Priming of Cardiopulmonary Bypass with Human Albumin Decreases Endothelial Dysfunction after Pulmonary Ischemia–Reperfusion in an Animal Model
Abstract
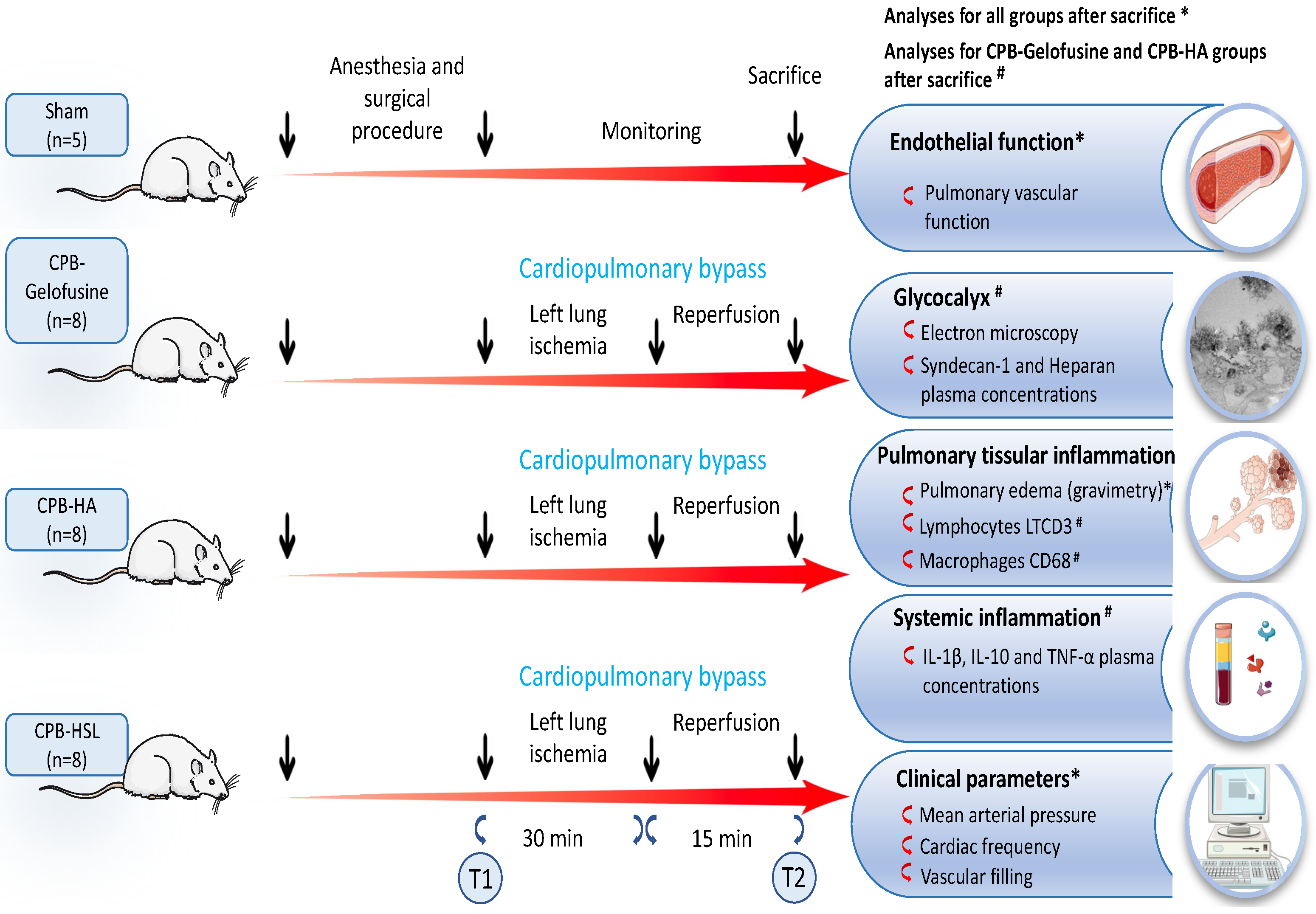
:1. Introduction
2. Results
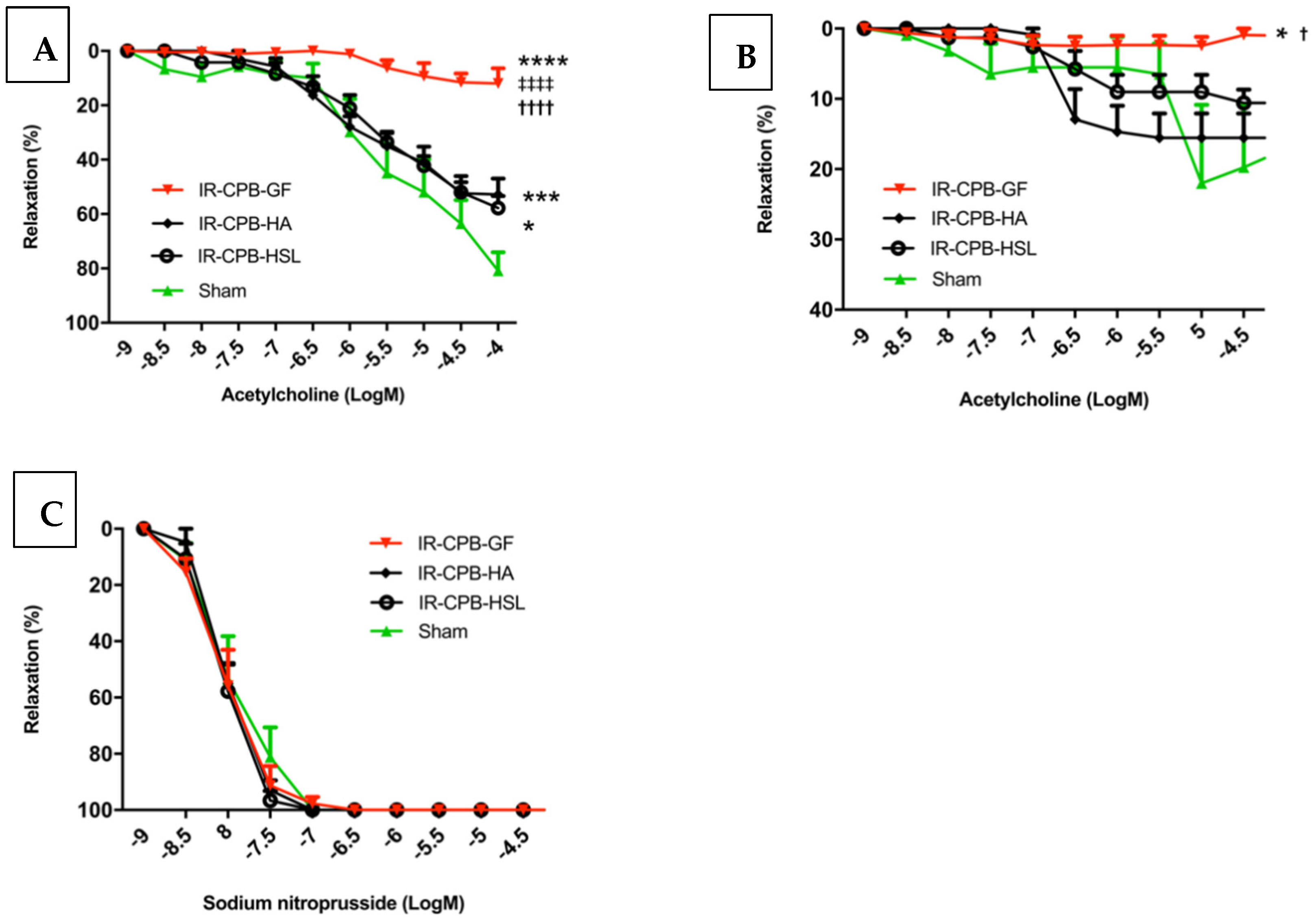
2.1. Effect of Priming Solution on Pulmonary Endothelial Function
2.2. Role of NO
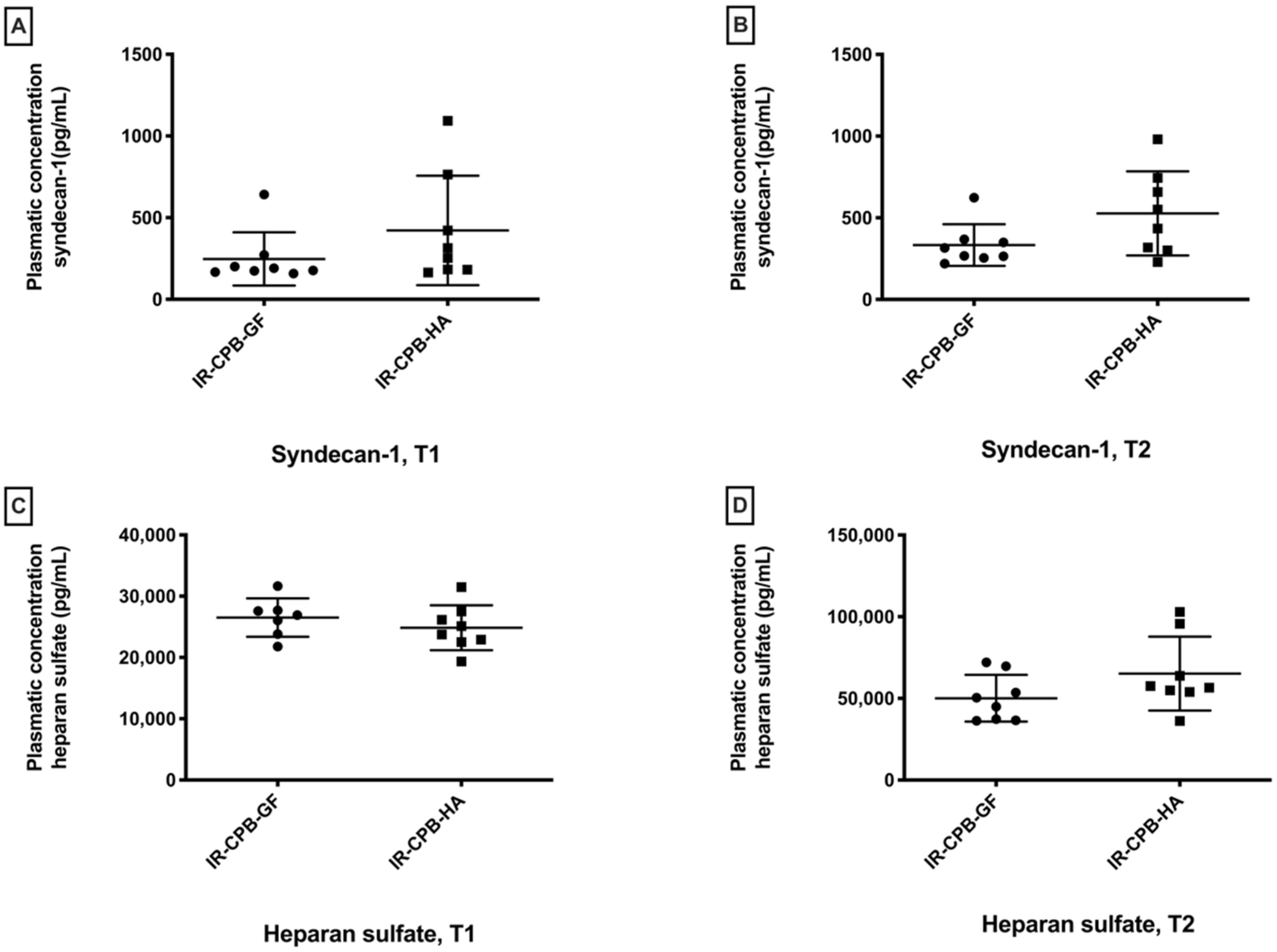
2.3. Glycocalyx Degradation
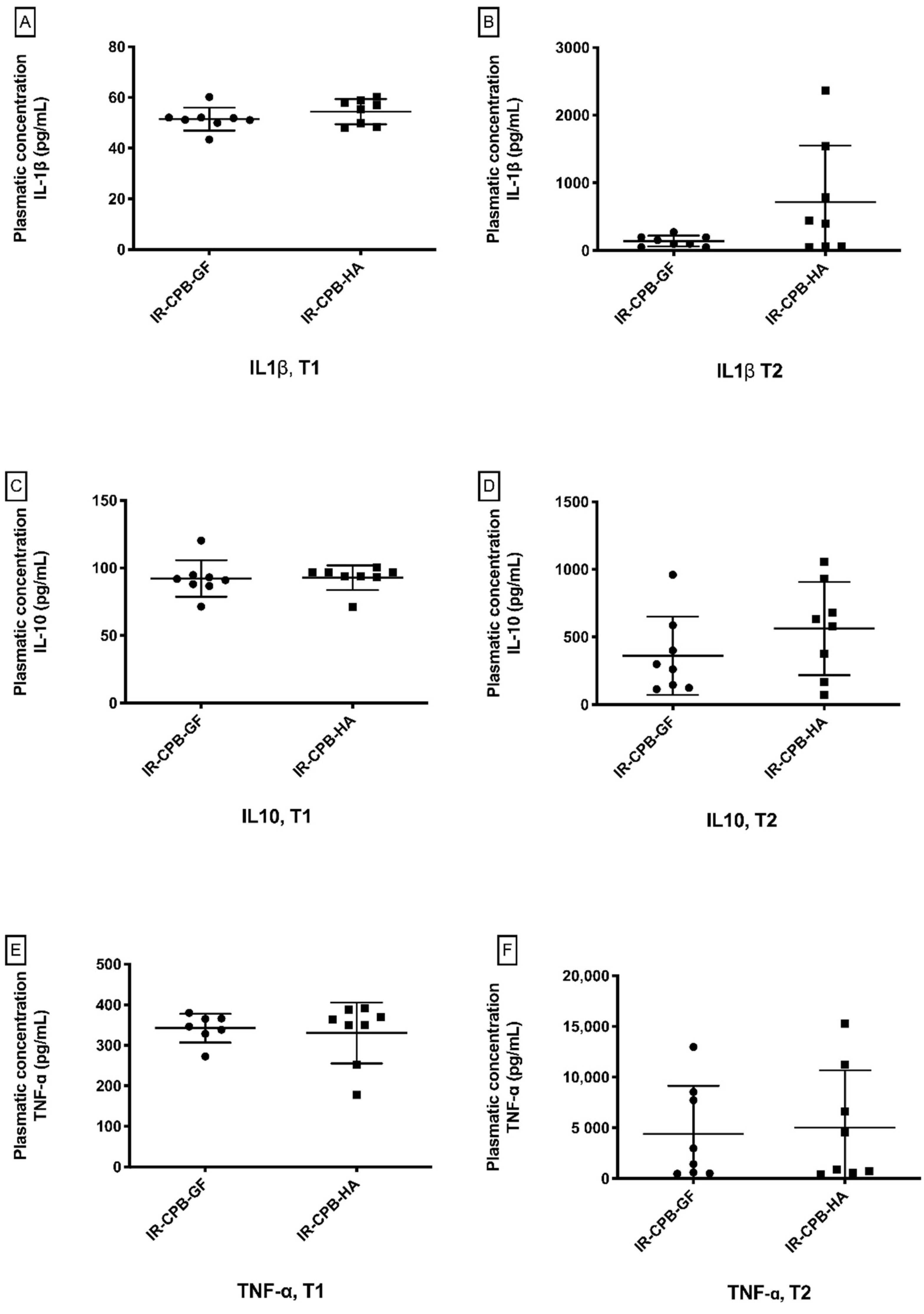
2.4. Inflammatory Response
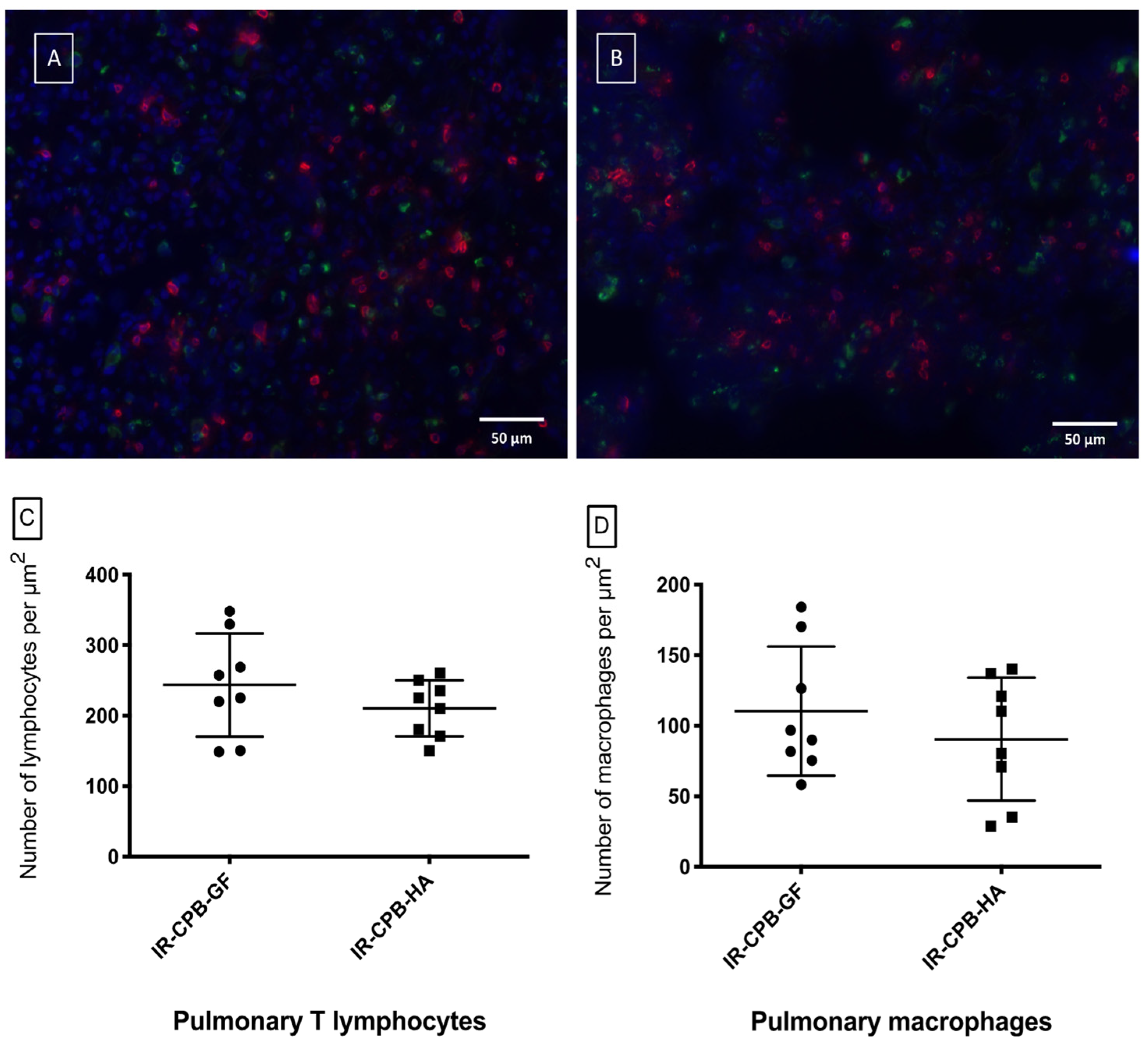
2.5. Histology
2.6. Clinical Parameters
3. Discussion
3.1. Fluid Therapy by Human Albumin
3.2. Fluid Therapy by Hypertonic Sodium Lactate
3.3. Limits
4. Materials and Methods
4.1. Animal Care and Study Groups
4.2. Surgical Procedure
4.3. Pulmonary Vascular Studies
4.4. Glycocalyx Analysis
4.5. Transmission Electron Microscopy (TEM)
4.6. Systemic Inflammation
4.7. Histology and Immunostaining Studies
4.8. Clinical Parameters
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.C.; Christie, J.D.; Keshavjee, S. Primary Graft Dysfunction: Definition, Risk Factors, Short- and Long-Term Outcomes. Semin. Respir. Crit. Care Med. 2010, 31, 161–171. [Google Scholar] [CrossRef]
- Christie, J.D.; Carby, M.; Bag, R.; Corris, P.; Hertz, M.; Weill, D. ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005, 24, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Porteous, M.K.; Lee, J.C. Primary Graft Dysfunction After Lung Transplantation. Clin. Chest Med. 2017, 38, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kiziltug, H.; Falter, F. Circulatory Support during Lung Transplantation. Curr. Opin. Anaesthesiol. 2020, 33, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Selim, J.; Hamzaoui, M.; Boukhalfa, I.; Djerada, Z.; Chevalier, L.; Piton, N.; Genty, D.; Besnier, E.; Clavier, T.; Dumesnil, A.; et al. Cardiopulmonary Bypass Increases Endothelial Dysfunction after Pulmonary Ischaemia-Reperfusion in an Animal Model. Eur. J. Cardiothorac. Surg. 2021, 59, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Meziani, F.; Kremer, H.; Tesse, A.; Baron-Menguy, C.; Mathien, C.; Mostefai, H.A.; Carusio, N.; Schneider, F.; Asfar, P.; Andriantsitohaina, R. Human Serum Albumin Improves Arterial Dysfunction during Early Resuscitation in Mouse Endotoxic Model via Reduced Oxidative and Nitrosative Stresses. Am. J. Pathol. 2007, 171, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Kremer, H.; Baron-Menguy, C.; Tesse, A.; Gallois, Y.; Mercat, A.; Henrion, D.; Andriantsitohaina, R.; Asfar, P.; Meziani, F. Human Serum Albumin Improves Endothelial Dysfunction and Survival during Experimental Endotoxemia: Concentration-Dependent Properties. Crit. Care Med. 2011, 39, 1414–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moret, E.; Jacob, M.W.; Ranucci, M.; Schramko, A.A. Albumin-Beyond Fluid Replacement in Cardiopulmonary Bypass Surgery: Why, How, and When? Semin. Cardiothorac Vasc. Anesth. 2014, 18, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Cuisinier, A.; Bouzat, P.; Batandier, C.; Lemasson, B.; Stupar, V.; Pernet-Gallay, K.; Crespy, T.; Barbier, E.L.; Payen, J.F. Hypertonic Sodium Lactate Reverses Brain Oxygenation and Metabolism Dysfunction after Traumatic Brain Injury. Br. J. Anaesth. 2018, 120, 1295–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnier, E.; Coquerel, D.; Kouadri, G.; Clavier, T.; Favory, R.; Duburcq, T.; Lesur, O.; Bekri, S.; Richard, V.; Mulder, P.; et al. Hypertonic Sodium Lactate Improves Microcirculation, Cardiac Function, and Inflammation in a Rat Model of Sepsis. Crit. Care 2020, 24, 354. [Google Scholar] [CrossRef] [PubMed]
- Doguet, F.; Tamion, F.; Le Guillou, V.; Bubenheim, M.; Thuillez, C.; Richard, V.; Bessou, J.P. Albumin Limits Mesenteric Endothelial Dysfunction and Inflammatory Response in Cardiopulmonary Bypass. Artif. Organs. 2012, 36, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Bruegger, D.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Contrasting Effects of Colloid and Crystalloid Resuscitation Fluids on Cardiac Vascular Permeability. Anesthesiology 2006, 104, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Milford, E.M.; Reade, M.C. Resuscitation Fluid Choices to Preserve the Endothelial Glycocalyx. Crit. Care 2019, 23, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, L.N.; Chung, K.K.; Salgado, C.L.; Dubick, M.A.; Torres Filho, I.P. Low-Volume Resuscitation with Normal Saline Is Associated with Microvascular Endothelial Dysfunction after Hemorrhage in Rats, Compared to Colloids and Balanced Crystalloids. Crit. Care 2017, 21, 160. [Google Scholar] [CrossRef]
- Lang, J.D.; Figueroa, M.; Chumley, P.; Aslan, M.; Hurt, J.; Tarpey, M.M.; Alvarez, B.; Radi, R.; Freeman, B.A. Albumin and Hydroxyethyl Starch Modulate Oxidative Inflammatory Injury to Vascular Endothelium. Anesthesiology 2004, 100, 51–58. [Google Scholar] [CrossRef]
- Tatara, T. The Contribution of Solute-Solvent Exchange at the Membrane Surface to the Reduction by Albumin of the Hydraulic Permeability Coefficient of an Artificial Semipermeable Membrane. Anesth. Analg. 2003, 97, 1137–1142. [Google Scholar] [CrossRef]
- Hanley, C.; Callum, J.; Karkouti, K.; Bartoszko, J. Albumin in Adult Cardiac Surgery: A Narrative Review. Can. J. Anaesth. 2021, 68, 1197–1213. [Google Scholar] [CrossRef]
- Pesonen, E.; Vlasov, H.; Suojaranta, R.; Hiippala, S.; Schramko, A.; Wilkman, E.; Eränen, T.; Arvonen, K.; Mazanikov, M.; Salminen, U.-S.; et al. Effect of 4% Albumin Solution vs Ringer Acetate on Major Adverse Events in Patients Undergoing Cardiac Surgery With Cardiopulmonary Bypass: A Randomized Clinical Trial. JAMA 2022, 328, 251–258. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Machuca, T.; Chen, M.; Singer, L.G.; Yasufuku, K.; de Perrot, M.; Pierre, A.; Waddell, T.K.; Keshavjee, S. Experience with the First 50 Ex Vivo Lung Perfusions in Clinical Transplantation. J. Thorac. Cardiovasc. Surg. 2012, 144, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boone, M.D.; Oren-Grinberg, A.; Robinson, T.M.; Chen, C.C.; Kasper, E.M. Mannitol or Hypertonic Saline in the Setting of Traumatic Brain Injury: What Have We Learned? Surg. Neurol. Int. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Orban, J.-C.; Ichai, C. Hyperosmolar Sodium-Lactate in the ICU: Vascular Filling and Cellular Feeding. Crit. Care 2014, 18, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebreton, G.; Tamion, F.; Bessou, J.-P.; Doguet, F. Cardiopulmonary Bypass Model in the Rat: A New Minimal Invasive Model with a Low Flow Volume. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 642–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doguet, F.; Litzler, P.-Y.; Tamion, F.; Richard, V.; Hellot, M.-F.; Thuillez, C.; Tabley, A.; Bouchart, F.; Bessou, J.P. Changes in Mesenteric Vascular Reactivity and Inflammatory Response after Cardiopulmonary Bypass in a Rat Model. Ann. Thorac. Surg. 2004, 77, 2130–2137. [Google Scholar] [CrossRef]
- Chevalier, L.; Selim, J.; Genty, D.; Baste, J.M.; Piton, N.; Boukhalfa, I.; Hamzaoui, M.; Pareige, P.; Richard, V. Electron Microscopy Approach for the Visualization of the Epithelial and Endothelial Glycocalyx. Morphologie 2017, 101, 55–63. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, J.; Hamzaoui, M.; Ghemired, A.; Djerada, Z.; Chevalier, L.; Piton, N.; Besnier, E.; Clavier, T.; Dumesnil, A.; Renet, S.; et al. Priming of Cardiopulmonary Bypass with Human Albumin Decreases Endothelial Dysfunction after Pulmonary Ischemia–Reperfusion in an Animal Model. Int. J. Mol. Sci. 2022, 23, 8938. https://doi.org/10.3390/ijms23168938
Selim J, Hamzaoui M, Ghemired A, Djerada Z, Chevalier L, Piton N, Besnier E, Clavier T, Dumesnil A, Renet S, et al. Priming of Cardiopulmonary Bypass with Human Albumin Decreases Endothelial Dysfunction after Pulmonary Ischemia–Reperfusion in an Animal Model. International Journal of Molecular Sciences. 2022; 23(16):8938. https://doi.org/10.3390/ijms23168938
Chicago/Turabian StyleSelim, Jean, Mouad Hamzaoui, Antoine Ghemired, Zoubir Djerada, Laurence Chevalier, Nicolas Piton, Emmanuel Besnier, Thomas Clavier, Anaïs Dumesnil, Sylvanie Renet, and et al. 2022. "Priming of Cardiopulmonary Bypass with Human Albumin Decreases Endothelial Dysfunction after Pulmonary Ischemia–Reperfusion in an Animal Model" International Journal of Molecular Sciences 23, no. 16: 8938. https://doi.org/10.3390/ijms23168938
APA StyleSelim, J., Hamzaoui, M., Ghemired, A., Djerada, Z., Chevalier, L., Piton, N., Besnier, E., Clavier, T., Dumesnil, A., Renet, S., Mulder, P., Doguet, F., Tamion, F., Veber, B., Bellien, J., Richard, V., & Baste, J.-M. (2022). Priming of Cardiopulmonary Bypass with Human Albumin Decreases Endothelial Dysfunction after Pulmonary Ischemia–Reperfusion in an Animal Model. International Journal of Molecular Sciences, 23(16), 8938. https://doi.org/10.3390/ijms23168938





