Immunomodulatory Properties of Polysaccharides from Lentinula edodes
Abstract
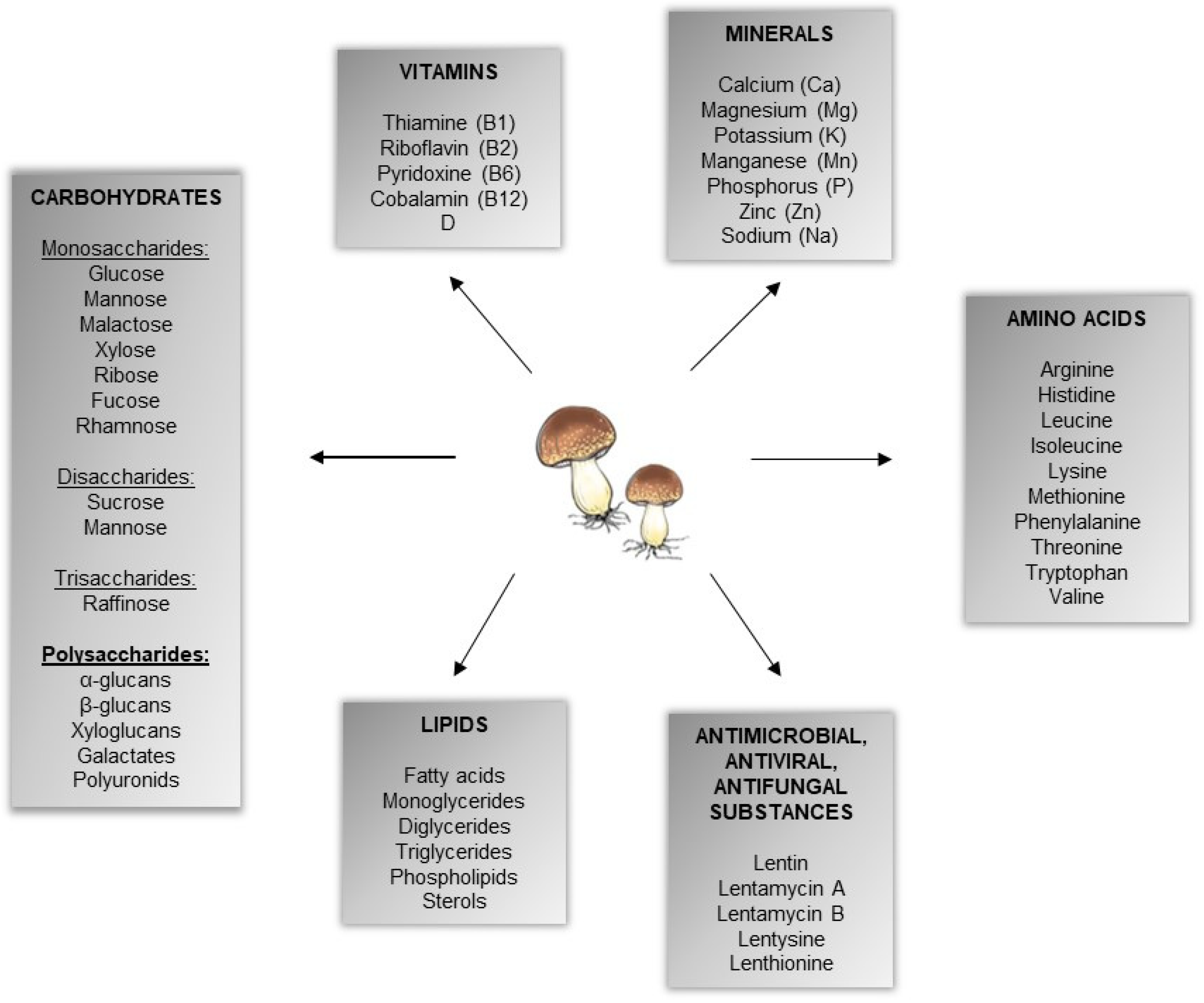
:1. Introduction
2. Polysaccharides from L. edodes—Immunomodulatory Properties
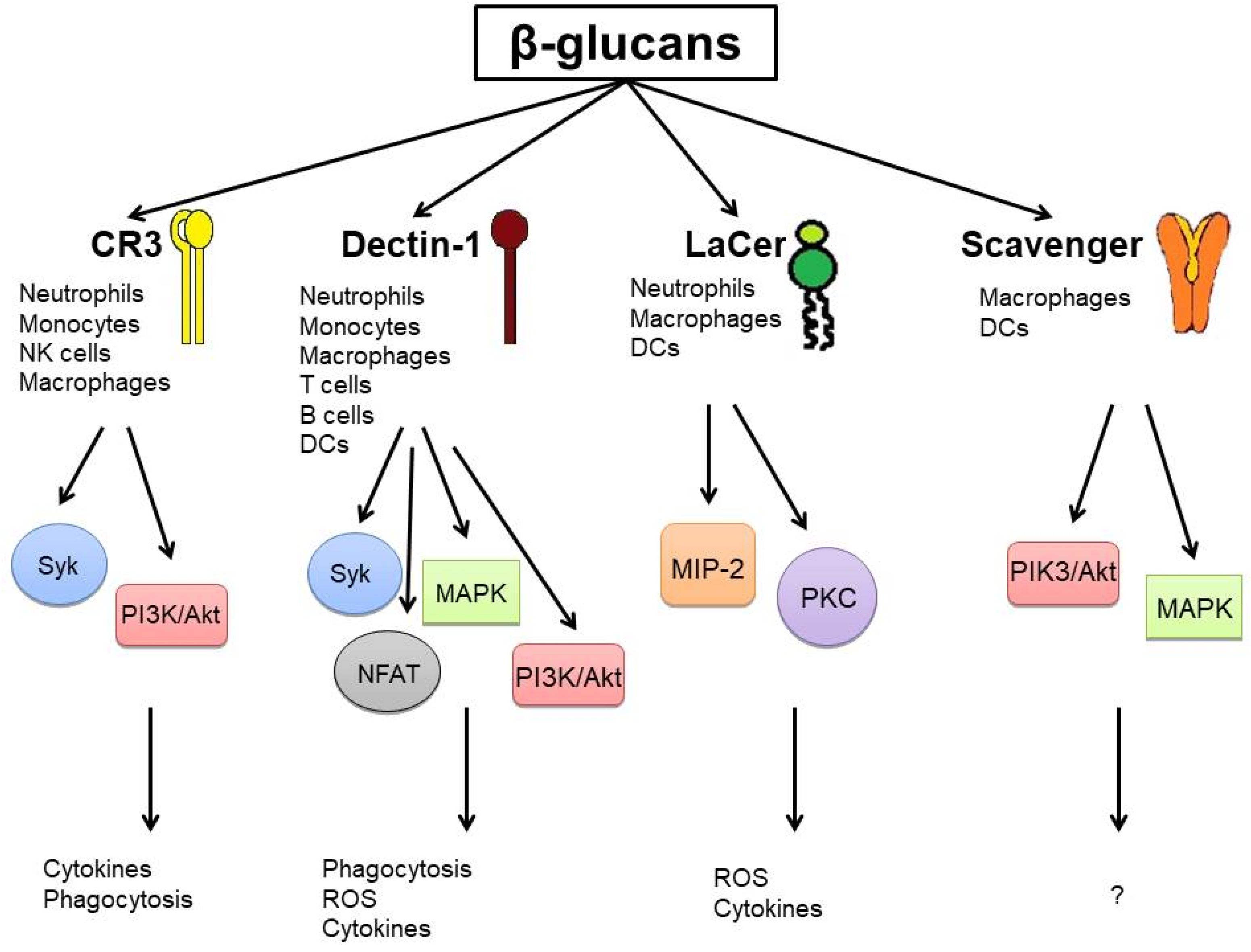
2.1. Surface Receptors and Signaling Pathways
2.2. Immunomodulatory Properties of Polysaccharides from L. edodes—Studies Performed in Animals
2.3. Immunomodulatory Properties of Polysaccharides from L. edodes—Studies Performed in Humans
3. Polysaccharides from L. edodes as Vaccine Adjuvants
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaitán-Hernández, R.; López-Peña, D.; Esqueda, M.; Gutiérrez, A. Review of Bioactive Molecules Production, Biomass, and Basidiomata of Shiitake Culinary-Medicinal Mushrooms, Lentinus edodes (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 841–850. [Google Scholar] [CrossRef]
- Muszyńska, B.; Pazdur, P.; Lazur, J.; Sułkowska-Ziaja, K. Lentinula edodes (Shiitake)—Biological activity. Med. Int. Rev. 2017, 27, 189–195. [Google Scholar]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Finimundy, T.; Dillon, A.; Henriques, J.; Ely, M. A Review on General Nutritional Compounds and Pharmacological Properties of the Lentinula edodes Mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar]
- Xu, X.; Yan, H.; Tang, J.; Chen, J.; Zhang, X. Polysaccharides in Lentinus edodes: Isolation, structure, immunomodulating activity and future prospective. Crit. Rev. Food Sci. Nutr. 2014, 54, 474–487. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, C.; Huang, X.; Hu, L.; Huang, Y.; Chen, H.; Fang, Q.; Dong, N.; Li, M.; Tang, W.; et al. Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts. J. Funct. Foods 2020, 66, 103791. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.; Liu, C.; Koon, J.C.; Fung, K.P. Polysaccharide biological response modifiers. Immunol. Lett. 2006, 105, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Chan, G.C.F.; Chan, W.K.; Sze, D.M.Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998, 273, 22014–22020. [Google Scholar] [CrossRef]
- Rice, P.J.; Kelley, J.L.; Kogan, G.; Ensley, H.E.; Kalbfleisch, J.H.; Browder, I.W.; Williams, D.L. Human monocyte scavenger receptors are pattern recognition receptors for (1-->3)-beta-D-glucans. J. Leukoc. Biol. 2002, 72, 140–146. [Google Scholar]
- Lin, Y.L.; Liang, Y.C.; Lee, S.S.; Chiang, B.L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by NF-kappa B and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef]
- Pan, W.; Jiang, P.; Zhao, J.; Shi, H.; Zhang, P.; Yang, X.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Park, S.O.; Lee, S.J.; Nam, S.H.; Friedman, M. A Polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against salmonellosis through upregulation of the Th1 immune reaction. J. Agric. Food Chem. 2014, 62, 2384–2391. [Google Scholar] [CrossRef]
- Han, D.; Lee, H.T.; Lee, J.B.; Kim, Y.; Lee, S.J.; Yoon, J.W. A Bioprocessed Polysaccharide from Lentinus edodes Mycelia Cultures with Turmeric Protects Chicks from a Lethal Challenge of Salmonella Gallinarum. J. Food Prot. 2017, 80, 245–250. [Google Scholar] [CrossRef]
- Wang, K.P.; Zhang, Q.L.; Liu, Y.; Wang, J.; Cheng, Y.; Zhang, Y. Structure and inducing tumor cell apoptosis activity of polysaccharides isolated from Lentinus edodes. J. Agric. Food Chem. 2013, 61, 9849–9858. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ren, Z.; Wang, X.; Jia, L.; Zhang, C. Antioxidant, anti-inflammatory and renoprotective effects of acidic-hydrolytic polysaccharides by spent mushroom compost (Lentinula edodes) on LPS-induced kidney injury. Int. J. Biol. Macromol. 2020, 151, 1267–1276. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Gu, P.; Bo, R.; Wusiman, A.; Liu, J.; Hu, Y.; Wang, D. Preparation of lentinan-calcium carbonate microspheres and their application as vaccine adjuvants. Carbohydr. Polym. 2020, 245, 116520. [Google Scholar] [CrossRef]
- Xing, J.; Liu, Z.; Huang, Y.; Qin, T.; Bo, R.; Zheng, S.; Luo, L.; Huang, Y.; Niu, Y.; Wang, D. Lentinan-Modified Carbon Nanotubes as an Antigen Delivery System Modulate Immune Response in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 19276–19283. [Google Scholar] [CrossRef]
- Wang, S.X.; Liu, Q.Y.; Li, Y. Lentinan ameliorates burn sepsis by attenuating CD4+ CD25+ Tregs. Burns 2016, 42, 1513–1521. [Google Scholar] [CrossRef]
- Djordjevic, B.; Skugor, S.; Jørgensen, S.M.; Overland, M.; Mydland, L.T.; Krasnov, A. Modulation of splenic immune responses to bacterial lipopolysaccharide in rainbow trout (Oncorhynchus mykiss) fed lentinan, a beta-glucan from mushroom Lentinula edodes. Fish Shellfish Immunol. 2009, 26, 201–209. [Google Scholar] [CrossRef]
- McCormack, E.; Skavland, J.; Mujic, M.; Bruserud, Ø.; Gjertsen, B.T. Lentinan: Hematopoietic, immunological, and efficacy studies in a syngeneic model of acute myeloid leukemia. Nutr. Cancer 2010, 62, 574–583. [Google Scholar] [CrossRef]
- Zheng, R.; Jie, S.; Hanchuan, D.; Moucheng, W. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int. Immunopharmacol. 2005, 5, 811–820. [Google Scholar] [CrossRef]
- Jeff, I.B.; Fan, E.; Tian, M.; Song, C.; Yan, J.; Zhou, Y. In vivo anticancer and immunomodulating activities of mannogalactoglucan-type polysaccharides from Lentinus edodes (Berkeley) Singer. Cent. Eur. J. Immunol. 2016, 41, 47–53. [Google Scholar] [CrossRef]
- Park, H.J.; Boo, S.; Park, I.; Shin, M.S.; Takahashi, T.; Takanari, J.; Homma, K.; Kang, I. AHCC®, a Standardized Extract of Cultured Lentinula Edodes Mycelia, Promotes the Anti-Tumor Effect of Dual Immune Checkpoint Blockade Effect in Murine Colon Cancer. Front. Immunol. 2022, 13, 875872. [Google Scholar] [CrossRef]
- Aoki, T. Lentinan. In Immune Modulation Agents and Their Mechanisms; Fenichel, R.L., Chirgis, M.A., Eds.; Marcel Dekker: New York, NY, USA, 1984; pp. 63–77. [Google Scholar]
- Tani, M.; Tanimura, H.; Yamaue, H.; Tsunoda, T.; Iwahashi, M.; Noguchi, K.; Tamai, M.; Hotta, T.; Mizobata, S. Augmentation of lymphokine-activated killer cell activity by lentinan. Anticancer Res. 1993, 13, 1773–1776. [Google Scholar]
- Arinaga, S.; Karimine, N.; Takamuku, K.; Nanbara, S.; Inoue, H.; Nagamatsu, M.; Ueo, H.; Akiyoshi, T. Enhanced induction of lymphokine-activated killer activity after lentinan administration in patients with gastric carcinoma. Int. J. Immunopharmacol. 1992, 14, 535–539. [Google Scholar] [CrossRef]
- Takeshita, K.; Watanuki, S.; Iida, M.; Saito, N.; Maruyama, M.; Sunagawa, M.; Habu, H.; Endo, M. Effect of lentinan on lymphocyte subsets of peripheral blood, lymph nodes, and tumor tissues in patients with gastric cancer. Surg. Today 1993, 23, 125–129. [Google Scholar] [CrossRef]
- Gaullier, J.M.; Sleboda, J.; Øfjord, E.S.; Ulvestad, E.; Nurminiemi, M.; Moe, C.; Tor, A.; Gudmundsen, O. Supplementation with a soluble β-glucan exported from Shiitake medicinal mushroom, Lentinus edodes (Berk.) singer mycelium: A crossover, placebo-controlled study in healthy elderly. Int. J. Med. Mushrooms 2011, 13, 319–326. [Google Scholar] [CrossRef]
- Morales, D.; Shetty, S.A.; López-Plaza, B.; Gómez-Candela, C.; Smidt, H.; Marín, F.R.; Soler-Rivas, C. Modulation of human intestinal microbiota in a clinical trial by consumption of a β-D-glucan-enriched extract obtained from Lentinula edodes. Eur. J. Nutr. 2021, 60, 3249–3265. [Google Scholar] [CrossRef]
- Dai, X.; Stanilka, J.M.; Rowe, C.A.; Esteves, E.A.; Nievesm, C., Jr.; Spaiserm, S.J.; Christman, M.C.; Langkamp-Henken, B.; Percival, S.S. Consuming Lentinula edodes (Shiitake) Mushrooms Daily Improves Human Immunity: A Randomized Dietary Intervention in Healthy Young Adults. J. Am. Coll. Nutr. 2015, 34, 478–487. [Google Scholar] [CrossRef]
- Wang, X.E.; Wang, Y.H.; Zhou, Q.; Peng, M.; Zhang, J.; Chen MMa, L.J.; Xie, G.M. Immunomodulatory Effect of Lentinan on Aberrant T Subsets and Cytokines Profile in Non-small Cell Lung Cancer Patients. Pathol. Oncol. Res. 2018, 26, 499–505. [Google Scholar] [CrossRef]
- Kaleta, B.; Górski, A.; Zagożdżon, R.; Cieślak, M.; Kaźmierczak-Barańska, J.; Nawrot, B.; Klimaszewska, M.; Malinowska, E.; Górska, S.; Turło, J. Selenium-containing polysaccharides from Lentinula edodes-Biological activity. Carbohydr. Polym. 2019, 223, 115078. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, M.; Górska, S.; Łapienis, G.; Kaleta, B.; Górska, S.; Kaszowska, M.; Dawidowski, M.; Gamian, A.; Zagożdżon, R.; Górski, A.; et al. Identification of the Primary Structure of Selenium-Containing Polysaccharides Selectively Inhibiting T-Cell Proliferation. Molecules 2021, 26, 5404. [Google Scholar] [CrossRef]
- Kaleta, B.; Roszczyk, A.; Zych, M.; Kniotek, M.; Zagożdżon, R.; Klimaszewska, M.; Malinowska, E.; Pac, M.; Turło, J. Selective Biological Effects of Selenium-Enriched Polysaccharide (Se-Le-30) Isolated from Lentinula edodes Mycelium on Human Immune Cells. Biomolecules 2021, 11, 1777. [Google Scholar] [CrossRef]
- Górska-Jakubowska, S.; Klimaszewska, M.; Podsadni, P.; Kaleta, B.; Zagożdżon, R.; Górska, S.; Gamian, A.; Strączek, T.; Kapusta, C.; Cieślak, M.; et al. Selenium-Containing Exopolysaccharides Isolated from the Culture Medium of Lentinula edodes: Structure and Biological Activity. Int. J. Mol. Sci. 2021, 22, 13039. [Google Scholar] [CrossRef]
- Shah, R.R.; Hassett, K.J.; Brito, L.A. Overview of Vaccine Adjuvants: Introduction, History, and Current Status. Methods Mol Biol. 2017, 1494, 1–13. [Google Scholar] [PubMed]
- Wan, X.; Yin, Y.; Zhou, C.; Hou, L.; Cui, Q.; Zhang, X.; Cai, X.; Wang, Y.; Wang, L.; Tian, J. Polysaccharides derived from Chinese medicinal herbs: A promising choice of vaccine adjuvants. Carboh. Polym. 2022, 276, 2022. [Google Scholar] [CrossRef]
- Drandarska, I.; Kussovski, V.; Nikolaeva, S.; Markova, N. Combined immunomodulating effects of BCG and lentinan after intranasal application in guinea pigs. Int. Immunopharmacol. 2005, 5, 795–803. [Google Scholar] [CrossRef]
- Jin, X.; Liu, X.; Ding, J.; Zhang, L.; Yang, Y.; Wang, X.; Yang, Y.; Liu, M. Lentinan improved the efficacy of vaccine against Trichinella spiralis in an NLRP3 dependent manner. PLoS Negl. Trop. Dis. 2020, 14, e0008632. [Google Scholar] [CrossRef] [PubMed]
| Sample | Animal Model | Immunomodulatory Effects | Reference |
|---|---|---|---|
| F1, F2, and F3 heteropolysaccharide fractions composed of glucose, galactose, and mannose in different proportions, isolated from L. edodes water extracts | BALB/c mice | 1. Increased thymus index and footpad thickness (F1, F2, and F3 fraction). 2. Increased proliferation of Con A- and LPS-stimulated splenocytes (F1, F2, F3 fraction). 3. Increased serum IgG and IgM levels (F1, F2, F3 fraction). 4. Enhanced cytotoxic activity of NK cells (F2 and F3 fraction). | Chen et al. [8] |
| 1, 3/1, 6-β-glucan from L. edodes | C57BL/6J mice | 1. Inhibited macrophage accumulation in the colon. 2. Inhibited expression of proinflammatory cytokines: IL-6, TNF-α, and IL-1β. | Pan et al. [17] |
| Bioprocessed polysaccharide (BPP)—β-glucan composed of glucose, galactose, rhamnose, fucose, mannose, and xylose, isolated from L. edodes liquid mycelial culture supplemented with black rice bran | BALB/c mice infected with Salmonella Typhimurium | 1. Upregulated spleen lymphocyte proliferation. 2. Increased serum IFN-α levels. 3. Increased IL-1β, IL-2, IL-6, and IL-12 production in splenocytes. | Kim et al. [18] |
| Bioprocessed polysaccharide (BPP)—β-glucan composed of glucose, galactose, rhamnose, fucose, mannose, and xylose, isolated from L. edodes liquid mycelial culture supplemented with tumeric | Chickens infected with Salmonella Gallinarum | 1. Reduced phagocytic activity of the chicken-derived macrophage cell line HD-11. 2. Increased expression of IL-1β, IL-10, TNF-α, iNOS. 3. Reduced expression of IL-4, IL-6, IFN-β, IFN-γ. | Han et al. [19] |
| SLNT1, SLNT2, JLNT1, JLNT2, and JLNT3 polysaccharides ((1→3)-β-glucans composed of glucose), isolated from L. edodes fruit body | H22-bearing mice | 1. Suppressed H22 tumor growth and enhanced tumor cell apoptosis (SLNT1 and JLNT1). 2. Increased IL-2 and TNF-α production (SLNT1 and JLNT1). | Wang et al. [20] |
| Polysaccharide (homogenous β-glucan composed of rhamnose, arabinose, galactose, and glucose) isolated from L. edodes spent compost (ASMCP) | Kunming strain mice with LPS-induced kidney injury | 1. Reduced the serum levels of TNF-α, IL-6 and IL-1β. 2. Reduced serum levels of urea nitrogen, creatinine, and uric acid. 3. Increased activity of superoxide dismutase, catalase, and malondialdehyde. | Song et al. [21] |
| Lentinan ((1→3)-β-glucan with (1→6) branches) encapsulated into CaCO3 microparticles | BALB/c mice | 1. Enhanced lymphocyte proliferation. 2. Enhanced activation of B cells. 3. Increased ratio of CD4+ to CD8+ T cells in spleen lymphocytes. 4. Increased serum IgG levels. 5. Increased IL-2, IL-4, IFN-γ, and TNF-α production. | Liu et al. [22] |
| Lentinan covalently attached to multiwalled carbon nanotubes | BALB/c mice | 1. Enhanced production of antiovalbumin IgG antibodies. 2. Increased production of IL-4, IL-6, TNF-α, and IFN-γ. 3. Upregulated percentage of CD4+ and CD8+ T cells in spleen | Xing et al. [23] |
| Lentinan | BALB/c mice | 1. Decreased IL-10 production. 2. Decreased FoxP3 expression of CD4+CD25+ Tregs. 3. Decreased LPS-induced IL-10 production in Tregs. 4. Attenuated LPS-stimulated Erk–FoxO1 activation. | Wang et al. [24] |
| Lentinan | Rainbow trout (Oncorhynchus mykiss) with LPS-induced inflammation | 1. Decreased expression of TNF- and IFN-related genes. | Djordjevic et al. [25] |
| Lentinan | Brown Norwegian rats | 1. Increased number of circulating monocytes. 2. Increased number of CD8+ T and decreased ratio of CD4/CD8 cells. 3. Decreased production of IL-4, IL-6, IL-10, and GM-CSF. | Jeff et al. [26] |
| L-II polysaccharide consisted of D-glucopyranose, isolated from L. edodes fruiting body | Sarcoma 180-bearing mice | 1. Increased the thymus and spleen weight. 2. Increased delayed-type hypersensitivity response. 3. Increased phagocytosis of macrophages. 4. Increased TNF-α and IFN-γ serum levels. 5. Increased NO production and catalase activity in macrophages. | Zheng et al. [27] |
| WPLE-N-2 and WPLE-A0.5-2 polysaccharides(α-glucans composed of glucose, galactose, and mannose), isolated from L. edodes fruiting body | Sarcoma 180-bearing mice | 1. Increased NO production in peritoneal macrophages. 2. Increased phagocytosis of macrophages. 3. Enhanced Con-A and LPS-induced splenocytes proliferation. | Jeff et al. [28] |
| AHCC®, a standardized extract of cultured L. edodes mycelia containing α- and β-glucans, starches, sugars, amino acids, and minerals | C57BL/6 mice | 1. Reduced tumor growth. 2. Increased granzyme B and Ki-67 expression by tumor-infiltrating CD8+ T cells. | Park et al. [29] |
| Sample | Participants | Immunomodulatory Effects | Reference |
|---|---|---|---|
| Lentinan used in PBMCs culture | Healthy humans | 1. Enhanced proliferation of PBMCs. 2. Increased production of lymphokine-activated killer cell. 3. Increased NK cells activity. | Aoki [30] Tani et al. [31] |
| Lentinan administered intravenously with chemiotherapy | Gastric cancer patients | 1. Enhanced proliferation of PBMCs. 2. Increased production of lymphokine-activated killer cell. 3. Increased NK cells activity. 4. Increased IL-1α, IL-1β, and TNF-α production by PBMCs. 5. Increased ratio of activated T cells and cytotoxic T cells in the spleen. 6. Elevated number of CD4 cells in lymph nodes 7. Increased number of tumor-inflitrating CD4, Leu11, and LeuM3 cells. | Tani et al. [31] Arinaga et al. [32] Takeshita et al. [33] |
| Lentinan administered orally | Healthy eldery humans | 1. Higher number of total CD3+ and CD4+ T cells. 2. Increased number of B cells (nonsignificantly). | Gaullier et al. [34] |
| β-D-glucan-enriched mixture obtained from L. edodes fruiting bodies incorporated in food products | Patients with hypercholesterolemia | 1. No changes in IL-1β, IL-6, and TNF-α production. | Morales et al. [35] |
| Whole dried L. edodes | Healthy young humans | 1. Increased ex vivo proliferation of γδ-T cells and NK-T cells. 2. Increased expression of activation receptors on γδ-T cells and NK-T cells. 3. Increased level of secretory IgA in saliva. 4. Decreased level of CRP in serum. 5. Upregulated production of IL-4, IL-10, TNF-α, and IL-1α by PBMCs. 6. Downregulated production of MIP-1α/CCL3 by PBMCs. | Dai et al. [36] |
| Lentinan in combined therapy with vinorelbin and cisplatin | Patients with non-small cell lung cancer (NSCLC) | 1. Increased number of CD3+CD56+ NKT cells. 2. Increased number of CD3+CD8+ T cells. 3. Increased number of CD3+CD4+ T cells. 4. Derceased number of CD4+CD25+ Tregs. 5. Increased production of IL-10, TGF-β1, IFN-γ, TNF-α, and IL-12. | Wang et al. [37] |
| Polysaccharides (Se-L and L) isolated from L. edodes mycelium used in PBMCs and granulocytes cultures | Healthy humans | 1. Decreased proliferation of anti-CD3 Ab and PHA-stimulated PBMCs. 2. No effects on production of O2− by granulocytes. | Kaleta et al. [38] |
| Homogenous polysaccharide fraction (Se-Le-30) isolated from L. edodes mycelium used in PBMCs and granulocytes cultures | Healthy humans | 1. Decreased proliferation of anti-CD3 Ab-stimulated PBMCs. 2. Decreased proliferation of allostimulated PBMCs. 3. Decreased production of TNF-α by CD3+ T cells. 4. No effects on production of O2- by granulocytes. | Kaleta et al. [39,40] |
| Exopolisaccharide isolated from a postculture medium of L. edodes | BALB/c mice | 1. Decreased proliferation of anti-CD3 Ab-stimulated PBMCs. 2. No effects on production of O2- by granulocytes. | Górska-Jakubowska et al. [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. https://doi.org/10.3390/ijms23168980
Roszczyk A, Turło J, Zagożdżon R, Kaleta B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. International Journal of Molecular Sciences. 2022; 23(16):8980. https://doi.org/10.3390/ijms23168980
Chicago/Turabian StyleRoszczyk, Aleksander, Jadwiga Turło, Radosław Zagożdżon, and Beata Kaleta. 2022. "Immunomodulatory Properties of Polysaccharides from Lentinula edodes" International Journal of Molecular Sciences 23, no. 16: 8980. https://doi.org/10.3390/ijms23168980
APA StyleRoszczyk, A., Turło, J., Zagożdżon, R., & Kaleta, B. (2022). Immunomodulatory Properties of Polysaccharides from Lentinula edodes. International Journal of Molecular Sciences, 23(16), 8980. https://doi.org/10.3390/ijms23168980






