Effects of Fructose and Stress on Rat Renal Copper Metabolism and Antioxidant Enzymes Function
Abstract
:1. Introduction
2. Results
2.1. Effects of Fructose and Stress on Plasma Glucose, Insulin and Copper Levels
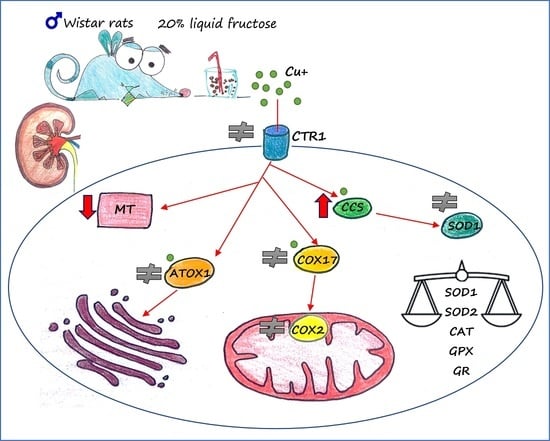
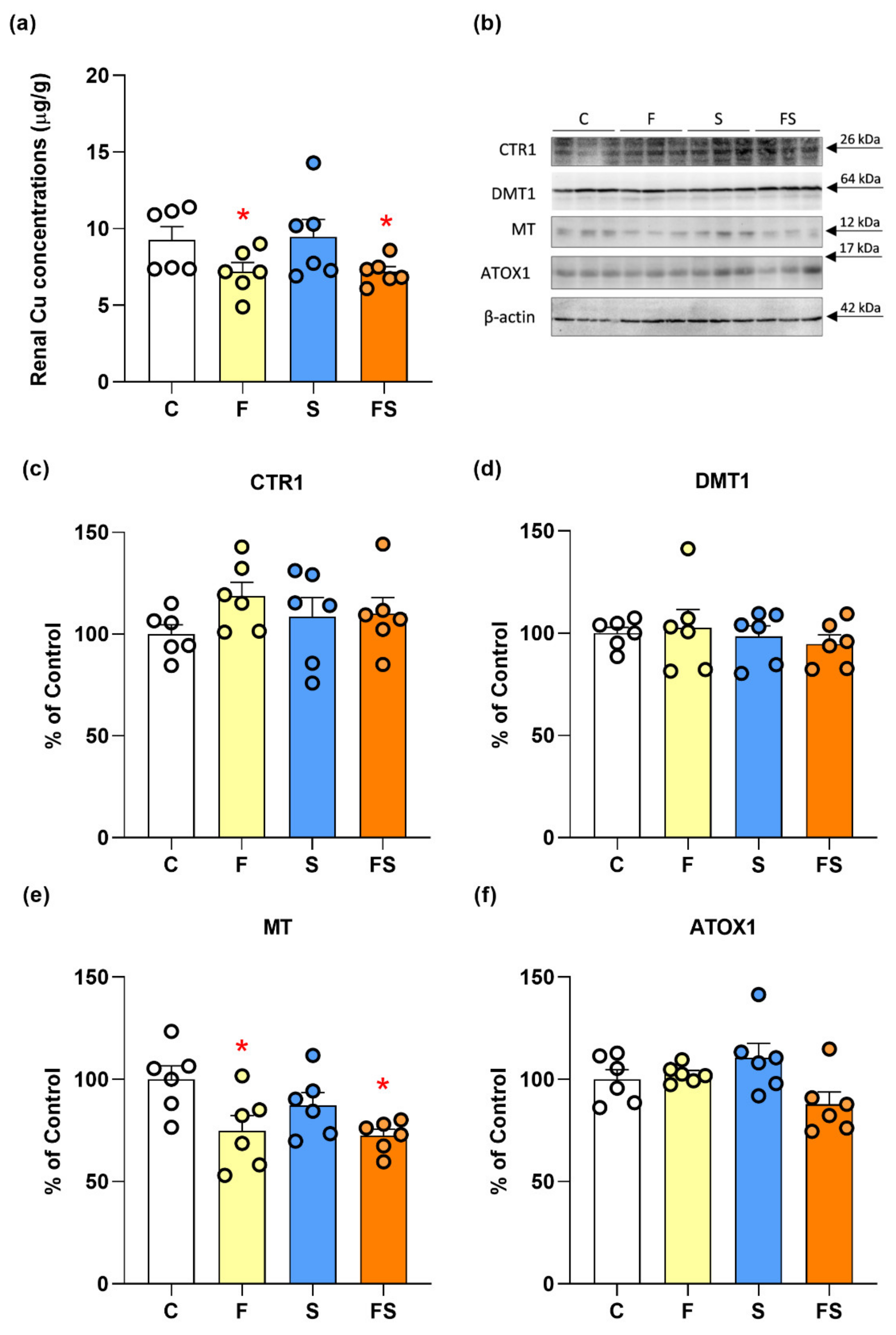
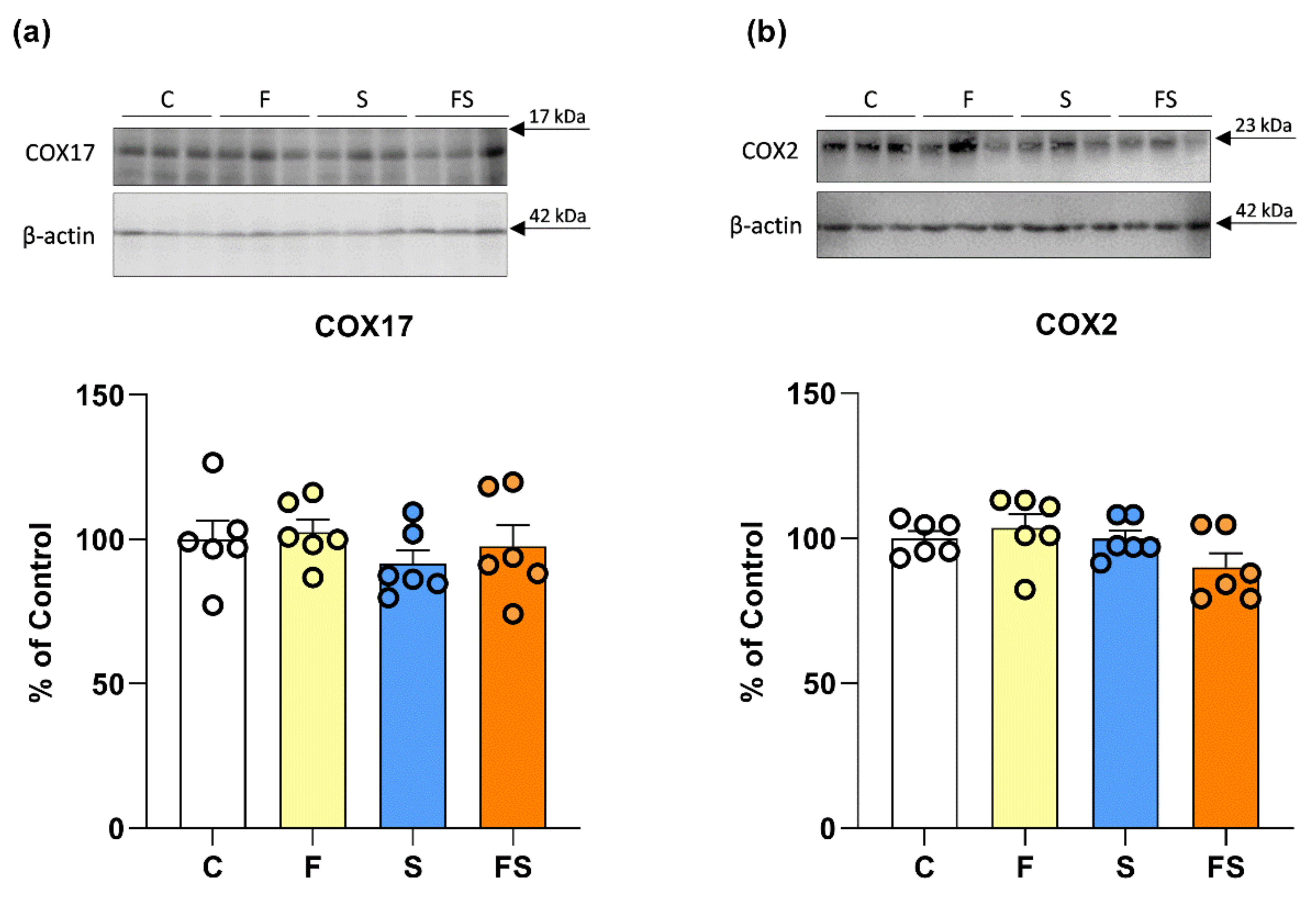
2.2. Effects of Fructose and Stress on Renal Copper Metabolism
2.3. Effects of Fructose and Stress on Renal Antioxidant Enzymes Function
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Biochemical Analysis
4.3. Tissue Preparation
4.4. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Immunoblotting
4.5. Antioxidant Enzymes Activity
4.6. Determination of Copper Concentrations in Plasma and Kidney
4.7. Data Presentation and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef]
- Bidwell, A.J. Chronic Fructose Ingestion as a Major Health Concern: Is a Sedentary Lifestyle Making It Worse? A Review. Nutrients 2017, 9, 549. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The effect of fructose on renal biology and disease. J. Am. Soc. Nephrol. 2010, 21, 2036–2039. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Packard, C.J.; Boren, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Helsley, R.N.; Moreau, F.; Gupta, M.K.; Radulescu, A.; DeBosch, B.; Softic, S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr. Diab. Rep. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Villeda-Gonzalez, J.D.; Gomez-Olivares, J.L.; Baiza-Gutman, L.A.; Manuel-Apolinar, L.; Damasio-Santana, L.; Millan-Pacheco, C.; Angeles-Mejia, S.; Cortes-Ginez, M.C.; Cruz-Lopez, M.; Vidal-Moreno, C.J.; et al. Nicotinamide reduces inflammation and oxidative stress via the cholinergic system in fructose-induced metabolic syndrome in rats. Life Sci. 2020, 250, 117585. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Spagnuolo, M.S.; Nazzaro, M.; Gatto, C.; Iossa, S.; Cigliano, L. Fructose Removal from the Diet Reverses Inflammation, Mitochondrial Dysfunction, and Oxidative Stress in Hippocampus. Antioxidants 2021, 10, 487. [Google Scholar] [CrossRef]
- Harder, N.H.O.; Hieronimus, B.; Stanhope, K.L.; Shibata, N.M.; Lee, V.; Nunez, M.V.; Keim, N.L.; Bremer, A.; Havel, P.J.; Heffern, M.C.; et al. Effects of Dietary Glucose and Fructose on Copper, Iron, and Zinc Metabolism Parameters in Humans. Nutrients 2020, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.; Fields, M.; Smith, J.C., Jr.; Reiser, S. Tissue distribution and excretion of copper-67 intraperitoneally administered to rats fed fructose or starch. J. Nutr. 1986, 116, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Morrell, A.; Tallino, S.; Yu, L.; Burkhead, J.L. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 2017, 69, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Schuschke, D.A.; Zhou, Z.; Chen, T.; Pierce, W.M., Jr.; Wang, R.; Johnson, W.T.; McClain, C.J. High fructose feeding induces copper deficiency in Sprague-Dawley rats: A novel mechanism for obesity related fatty liver. J. Hepatol. 2012, 56, 433–440. [Google Scholar] [CrossRef]
- Aigner, E.; Strasser, M.; Haufe, H.; Sonnweber, T.; Hohla, F.; Stadlmayr, A.; Solioz, M.; Tilg, H.; Patsch, W.; Weiss, G.; et al. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am. J. Gastroenterol. 2010, 105, 1978–1985. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Amarsingh, G.V.; Cheung, C.C.; Hogl, S.; Narayanan, U.; Zhang, L.; McHarg, S.; Xu, J.; Gong, D.; et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc. Diabetol. 2014, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Occurrence of copper proteins through the three domains of life: A bioinformatic approach. J. Proteome Res. 2008, 7, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014, 6, 143. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper transporters and copper chaperones: Roles in cardiovascular physiology and disease. Am. J. Physiol. Cell Physiol. 2018, 315, C186–C201. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Predki, P.F.; Sarkar, B. Effect of replacement of “zinc finger” zinc on estrogen receptor DNA interactions. J. Biol. Chem. 1992, 267, 5842–5846. [Google Scholar] [CrossRef]
- Bertinato, J.; L’Abbe, M.R. Maintaining copper homeostasis: Regulation of copper-trafficking proteins in response to copper deficiency or overload. J. Nutr. Biochem. 2004, 15, 316–322. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Nevitt, T.; Ohrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta 2012, 1823, 1580–1593. [Google Scholar] [CrossRef]
- Heacox, H.N.; Gillman, P.L.; Zwart, S.R.; Smith, S.M. Excretion of Zinc and Copper Increases in Men during 3 Weeks of Bed Rest, with or without Artificial Gravity. J. Nutr. 2017, 147, 1113–1120. [Google Scholar] [CrossRef]
- Prohaska, J.R.; Gybina, A.A. Intracellular copper transport in mammals. J. Nutr. 2004, 134, 1003–1006. [Google Scholar] [CrossRef]
- Timon-Gomez, A.; Nyvltova, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef]
- Casareno, R.L.; Waggoner, D.; Gitlin, J.D. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J. Biol. Chem. 1998, 273, 23625–23628. [Google Scholar] [CrossRef]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, A.L.; Jaime, H.B.; Escobar Villanueva, M.D.C.; Padilla, M.B.; Palacios, G.V.; Aguilar, F.J.A. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol. Behav. 2016, 161, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Gumuslu, S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav. Brain Res. 2004, 155, 241–248. [Google Scholar] [CrossRef]
- Sahin, E.; Gumuslu, S. Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 144, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Gumuslu, S. Stress-dependent induction of protein oxidation, lipid peroxidation and anti-oxidants in peripheral tissues of rats: Comparison of three stress models (immobilization, cold and immobilization-cold). Clin. Exp. Pharmacol. Physiol. 2007, 34, 425–431. [Google Scholar] [CrossRef]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Niciforovic, A.; Radojcic, M.B. Chronic stress differentially affects antioxidant enzymes and modifies the acute stress response in liver of Wistar rats. Physiol. Res. 2010, 59, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Radojcic, M.B. Chronic social isolation compromises the activity of both glutathione peroxidase and catalase in hippocampus of male wistar rats. Cell Mol. Neurobiol. 2010, 30, 693–700. [Google Scholar] [CrossRef]
- Che, Y.; Zhou, Z.; Shu, Y.; Zhai, C.; Zhu, Y.; Gong, S.; Cui, Y.; Wang, J.F. Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci. Lett. 2015, 584, 208–213. [Google Scholar] [CrossRef]
- Kovacevic, S.; Nestorov, J.; Matic, G.; Elakovic, I. Fructose-enriched diet induces inflammation and reduces antioxidative defense in visceral adipose tissue of young female rats. Eur. J. Nutr. 2017, 56, 151–160. [Google Scholar] [CrossRef]
- Lopes, A.; Vilela, T.C.; Taschetto, L.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Streck, E.L.; Ferreira, G.C.; Schuck, P.F. Evaluation of the effects of fructose on oxidative stress and inflammatory parameters in rat brain. Mol. Neurobiol. 2014, 50, 1124–1130. [Google Scholar] [CrossRef]
- Seraphim, D.C.C.; Punaro, G.R.; Fernandes, T.O.; Ginoza, M.; Lopes, G.S.; Higa, E.M.S. Assessment of fructose overload in the metabolic profile and oxidative/nitrosative stress in the kidney of senescent female rats. Exp. Gerontol. 2017, 99, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vojnovic Milutinovic, D.; Brkljacic, J.; Teofilovic, A.; Bursac, B.; Nikolic, M.; Gligorovska, L.; Kovacevic, S.; Djordjevic, A.; Preitner, F.; Tappy, L.; et al. Chronic Stress Potentiates High Fructose-Induced Lipogenesis in Rat Liver and Kidney. Mol. Nutr. Food Res. 2020, 64, e1901141. [Google Scholar] [CrossRef]
- Shirif, A.Z.; Kovacevic, S.; Brkljacic, J.; Teofilovic, A.; Elakovic, I.; Djordjevic, A.; Matic, G. Decreased Glucocorticoid Signaling Potentiates Lipid-Induced Inflammation and Contributes to Insulin Resistance in the Skeletal Muscle of Fructose-Fed Male Rats Exposed to Stress. Int. J. Mol. Sci. 2021, 22, 7206. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, S.; Nestorov, J.; Matic, G.; Elakovic, I. Fructose and stress induce opposite effects on lipid metabolism in the visceral adipose tissue of adult female rats through glucocorticoid action. Eur. J. Nutr. 2017, 56, 2115–2128. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, S.; Aulia, A.; Soetikno, V. Development of rat metabolic syndrome models: A review. Vet. World 2021, 14, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Vos, M.B.; McClain, C.J. Copper-Fructose Interactions: A Novel Mechanism in the Pathogenesis of NAFLD. Nutrients 2018, 10, 1815. [Google Scholar] [CrossRef]
- Tang, Z.; Gasperkova, D.; Xu, J.; Baillie, R.; Lee, J.H.; Clarke, S.D. Copper deficiency induces hepatic fatty acid synthase gene transcription in rats by increasing the nuclear content of mature sterol regulatory element binding protein 1. J. Nutr. 2000, 130, 2915–2921. [Google Scholar] [CrossRef]
- Wilson, J.; Kim, S.; Allen, K.G.; Baillie, R.; Clarke, S.D. Hepatic fatty acid synthase gene transcription is induced by a dietary copper deficiency. Am. J. Physiol. 1997, 272, E1124–E1129. [Google Scholar] [CrossRef]
- Bier, A.; Shapira, E.; Khasbab, R.; Sharabi, Y.; Grossman, E.; Leibowitz, A. High-Fructose Diet Increases Renal ChREBPbeta Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome. Biology 2022, 11, 618. [Google Scholar] [CrossRef]
- Kovacevic, S.; Nestorov, J.; Matic, G.; Elakovic, I. Chronic Stress Combined with a Fructose Diet Reduces Hypothalamic Insulin Signaling and Antioxidative Defense in Female Rats. Neuroendocrinology 2019, 108, 278–290. [Google Scholar] [CrossRef]
- O’Dell, B.L. Dietary carbohydrate source and copper bioavailability. Nutr. Rev. 1990, 48, 425–434. [Google Scholar] [CrossRef]
- Pabla, N.; Murphy, R.F.; Liu, K.; Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2009, 296, F505–F511. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Gybina, A.A.; Pyatskowit, J.W.; Gitschier, J.; Prohaska, J.R. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J. Nutr. 2006, 136, 21–26. [Google Scholar] [CrossRef]
- Molloy, S.A.; Kaplan, J.H. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J. Biol. Chem. 2009, 284, 29704–29713. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; L’Abbe, M.R. Copper modulates the degradation of copper chaperone for Cu,Zn superoxide dismutase by the 26 S proteosome. J. Biol. Chem. 2003, 278, 35071–35078. [Google Scholar] [CrossRef]
- Bertinato, J.; Iskandar, M.; L’Abbe, M.R. Copper deficiency induces the upregulation of the copper chaperone for Cu/Zn superoxide dismutase in weanling male rats. J. Nutr. 2003, 133, 28–31. [Google Scholar] [CrossRef]
- Bertinato, J.; Sherrard, L.; Plouffe, L.J. Decreased erythrocyte CCS content is a biomarker of copper overload in rats. Int. J. Mol. Sci. 2010, 11, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Getz, J.; Lin, D.; Medeiros, D.M. The cardiac copper chaperone proteins Sco1 and CCS are up-regulated, but Cox 1 and Cox4 are down-regulated, by copper deficiency. Biol. Trace Elem. Res. 2011, 143, 368–377. [Google Scholar] [CrossRef]
- Danzeisen, R.; Araya, M.; Harrison, B.; Keen, C.; Solioz, M.; Thiele, D.; McArdle, H.J. How reliable and robust are current biomarkers for copper status? Br. J. Nutr. 2007, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.J.; McArdle, H.J. Biomarkers of copper status: A brief update. Br. J. Nutr. 2008, 99 (Suppl. S3), S10–S13. [Google Scholar] [CrossRef]
- Banks, C.J.; Andersen, J.L. Mechanisms of SOD1 regulation by post-translational modifications. Redox Biol. 2019, 26, 101270. [Google Scholar] [CrossRef]
- Eleutherio, E.C.A.; Silva Magalhaes, R.S.; de Araujo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Curcumin Attenuates Oxidative Stress and Activation of Redox-Sensitive Kinases in High Fructose- and High-Fat-Fed Male Wistar Rats. Sci. Pharm. 2015, 83, 159–175. [Google Scholar] [CrossRef]
- Sivakumar, A.S.; Viswanathan, P.; Anuradha, C.V. Dose-dependent effect of galangin on fructose-mediated insulin resistance and oxidative events in rat kidney. Redox Rep. 2010, 15, 224–232. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Zeng, H.; Saari, J.T.; Johnson, W.T. Copper deficiency decreases complex IV but not complex I, II, III, or V in the mitochondrial respiratory chain in rat heart. J. Nutr. 2007, 137, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Elmore, B.O.; Bollinger, J.A.; Dooley, D.M. Human kidney diamine oxidase: Heterologous expression, purification, and characterization. J. Biol. Inorg. Chem. 2002, 7, 565–579. [Google Scholar] [CrossRef]
- Guo, H.; Xu, D.; Kuroki, M.; Lu, Z.; Xu, X.; Geurts, A.; Osborn, J.W.; Chen, Y. Kidney failure, arterial hypertension and left ventricular hypertrophy in rats with loss of function mutation of SOD3. Free Radic. Biol. Med. 2020, 152, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Petrak, J.; Vyoral, D. Hephaestin—A ferroxidase of cellular iron export. Int. J. Biochem. Cell Biol. 2005, 37, 1173–1178. [Google Scholar] [CrossRef]
- Hatori, Y.; Lutsenko, S. The Role of Copper Chaperone Atox1 in Coupling Redox Homeostasis to Intracellular Copper Distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef]
- Fabisiak, J.P.; Tyurin, V.A.; Tyurina, Y.Y.; Borisenko, G.G.; Korotaeva, A.; Pitt, B.R.; Lazo, J.S.; Kagan, V.E. Redox regulation of copper-metallothionein. Arch. Biochem. Biophys. 1999, 363, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Dundjerski, J.; Butorovic, B.; Kipic, J.; Trajkovic, D.; Matic, G. Cadmium affects the activity of rat liver tyrosine aminotransferase and its induction by dexamethasone. Arch. Toxicol. 1996, 70, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, L.; Bai, Q.; Zhu, X.; Zhang, J.; Wei, Q.; Li, D.; Gao, C.; Li, J.; Zhang, Z.; et al. Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J. Biol. Chem. 2014, 289, 22413–22426. [Google Scholar] [CrossRef]
- Kang, M.; Zhao, L.; Ren, M.; Deng, M.; Li, C. Reduced metallothionein expression induced by Zinc deficiency results in apoptosis in hepatic stellate cell line LX-2. Int. J. Clin. Exp. Med. 2015, 8, 20603–20609. [Google Scholar] [PubMed]
- Szczurek, E.I.; Bjornsson, C.S.; Taylor, C.G. Dietary zinc deficiency and repletion modulate metallothionein immunolocalization and concentration in small intestine and liver of rats. J. Nutr. 2001, 131, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Saari, J.T.; Kang, J.Y. Copper deficiency increases metallothionein-I mRNA content selectively in rat liver. J. Nutr. Biochem. 1995, 6, 572–576. [Google Scholar] [CrossRef]
- Baydas, G.; Gursu, M.F.; Yilmaz, S.; Canpolat, S.; Yasar, A.; Cikim, G.; Canatan, H. Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci. Lett. 2002, 323, 195–198. [Google Scholar] [CrossRef]
- Teofilovic, A.; Brkljacic, J.; Djordjevic, A.; VojnovicMilutinovic, D.; Tappy, L.; Matic, G.; Velickovic, N. Impact of insulin and glucocorticoid signalling on hepatic glucose homeostasis in the rat exposed to high-fructose diet and chronic stress. Int. J. Food Sci. Nutr. 2020, 71, 815–825. [Google Scholar] [CrossRef]
- Joels, M.; Karst, H.; Alfarez, D.; Heine, V.M.; Qin, Y.; van Riel, E.; Verkuyl, M.; Lucassen, P.J.; Krugers, H.J. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 2004, 7, 221–231. [Google Scholar] [CrossRef]
- Nikolic-Kokic, A.; Mijuskovic, A.; Tatalovic, N.; Nestorov, J.; Miler, M.; Orescanin-Dusic, Z.; Nikolic, M.; Milosevic, V.; Blagojevic, D.; Spasic, M.; et al. Effects of antipsychotic drug administration on antioxidative defense enzymes in male rat kidney. J. Toxicol. Environ. Health A 2016, 79, 905–911. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972, 247, 6960–6962. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism—A Manual of Biochemical Methods; Grune and Stratton: New York, NY, USA, 1982. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control | Fructose | Stress | Fructose + Stress |
|---|---|---|---|---|
| Glucose (mmol/L) | 5.68 ± 0.16 | 7.14 ± 0.33 ** | 6.34 ± 0.14 | 6.59 ± 0.29 ** |
| Insulin (ng/mL) | 2.47 ± 0.31 | 4.03 ± 0.50 | 3.33 ± 0.47 | 3.17 ± 0.55 |
| HOMA | 0.63 ± 0.09 | 1.28 ± 0.17 * | 0.94 ± 0.14 | 1.03 ± 0.13 * |
| Cu (μg/g) | 1.47 ± 0.08 | 1.48 ± 0.14 | 1.28 ± 0.06 | 1.33 ± 0.07 |
| Enzyme | Control | Fructose | Stress | Fructose + Stress |
|---|---|---|---|---|
| SOD1 | 22.6 ± 1.1 | 24.3 ± 2.2 | 20.9 ± 1.8 | 21.8 ± 2.1 |
| SOD2 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.2 |
| CAT | 63.7 ± 5.8 | 71.3 ± 2.4 | 75.7 ± 2.8 | 72.5 ± 2.4 |
| GPX | 377.7 ± 17.9 | 427.7 ± 29.1 | 397.1 ± 19.6 | 428.5 ± 15.2 |
| GR | 183.9 ± 14.5 | 195.3 ± 9.2 | 180.4 ± 19.6 | 201.4 ± 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasić, D.; Opačić, M.; Kovačević, S.; Nikolić Kokić, A.; Dimitrijević, M.; Nikolić, D.; Vojnović Milutinović, D.; Blagojević, D.; Djordjevic, A.; Brkljačić, J. Effects of Fructose and Stress on Rat Renal Copper Metabolism and Antioxidant Enzymes Function. Int. J. Mol. Sci. 2022, 23, 9023. https://doi.org/10.3390/ijms23169023
Tasić D, Opačić M, Kovačević S, Nikolić Kokić A, Dimitrijević M, Nikolić D, Vojnović Milutinović D, Blagojević D, Djordjevic A, Brkljačić J. Effects of Fructose and Stress on Rat Renal Copper Metabolism and Antioxidant Enzymes Function. International Journal of Molecular Sciences. 2022; 23(16):9023. https://doi.org/10.3390/ijms23169023
Chicago/Turabian StyleTasić, Danica, Miloš Opačić, Sanja Kovačević, Aleksandra Nikolić Kokić, Milena Dimitrijević, Dušan Nikolić, Danijela Vojnović Milutinović, Duško Blagojević, Ana Djordjevic, and Jelena Brkljačić. 2022. "Effects of Fructose and Stress on Rat Renal Copper Metabolism and Antioxidant Enzymes Function" International Journal of Molecular Sciences 23, no. 16: 9023. https://doi.org/10.3390/ijms23169023
APA StyleTasić, D., Opačić, M., Kovačević, S., Nikolić Kokić, A., Dimitrijević, M., Nikolić, D., Vojnović Milutinović, D., Blagojević, D., Djordjevic, A., & Brkljačić, J. (2022). Effects of Fructose and Stress on Rat Renal Copper Metabolism and Antioxidant Enzymes Function. International Journal of Molecular Sciences, 23(16), 9023. https://doi.org/10.3390/ijms23169023