New Progress in Early Diagnosis of Atherosclerosis
Abstract
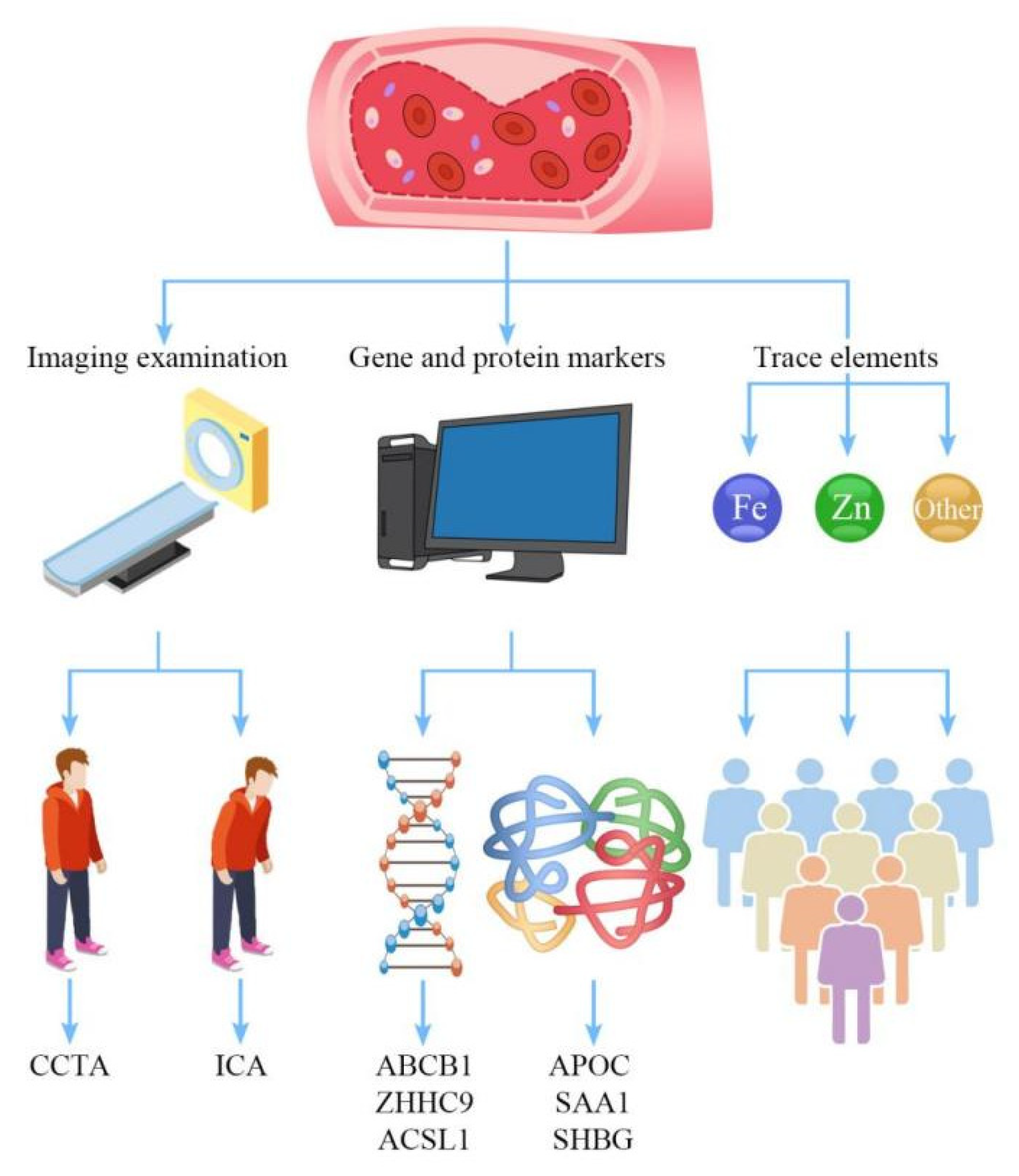
1. Introduction
2. Imaging Examination
3. Gene and Protein Markers
3.1. Gene Level
3.2. Protein Levels
4. Trace Elements
4.1. Zinc Ion
4.2. Iron Ion
4.3. Other Trace Elements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| LDL | Low-density lipoprotein |
| ICA | Invasive coronary angiography |
| CCTA | Computed tomography coronary angiography |
| CAD | Coronary artery disease |
| ACS | Acute coronary syndrome |
| MACE | Major cardiovascular adverse events |
| MSCT | Multi-slice spiral CT |
| DSCT | Dual-source computed tomography |
| IVUS | Intravascular ultrasound |
| MRI | Magnetic resonance imaging |
| 18-F-FDG | 18 -F-fluorodeoxyglucose |
| PET | Positron emission tomograph |
| NIR | Near-infrared imaging |
| ICG | Indocyanine green |
| miRNA | MicroRNA |
| CV | Coefficient of variation |
| ABCA1 | ATP binding cassette A1 |
| HDL | High-density lipoprotein |
| LDLC | Low-density lipoprotein cholesterol |
| HDLC | High-density lipoprotein cholesterol |
| VCAM | Vascular adhesion molecule |
| ICAM | Intracellular adhesion molecule |
| NF | Nuclear factor |
| ACS | Acute coronary syndrome |
| HF | Heart failure |
| PK/PD | Pharmacokinetics/pharmacodynamics |
| AMI | Acute myocardial infarction |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.E.; Kramer, F.; Barnhart, S.; Averill, M.M.; Vivekanandan-Giri, A.; Vickery, T.; Li, L.O.; Becker, L.; Yuan, W.; Chait, A.; et al. Diabetes promotes an inflammatory macrophage phenotype and Atherosclerosis through acyl-CoA synthetase 1. Proc. Natl. Acad. Sci. USA 2012, 109, E715–E724. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, J.L. Inflammation may be a bridge connecting hypertension and Atherosclerosis. Med. Hypotheses 2005, 64, 925–929. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Miname, M.H.; Santos, R.D. Reducing cardiovascular risk in patients with familial hypercholesterolemia: Risk prediction and lipid management. Prog. Cardiovasc. Dis. 2019, 62, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.F.; Sobenin, I.A.; Orekhov, A.N. The atherogenic role of circulating modified lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Inflammation in Atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef]
- Cheng, F.; Torzewski, M.; Degreif, A.; Rossmann, H.; Canisius, A.; Lackner, K.J. Impact of glutathione peroxidase-1 deficiency on macrophage foam cell formation and proliferation: Implications for atherogenesis. PLoS ONE 2013, 8, e72063. [Google Scholar] [CrossRef]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef]
- Rognoni, A.; Cavallino, C.; Veia, A.; Bacchini, S.; Rosso, R.; Facchini, M.; Secco, G.G.; Lupi, A.; Nardi, F.; Rametta, F.; et al. Pathophysiology of atherosclerotic plaque development. Cardiovasc. Hematol. Agents Med. Chem. 2015, 13, 10–13. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of Atherosclerosis’s initial, fatty streak, and intermediate lesions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in Diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Matsumura, M.; Mintz, G.S.; Lee, T.; Zhang, W.; Cao, Y.; Fujino, A.; Lin, Y.; Usui, E.; Kanaji, Y.; et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc. Imaging 2017, 10, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Rochitte, C.E.; Dewey, M.; Arbab-Zadeh, A.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 2008, 359, 2324–2336. [Google Scholar] [CrossRef] [PubMed]
- Assante, R.; Klain, M.; Acampa, W. Use coronary artery calcium scanning as a triage for invasive coronary angiography. J. Nucl. Cardiol. 2019, 26, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.D.; Gunn, J.P. Computing Fractional Flow Reserve From Invasive Coronary Angiography: Getting Closer. Circ. Cardiovasc. Interv. 2017, 10, e005806. [Google Scholar] [CrossRef]
- Minhas, A.; Dewey, M.; Vavere, A.L.; Tanami, Y.; Ostovaneh, M.R.; Laule, M.; Rochitte, C.E.; Niinuma, H.; Kofoed, K.F.; Geleijns, J.; et al. Patient Preferences for Coronary CT Angiography with Stress Perfusion, SPECT, or Invasive Coronary Angiography. Radiology 2019, 291, 340–348. [Google Scholar] [CrossRef]
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef]
- Collet, C.; Onuma, Y.; Andreini, D.; Sonck, J.; Pompilio, G.; Mushtaq, S.; La Meir, M.; Miyazaki, Y.; de Mey, J.; Gaemperli, O.; et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 2018, 39, 3689–3698. [Google Scholar] [CrossRef]
- Mushtaq, S.; Andreini, D.; Pontone, G.; Bertella, E.; Bartorelli, A.L.; Conte, E.; Baggiano, A.; Annoni, A.; Formenti, A.; Trabattoni, D.; et al. Prognostic value of coronary CTA in coronary bypass patients: A long-term follow-up study. JACC Cardiovasc. Imaging 2014, 7, 580–589. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Clinical Excellence. Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin; Royal College of Physicians: London, UK, 2010. [Google Scholar]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D.; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Cardiovasc. Comput. Tomogr. 2010, 4, 407. [Google Scholar] [PubMed]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary atherosclerosis imaging by coronary CT angiography: Current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc. Imaging 2011, 4, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Chinnaiyan, K.M.; Abidov, A. CT-STAT Investigators The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J. Am. Coll. Cardiol. 2011, 58, 1414–1422. [Google Scholar] [CrossRef]
- Litt, H.I.; Gatsonis, C.; Snyder, B.; Singh, H.; Miller, C.D.; Entrikin, D.W.; Leaming, J.M.; Gavin, L.J.; Pacella, C.B.; Hollander, J.E. CT angiography for safe discharge of patients with possible acute coronary syndromes. N. Engl. J. Med. 2012, 366, 1393–1403. [Google Scholar] [CrossRef]
- Hoffmann, U.; Truong, Q.A.; Schoenfeld, D.A. ROM ICAT-II Investigators Coronary CT angiography versus standard evaluation in acute chest pain. N. Engl. J. Med. 2012, 367, 299–308. [Google Scholar] [CrossRef]
- Hamilton-Craig, C.; Fifoot, A.; Hansen, M.; Pincus, M.; Chan, J.; Walters, D.L.; Branch, K.R. Diagnostic performance and cost of CT angiography versus stress ECG—A randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE). Int. J. Cardiol. 2014, 177, 867–873. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Miller, J.M.; Rochitte, C.E.; Dewey, M.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (coronary artery evaluation using 64-row multidetector computed tomography angiography) International Multicenter Study. J. Am. Coll. Cardiol. 2012, 59, 379–387. [Google Scholar]
- Kuchynka, P.; Lambert, L.; Černý, V.; Marek, J.; Ambrož, D.; Danek, B.A.; Linhart, A. Coronary CT angiography. Cor Vasa 2015, 57, e425–e432. [Google Scholar] [CrossRef]
- Halliburton, S.S.; Abbara, S.; Chen, M.Y.; Gentry, R.; Mahesh, M.; Raff, G.L.; Shaw, L.J.; Hausleiter, J.; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J. Cardiovasc. Comput. Tomogr. 2011, 5, 198–224. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, L.; Song, X.; Li, Y.N.; Jiang, Y.; Zhang, X.H.; Ju, H.Y.; Wu, J.; Chang, R.P. Feasibility study of low tube voltage (80 kVp) coronary CT angiography combined with contrast medium reduction using iterative model reconstruction (IMR) on standard BMI patients. Br. J. Radiol. 2016, 89, 20150766. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Thompson, A.G.; Lee, K.; Precious, B.; Yang, T.H.; Berger, A.; Taylor, C.; Heilbron, B.; Nguyen, G.; Earls, J.; et al. Reduced iodine load with CT coronary angiography using dual-energy imaging: A prospective randomized trial compared with standard coronary CT angiography. J. Cardiovasc. Comput. Tomogr. 2014, 8, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ulzheimer, S.; Flohr, T. Multislice CT: Current Technology and Future Developments. In Medical Radiology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–23. [Google Scholar]
- Tsiknakis, N.; Spanakis, C.; Tsompou, P.; Karanasiou, G.; Karanasiou, G.; Sakellarios, A.; Rigas, G.; Kyriakidis, S.; Papafaklis, M.; Nikopoulos, S.; et al. IVUS Longitudinal and Axial Registration for Atherosclerosis Progression Evaluation. Diagnostics 2021, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Lavin, B.; Andia, M.E.; Saha, P.; Botnar, R.M.; Phinikaridou, A. Quantitative MRI of Endothelial Permeability and (Dys)function in Atherosclerosis. J. Vis. Exp. 2021, 178, e62724. [Google Scholar] [CrossRef] [PubMed]
- Sriranjan, R.S.; Tarkin, J.M.; Evans, N.R.; Le, E.P.V.; Chowdhury, M.M.; Rudd, J.H.F. Atherosclerosis imaging using PET: Insights and applications. Br. J. Pharmacol. 2021, 178, 2186–2203. [Google Scholar] [CrossRef]
- Verjans, J.W.; Osborn, E.A.; Ughi, G.J.; Calfon Press, M.A.; Hamidi, E.; Antoniadis, A.P.; Papafaklis, M.I.; Conrad, M.F.; Libby, P.; Stone, P.H.; et al. Targeted Near-Infrared Fluorescence Imaging of Atherosclerosis: Clinical and Intracoronary Evaluation of Indocyanine Green. JACC Cardiovasc. Imaging 2016, 9, 1087–1095. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef]
- Seronde, M.F.; Vausort, M.; Gayat, E.; Goretti, E.; Ng, L.L.; Squire, I.B.; Vodovar, N.; Sadoune, M.; Samuel, J.-L.; Thum, T.; et al. Circulating microRNAs and Outcome in Patients with Acute Heart Failure. PLoS ONE 2015, 10, e0142237. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernández-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Elmen, J.; Lindow, M.; Schutz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjarn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Elmen, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjarn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Landstreet, S.R.; Levin, M.G.; Shoucri, B.M.; Toth, C.L.; Taylor, R.C.; Palmisano, B.T.; Tabet, F.; Cui, H.L.; Rye, K.A.; et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 14518–14523. [Google Scholar] [CrossRef]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of Atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Suarez, Y.; Wang, C.; Manes, T.D.; Pober, J.S. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: Feedback control of inflammation. J. Immunol. 2010, 184, 21–25. [Google Scholar] [CrossRef]
- Shi, Z.G.; Sun, Y.; Wang, K.S.; Jia, J.D.; Yang, J.; Li, Y.N. Effects of miR-26a/miR-146a/miR-31 on airway inflammation of asthma mice and asthma children. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5432–5440. [Google Scholar]
- Zhou, F.; Liu, P.; Lv, H.; Gao, Z.; Chang, W.; Xu, Y. miR-31 attenuates murine allergic rhinitis by suppressing interleukin-13-induced nasal epithelial inflammatory responses. Mol. Med. Rep. 2021, 23, 42. [Google Scholar] [CrossRef]
- An, J.H.; Chen, Z.Y.; Ma, Q.L.; Wang, H.J.; Zhang, J.Q.; Shi, F.W. LncRNA SNHG16 promoted proliferation and inflammatory response of macrophages through miR-17-5p/NF-κB signaling pathway in patients with Atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8665–8677. [Google Scholar]
- Sun, X.; Belkin, N.; Feinberg, M.W. Endothelial microRNAs and Atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 372. [Google Scholar] [CrossRef]
- Zhelankin, A.; Stonogina, D.; Vasiliev, S.; Babalyan, K.; Sharova, E.; Doludin, Y.; Shchekochikhin, D.; Generozov, E.; Akselrod, A. Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules 2021, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Mackin, S.T.; Schlosser, K.; Wong, F.L.; Elharram, M.; Delles, C.; Stewart, D.J.; Dayan, N.; Landry, T.; Pilote, L. Systematic review of microRNA biomarkers in acute coronary syndrome and stable coronary artery disease. Cardiovasc. Res. 2020, 116, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Viereck, J.; Thum, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyöngyösi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Melman, Y.F.; Shah, R.; Das, S. MicroRNAs in heart failure: Is the picture becoming less miRky? Circ. Heart Fail. 2014, 7, 203–214. [Google Scholar] [CrossRef]
- Wright, K.; de Silva, K.; Purdie, A.C.; Plain, K.M. Comparison of methods for miRNA isolation and quantification from ovine plasma. Sci. Rep. 2020, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo-Calvo, D.; Pérez-Boza, J.; Curado, J.; Devaux, Y.; EU-CardioRNA COST Action CA17129. Challenges of microRNA-based biomarkers in clinical application for cardiovascular diseases. Clin. Transl. Med. 2022, 12, e585. [Google Scholar] [CrossRef]
- McPherson, R. Chromosome 9p21 and coronary artery disease. N. Engl. J. Med. 2010, 362, 1736–1737. [Google Scholar] [CrossRef]
- Ardissino, D.; Berzuini, C.; Merlini, P.A.; Mannucci, P.M.; Surti, A.; Burtt, N.; Voight, B.; Tubaro, M.; Peyvandi, F.; Spreafico, M.; et al. Influence of 9p21.3 genetic variants on clinical and angiographic outcomes in early-onset myocardial infarction. J. Am. Coll. Cardiol. 2011, 58, 426–434. [Google Scholar] [CrossRef]
- Aziz, H.; Zaas, A.; Ginsburg, G.S. Peripheral blood gene expression profiling for cardiovascular disease assessment. Genom. Med. 2007, 1, 105–112. [Google Scholar] [CrossRef]
- Meng, H.; Wang, Y.; Ruan, J.; Chen, Y.; Wang, X.; Zhou, F.; Meng, F. Decreased iron ion concentrations in the peripheral blood correlate with coronary Atherosclerosis. Nutrients 2022, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wang, X.; Ruan, J.; Chen, W.; Meng, F.; Yang, P. High expression levels of the SOCS3 gene are associated with acute myocardial infarction. Genet. Test. Mol. Biomark. 2020, 24, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Meng, H.; Wang, X.; Chen, W.; Tian, X.; Meng, F. Low expression of FFAR2 in peripheral white blood cells may Be a genetic marker for early diagnosis of acute myocardial infarction. Cardiol. Res. Pract. 2020, 2020, 3108124. [Google Scholar] [CrossRef]
- Tan, B.; Liu, L.; Yang, Y.; Liu, Q.; Yang, L.; Meng, F. Low CPNE3 expression is associated with risk of acute myocardial infarction: A feasible genetic marker of acute myocardial infarction in patients with stable coronary artery disease. Cardiol. J. 2019, 26, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Ruan, J.; Chen, Y.; Yan, Z.; Shi, K.; Li, X.; Yang, P.; Meng, F. Investigation of specific proteins related to different types of coronary atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 758035. [Google Scholar] [CrossRef]
- Elashoff, M.R.; Wingrove, J.A.; Beineke, P.; Daniels, S.E.; Tingley, W.G.; Rosenberg, S.; Voros, S.; Kraus, W.E.; Ginsburg, G.S.; Schwartz, R.S.; et al. Development of a blood-based gene expression algorithm for assessing obstructive coronary artery disease in non-diabetic patients. BMC Med. Genom. 2011, 4, 26. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Kadoglou, N.; Tsiotra, P.C.; Kollias, A.; Palios, I.; Fountoulaki, K.; Halvatsiotis, I.; Maratou, E.; Dimitriadis, G.; Kremastinos, D.T.; et al. Arterial stiffness is associated with increased monocyte expression of adiponectin receptor mRNA and protein in patients with coronary artery disease. Am. J. Hypertens. 2012, 25, 746–755. [Google Scholar] [CrossRef][Green Version]
- Meng, H.; Li, L.; Ruan, J.; Chen, Y.; Yan, Z.; Liu, J.; Li, X.; Mao, C.; Yang, P. Association of Low Expression of NUMB in Peripheral Blood with Acute Myocardial Infarction. Cardiol. Res. Pract. 2022, 2022, 7981637. [Google Scholar] [CrossRef]
- Ruan, J.; Meng, H.; Chen, Y.; Yan, Z.; Li, X.; Meng, F. Expression of ATP-binding cassette subfamily B member 1 gene in peripheral blood patients with acute myocardial infarction. Bioengineered 2022, 13, 11095–11105. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Meng, H.; Chen, L.; Meng, F. ACSL1 affects Triglyceride Levels through the PPARγ Pathway. Int. J. Med. Sci. 2020, 17, 720–727. [Google Scholar] [CrossRef]
- Li, L.; Meng, H.; Wang, X.; Ruan, J.; Tian, X.; Meng, F. Low ZCCHC9 Gene Expression in Peripheral Blood May Be an Acute Myocardial Infarction Genetic Molecular Marker in Patients with Stable Coronary Atherosclerotic Disease. Int. J. Med. Sci. 2022, 15, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.H.; Elenbaas, J.S.; Alisio, A.; Santana, K.; Young, E.P.; Kang, C.J.; Kachroo, P.; Lavine, K.J.; Razani, B.; Mecham, R.P.; et al. SVEP1 is a human coronary artery disease locus that promotes Atherosclerosis. Sci. Transl. Med. 2021, 13, eabe0357. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.H.; von Känel, R. Psychological stress, inflammation, and coronary heart disease. Curr. Cardiol. Rep. 2017, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, E. Obesity and cardiovascular disease. Minerva Pediatr. 2015, 67, 25–32. [Google Scholar]
- Khot, U.N.; Khot, M.B.; Bajzer, C.T.; Sapp, S.K.; Ohman, E.M.; Brener, S.J.; Ellis, S.G.; Lincoff, A.M.; Topol, E.J. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003, 290, 898–904. [Google Scholar] [CrossRef]
- Jayashree, S.; Arindam, M.; Vijay, K.V. Genetic epidemiology of coronary artery disease: An Asian Indian perspective. J. Genet. 2015, 94, 539–549. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Smith, J.G.; Gerszten, R.E. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation 2017, 135, 1651–1664. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Vaisar, T.; Davidson, W.S.; Bornfeldt, K.E.; Probstfield, J.L.; O’Brien, K.D.; Zhao, X.-Q.; Heinecke, J.W. Niacin Increases Atherogenic Proteins in High-Density Lipoprotein of Statin-Treated Subjects. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2330–2341. [Google Scholar] [CrossRef]
- Stitziel, N.O.; Kanter, J.E.; Bornfeldt, K.E. Emerging Targets for Cardiovascular Disease Prevention in Diabetes. Trends Mol. Med. 2020, 26, 744–757. [Google Scholar] [CrossRef]
- Zha, Y.; Lu, Y.; Zhang, T.; Yan, K.; Zhuang, W.; Liang, J.; Cheng, Y.; Wang, Y. CRISPR/Cas9-mediated knockout of APOC3 stabilizes plasma lipids and inhibits Atherosclerosis in rabbits. Lipids Health Dis. 2021, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of obesity management: An Endocrine Society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Zhu, K.F.; Wang, Y.M.; Zhu, J.Z.; Zhou, Q.Y.; Wang, N.F. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur. J. Prev. Cardiol. 2016, 23, 530–543. [Google Scholar] [CrossRef]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, D.; Yin, X.; Zheng, F.; Li, H. Socioeconomic status is associated with global diabetes prevalence. Oncotarget 2017, 8, 44434–44439. [Google Scholar] [CrossRef]
- Argent, A.C.; Balachandran, R.; Vaidyanathan, B.; Khan, A.; Kumar, R.K. Management of undernutrition and failure to thrive in children with congenital heart disease in low- and middle-income countries. Cardiol. Young 2017, 27, S22–S30. [Google Scholar] [CrossRef]
- Mardones-Santander, F.; Rosso, P.; Stekel, A.; Ahumada, E.; Llaguno, S.; Pizarro, F.; Salinas, J.; Vial, I.; Walter, T. Effect of a milk-based food supplement on maternal nutritional status and fetal growth in underweight Chilean women. Am. J. Clin. Nutr. 1988, 47, 413–419. [Google Scholar] [CrossRef]
- Krachler, M.; Lindschinger, M.; Eber, B.; Watzinger, N.; Wallner, S. Trace elements in coronary heart disease: Impact of intensi-fied lifestyle modification. Biol. Trace Elem. Res. 1997, 60, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Hernández-Aguilera, A.; Luciano-Mateo, F.; Cabré, N.; Baiges-Gaya, G.; Nadal, M.; Martín-Paredero, V.; Camps, J.; Joven, J.; Domingo, J.L. Trace elements and Paraoxonase-1 activity in lower extremity artery disease. Biol. Trace Elem. Res. 2018, 186, 74–84. [Google Scholar] [CrossRef]
- Strain, J.J. Putative role of dietary trace elements in coronary heart disease and cancer. Br. J. Biomed. Sci. 1994, 51, 241–251. [Google Scholar] [PubMed]
- Shokrzadeh, M.; Ghaemian, A.; Salehifar, E.; Aliakbari, S.; Saravi, S.S.; Ebrahimi, P. Serum zinc and copper levels in ischemic cardiomyopathy. Biol. Trace Elem. Res. 2009, 127, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Shah, M.H. Abnormalities of selected trace elements in patients with coronary artery disease. Acta Cardiol. Sin. 2015, 31, 518–527. [Google Scholar]
- Hughes, S.; Samman, S. The effect of zinc supplementation in humans on plasma lipids, antioxidant status and thrombogenesis. J. Am. Coll. Nutr. 2006, 25, 285–291. [Google Scholar] [CrossRef]
- Meng, H.; Wang, Y.; Zhou, F.; Ruan, J.; Duan, M.; Wang, X.; Yu, Q.; Yang, P.; Chen, W.; Meng, F. Reduced Serum Zinc Ion Concentration Is Associated with Coronary Heart Disease. Biol. Trace Elem. Res. 2021, 199, 4109–4118. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A red carpet for iron metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New insights into the role of iron in inflammation and Atherosclerosis. EBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.A.; Hassan, K.A.; Paulsen, I.T.; Brown, M.H. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genom. 2011, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 1, 1293–1294. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A possible physiological mediator of low-density lipoprotein oxidation and endothelial injury. Arterioscler. Thromb. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Jelani, Q.U.; Harchandani, B.; Cable, R.G.; Guo, Y.; Zhong, H.; Hilbert, T.; Newman, J.D.; Katz, S.D. Effects of serial phlebotomy on vascular endothelial function: Results of a prospective, double-blind, randomized study. Cardiovasc. Ther. 2018, 36, 1755–5922. [Google Scholar] [CrossRef]
- Klingler, K.R.; Zech, D.; Wielckens, K. Haemochromatosis: Automated detection of the two-point mutations in the HFE gene: Cys282Tyr and His63Asp. Clin. Chem. Lab. Med. 2000, 38, 1225–1230. [Google Scholar] [CrossRef]
- Aalbers, T.G.; Houtman, J.P. Relationships between trace elements and Atherosclerosis. Sci. Total Environ. 1985, 43, 255–283. [Google Scholar] [CrossRef]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef]
- Kodali, H.P.; Pavilonis, B.T.; Schooling, C.M. Effects of copper and zinc on ischemic heart disease and myocardial infarction: A Mendelian randomization study. Am. J. Clin. Nutr. 2018, 108, 237–242. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Bu, J. Relationship between selected serum metallic elements and obesity in children and adolescents in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A.; Savage, P.D. Obesity in coronary heart disease: An unaddressed behavioural risk factor. Prev. Med. 2017, 104, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Kalita, H.; Hazarika, A.; Devi, R. Withdrawal of high-carbohydrate, high-fat diet alters the status of trace elements to ameliorate metabolic syndrome in rats with type 2 diabetes mellitus. Can. J. Diabetes 2020, 44, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zou, Y.; Shen, Z.; Xiong, Y.; Zhang, W.; Liu, C.; Chen, S. Trace elements, PPARs, and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2612. [Google Scholar] [CrossRef]
- Li, J.; Lo, K.; Shen, G.; Feng, Y.Q.; Huang, Y.Q. Gender difference in the association of serum selenium with all-cause and cardiovascular mortality. Postgrad. Med. 2020, 132, 148–155. [Google Scholar] [CrossRef]
| Name | miR-122 miR-223 miR-27 miR-33 miR-128 miR-148a | miR-17 miR-31 | miR-181 miR-146 | miR-146 miR-21 miR-1 miR-133a miR-208a miR-499 | miR-132 | miR-1 miR-133 miR-328 | miR-21 miR-29 | miR-208 miR-133 |
| Role | Lipid metabolism | Inflammatory | Proliferation and differentiation | ACS | Heart failure | Arrhythmias | Fibrosis | Ventricular hypertrophy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Ruan, J.; Yan, Z.; Chen, Y.; Liu, J.; Li, X.; Meng, F. New Progress in Early Diagnosis of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 8939. https://doi.org/10.3390/ijms23168939
Meng H, Ruan J, Yan Z, Chen Y, Liu J, Li X, Meng F. New Progress in Early Diagnosis of Atherosclerosis. International Journal of Molecular Sciences. 2022; 23(16):8939. https://doi.org/10.3390/ijms23168939
Chicago/Turabian StyleMeng, Heyu, Jianjun Ruan, Zhaohan Yan, Yanqiu Chen, Jinsha Liu, Xiangdong Li, and Fanbo Meng. 2022. "New Progress in Early Diagnosis of Atherosclerosis" International Journal of Molecular Sciences 23, no. 16: 8939. https://doi.org/10.3390/ijms23168939
APA StyleMeng, H., Ruan, J., Yan, Z., Chen, Y., Liu, J., Li, X., & Meng, F. (2022). New Progress in Early Diagnosis of Atherosclerosis. International Journal of Molecular Sciences, 23(16), 8939. https://doi.org/10.3390/ijms23168939






