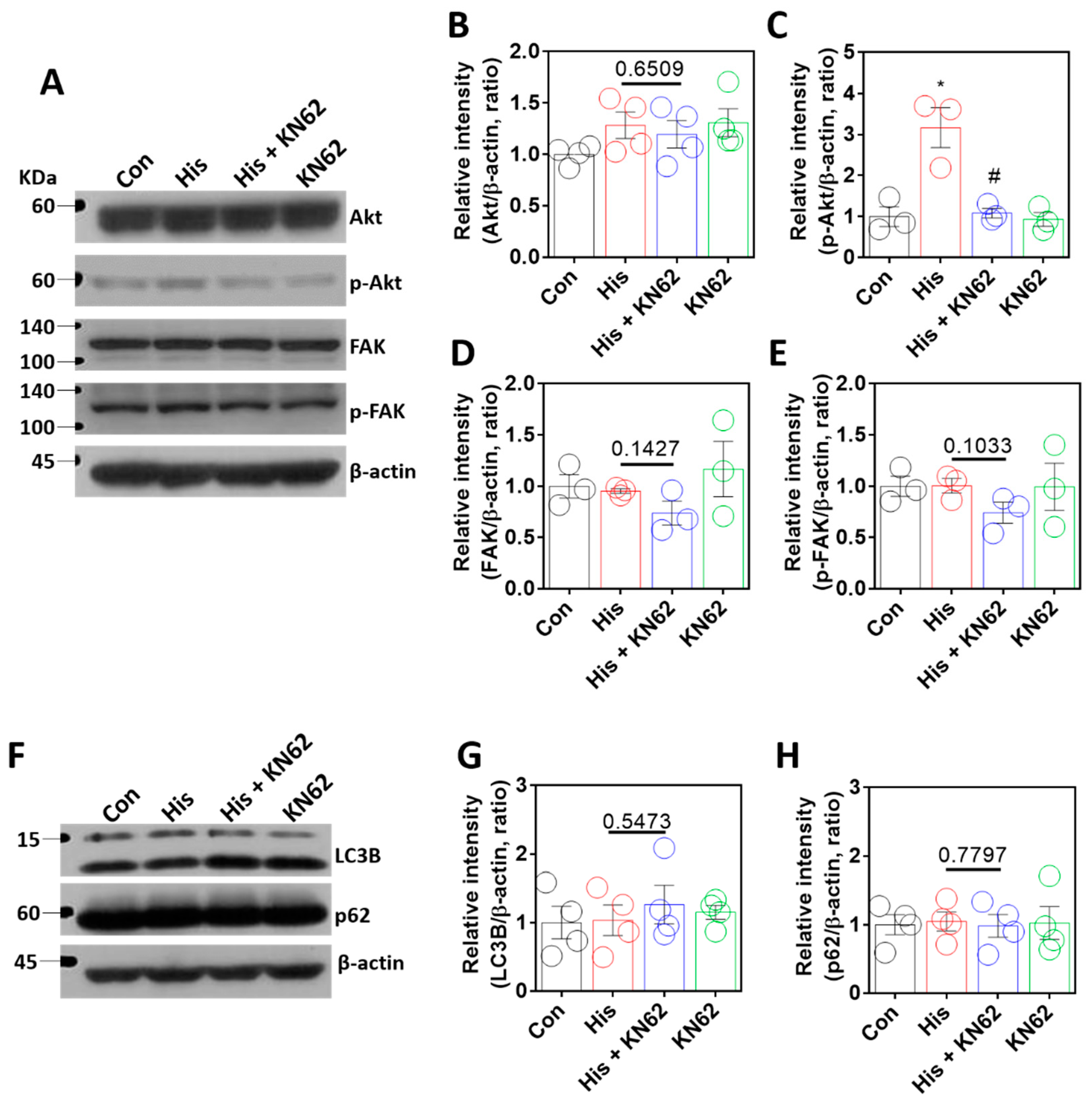
2.5. Histamine-Mediated Lamin-A/C Expression Was Independent of Akt/FAK or Autophagy Signaling
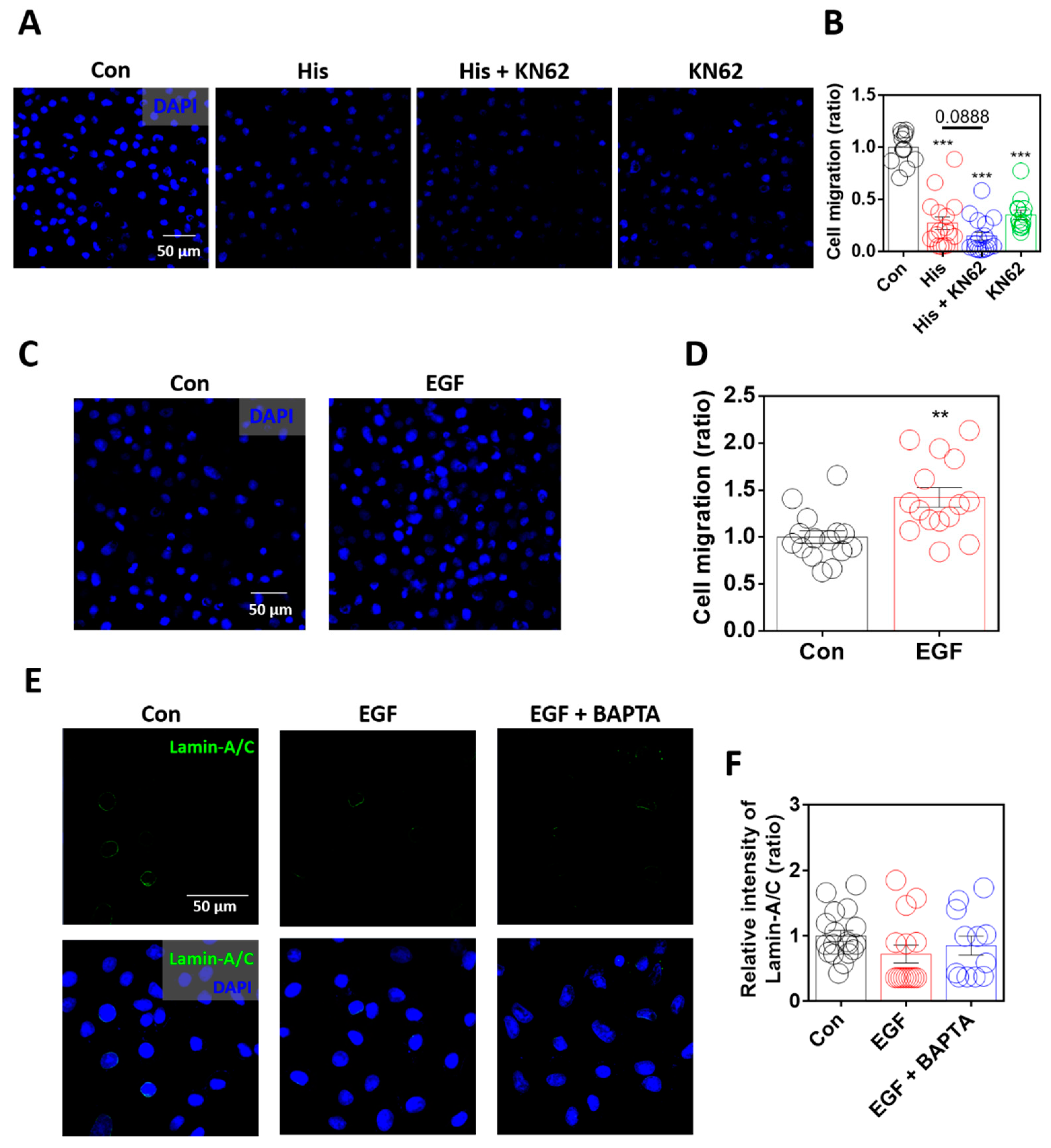
2.6. Enhanced Lamin-A/C Expression Was Associated with Reduced Cellular Motility
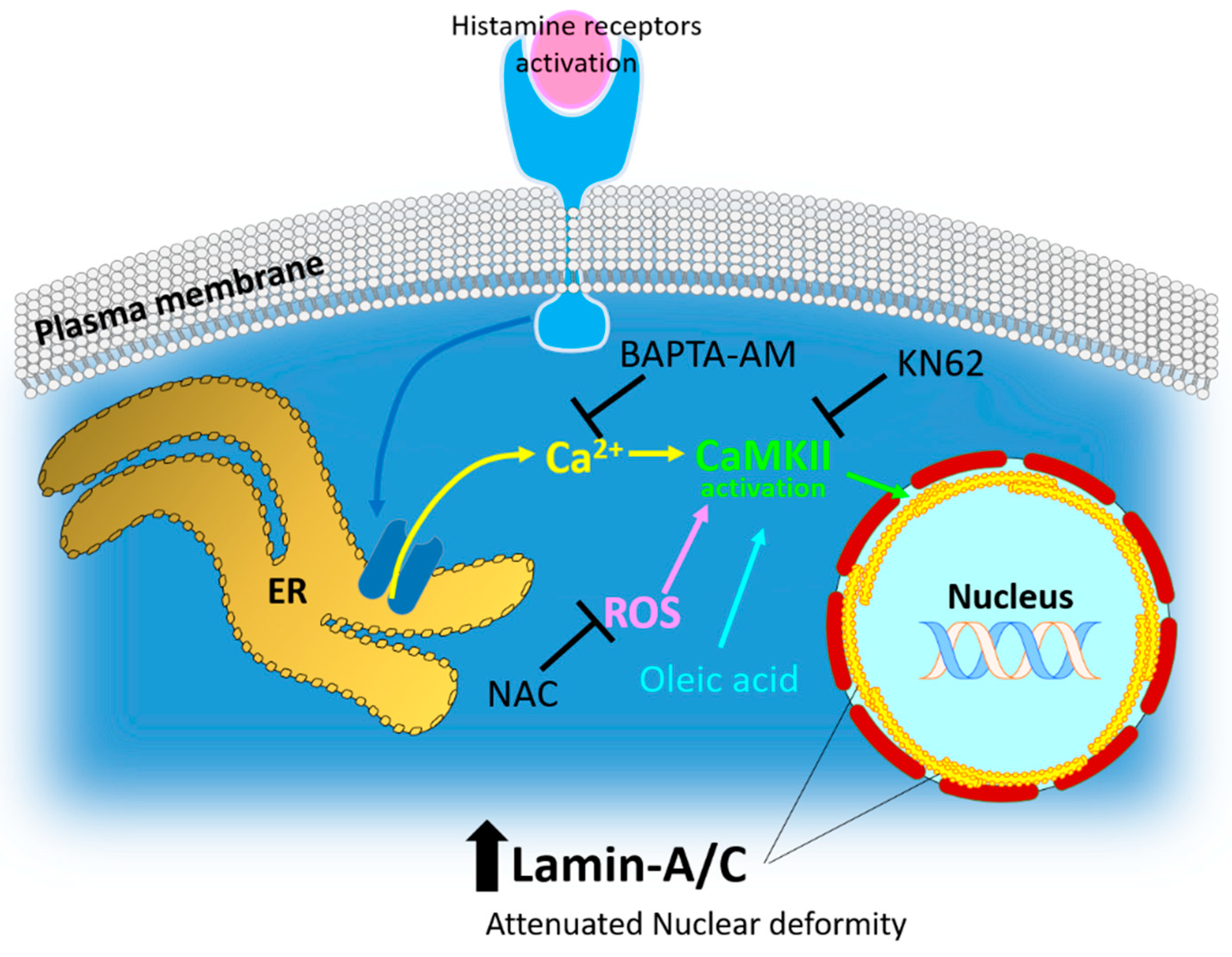
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunofluorescence and Confocal Imaging
4.3. Western Blot
4.4. Measurement of Intracellular Ca2+ Concentration
4.5. Migration Assay
4.6. MTT Assay
4.7. Total RNA Extraction and Quantitative Real Time-Polymerase Chain Reaction (RT-qPCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lund, E.; Oldenburg, A.R.; Delbarre, E.; Freberg, C.T.; Duband-Goulet, I.; Eskeland, R.; Buendia, B.; Collas, P. Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Res. 2013, 23, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Osmanagic-Myers, S.; Dechat, T.; Foisner, R. Lamins at the crossroads of mechanosignaling. Gene Dev. 2015, 29, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Nmezi, B.; Xu, J.Q.; Fu, R.; Armiger, T.J.; Rodriguez-Bey, G.; Powell, J.S.; Ma, H.Q.; Sullivan, M.; Tu, Y.P.; Chen, N.Y.; et al. Concentric organization of A- and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina. Proc. Natl. Acad. Sci. USA 2019, 116, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and Lamin A/C Sequentially Tether Peripheral Heterochromatin and Inversely Regulate Differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef]
- Guinde, J.; Frankel, D.; Perrin, S.; Delecourt, V.; Levy, N.; Barlesi, F.; Astoul, P.; Roll, P.; Kaspi, E. Lamins in Lung Cancer: Biomarkers and Key Factors for Disease Progression through miR-9 Regulation? Cells 2018, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Dubik, N.; Mai, S. Lamin A/C: Function in Normal and Tumor Cells. Cancers 2020, 12, 3688. [Google Scholar] [CrossRef]
- Moiseeva, O.; Lessard, F.; Acevedo-Aquino, M.; Vernier, M.; Tsantrizos, Y.S.; Ferbeyre, G. Mutant lamin A links prophase to a p53 independent senescence program. Cell Cycle 2015, 14, 2408–2421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broers, J.L.; Ramaekers, F.C. The role of the nuclear lamina in cancer and apoptosis. Adv. Exp. Med. Biol. 2014, 773, 27–48. [Google Scholar]
- Wang, X.; Zabell, A.; Koh, W.; Tang, W.H. Lamin A/C Cardiomyopathies: Current Understanding and Novel Treatment Strategies. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Muchir, A.; van Engelen, B.G.; Lammens, M.; Mislow, J.M.; McNally, E.; Schwartz, K.; Bonne, G. Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp. Cell Res. 2003, 291, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Yoon, M.H.; Park, B.J. Laminopathies; Mutations on single gene and various human genetic diseases. Bmb. Rep. 2018, 51, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.T.; Muchir, A.; Nagy, P.L.; Worman, H.J. LMNA cardiomyopathy: Cell biology and genetics meet clinical medicine. Dis. Model Mech. 2011, 4, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mounkes, L.C.; Kozlov, S.; Hernandez, L.; Sullivan, T.; Stewart, C.L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 2003, 423, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lazarte, J.; Hegele, R.A. Lamin A/C missense variants: From discovery to functional validation. NPJ Genom. Med. 2021, 6, 102. [Google Scholar] [CrossRef]
- Cenni, V.; Bertacchini, J.; Beretti, F.; Lattanzi, G.; Bavelloni, A.; Riccio, M.; Ruzzene, M.; Marin, O.; Arrigoni, G.; Parnaik, V.; et al. Lamin A Ser404 Is a Nuclear Target of Akt Phosphorylation in C2C12 Cells. J. Proteome Res. 2008, 7, 4727–4735. [Google Scholar] [CrossRef]
- Chuang, H.H.; Wang, P.H.; Niu, S.W.; Zhen, Y.Y.; Huang, M.S.; Hsiao, M.; Yang, C.J. Inhibition of FAK Signaling Elicits Lamin A/C-Associated Nuclear Deformity and Cellular Senescence. Front. Oncol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Swift, J.; Irianto, J.; Spinler, K.R.; Dingal, P.C.; Athirasala, A.; Kao, Y.R.; Cho, S.; Harada, T.; Shin, J.W.; et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014, 24, 1909–1917. [Google Scholar] [CrossRef]
- Qi, Y.X.; Yao, Q.P.; Huang, K.; Shi, Q.; Zhang, P.; Wang, G.L.; Han, Y.; Bao, H.; Wang, L.; Li, H.P.; et al. Nuclear envelope proteins modulate proliferation of vascular smooth muscle cells during cyclic stretch application. Proc. Natl. Acad. Sci. USA 2016, 113, 5293–5298. [Google Scholar] [CrossRef]
- Urciuoli, E.; D’Oria, V.; Petrini, S.; Peruzzi, B. Lamin A/C Mechanosensor Drives Tumor Cell Aggressiveness and Adhesion on Substrates With Tissue-Specific Elasticity. Front. Cell Dev. Biol. 2021, 9, 712377. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, Y.; Fan, Z.; Cheng, L.; Han, S.; Che, H. Apigenin Inhibits Histamine-Induced Cervical Cancer Tumor Growth by Regulating Estrogen Receptor Expression. Molecules 2020, 25, 1960. [Google Scholar] [CrossRef]
- Kaspi, E.; Frankel, D.; Guinde, J.; Perrin, S.; Laroumagne, S.; Robaglia-Schlupp, A.; Ostacolo, K.; Harhouri, K.; Tazi-Mezalek, R.; Micallef, J.; et al. Low lamin A expression in lung adenocarcinoma cells from pleural effusions is a pejorative factor associated with high number of metastatic sites and poor Performance status. PLoS ONE 2017, 12, e0183136. [Google Scholar] [CrossRef]
- Roncato, F.; Regev, O.; Feigelson, S.W.; Yadav, S.K.; Kaczmarczyk, L.; Levi, N.; Drago-Garcia, D.; Ovadia, S.; Kizner, M.; Addadi, Y.; et al. Reduced Lamin A/C Does Not Facilitate Cancer Cell Transendothelial Migration but Compromises Lung Metastasis. Cancers 2021, 13, 2383. [Google Scholar] [CrossRef] [PubMed]
- Massari, N.A.; Nicoud, M.B.; Medina, V.A. Histamine receptors and cancer pharmacology: An update. Br. J. Pharmacol. 2020, 177, 516–538. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Granata, A.; Familiari, D.; Zirilli, A.; Cimino, F.; Tomaino, A. Histamine and selenium in lung cancer. Anticancer Res. 2006, 26, 2937–2942. [Google Scholar] [PubMed]
- Stoyanov, E.; Uddin, M.; Mankuta, D.; Dubinett, S.M.; Levi-Schaffer, F. Mast cells and histamine enhance the proliferation of non-small cell lung cancer cells. Lung Cancer 2012, 75, 38–44. [Google Scholar] [CrossRef]
- Clauzure, M.; Taquez Delgado, M.A.; Phillip, J.M.; Revuelta, M.V.; Cerchietti, L.; Medina, V.A. Histamine H4 Receptor Agonism Induces Antitumor Effects in Human T-Cell Lymphoma. Int. J. Mol. Sci. 2022, 23, 1378. [Google Scholar] [CrossRef]
- Machiels, B.M.; Broers, J.L.; Raymond, Y.; de Ley, L.; Kuijpers, H.J.; Caberg, N.E.; Ramaekers, F.C. Abnormal A-type lamin organization in a human lung carcinoma cell line. Eur. J. Cell Biol. 1995, 67, 328–335. [Google Scholar]
- Desai, P.; Thurmond, R.L. Histamine H-4 receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur. J. Immunol. 2011, 41, 1764–1773. [Google Scholar] [CrossRef]
- Obara, I.; Telezhkin, V.; Alrashdi, I.; Chazot, P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2020, 177, 580–599. [Google Scholar] [CrossRef]
- Vollesen, L.H.; Guo, S.; Andersen, M.R.; Ashina, M. Effect of the H-1-antihistamine clemastine on PACAP38 induced migraine. Cephalalgia 2019, 39, 597–607. [Google Scholar] [CrossRef]
- Sihra, T.S.; Pearson, H.A. Ca/calmodulin-dependent kinase II inhibitor KN62 attenuates glutamate release by inhibiting voltage-dependent Ca(2+)-channels. Neuropharmacology 1995, 34, 731–741. [Google Scholar] [CrossRef]
- Clyne, C.D.; Nguyen, A.; Rainey, W.E. The effects of KN62, a Ca2+/calmodulin-dependent protein kinase II inhibitor, on adrenocortical cell aldosterone production. Endocr. Res. 1995, 21, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kontny, E.; Kurowska, M.; Szczepanska, K.; Maslinski, W. Rottlerin, a PKC isozyme-selective inhibitor, affects signaling events and cytokine production in human monocytes. J. Leukoc. Biol. 2000, 67, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Kim, S.H.; Jeong, M.W.; Baek, N.I.; Kim, K.T. Effect of rottlerin, a PKC-delta inhibitor, on TLR-4-dependent activation of murine microglia. Biochem. Biophys. Res. Commun. 2005, 337, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Huang, W.; Cai, J.; Ba, J.; Wang, Y.; Ke, Q.; Huang, Y.; Liu, X.; Qiu, Y.; et al. Nuclear Nestin deficiency drives tumor senescence via lamin A/C-dependent nuclear deformation. Nat. Commun. 2018, 9, 3613. [Google Scholar] [CrossRef]
- Wang, J.C.; Lu, Q.Y.; Cai, J.Y.; Wang, Y.; Lai, X.F.; Qiu, Y.; Huang, Y.N.; Ke, Q.; Zhang, Y.N.; Guan, Y.J.; et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1-Nrf2 feedback loop. Nat. Commun. 2019, 10, 5043. [Google Scholar] [CrossRef]
- Chen, X.C.; Weng, Y.L.; Li, Y.; Fu, W.K.; Huang, Z.W.; Pan, Y.H.; Hong, W.Q.; Lin, W.Z.; Lin, X.D.; Qiu, S.F. Upregulation of PNCK Promotes Metastasis and Angiogenesis via Activating NF-kappa B/VEGF Pathway in Nasopharyngeal Carcinoma. J. Oncol. 2022, 2022, 8541582. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Nishio, Y.; Sakai, H.; Asagiri, M.; Yoshimura, K.; Inui, M.; Kuramasu, A. Calcium/calmodulin-dependent regulation of Rac GTPases and Akt in histamine-induced chemotaxis of mast cells. Cell Signal 2021, 83, 109973. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Schlafli, A.M.; Berezowska, S.; Adams, O.; Langer, R.; Tschan, M.P. Reliable LC3 and p62 autophagy marker detection in formalin fixed paraffin embedded human tissue by immunohistochemistry. Eur. J. Histochem. 2015, 59, 2481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Zhang, Y.; Gao, Z.; Liu, Y.; Hu, J.; Hu, X.; Li, L.; Shi, J.; Gao, N. Nuclear accumulation of UBC9 contributes to SUMOylation of lamin A/C and nucleophagy in response to DNA damage. J. Exp. Clin. Cancer Res. 2019, 38, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, J.; He, L.; Gong, G.; Wu, X. Effect of lamin-A expression on migration and nuclear stability of ovarian cancer cells. Gynecol. Oncol. 2019, 152, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Nicoud, M.B.; Formoso, K.; Medina, V.A. Pathophysiological Role of Histamine H4 Receptor in Cancer: Therapeutic Implications. Front. Pharmacol. 2019, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yu, Z.X.; Ferrans, V.J.; Takeda, K.; Irani, K.; Ziegelstein, R.C. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J. Biol. Chem. 2002, 277, 32546–32551. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, O.; Lojek, A.; Jancinova, V.; Nosal, R.; Ciz, M. Role of histamine receptors in the effects of histamine on the production of reactive oxygen species by whole blood phagocytes. Life Sci. 2014, 100, 67–72. [Google Scholar] [CrossRef]
- Clark, R.A.; Sandler, J.A.; Gallin, J.I.; Kaplan, A.P. Histamine modulation of eosinophil migration. J. Immunol. 1977, 118, 137–145. [Google Scholar]
- Kohyama, T.; Yamauchi, Y.; Takizawa, H.; Kamitani, S.; Kawasaki, S.; Nagase, T. Histamine stimulates human lung fibroblast migration. Mol. Cell Biochem. 2010, 337, 77–81. [Google Scholar] [CrossRef]
- Kay, L.J.; Suvarna, S.K.; Peachell, P.T. Histamine H4 receptor mediates chemotaxis of human lung mast cells. Eur. J. Pharmacol. 2018, 837, 38–44. [Google Scholar] [CrossRef]
- Kennedy, L.; Hodges, K.; Meng, F.; Alpini, G.; Francis, H. Histamine and histamine receptor regulation of gastrointestinal cancers. Transl. Gastrointest. Cancer 2012, 1, 215–227. [Google Scholar]
- Cai, W.K.; Hu, J.; Li, T.; Meng, J.R.; Ma, X.; Yin, S.J.; Zhao, C.H.; He, G.H.; Xu, G.L. Activation of histamine H4 receptors decreases epithelial-to-mesenchymal transition progress by inhibiting transforming growth factor-beta1 signalling pathway in non-small cell lung cancer. Eur. J. Cancer 2014, 50, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Salvermoser, M.; Begandt, D.; Alon, R.; Walzog, B. Nuclear Deformation During Neutrophil Migration at Sites of Inflammation. Front. Immunol. 2018, 9, 2680. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Vong, J.S.; Asafova, A.; Garvalov, B.K.; Caputo, L.; Cordero, J.; Singh, A.; Boettger, T.; Gunther, S.; Fink, L.; et al. Lamin B1 loss promotes lung cancer development and metastasis by epigenetic derepression of RET. J. Exp. Med. 2019, 216, 1377–1395. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Mabry, M.; Jasti, R.; Shaper, J.H. Differential expression of nuclear envelope lamins A and C in human lung cancer cell lines. Cancer Res. 1991, 51, 581–586. [Google Scholar] [PubMed]
- Broers, J.L.; Raymond, Y.; Rot, M.K.; Kuijpers, H.; Wagenaar, S.S.; Ramaekers, F.C. Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am. J. Pathol. 1993, 143, 211–220. [Google Scholar] [PubMed]
- Gu, Y.; Chen, T.; Meng, Z.P.; Gan, Y.C.; Xu, X.H.; Lou, G.Y.; Li, H.Z.; Gan, X.X.; Zhou, H.; Tang, J.F.; et al. CaMKII gamma, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood 2012, 120, 4829–4839. [Google Scholar] [CrossRef]
- Meng, Z.; Li, T.; Ma, X.; Wang, X.; Van Ness, C.; Gan, Y.; Zhou, H.; Tang, J.; Lou, G.; Wang, Y.; et al. Berbamine inhibits the growth of liver cancer cells and cancer-initiating cells by targeting Ca(2+)/calmodulin-dependent protein kinase II. Mol. Cancer Ther. 2013, 12, 2067–2077. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, P.C.W.; Hong, J.H. Lamin-A/C Is Modulated by the Involvement of Histamine-Mediated Calcium/Calmodulin-Dependent Kinase II in Lung Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9075. https://doi.org/10.3390/ijms23169075
Kim H-J, Lee PCW, Hong JH. Lamin-A/C Is Modulated by the Involvement of Histamine-Mediated Calcium/Calmodulin-Dependent Kinase II in Lung Cancer Cells. International Journal of Molecular Sciences. 2022; 23(16):9075. https://doi.org/10.3390/ijms23169075
Chicago/Turabian StyleKim, Hyeong-Jae, Peter C. W. Lee, and Jeong Hee Hong. 2022. "Lamin-A/C Is Modulated by the Involvement of Histamine-Mediated Calcium/Calmodulin-Dependent Kinase II in Lung Cancer Cells" International Journal of Molecular Sciences 23, no. 16: 9075. https://doi.org/10.3390/ijms23169075
APA StyleKim, H.-J., Lee, P. C. W., & Hong, J. H. (2022). Lamin-A/C Is Modulated by the Involvement of Histamine-Mediated Calcium/Calmodulin-Dependent Kinase II in Lung Cancer Cells. International Journal of Molecular Sciences, 23(16), 9075. https://doi.org/10.3390/ijms23169075






