Tailoring Nylon 6/Acrylonitrile-Butadiene-Styrene Nanocomposites for Application against Electromagnetic Interference: Evaluation of the Mechanical, Thermal and Electrical Behavior, and the Electromagnetic Shielding Efficiency
Abstract
1. Introduction
2. Results and Discussion
2.1. Rheological Properties
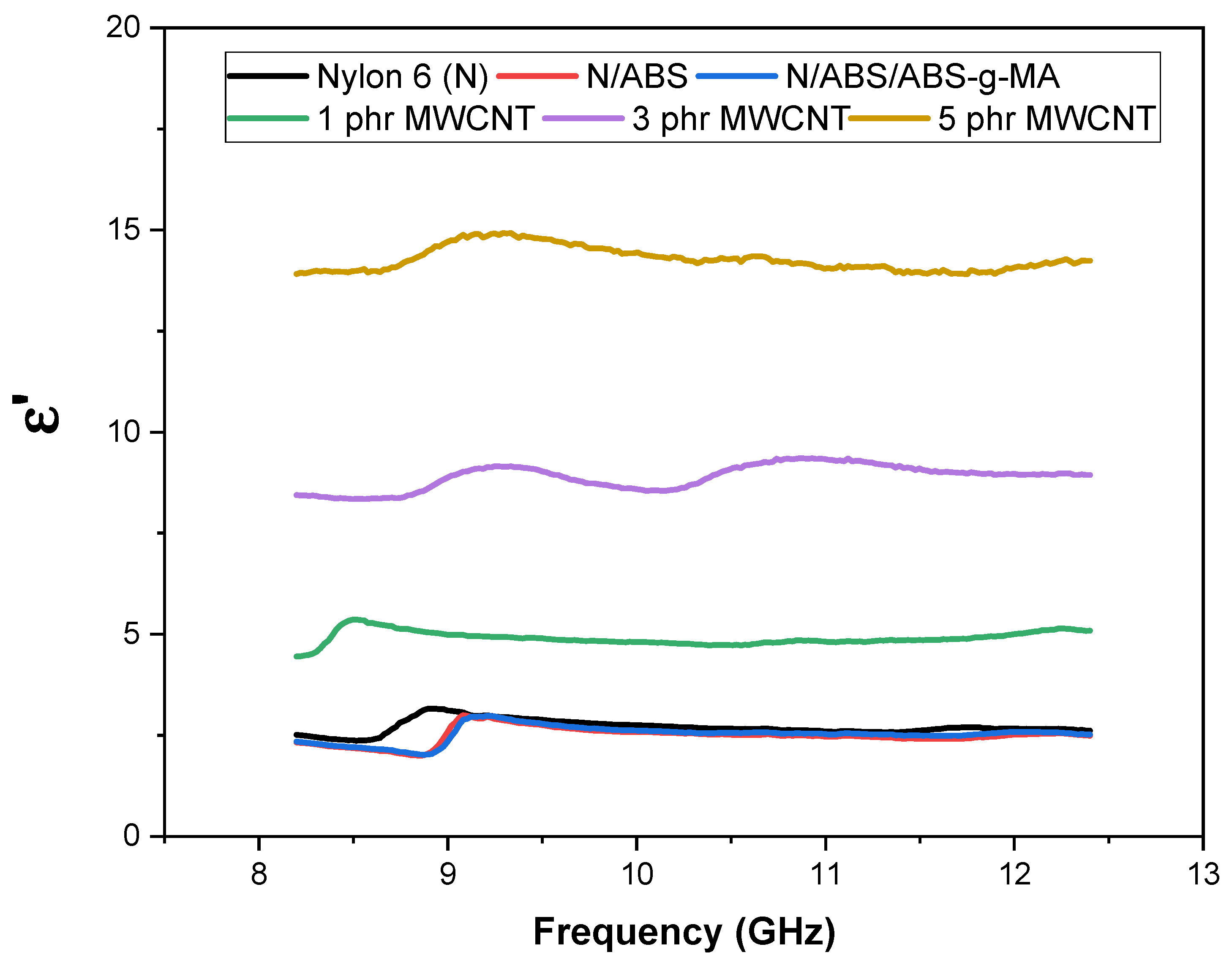
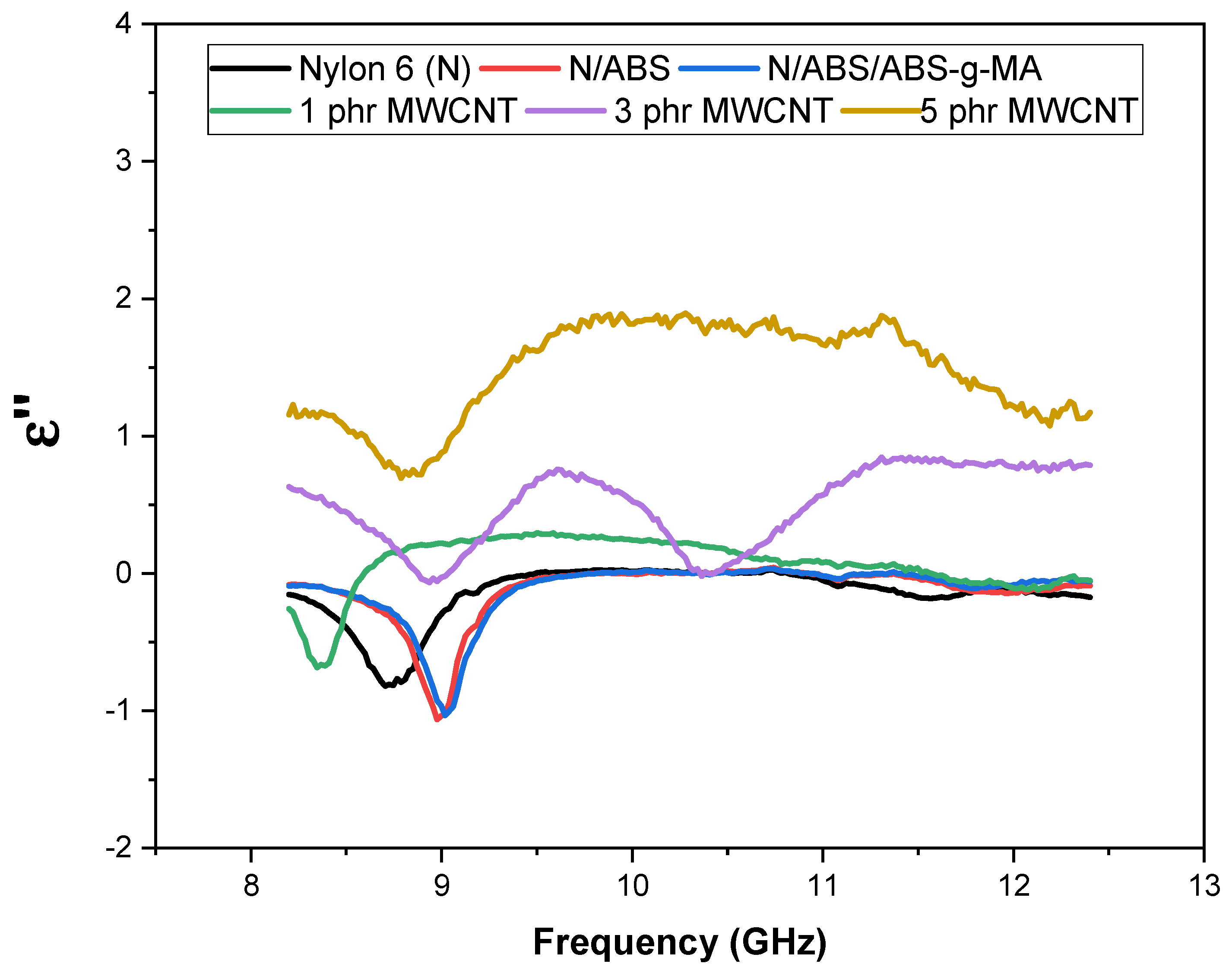
2.2. Electrical Properties
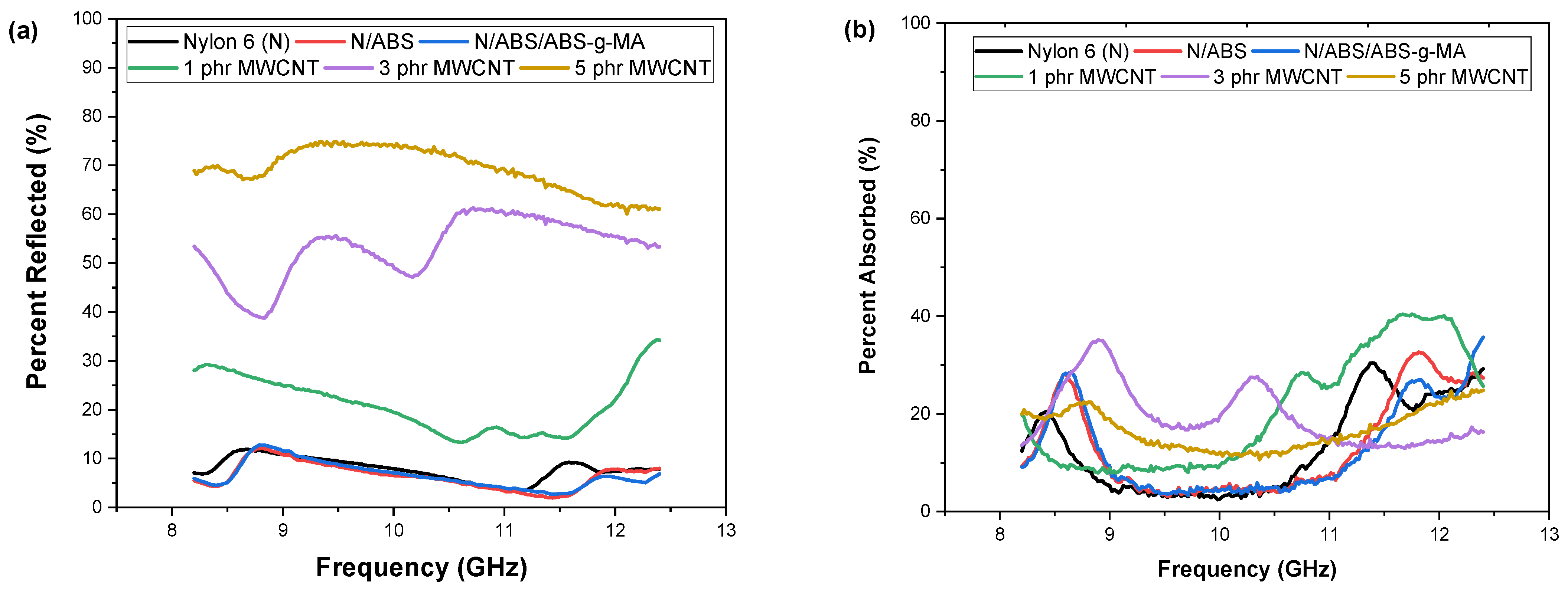
2.3. Electromagnetic Shielding
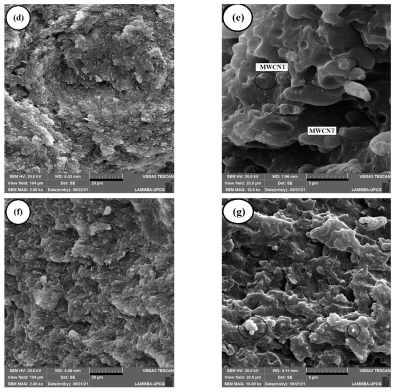
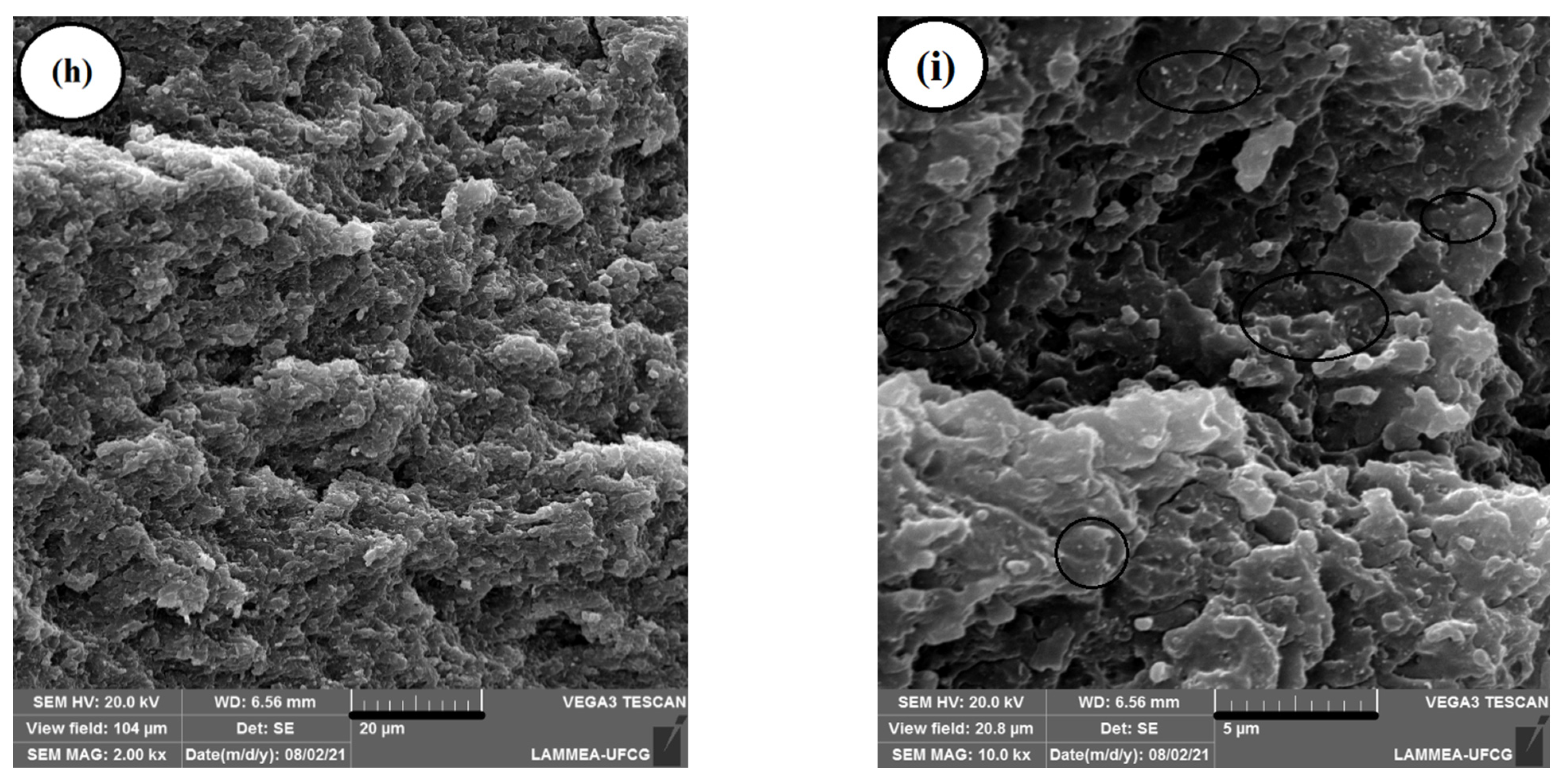
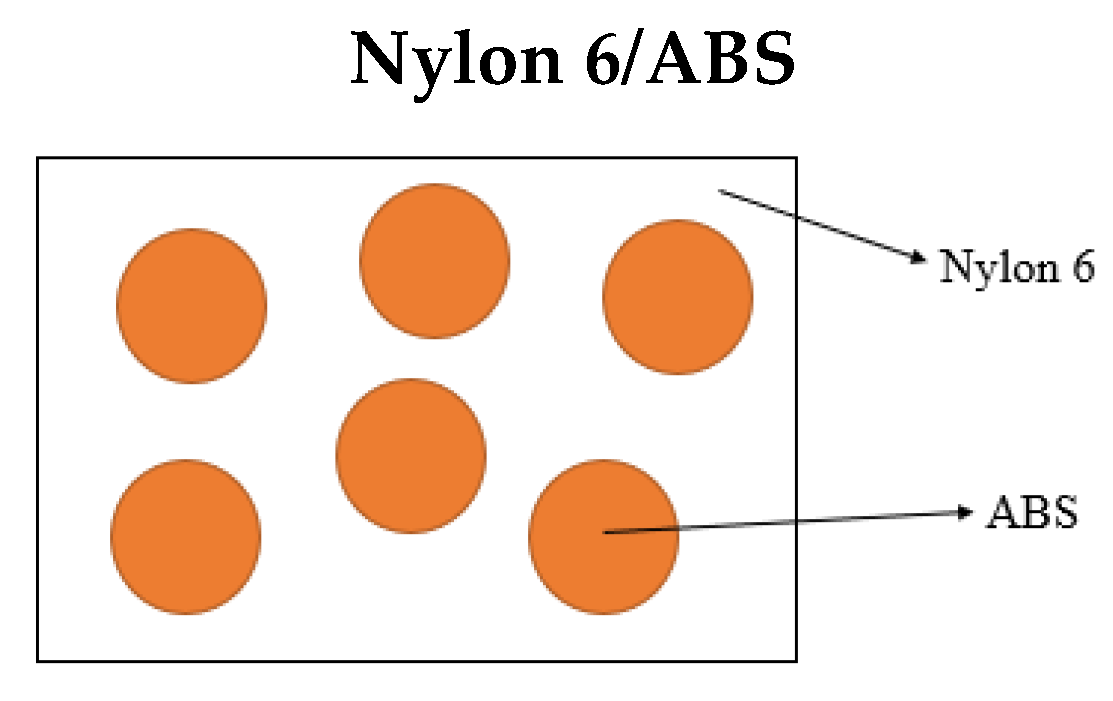
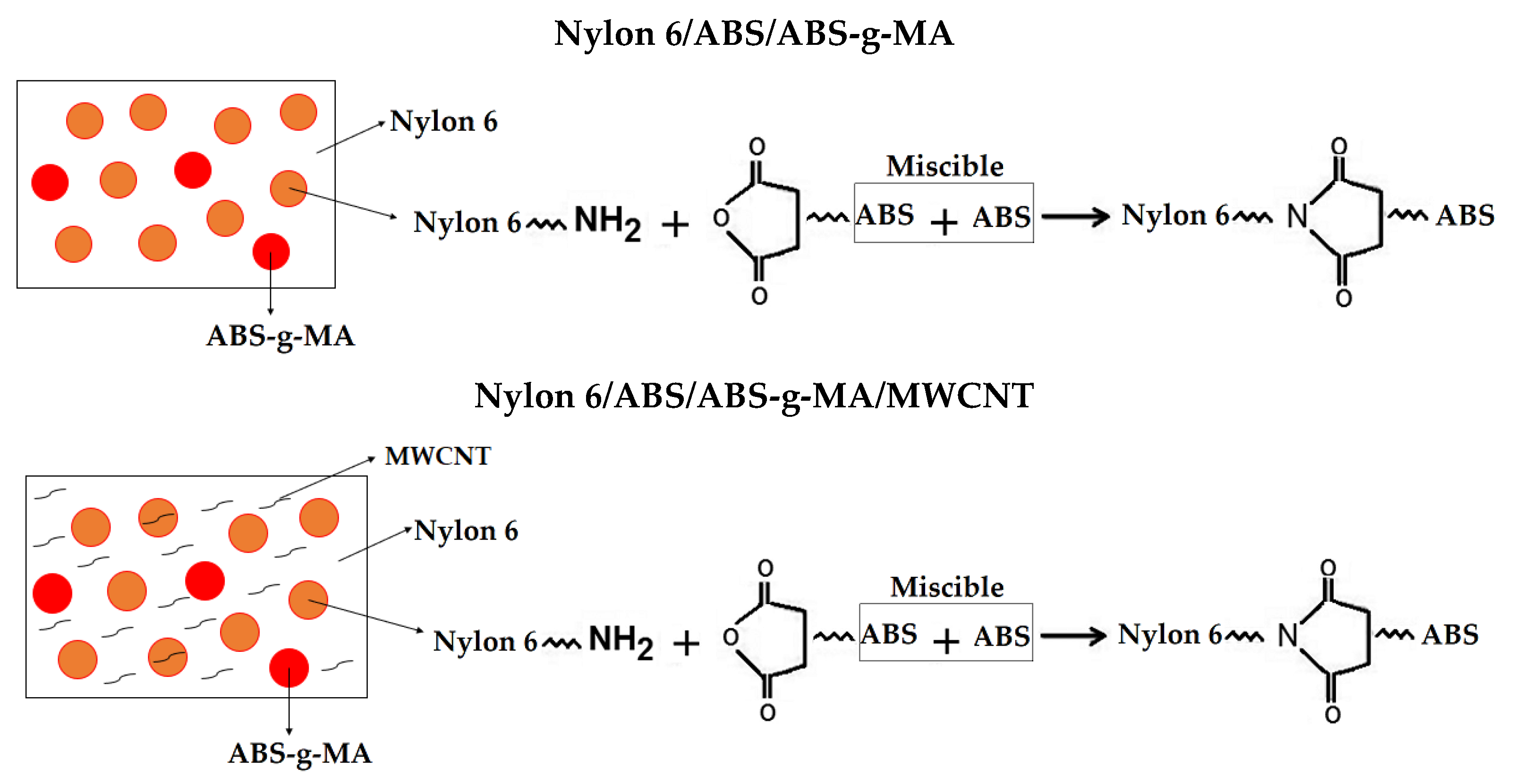
2.4. Scanning Electron Microscopy (SEM)
2.5. Mechanical Properties
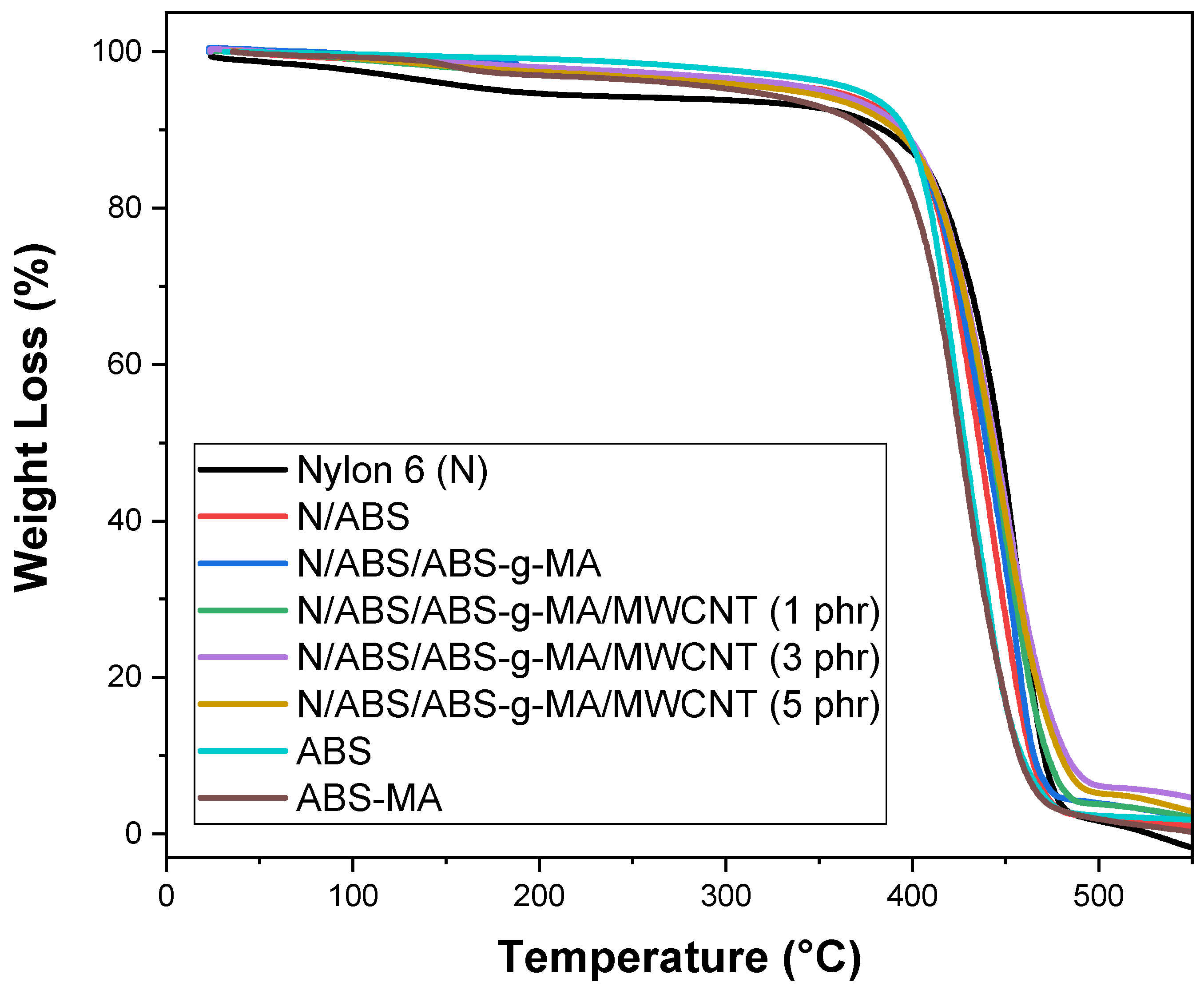
2.6. Thermogravimetry (TG)
3. Materials and Methods
3.1. Materials
3.2. Methods
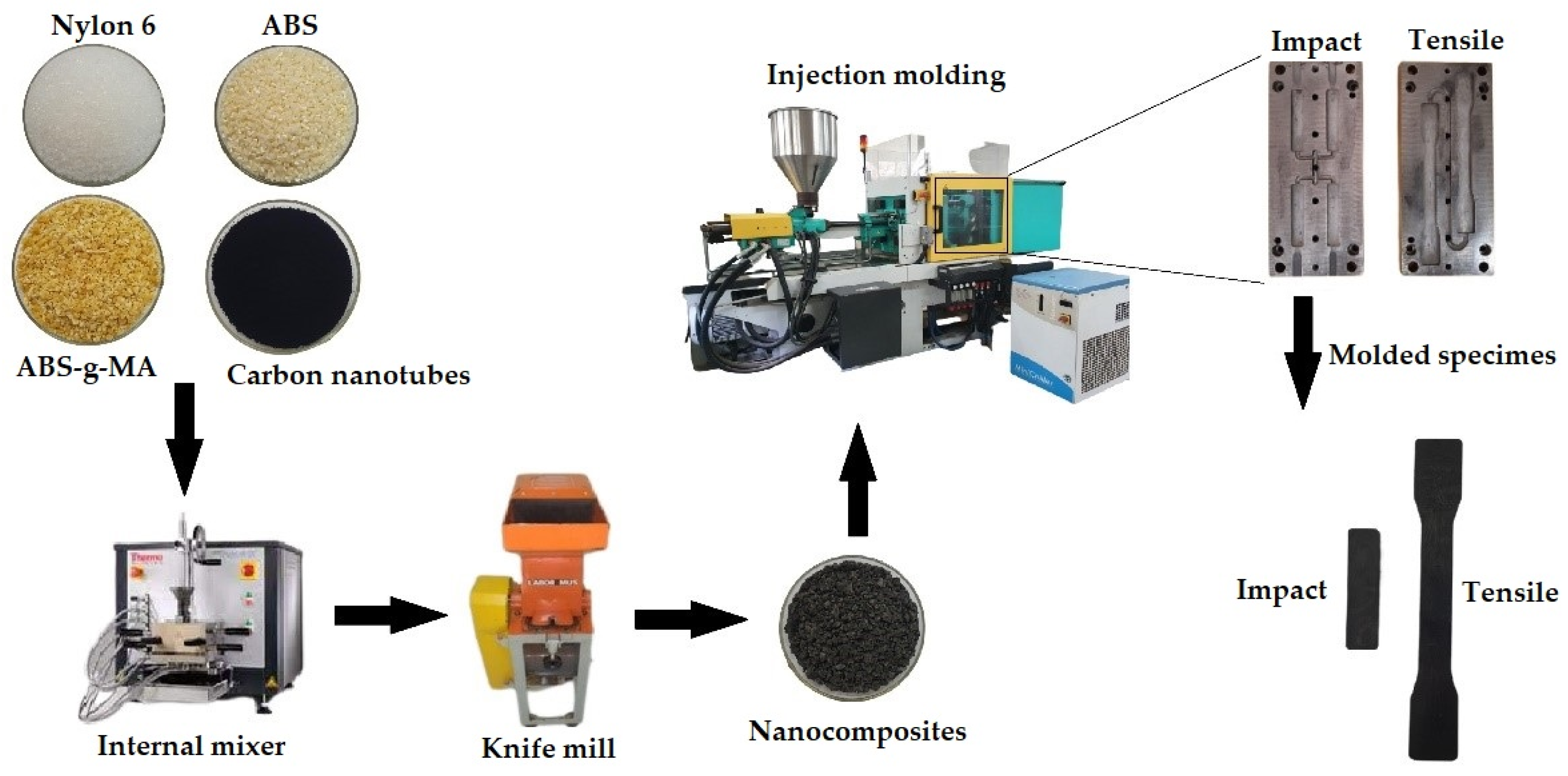
3.2.1. Processing of Materials
3.2.2. Characterization of Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shameen, M.M.; Sasikanth, S.M.; Annamalai, R.; Raman, R.G. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar]
- Shukla, P.; Saxena, P. Polymer Nanocomposites in Sensor Applications: A Review on Present Trends and Future Scope. Chin. J. Polym. Sci. 2021, 39, 665–691. [Google Scholar] [CrossRef]
- Su, L.S.; Tsai, J.L. Characterizing the mechanical properties of nanocomposites with aligned graphene. Polym. Compos. 2021, 42, 4005–4014. [Google Scholar] [CrossRef]
- George, E.; Joy, J.; Anas, S. Acrylonitrile-based polymer/graphene nanocomposites: A review. Polym. Compos. 2021, 42, 4961–4980. [Google Scholar] [CrossRef]
- Ezenkwa, O.E.; Hassan, A.; Samsudin, S.A. Comparison of mechanical properties and thermal stability of graphene-based materials and halloysite nanotubes reinforced maleated polymer compatibilized polypropylene nanocomposites. Polym. Compos. 2022, 43, 1852–1863. [Google Scholar] [CrossRef]
- Oliveira, S.V.; Araújo, E.M.; Pereira, C.M.C.; Leite, A.M.D. Polyethylene/bentonite clay nanocomposite with flame retardant properties. Polímeros 2017, 27, 91–98. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Silva, A.L.; Siqueira, D.D.; Filho, E.A.S.; Araújo, E.M.; Nascimento, E.P.; Costa, A.C.F.M. Preparation of flexible and magnetic PA6/SEBS-MA nanocomposites reinforced with Ni-Zn ferrite. Polym. Compos. 2022, 43, 68–83. [Google Scholar] [CrossRef]
- Farzaneh, A.; Rostami, A.; Nazockdast, H. Thermoplastic polyurethane/multiwalled carbon nanotubes nanocomposites: Effect of nanoparticle content, shear, and thermal processing. Polym. Compos. 2021, 42, 4804–4813. [Google Scholar] [CrossRef]
- Orawiec, M.; Belton, D.; Telford, R.; Surtees, A. Application of semi-in situ liquid exfoliation of graphite to the scalable production of graphene-epoxy nanocomposites. Polym. Compos. 2020, 41, 4933–4944. [Google Scholar] [CrossRef]
- Dhawan, A.; Jindal, P. Mechanical behavior of carboxylic functionalized graphene reinforced polyurethane nanocomposites under static and dynamic loading. Polym. Compos. 2021, 42, 4911–4922. [Google Scholar] [CrossRef]
- Jaffal, D.; Daniels, S.; Tang, H.Y.; Ghadimi, H.; Monty, C.N. Electroconductive nylon-6/multi-walled carbon nanotube nanocomposite for sodium sensing applications. Compos. Part C Open Access 2021, 4, 100116. [Google Scholar] [CrossRef]
- Rodríguez, R.L.; Hernandez, E.H.; Macias, R.Y.; Hernandez, Z.G.; Zuniga, G.Y.R.; Falcon, M.G.G.; Vega, C.G.; Morones, P.G. Study of the dielectric heating of graphite oxide and its effect on the microwave-assisted synthesis of Nylon-6/graphite oxide polymeric hybrid nanocomposites. J. Appl. Polym. Sci. 2022, 139, 51567. [Google Scholar] [CrossRef]
- Aparna, S.; Purnima, D.; Adusumalli, R.B. Review on various compatibilizers and its effect on mechanical properties of compatibilized nylon blends. Polym. Plast. Technol. Eng. 2017, 56, 617–634. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Filho, E.A.S.; Siqueira, D.D.; Araújo, E.M.; Nascimento, E.P.; Mélo, T.J.A. Influence of Small Amounts of ABS and ABS-MA on PA6 Properties: Evaluation of Torque Rheometry, Mechanical, Thermomechanical, Thermal, Morphological, and Water Absorption Kinetics Characteristics. Materials 2022, 15, 2502. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Trends in graphene reinforced polyamide nanocomposite for functional application: A review. Polym. Plast Technol. Eng. 2018, 58, 917–933. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Shentu, B.; Weng, Z. In Situ Formation of the Core–Shell Particles and Their Function in Toughening PA6/SEBS-g-MA/PP Blends. Ind. Eng. Chem. Res. 2017, 56, 11657–11663. [Google Scholar] [CrossRef]
- Oliveira, A.D.; Castro, L.D.C.; Jung, M.K.; Pessan, L.A. Influência da modificação da argila montmorilonita nas propriedades mecânicas, termo-mecânicas e morfológicas de nanocompósitos de blendas de poliamida 6/Acrilonitrila-EPDM-estireno. Polímeros 2015, 25, 219–229. [Google Scholar] [CrossRef]
- Oliveira, A.D.; Larocca, N.M.; Paul, D.R.; Pessan, L.A. Effects of mixing protocol on the performance of nanocomposites based on polyamide 6/acrylonitrile– butadiene–styrene blends. Polym. Eng Sci. 2012, 52, 1909–1919. [Google Scholar] [CrossRef]
- Castro, L.D.C.; Oliveira, A.D.; Kersch, M.; Altsradt, V.; Pessan, L.A. Effect of organoclay incorporation and blending protocol on performance of PA6/ABS nanocomposites compatibilized with SANMA. Polym. Eng. Sci. 2017, 57, 1147–1154. [Google Scholar] [CrossRef]
- Arigbabowo, O.K.; Tate, J.S. Additive manufacturing of polyamide nanocomposites for electrostatic charge dissipation applications. Mater. Sci. Eng. B 2021, 271, 115251. [Google Scholar] [CrossRef]
- Braga, N.F.; Zaggo, H.M.; Montagna, L.S.; Passador, F.R. Effect of Carbon Nanotubes (CNT) Functionalization and Maleic Anhydride-Grafted Poly(trimethylene terephthalate) (PTT-g-MA) on the Preparation of Antistatic Packages of PTT/CNT Nanocomposites. J. Compos. Sci. 2020, 4, 44. [Google Scholar] [CrossRef]
- Kaushal, A.; Singh, V. Electromagnetic interference shielding response of multiwall carbon nanotube/polypropylene nanocomposites prepared via melt processing technique. Polym. Compos. 2021, 42, 1148–1154. [Google Scholar] [CrossRef]
- Jiang, D.; Murugadoss, V.; Wang, Y.; Lin, J.; Ding, T.; Wang, Z.; Shao, Q.; Wang, C.; Liu, H.; Liu, H.; et al. Electromagnetic interference shielding polymers and nanocomposites-A review. Polym. Rev. 2019, 59, 280–337. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Tian, G.; Wang, Y.; Gao, J.; Wang, C.; Xu, L.; Zhang, H. Morphology evolution to form double percolation polylactide/polycaprolactone/MWCNT Ts nanocomposites with ultralow percolation threshold and excellent EMI shielding. Compos. Sci. Technol. 2021, 214, 108956. [Google Scholar] [CrossRef]
- Yao, Y.; Jin, S.; Zou, H.; Li, L.; Ma, X.; Lv, G.; Gao, F.; Lv, X.; Shu, Q. Polymer-based lightweight materials for electromagnetic interference shielding: A review. J. Mater. Sci. 2021, 56, 6549–6580. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.H. Carbon materials and their composites for electromagnetic interference shielding effectiveness in X-band. Carbon 2019, 152, 159–187. [Google Scholar] [CrossRef]
- Zachariah, S.M.; Grohens, Y.; Kalarikkal, N.; Thomas, S. Hybrid materials for electromagnetic shielding: A review. Polym. Compos. 2022, 43, 2507–2544. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Jerome, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R Rep. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, P.; Wang, Q.; Li, L.; Dong, S.; Liu, J.; Rao, W. Stretchable liquid metal electromagnetic interference shielding coating materials with superior effectiveness. J. Mater. Chem. C 2019, 33, 10331–10337. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress on carbon-based composite materials for microwave electromagnetic interference shielding. Carbon 2021, 177, 304–331. [Google Scholar] [CrossRef]
- Saleh, M.H.A.; Saadeh, W.H.; Sundararaj, U. EMI shielding effectiveness of carbon based nanostructured polymeric materials: A comparative study. Carbon 2013, 60, 146–156. [Google Scholar] [CrossRef]
- Mathews, S.A.; Babu, D.R. Analysis of the role of M-type hexaferrite-based materials in electromagnetic interference shielding. Curr. Appl. Phys. 2021, 29, 39–53. [Google Scholar] [CrossRef]
- Sarmad, M.P.; Noroozi, M.; Abrisham, M.; Eghbalinia, S.; Teimoury, F.; Bahramian, A.R.; Dehghan, P.; Sadri, M.; Goodarzi, V. A Comprehensive review on carbon-based polymer nanocomposite foams as electromagnetic interference shields and piezoresistive sensors. ACS Appl. Electron. Mater. 2020, 2, 2318–2350. [Google Scholar] [CrossRef]
- Cheng, M.; Ren, W.; Li, H.; Liu, X.; Bandaru, S.; Zhang, J.; Zhang, X. Multiscale collaborative coupling of wood-derived porous carbon modified by three-dimensional conductive magnetic networks for electromagnetic interference shielding. Compos. B Eng. 2021, 224, 109169. [Google Scholar] [CrossRef]
- Chung, D.D.L. Materials for electromagnetic interference shielding. Mater. Chem. Phys. 2020, 255, 123587. [Google Scholar] [CrossRef]
- Guo, Y.L.; Zhang, R.Z.; Wu, K.; Chen, F.; Fu, Q. Preparation of nylon MXD6/EG/CNTs ternary composites with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Chin. J. Polym. Sci. 2017, 35, 1497–1507. [Google Scholar] [CrossRef]
- Nasouri, K.; Valipour, P. Fabrication of polyamide 6/carbon nanotubes composite electrospun nanofibers for microwave absorption application. Polym. Sci. A 2015, 57, 359–364. [Google Scholar] [CrossRef]
- Duan, H.; Zhu, H.; Yang, Y.; Hou, T.; Zhao, G.; Liu, Y. Facile and economical fabrication of conductive polyamide 6 composites with segregated expanded graphite networks for efficient electromagnetic interference shielding. J. Mater. Sci. Mater. Electron. 2018, 29, 1058–1064. [Google Scholar] [CrossRef]
- Guerreiro, M.; Rompante, J.; Leite, A.C.; Fernandes, L.P.; Santos, R.M.; Paiva, M.C.; Covas, J.A. Development of electrically conductive polymer nanocomposites for the automotive cable industry. Polímeros 2021, 31, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Du, D.; Guo, L.; Guo, Y.; Ooyang, J. Stretchable and conductive polymer films for high-performance electromagnetic interference shielding. J. Mater. Chem. C 2016, 4, 6525–6532. [Google Scholar] [CrossRef]
- Lin, T.; Yu, H.; Wang, L.; Fahad, S.; Khan, A.; Naveed, K.U.R.; Haq, F.; Nazir, A.; Amin, B.U. A review of recent advances in the preparation of polyaniline-based composites and their electromagnetic absorption properties. J. Mater. Sci. 2021, 56, 5449–5478. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.R.; Bondre, A.P.; Kulkarni, A.R.; Potschke, P. Rheology, electrical conductivity, and the phase behavior of cocontinuous PA6/ABS blends with MWNT: Correlating the aspect ratio of MWNT with the percolation threshold. J. Polym. Sci. B Polym. Phys. 2008, 46, 1619–1631. [Google Scholar] [CrossRef]
- Agrawal, P.; Rodrigues, A.W.B.; Araújo, E.M.; MÉLO, T.J.A. Influence of reactive compatibilizers on the rheometrical and mechanical properties of PA6/LDPE and PA6/HDPE blends. J. Mater. Sci. 2010, 45, 496–502. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Ferreira, E.S.B.; Araújo, E.M.; Wellen, R.M.R. Reactive processing of PA6/EPDM-MA blends as modifier for application and development of high-performance polypropylene. J. Vinyl Addit. Technol. 2021, 27, 736–756. [Google Scholar] [CrossRef]
- Kasgoz, A.; Akin, D.; Durmus, A. Rheological and electrical properties of carbon black and carbon fiber filled cyclic olefin copolymer composites. Compos. B Eng. 2014, 62, 113–120. [Google Scholar] [CrossRef]
- Hassanabadi, H.M.; Wilhelm, M.; Rodrigues, D. A rheological criterion to determine the percolation threshold in polymer nano-composites. Rheol. Acta 2014, 53, 869–882. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Pan, Y.; Schubert, D.W.; Liu, C. Shear-induced rheological and electrical properties of molten poly(methyl methacrylate)/carbon black nanocomposites. Compos. B Eng. 2019, 164, 37–44. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T. Electrical and rheological properties of carbon black and carbon fiber filled low-density polyethylene/ethylene vinyl acetate composites. Sci. Eng. Compos 2017, 25, 715–723. [Google Scholar] [CrossRef]
- Ribeiro, B.; Botelho, E.C.; Costa, M.L.; Bandeira, C.F. Carbon nanotube buckypaper reinforced polymer composites: A review. Polímeros 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Alves, A.M.; Cavalcanti, S.N.; Silva, M.P.; Freitas, D.M.G.; Agrawal, P.; Mélo, T.J.A. Electrical, rheological, and mechanical properties copolymer/carbon black composites. J. Vinyl Addit. Technol. 2021, 27, 445–458. [Google Scholar] [CrossRef]
- Shang, M.; Gao, Z.; Cheng, H.; Shentu, B. Comparative Study of Poly(butylene terephthalate)/Carbon Nanotube Nanocomposites with Non-reactive and Reactive Elastomers: Morphology and Properties. Ind. Eng. Chem. Res. 2020, 59, 14306–14314. [Google Scholar] [CrossRef]
- Cardinaud, R.; Mcnally, T. Localization of MWCNT Ts in PET/LDPE blends. Eur. Polym. J. 2013, 49, 1287–1297. [Google Scholar] [CrossRef]
- Stanciu, N.V.; Stan, F.; Sandu, I.L.; Fetecau, C.; Turcanu, A.M. Thermal, Rheological, Mechanical, and Electrical Properties of Polypropylene/Multi-Walled Carbon Nanotube Nanocomposites. Polymers 2021, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, E.; Pereira, E.C.L.; Silva, A.A.; Soares, B.G. Polypropylene/poly(lactic acid)/carbon nanotube semi-biodegradable nanocomposites: The effect of sequential mixing approach and compatibilization on morphology, rheology and electrical conductivity. J. Appl. Polym. Sci. 2021, 138, 51195. [Google Scholar] [CrossRef]
- Soares, B.G.; Cordeiro, E.; Maia, J.; Pereira, E.C.L.; Silva, A.A. The effect of the noncovalent functionalization of CNT by ionic liquid on electrical conductivity and electromagnetic interference shielding effectiveness of semi-biodegradable polypropylene/poly(lactic acid) composites. Polym. Compos. 2020, 41, 82–93. [Google Scholar] [CrossRef]
- Silva, M.P.; Cavalcanti, S.N.; Alves, A.M.; Freitas, D.M.G.; Agrawal, P.; Vilar, E.O.; Mélo, T.J.A. Evaluation of the rheological and electrical percolation of high-density polyethylene/carbon black composites using mathematical models. Polym Eng. Sci. 2021, 61, 2105–2116. [Google Scholar]
- Potschke, P.; Goad, M.A.; Alig, I.; Dukin, S.; Lellinger, D. Rheological and dielectrical characterization of melt mixed polycarbonate-multiwalled carbon nanotube composites. Polymer 2004, 45, 8863–8870. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing Carbon Nanotubes by Grafting on Intumescent Flame Retardant: Nanocomposite Synthesis, Morphology, Rheology, and Flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Khan, T.; Irfan, M.S.; Ali, M.; Dong, Y.; Ramakrissna, S.; Umer, R. Insights to low electrical percolation thresholds of carbon-based polypropylene nanocomposites. Carbon 2021, 176, 602–631. [Google Scholar] [CrossRef]
- Vieira, L.S.; Anjos, E.G.R.; Verginio, G.E.A.; Oyama, I.C.; Braga, N.F.; Silva, T.F.; Montagna, L.S.; Rezende, M.C.; Passador, F.R. Carbon-based materials as antistatic agents for the production of antistatic packaging: A review. J. Mater. Sci. Mater. Electron. 2021, 32, 3929–3947. [Google Scholar] [CrossRef]
- Vieira, L.d.S.; Anjos, E.G.R.d.; Verginio, G.E.A.; Oyama, I.C.; Braga, N.F.; Silva, T.F.d.; Montagna, L.S.; Passador, F.R. A review concerning the main factors that interfere in the electrical percolation threshold content of polymeric antistatic packaging with carbon fillers as antistatic agente. Nano Sel. 2021, 3, 248–260. [Google Scholar] [CrossRef]
- Ribeiro, B.; Botelho, E.C.; Costa, M.L. Estudo das propriedades elétricas e térmicas de compósitos nanoestruturados de poli(sulfeto de fenileno) reforçados com nanotubos de carbono. Polímeros 2015, 25, 94–100. [Google Scholar] [CrossRef][Green Version]
- Silva, T.F.; Menezes, F.; Stieven, M.L.; Lemes, A.P.; Passador, F.R. Efeito sinérgico da adição de lignina e negro de fumo em poli (ácido lático). Polímeros 2020, 30, e2020002. [Google Scholar]
- Chikyu, N.; Nakano, T.; Kletetschka, G.; Inouue, Y. Excellent electromagnetic interference shielding characteristics of a unidirectionally oriented thin multiwalled carbon nanotube/polyethylene film. Mater. Des. 2020, 195, 108918. [Google Scholar] [CrossRef]
- Souto, L.F.C.; Cossa, M.M.; Soares, B.G.; Siqueira, A.S. Estudo das propriedades reológicas, mecânicas e de blindagem eletromagnética de misturas elastoméricas envolvendo borracha nitrílica (NBR) e borracha nitrílica carboxilada (XNBR). Polímeros 2017, 27, 14–19. [Google Scholar] [CrossRef][Green Version]
- Soares, B.G.; Barra, G.M.O.; Indrussiak, T. Conducting Polymeric Composites Based on Intrinsically Conducting Polymers as Electromagnetic Interference Shielding/Microwave Absorbing Materials—A Review. J. Compos. Sci. 2021, 5, 173. [Google Scholar] [CrossRef]
- Ecco, L.G.; Dul, S.; Schmitz, D.P.; Barra, G.M.O.; Soares, B.G.; Fambri, L.; Pegoretti, A. Rapid Prototyping of Efficient Electromagnetic Interference Shielding Polymer Composites via Fused Deposition Modeling. Appl. Sci. 2019, 9, 37. [Google Scholar] [CrossRef]
- Yusoff, A.N.; Abdullah, M.H.; Ahmad, S.H.; Jusoh, S.F.; Mansor, A.A.; Hamid, A.S.S. Electromagnetic and absorption properties of some microwave absorbers. J. Appl. Phys. 2022, 92, 876–882. [Google Scholar] [CrossRef]
- Sui, X.; Xie, X.M. Creating super-tough and strong PA6/ABS blends using multi-phase compatibilizers. Chin. Chem. Lett. 2019, 30, 149–152. [Google Scholar] [CrossRef]
- Majumdar, B.; Keskkula, H.; Paul, D.R. Effects of the nature of the polyamide on the properties and morphology of compatibilized nylon 6/ABS blends. Polymer 1994, 35, 5468–5477. [Google Scholar] [CrossRef]
- Koning, C.; Duin, M.V.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; Nascimento, E.P.; Melo, J.B.C.A. Evaluation of the SEBS copolymer in the compatibility of PP/ABS blends through mechanical, thermal, thermomechanical properties, and morphology. Polym. Adv. Technol. 2022, 33, 111–124. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Micusik, M.; Omastová, M.; Krupa, I.; Prokes, J.; Pissis, P.; Logakis, E.; Pandis, C.; Potsckke, P.; Piontech, J. A comparative study on the Electrical and mechanical behaviour of multi-walled carbon nanotube composites prepared by diluting a masterbatch with various types of polypropylenes. J. Appl. Polym. Sci. 2009, 113, 2536–2551. [Google Scholar] [CrossRef]
- Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawzzak, P.; Dupin, G.; Claes, M. Masterbatch-based multi-walled carbon nanotube filled polypropylene nanocomposites: Assessment of rheological and mechanical properties. Compos. Sci. Technol. 2009, 69, 1756–1763. [Google Scholar] [CrossRef]
- Chiu, F.C.; Kao, G.F. Polyamide 46/multi-walled carbon nanotube nanocomposites with enhanced thermal, electrical, and mechanical properties. Compos. A Appl. Sci. Manuf. 2012, 43, 208–218. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; Wellen, R.M.R.; de Mélo, T.J.A. Approaches on the acrylonitrile-butadiene-styrene functionalization through maleic anhydride and dicumyl peroxide. J. Vinyl Addit. Technol. 2021, 27, 308–318. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.V.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
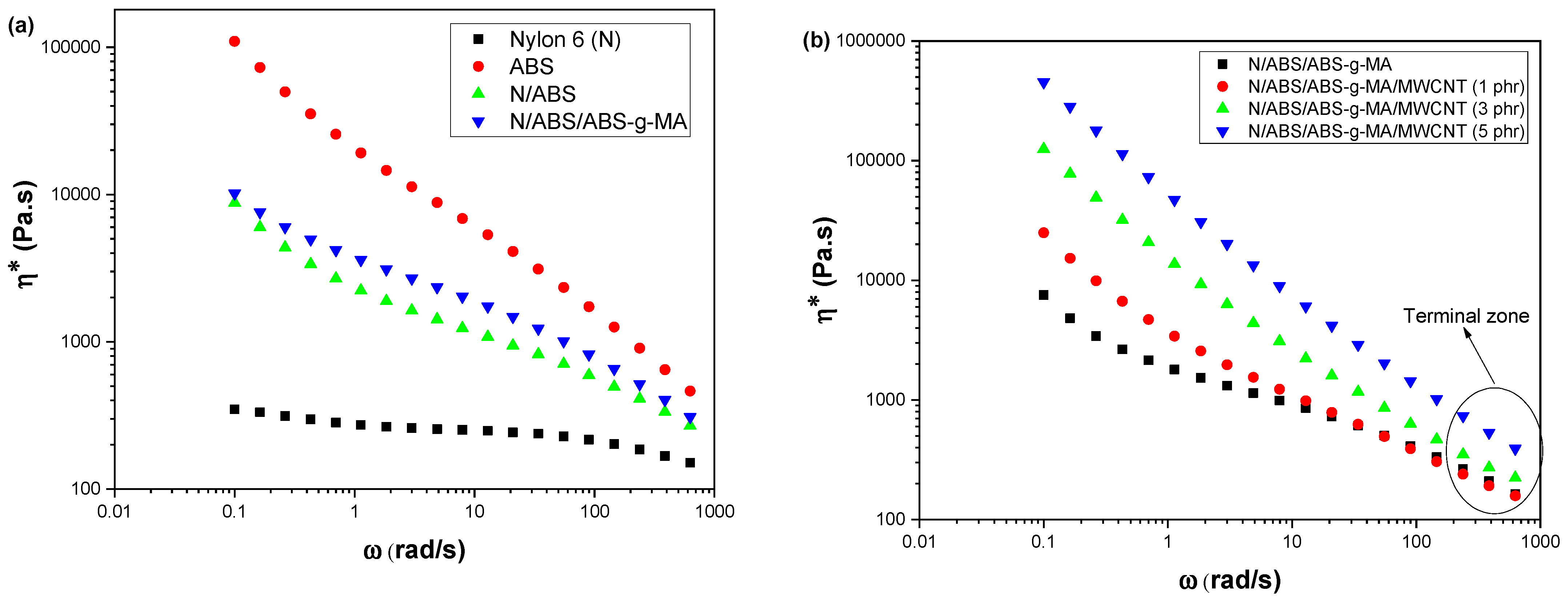
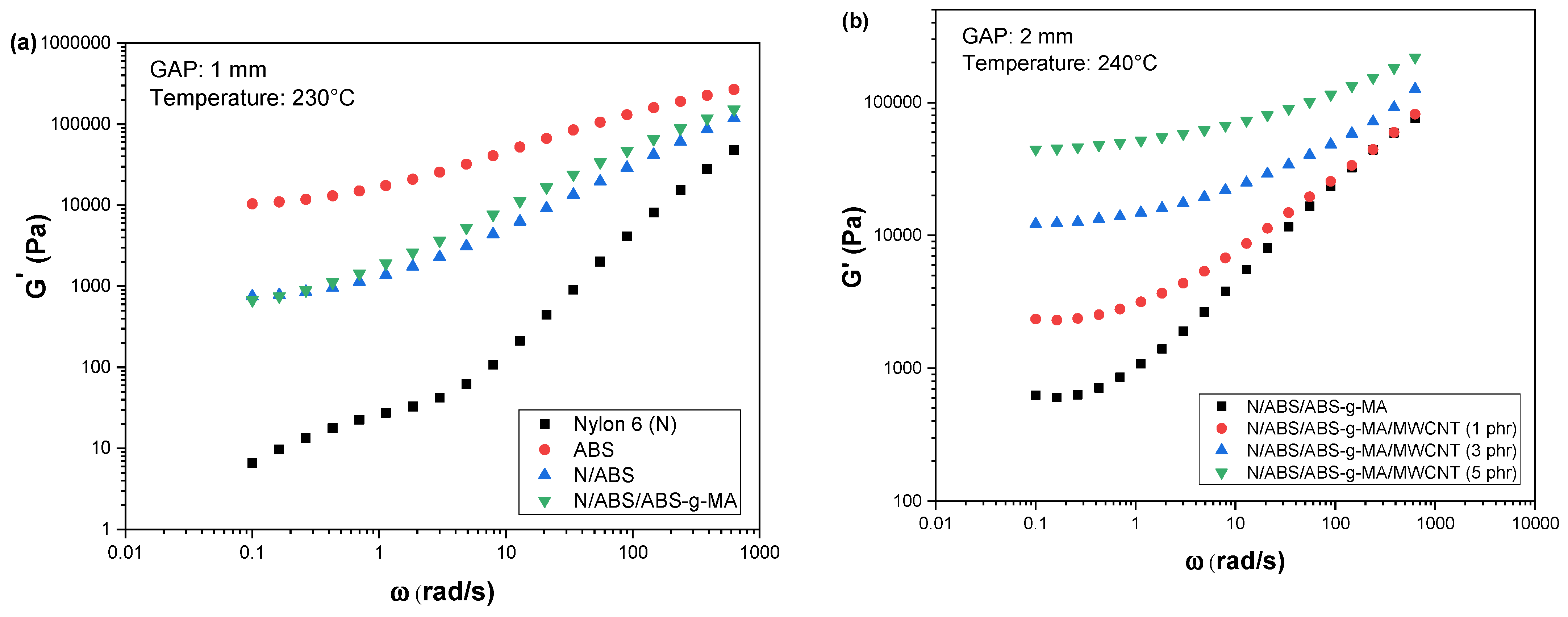
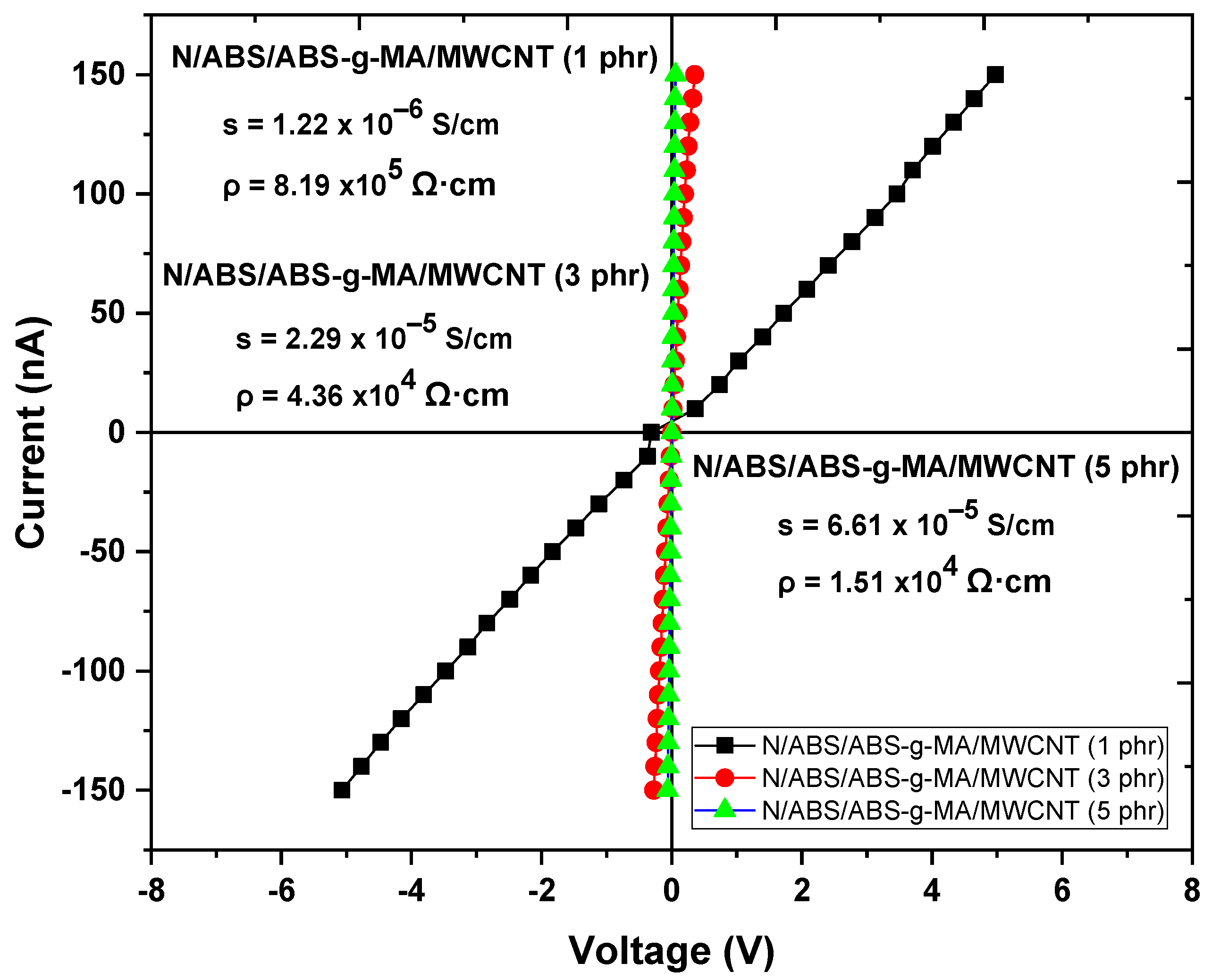
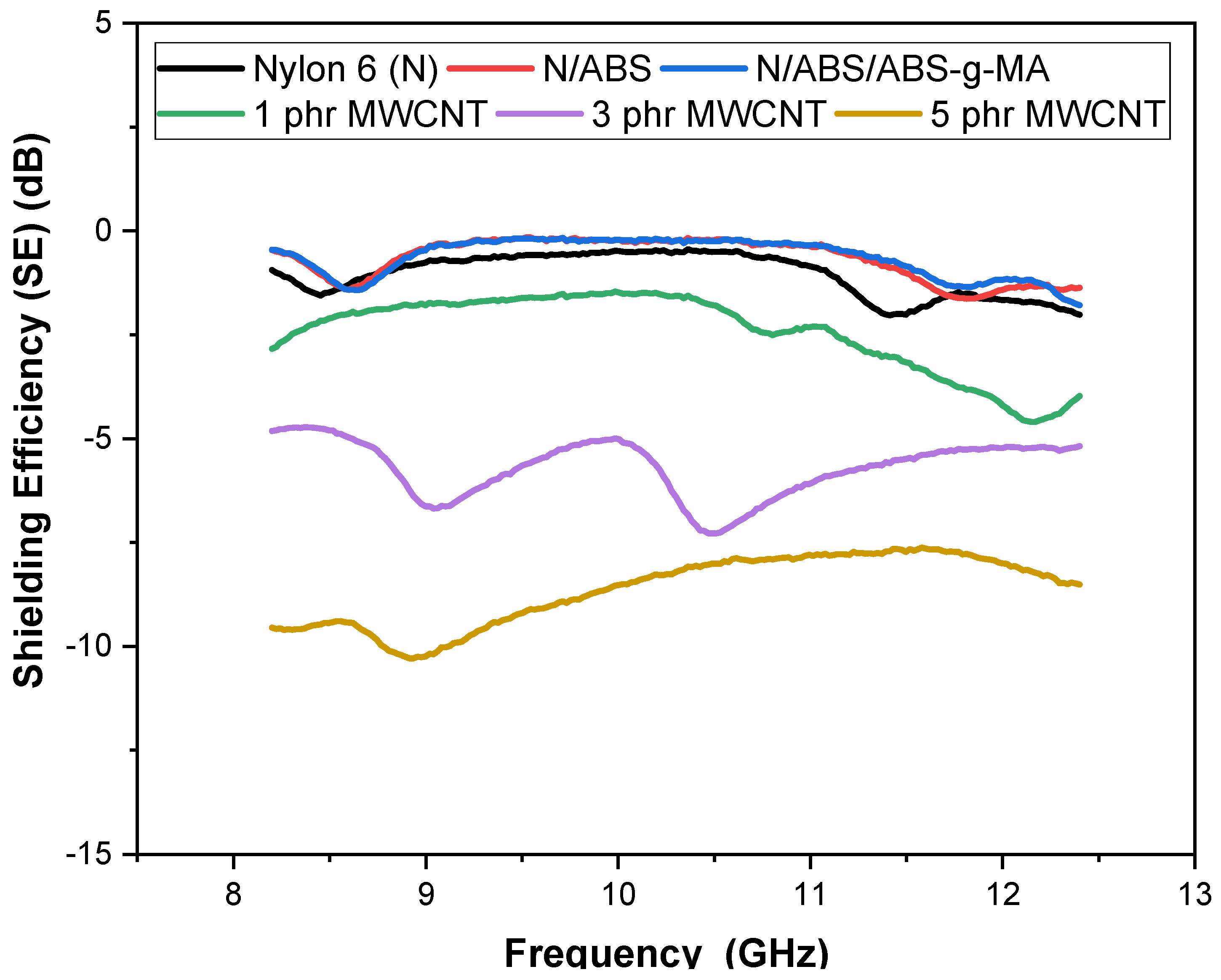

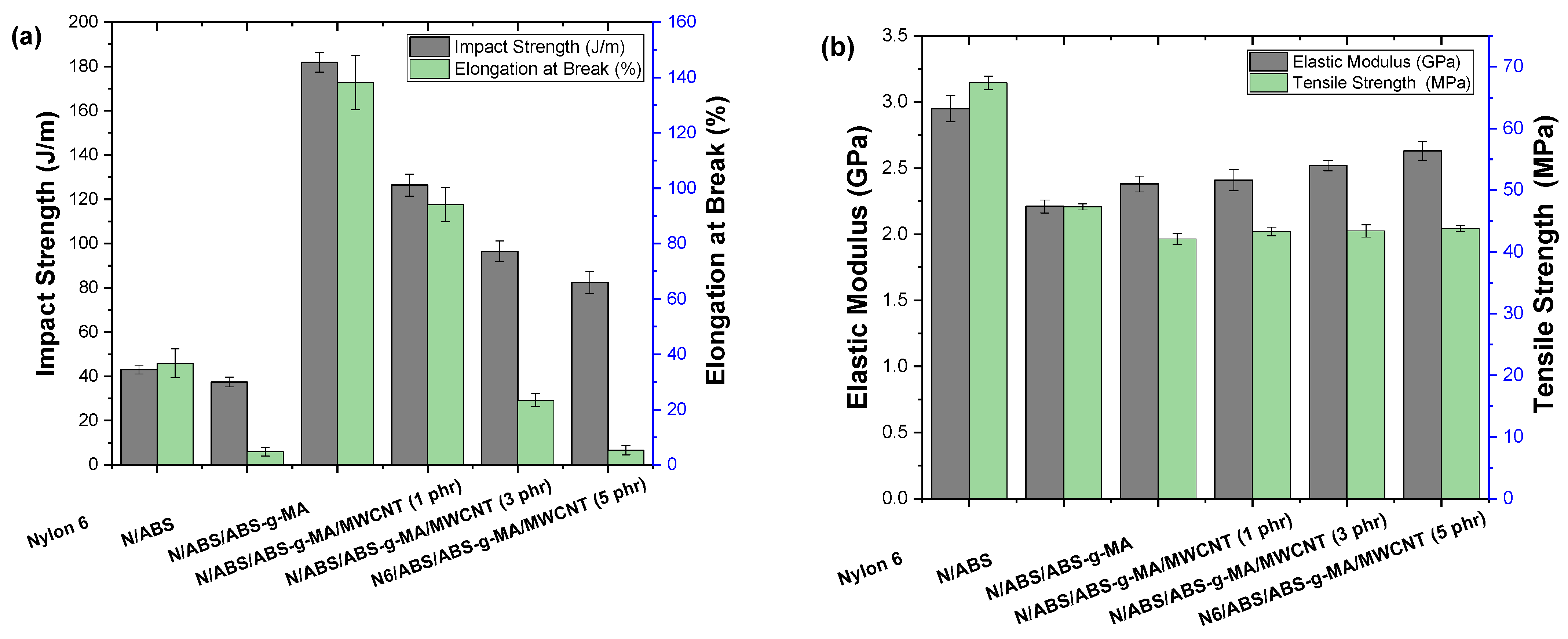
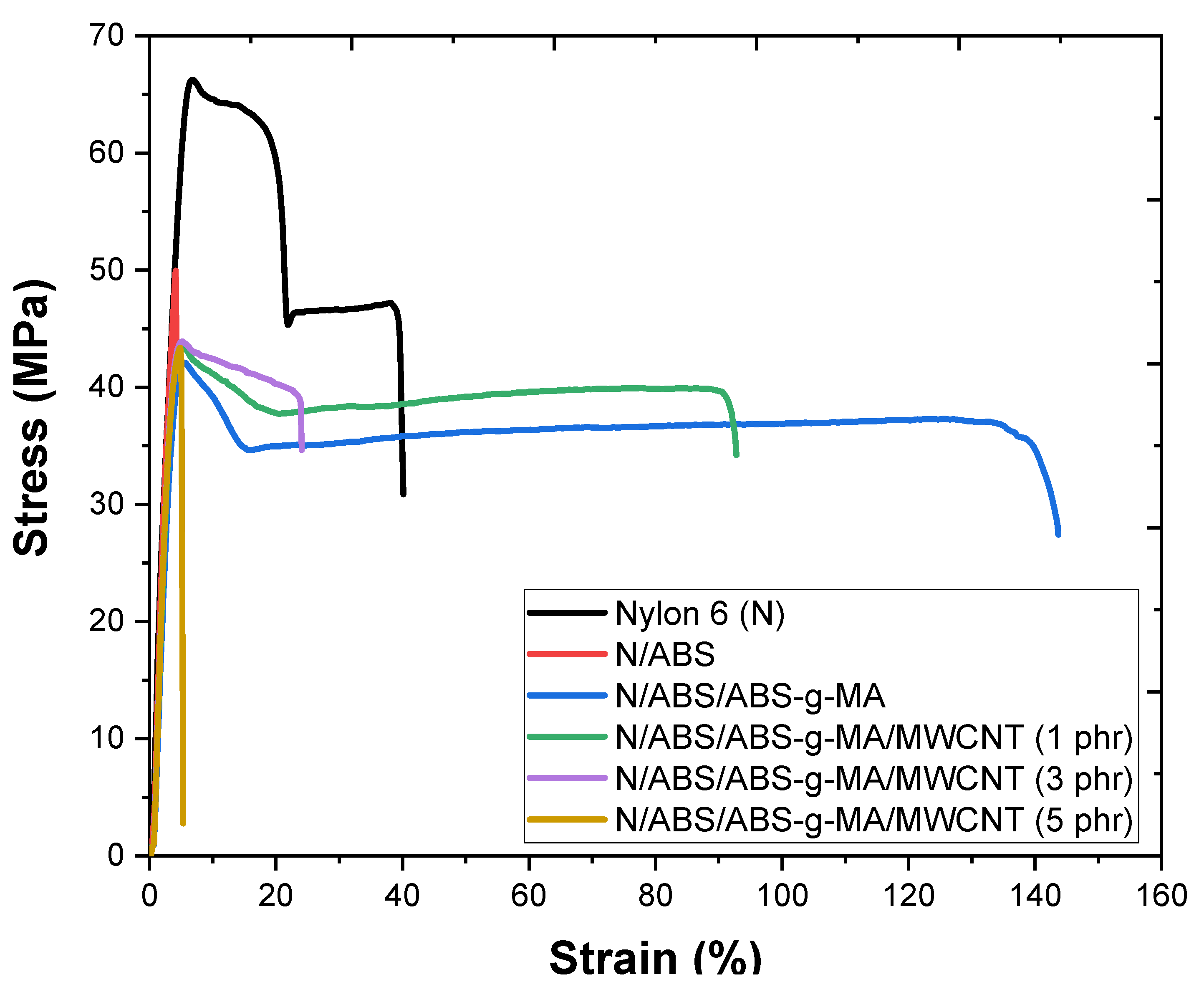
| Samples | IS (J/m) | E (GPa) | TS (MPa) | EB (%) |
|---|---|---|---|---|
| Nylon 6 (N) | 43.0 ± 2.0 | 2.95 ± 0.1 | 67.4 ± 1.1 | 36.7 ± 5.2 |
| N/ABS | 37.4 ± 2.2 | 2.21 ± 0.05 | 47.3 ± 0.5 | 4.8 ± 1.6 |
| N/ABS/ABS-g-MA | 181.9 ± 4.5 | 2.38 ± 0.06 | 42.1 ± 0.9 | 138.3 ± 9.8 |
| N/ABS/ABS-g-MA/MWCNT (1 phr) | 126.4 ± 5.0 | 2.41 ± 0.08 | 43.3 ± 0.7 | 94.1 ± 6.2 |
| N/ABS/ABS-g-MA/MWCNT (3 phr) | 96.5 ± 4.7 | 2.52 ± 0.04 | 43.4 ± 1.0 | 23.4 ± 2.3 |
| N/ABS/ABS-g-MA/MWCNT (5 phr) | 82.4 ± 5.0 | 2.63 ± 0.07 | 43.8 ± 0.5 | 5.3 ± 1.7 |
| Samples | T0.1 (°C) | T0.5 (°C) |
|---|---|---|
| Nylon 6 (N) | 384.6 | 446.1 |
| ABS | 396.2 | 427.9 |
| ABS-MA | 376.1 | 426.1 |
| N/ABS | 394.8 | 436.1 |
| N/ABS/ABS-g-MA | 391.5 | 439.6 |
| N/ABS/ABS-g-MA/MWCNT (1 phr) | 397.3 | 444.9 |
| N/ABS/ABS-g-MA/MWCNT (3 phr) | 398.4 | 445.6 |
| N/ABS/ABS-g-MA/MWCNT (5 phr) | 399.1 | 445.5 |
| Samples | Nylon 6 (%) | ABS (%) | ABS-g-MA (%) | MWCNT (phr) |
|---|---|---|---|---|
| Nylon 6 (N) | 100 | - | - | - |
| N/ABS | 60 | 40 | - | - |
| N/ABS/ABS-g-MA | 60 | 30 | 10 | - |
| N/ABS/ABS-g-MA/MWCNT | 60 | 30 | 10 | 1 |
| N/ABS/ABS-g-MA/MWCNT | 60 | 30 | 10 | 3 |
| N/ABS/ABS-g-MA/MWCNT | 60 | 30 | 10 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, C.B.B.; do Nascimento, E.P.; Siqueira, D.D.; Soares, B.G.; Agrawal, P.; de Mélo, T.J.A.; Araújo, E.M. Tailoring Nylon 6/Acrylonitrile-Butadiene-Styrene Nanocomposites for Application against Electromagnetic Interference: Evaluation of the Mechanical, Thermal and Electrical Behavior, and the Electromagnetic Shielding Efficiency. Int. J. Mol. Sci. 2022, 23, 9020. https://doi.org/10.3390/ijms23169020
Luna CBB, do Nascimento EP, Siqueira DD, Soares BG, Agrawal P, de Mélo TJA, Araújo EM. Tailoring Nylon 6/Acrylonitrile-Butadiene-Styrene Nanocomposites for Application against Electromagnetic Interference: Evaluation of the Mechanical, Thermal and Electrical Behavior, and the Electromagnetic Shielding Efficiency. International Journal of Molecular Sciences. 2022; 23(16):9020. https://doi.org/10.3390/ijms23169020
Chicago/Turabian StyleLuna, Carlos Bruno Barreto, Emanuel Pereira do Nascimento, Danilo Diniz Siqueira, Bluma Guenther Soares, Pankaj Agrawal, Tomás Jeferson Alves de Mélo, and Edcleide Maria Araújo. 2022. "Tailoring Nylon 6/Acrylonitrile-Butadiene-Styrene Nanocomposites for Application against Electromagnetic Interference: Evaluation of the Mechanical, Thermal and Electrical Behavior, and the Electromagnetic Shielding Efficiency" International Journal of Molecular Sciences 23, no. 16: 9020. https://doi.org/10.3390/ijms23169020
APA StyleLuna, C. B. B., do Nascimento, E. P., Siqueira, D. D., Soares, B. G., Agrawal, P., de Mélo, T. J. A., & Araújo, E. M. (2022). Tailoring Nylon 6/Acrylonitrile-Butadiene-Styrene Nanocomposites for Application against Electromagnetic Interference: Evaluation of the Mechanical, Thermal and Electrical Behavior, and the Electromagnetic Shielding Efficiency. International Journal of Molecular Sciences, 23(16), 9020. https://doi.org/10.3390/ijms23169020







