Examination of Trace Metals and Their Potential Transplacental Transfer in Pregnancy
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Toxic Trace Metals
3.2. Essential Trace Metals
4. Materials and Methods
4.1. Collection of Samples
4.2. Trace Element Analysis
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Malinowski, W.; Szymański, S.; Mularczyk, M.; Tomska, N.; Rotter, I. interactions between 14 elements in the human placenta, fetal membrane and umbilical cord. Int. J. Environ. Res. Public Health 2019, 16, 1615. [Google Scholar] [CrossRef] [Green Version]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal heavy metal exposure and adverse birth outcomes in Myanmar: A birth-cohort study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef] [Green Version]
- Shah-Kulkarni, S.; Lee, S.; Jeong, K.S.; Hong, Y.C.; Park, H.; Ha, M.; Kim, Y.; Ha, E.H. Prenatal exposure to mixtures of heavy metals and neurodevelopment in infants at 6 months. Environ. Res. 2020, 182, 109122. [Google Scholar] [CrossRef]
- Turdi, M.; Yang, L. Trace elements contamination and human health risk assessment in drinking water from the agricultural and pastoral areas of bay county, Xinjiang, China. Int. J. Environ. Res. Public Health 2016, 13, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, C.V.; Lewin, M.; Ragin-Wilson, A.; Jones, R.; Jarrett, J.M.; Wallon, K.; Ward, C.; Hilliard, N.; Irvin-Barnwell, E. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999-2016. Environ. Res. 2020, 183, 109208. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ Res. 2019, 177, 108599. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Storey, K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. EXCLI J. 2021, 20, 956–967. [Google Scholar] [CrossRef]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS ONE 2019, 14, e0218437. [Google Scholar] [CrossRef]
- Ashrap, P.; Watkins, D.J.; Milne, G.L.; Ferguson, K.; Loch-Caruso, R.; Fernandez, J.; Rosario, Z.; Vélez-Vega, C.; Alshawabkeh, A.; Cordero, J.; et al. Maternal urinary metal and metalloid concentrations in association with oxidative stress biomarkers. Antioxidants 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Mohamed, G.E.D.; Rabah, A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int. J. Hyg. Environ. Health 2011, 214, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Shimada, M.; Kameo, S.; Nakai, K.; Yaginuma-Sakurai, K.; Tatsuta, N.; Kurokawa, N.; Nakayama, S.F.; Satoh, H. Exposure profile of mercury, lead, cadmium, arsenic, antimony, copper, selenium and zinc in maternal blood, cord blood and placenta: The Tohoku Study of Child Development in Japan. Environ. Health Prev. Med. 2019, 24, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Li, Z.; Xia, X.; Wang, Q.; Tao, R.; Tao, Y.; Xiang, H.; Tong, S.; Tao, F. Determine multiple elements simultaneously in the sera of umbilical cord blood samples-a very simple method. Biol. Trace Elem. Res. 2017, 177, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Kirman, C.R.; Marchitti, S.A.; Kenneke, J.F.; English, C.; Mattison, D.R.; Becker, R.A. Relationships of chemical concentrations in maternal and cord blood: A review of available data. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 175–203. [Google Scholar] [CrossRef]
- Punshon, T.; Li, Z.; Marsit, C.J.; Jackson, B.P.; Baker, E.R.; Karagas, M.R. Placental metal concentrations in relation to maternal and infant toenails in a U.S. cohort. Environ. Sci. Technol. 2016, 50, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Cerrillos, L.; Fernández, R.; Machado, M.J.; Morillas, I.; Dahiri, B.; Paz, S.; Gonzalez-Weller, D.; Gutiérrez, A.; Rubio, C.; Hardisson, A.; et al. Placental levels of metals and associated factors in urban and sub-urban areas of Seville (Spain). J. Trace Elem. Med. Biol. 2019, 54, 21–26. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Kim, S.; Park, J.; Kim, H.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Kim, S.; et al. Placental transfer of persistent organic pollutants and feasibility using the placenta as a non-invasive biomonitoring matrix. Sci. Total Environ. 2018, 612, 1498–1505. [Google Scholar] [CrossRef]
- Maharajan, N.; Cho, G.W.; Choi, J.H.; Jang, C.H. Regenerative therapy using umbilical cord serum. In Vivo 2021, 35, 699–705. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Reddy, R.C.; Chadchan, K.S.; Patil, A.J.; Biradar, M.S.; Das, K.K. Nickel and oxidative stress: Cell signaling mechanisms and protective role of vitamin C. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.L.; Hu, H.; Xu, F.P.; Liu, L.Y.; Peng, J.; Dong, X.D. Pregnancy complications effect on the nickel content in maternal blood, placenta blood and umbilical cord blood during pregnancy. World J. Clin. Cases 2021, 9, 8340–8348. [Google Scholar] [CrossRef] [PubMed]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Kopplin, M.J.; Wollenberg, M.L. Oxidation and detoxification of trivalent arsenic species. Toxicol. Appl. Pharmacol. 2003, 193, 1–8. [Google Scholar] [CrossRef]
- Tam, L.M.; Price, N.E.; Wang, Y. Molecular mechanisms of arsenic-induced disruption of DNA repair. Chem Res Toxicol. 2020, 33, 709–726. [Google Scholar] [CrossRef]
- Navasumrit, P.; Chaisatra, K.; Promvijit, J.; Parnlob, V.; Waraprasit, S.; Chompoobut, C.; Binh, T.T.; Hai, D.N.; Bao, N.D.; Hai, N.K.; et al. Exposure to arsenic in utero is associated with various types of DNA damage and micronuclei in newborns: A birth cohort study. Environ. Health 2019, 18, 68. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhuang, T.; Shi, J.; Liang, Y.; Song, M. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. J. Environ. Sci. (China) 2019, 80, 99–106. [Google Scholar] [CrossRef]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [Green Version]
- Esteban-Vasallo, M.D.; Aragonés, N.; Pollan, M.; López-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef] [Green Version]
- Goyer, R.A.; Cherian, M.G. Role of metallothionein in human placenta and rats exposed to cadmium. IARC Sci. Publ. 1992, 118, 239–247. [Google Scholar]
- Migliore, L.; Frenzilli, G.; Nesti, C.; Fortaner, S.; Sabbioni, E. Cytogenetic and oxidative damage induced in human lymphocytes by platinum, rhodium and palladium compounds. Mutagenesis 2002, 17, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carozzi, V.A.; Marmiroli, P.; Cavaletti, G. The role of oxidative stress and anti-oxidant treatment in platinum-induced peripheral neurotoxicity. Curr. Cancer Drug Targets 2010, 10, 670–682. [Google Scholar] [CrossRef]
- Sengupta, P.; Banerjee, R.; Nath, S.; Das, S.; Banerjee, S. Metals and female reproductive toxicity. Hum. Exp. Toxicol. 2015, 34, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RÍsovÁ, V. The pathway of lead through the mother’s body to the child. Interdiscip Toxicol. 2019, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; Siddiqui, M.K. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 2007, 383, 57–64. [Google Scholar] [CrossRef]
- Iqbal, S.; Ali, I.; Rust, P.; Kundi, M.; Ekmekcioglu, C. Selenium, zinc, and manganese status in pregnant women and its relation to maternal and child complications. Nutrients 2020, 12, 725. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Chartrand, M.S.; Aaseth, J. Manganese exposure and neurotoxic effects in children. Environ Res. 2017, 155, 380–384. [Google Scholar] [CrossRef]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Ettinger, A.S.; Shapiro, G.D.; Fisher, M.; Monnier, P.; Morisset, A.-S.; Fraser, W.D.; Bouchard, M.F. Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int.J. Hyg. Environ. Health 2018, 221, 876–882. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D. MIREC study group. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 3, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandakumaran, M.; Al-Sannan, B.; Al-Sarraf, H.; Al-Shammari, M. Maternal-fetal transport kinetics of manganese in perfused human placental lobule in vitro. J. Matern. Fetal Neonatal. Med. 2016, 29, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Birch, C.S.; Brasch, N.E.; McCaddon, A.; Williams, J.H. A novel role for vitamin B(12): Cobalamins are intracellular antioxidants in vitro. Free Radic. Biol. Med. 2009, 47, 184–188. [Google Scholar] [CrossRef]
- Callan, A.C.; Hinwood, A.L.; Ramalingam, M.; Boyce, M.; Heyworth, J.; McCafferty, P.; Odland, J.Ø. Maternal exposure to metals--concentrations and predictors of exposure. Environ. Res. 2013, 126, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Liang, C.M.; Xia, X.; Huang, K.; Yan, S.-Q.; Tao, R.-W.; Pan, W.-J.; Sheng, J.; Tao, Y.-R.; Xiang, H.-Y.; et al. Association between maternal and umbilical cord serum cobalt concentration during pregnancy and the risk of preterm birth: The Ma’anshan birth cohort (MABC) study. Chemosphere 2019, 218, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N. The role of trace elements on hepatitis virus infections: A review. J. Trace Elem. Med. Biol. 2011, 25, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, S.M.; Abu-Elteen, K.H.; Elkarmi, A.Z.; Qaraein, S.H.; Salem, N.M.; Mubarak, M.S. Maternal and cord blood serum levels of zinc, copper, and iron in healthy pregnant Jordanian women. J. Trace Elem. Exp. Med. 2004, 17, 1–8. [Google Scholar] [CrossRef]
- McArdle, H.J.; Andersen, H.S.; Jones, H.; Gambling, L. Copper and iron transport across the placenta: Regulation and interactions. J. Neuroendocrinol. 2008, 20, 427–431. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Al-Saleh, E.; Nandakumaran, M.; Al-Shammari, M.; Al-Falah, F.; Al-Harouny, A. Assessment of maternal-fetal status of some essential trace elements in pregnant women in late gestation: Relationship with birth weight and placental weight. J. Matern. Fetal Neonatal Med. 2004, 16, 9–14. [Google Scholar] [CrossRef]
- Kucukaydin, Z.; Kurdoglu, M.; Kurdoglu, Z.; Demir, H.; Yoruk, I.H. Selected maternal, fetal and placental trace element and heavy metal and maternal vitamin levels in preterm deliveries with or without preterm premature rupture of membranes. J. Obstet. Gynaecol. Res. 2018, 44, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Chan, H.M.; Domingo, J.L.; Koriyama, C.; Murata, K. Placental transfer and levels of mercury, selenium, vitamin E, and docosahexaenoic acid in maternal and umbilical cord blood. Environ. Int. 2018, 111, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Varsi, K.; Bolann, B.; Torsvik, I.; Rosvold Eik, T.C.; Høl, P.J.; Bjørke-Monsen, A.L. Impact of maternal selenium status on infant outcome during the first 6 months of life. Nutrients 2017, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Pieczyńska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
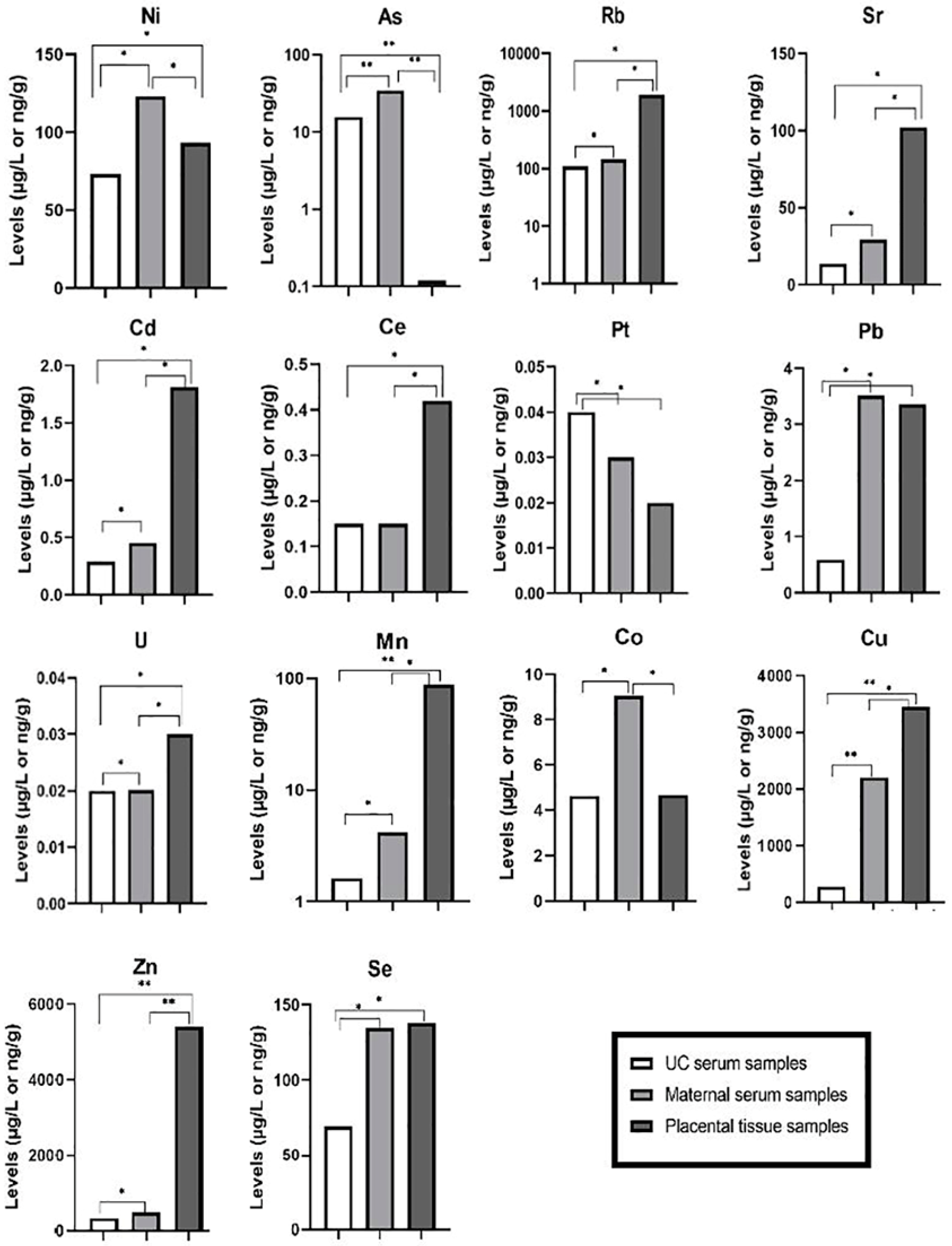
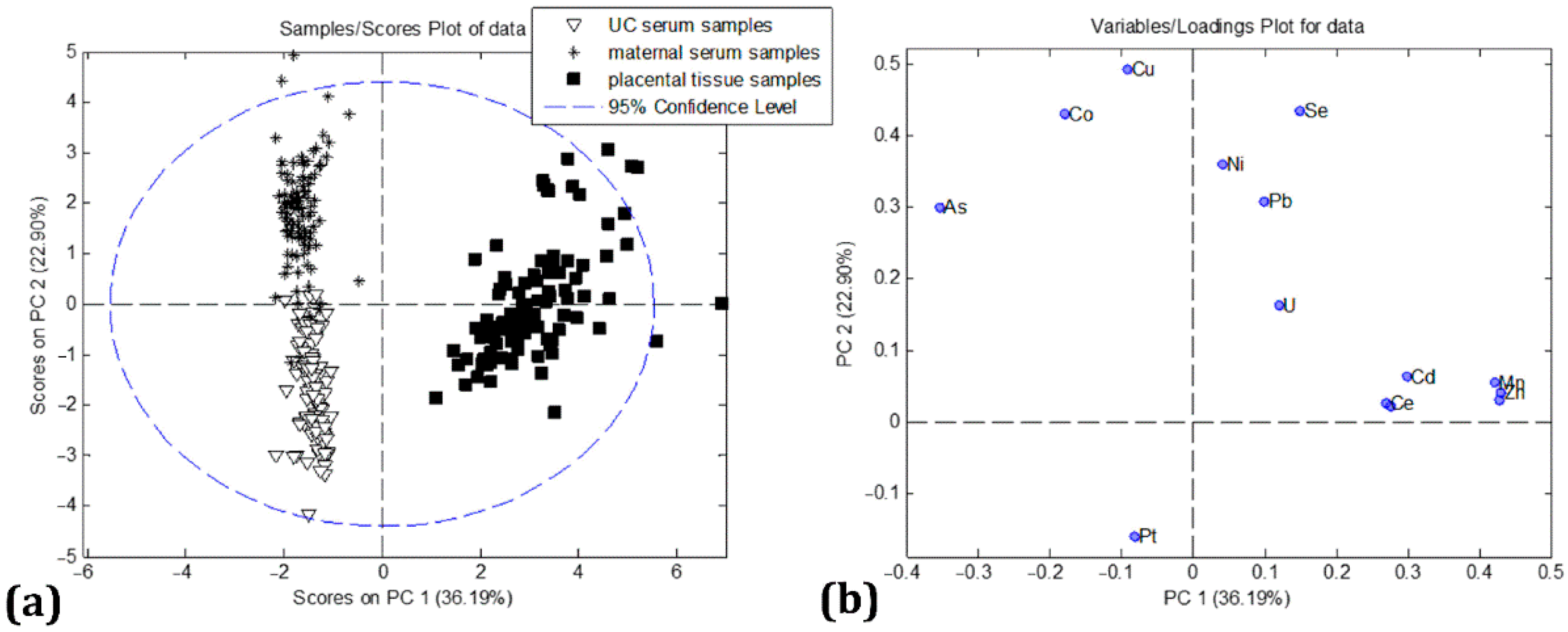
| Ni | As | Rb | Sr | Cd | Ce | Pt | Pb | U | Mn | Co | Cu | Zn | Se | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Umbilical cord serum (µg/L) | Median | 73.2 | 15.70 | 110.0 | 13.50 | 0.29 | 0.15 | 0.040 | 0.59 | 0.020 | 1.61 | 4.63 | 273.0 | 338.0 | 69.3 |
| IQR | 38.3 | 11.40 | 85.2 | 8.73 | 0.20 | 0.11 | 0.050 | 0.50 | 0.020 | 1.04 | 5.99 | 151.0 | 180.0 | 32.1 | |
| Min | 21.3 | 2.51 | 20.7 | 3.92 | 0.01 | 0.01 | 0.003 | 0.01 | 0.003 | 0.12 | 1.21 | 56.1 | 95.3 | 23.4 | |
| Max | 184.0 | 39.60 | 295.0 | 41.10 | 0.77 | 0.39 | 0.720 | 4.14 | 0.090 | 4.34 | 8.69 | 634.0 | 755.0 | 146.0 | |
| Maternal serum (µg/L) | Median | 123.0 | 34.60 | 144.0 | 29.20 | 0.45 | 0.15 | 0.030 | 3.51 | 0.020 | 4.21 | 9.05 | 2200.0 | 472.0 | 135.0 |
| IQR | 19.4 | 8.01 | 39.7 | 11.30 | 0.24 | 0.13 | 0.030 | 5.29 | 0.030 | 1.02 | 1.31 | 507.0 | 92.9 | 35.9 | |
| Min | 74.8 | 16.90 | 63.6 | 15.40 | 0.04 | 0.01 | 0.003 | 0.11 | 0.010 | 1.69 | 5.42 | 547.0 | 180.0 | 55.1 | |
| Max | 168.0 | 60.80 | 246.0 | 69.30 | 2.65 | 0.67 | 0.080 | 19.20 | 0.310 | 9.23 | 12.10 | 3446.0 | 1420.0 | 247.0 | |
| Placental tissue (ng/g) | Median | 93.3 | 0.12 | 1923.0 | 102.00 | 1.81 | 0.42 | 0.020 | 3.36 | 0.030 | 87.80 | 4.09 | 859.0 | 5401.0 | 138.0 |
| IQR | 85.2 | 0.11 | 527.0 | 78.80 | 1.43 | 0.53 | 0.020 | 3.26 | 0.040 | 31.20 | 2.48 | 198.0 | 1440.0 | 32.2 | |
| Min | 14.6 | 0.02 | 664.0 | 28.80 | 0.33 | 0.02 | 0.001 | 0.13 | 0.001 | 49.40 | 1.36 | 401.0 | 2840.0 | 34.1 | |
| Max | 466.0 | 2.51 | 3306.0 | 650.00 | 16.70 | 5.76 | 0.090 | 31.10 | 0.280 | 237.00 | 20.40 | 1616.0 | 10171.0 | 188.0 |
| Ni | As | Rb | Sr | Cd | Ce | Pt | ||
|---|---|---|---|---|---|---|---|---|
| UC serum | Maternal serum | −0.1598 | −0.2070 | −0.1154 | −0.2494 | 0.1158 | −0.0427 | 0.4162 |
| UC serum | Placental tissue | 0.1375 | 0.2872 | −0.1170 | 0.1735 | 0.1106 | −0.2208 | −0.0493 |
| Maternal serum | Placental tissue | −0.2244 | −0.1998 | 0.0083 | −0.0289 | −0.0227 | −0.0348 | 0.0428 |
| Pb | U | Mn | Co | Cu | Zn | Se | ||
| UC serum | Maternal serum | 0.0808 | 0.0423 | −0.0414 | 0.1410 | −0.1396 | −0.2182 | −0.1232 |
| UC serum | Placental tissue | 0.1284 | −0.0807 | −0.0675 | 0.1494 | −0.1411 | −0.0888 | −0.0537 |
| Maternal serum | Placental tissue | −0.0876 | −0.0043 | 0.0011 | −0.1374 | 0.0829 | −0.0198 | 0.1056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagodić, J.; Pavlović, S.; Borković-Mitić, S.; Perović, M.; Miković, Ž.; Đurđić, S.; Manojlović, D.; Stojsavljević, A. Examination of Trace Metals and Their Potential Transplacental Transfer in Pregnancy. Int. J. Mol. Sci. 2022, 23, 8078. https://doi.org/10.3390/ijms23158078
Jagodić J, Pavlović S, Borković-Mitić S, Perović M, Miković Ž, Đurđić S, Manojlović D, Stojsavljević A. Examination of Trace Metals and Their Potential Transplacental Transfer in Pregnancy. International Journal of Molecular Sciences. 2022; 23(15):8078. https://doi.org/10.3390/ijms23158078
Chicago/Turabian StyleJagodić, Jovana, Slađan Pavlović, Slavica Borković-Mitić, Milan Perović, Željko Miković, Slađana Đurđić, Dragan Manojlović, and Aleksandar Stojsavljević. 2022. "Examination of Trace Metals and Their Potential Transplacental Transfer in Pregnancy" International Journal of Molecular Sciences 23, no. 15: 8078. https://doi.org/10.3390/ijms23158078
APA StyleJagodić, J., Pavlović, S., Borković-Mitić, S., Perović, M., Miković, Ž., Đurđić, S., Manojlović, D., & Stojsavljević, A. (2022). Examination of Trace Metals and Their Potential Transplacental Transfer in Pregnancy. International Journal of Molecular Sciences, 23(15), 8078. https://doi.org/10.3390/ijms23158078









