Analysis of the Chemical Composition and Morphological Characterization of Tissue Osseointegrated to a Dental Implant after 5 Years of Function
Abstract
:1. Introduction
2. Results
2.1. Case Report
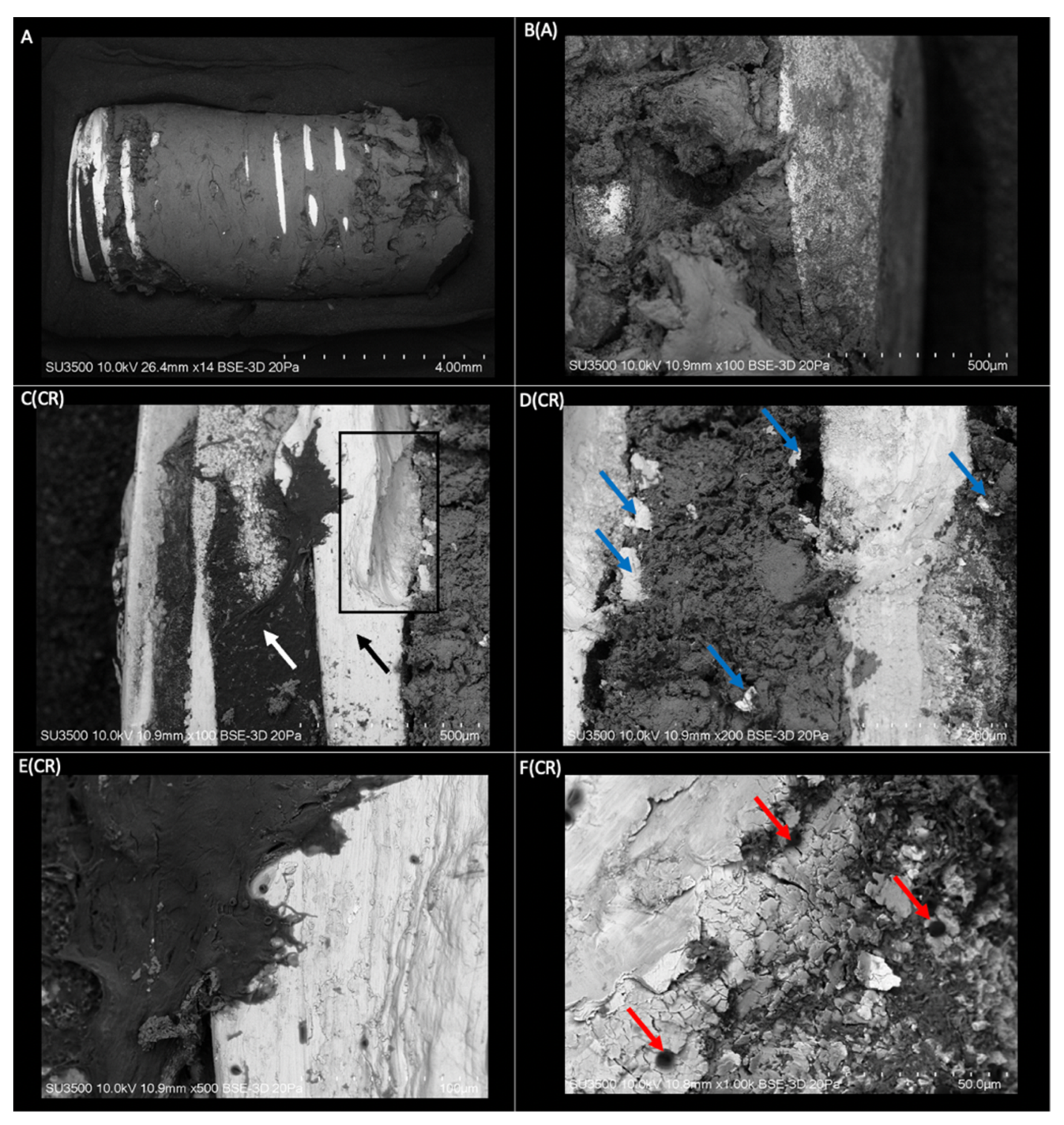
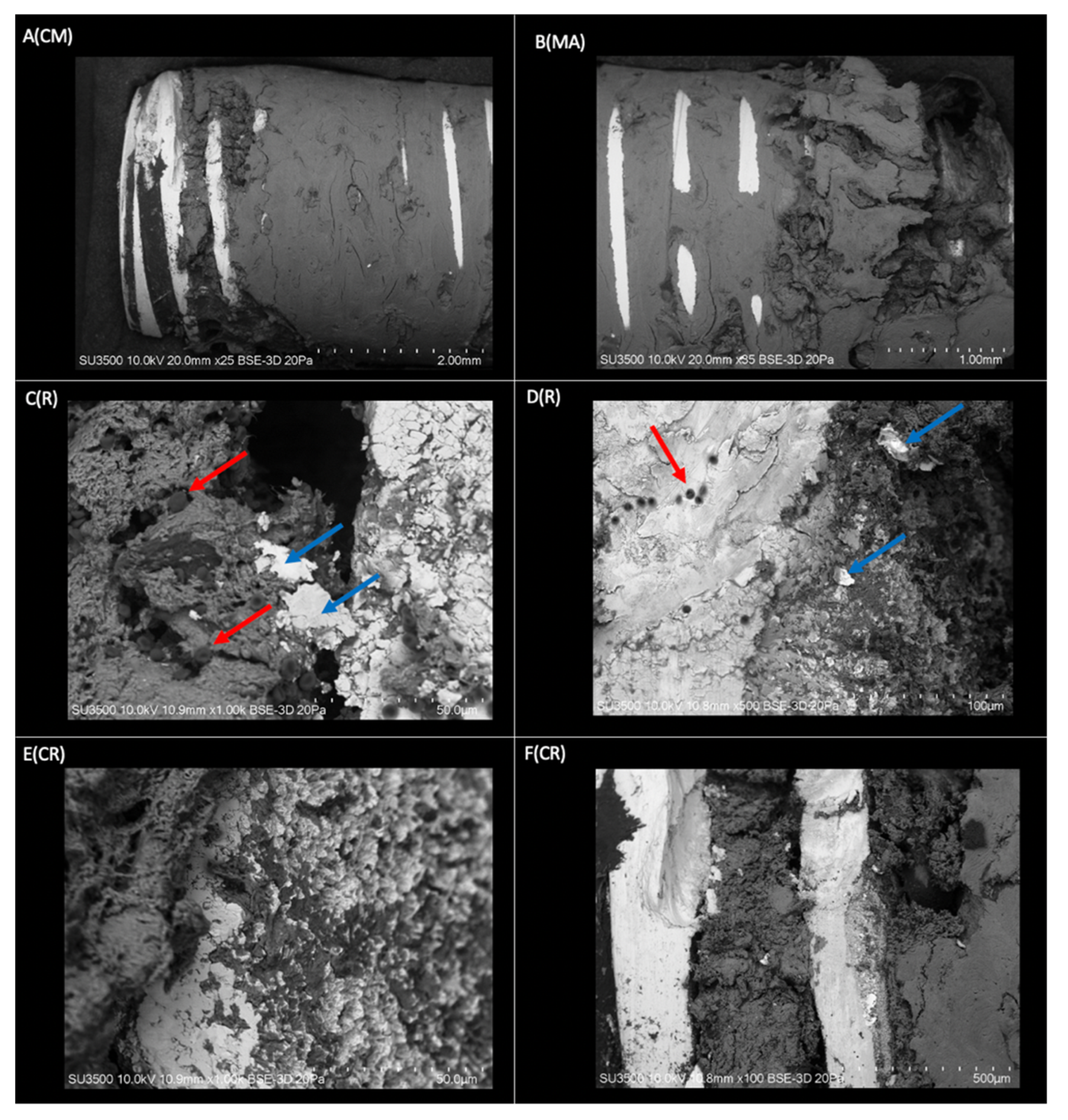
2.2. Surface Morphological Analysis of Osseointegrated Dental Implants
2.2.1. Morphological Analysis of the Dental Implant Surface
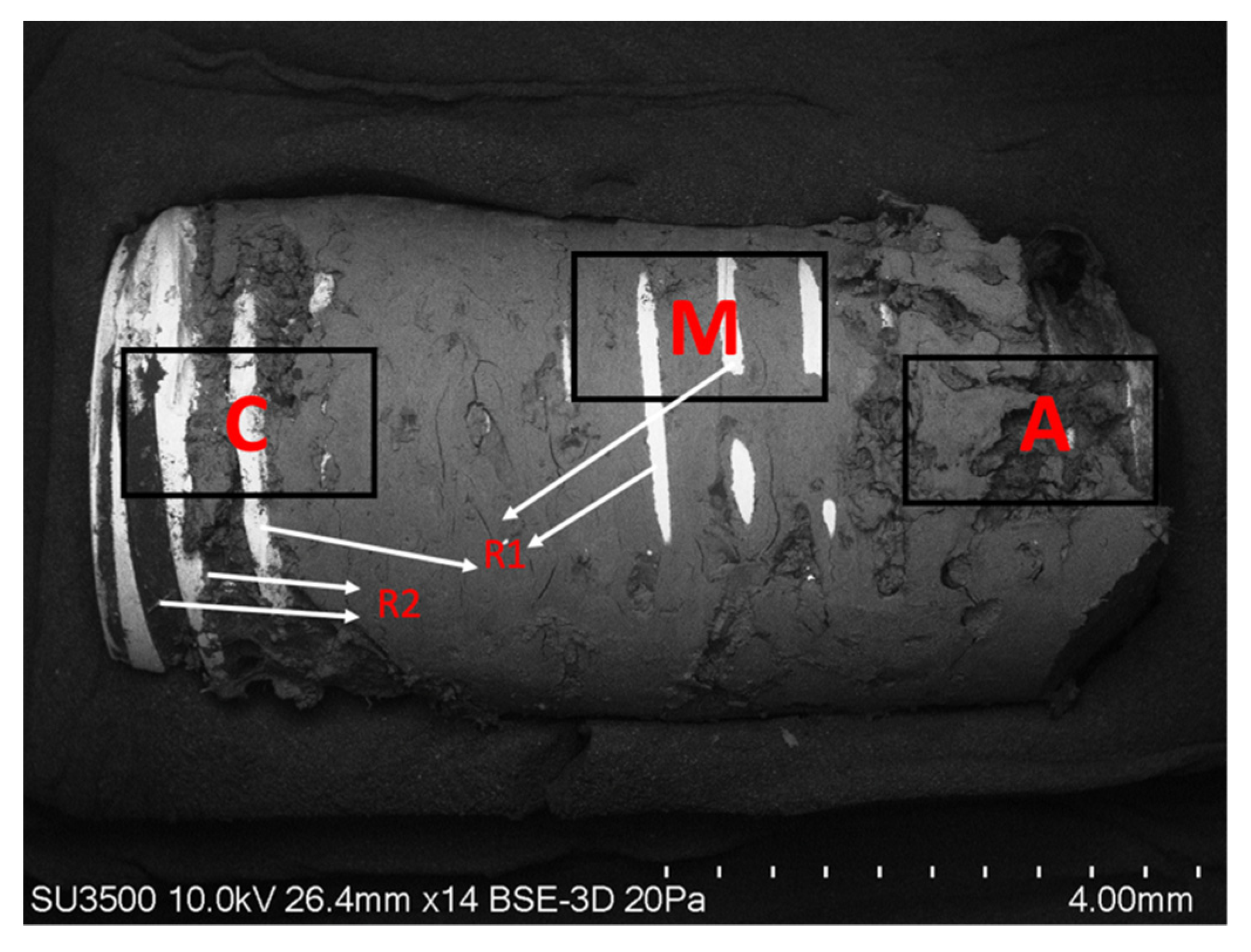
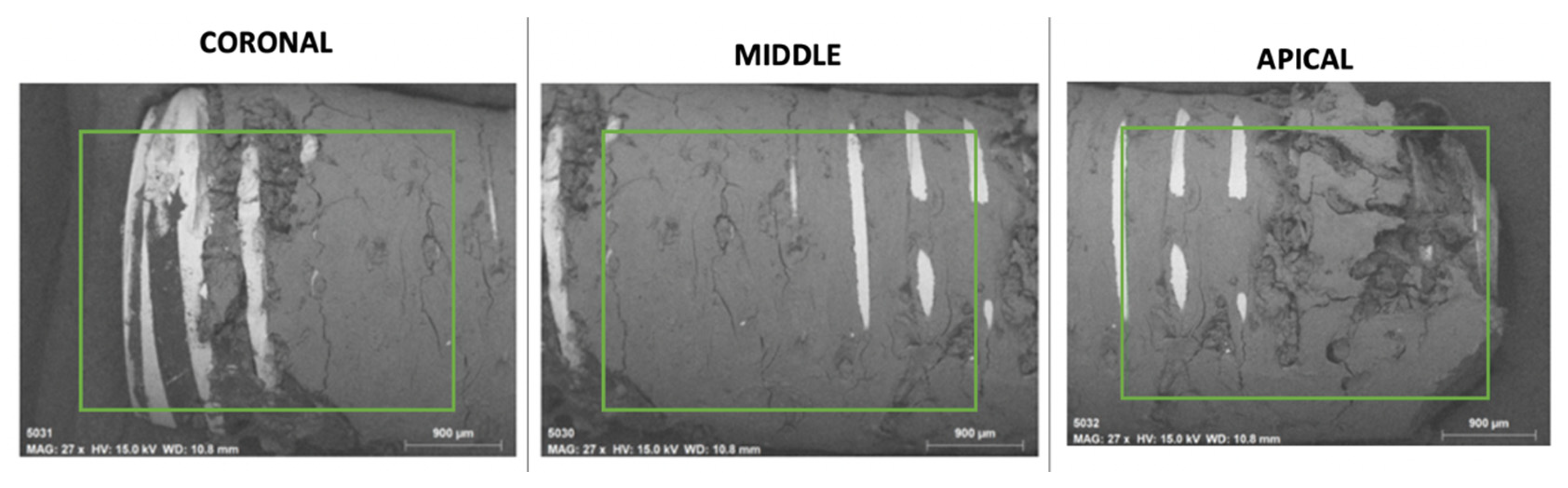
2.2.2. Superficial Morphological Analysis of Bone Tissue Associated with Dental Implants
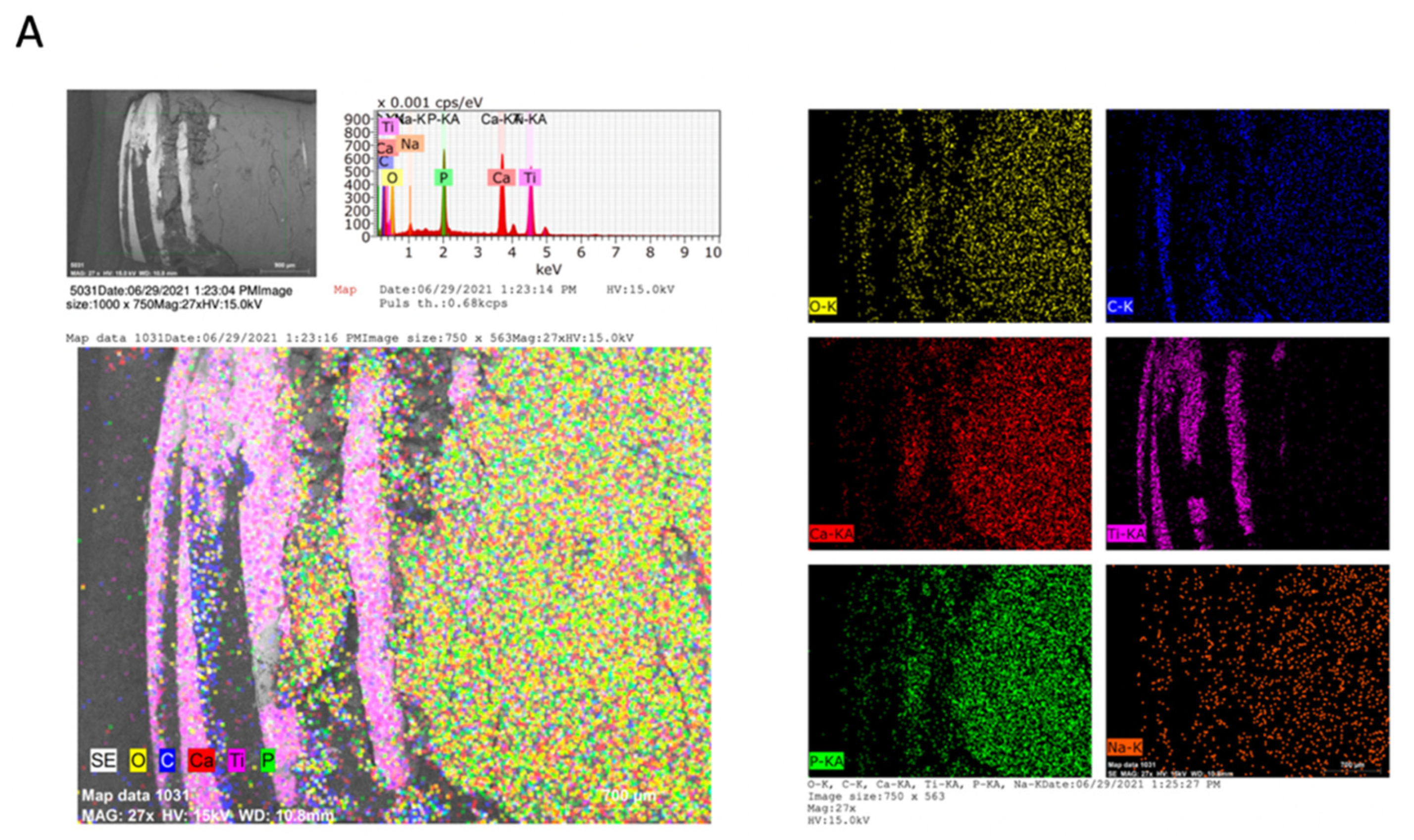
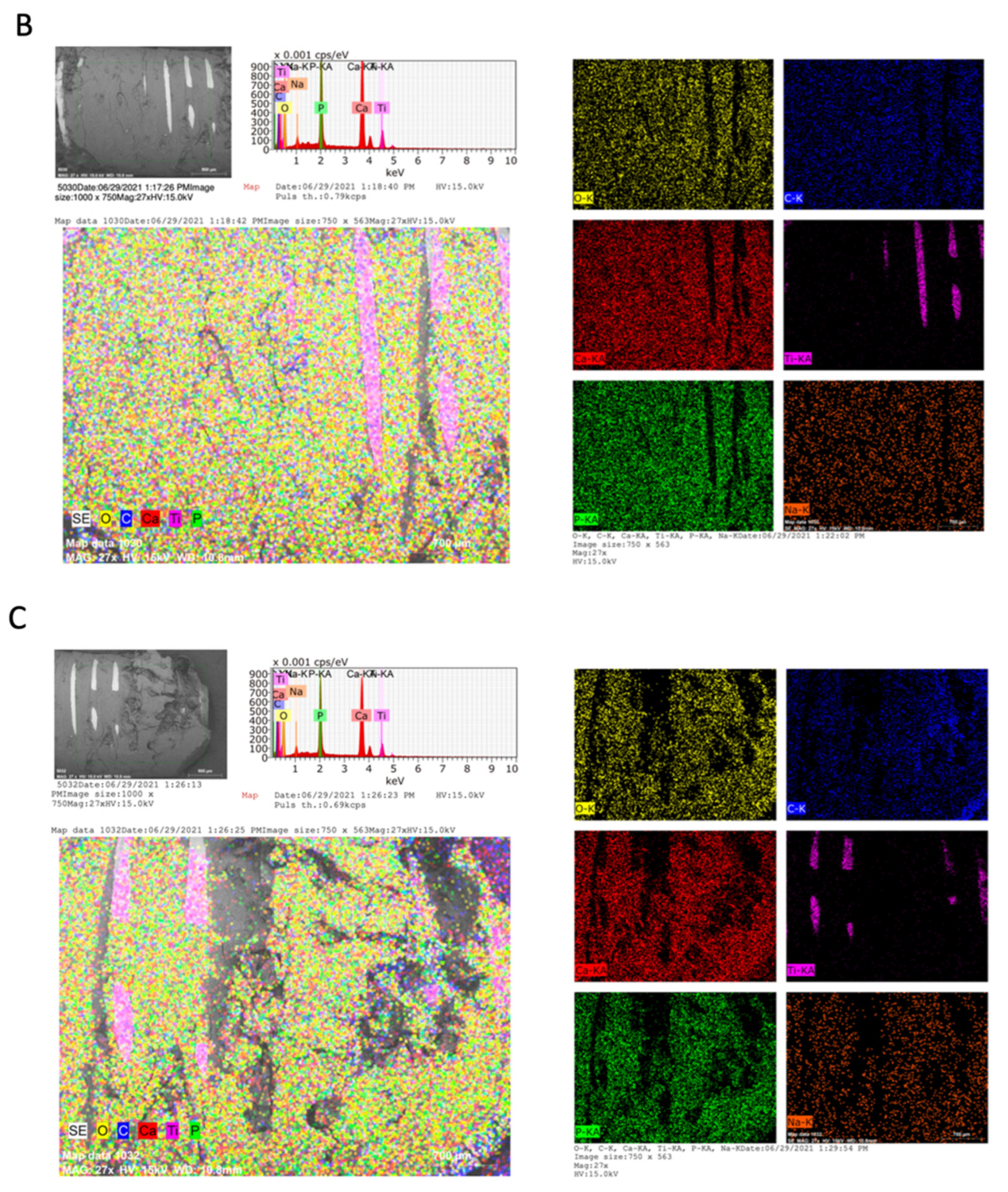
2.3. Chemical Analysis of Dental Implants and Bone Tissue
3. Discussion
4. Materials and Methods
4.1. Case Report
4.2. Surface Morphological Analysis of Osseointegrated Dental Implants
4.3. Chemical Analysis of Osseointegrated Dental Implants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lafita, J. Physiology and bone physiopathology. An. Sist. Sanit. Navar. 2003, 6, 7–15. [Google Scholar]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vengas, J.C.; Garzón-Alvarado, D.; Casale, M. Interaction between osteoblasts and titanium surfaces: Application in dental implants. Rev. Cuba. Investig. Bioméd. 2010, 20, 1561–3011. [Google Scholar]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Branemark, P.I. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11, 1–82. [Google Scholar]
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2010, 39, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Guler, B.; Uraz, A.; Çetiner, D. The chemical surface evaluation of black and white porous titanium granules and different commercial dental implants with energy-dispersive x-ray spectroscopy analysis. Clin. Implant Dent. Relat. Res. 2019, 21, 352–359. [Google Scholar] [CrossRef]
- Pearce, A.; Richards, R.; Milz, S.; Schneider, E.; Pearce, S. Animal models for implant biomaterial research in bone: A review. Eur. Cell. Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 4, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicinas 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Thalji, G.; Gretzer, C.; Cooper, L.F. Comparative molecular assessment of early osseointegration in implant-adherent cells. Bone 2013, 52, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Almas, K.; Smith, S.; Kutkut, A. What is the Best Micro and Macro Dental Implant Topography? Dent. Clin. N. Am. 2019, 63, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Caeiro Rey, J.R.; Dapía Robleda, S.; Vaquero Cervino, E.; Roca Ruiz, L.; Blanco Ramos, M.A. Factores determinantes de la resistencia ósea. Rev. Esp. Enferm. Metab. Óseas. 2005, 14, 67–74. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Isidor, F. Influence of forces on peri-implant bone. Clin. Oral Implant. Res. 2006, 17, 8–18. [Google Scholar] [CrossRef]
- Flores Renteria, M.A.; Ayala Ruiz, A. Efectos en la Remodelación Ósea Debido a la Aplicación de Tornillos en Fémur Proximal. Ingenier. Mecáni. Tecnolog. 2012, 4, 43–50. [Google Scholar]
- Glauser, R. Implants with an oxidized surface placed predominately in soft bone quality and subjected to immediate occlusal loading: Results from a 7-year clinical follow-up. Clin. Implant. Dent. Relat. Res. 2013, 15, 322–331. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Venegas, J.C.; Landinez, N.S.; Garzón-Alvarado, D. Basic principles of bone-dental implant interphase. Rev. Cuba. Invest. Bioméd. 2009, 28, 130–146. [Google Scholar]
- Dohan Ehrenfest, D.M.; Vazquez, L.; Park, Y.J.; Sammartino, G.; Bernard, J.P. Identification card and codification of the chemical and morphological characteristics of 14 dental implant surfaces. J. Oral Implantol. 2011, 37, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta. Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Blanco Lopez, P.; Monsalve Guil, L.; Matos Garrido, N.; Moreno Muñoz, J.; Nuñez Marquez, E.; Velasco Ortega, E. Osseointegration of titanium implant with several rough surfaces. Av. Odontoestomatol. 2018, 34, 141–149. [Google Scholar]
- McCafferty, E.; Wightman, J.P. An X-ray photoelectron spectroscopy sputter profile study of the native air-formed oxide film on titanium. Appl. Surf. Sci. 1999, 143, 92–100. [Google Scholar] [CrossRef]
- Prati, C.; Zamparini, F.; Botticelli, D.; Ferri, M.; Yonezawa, D.; Piattelli, A.; Gandolfi, M.G. The Use of ESEM-EDX as an Innovative Tool to Analyze the Mineral Structure of Peri-Implant Human Bone. Materials 2020, 13, 1671. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.G.; Zamparini, F.; Iezzi, G.; Degidi, M.; Botticelli, D.; Piattelli, A.; Prati, C. Microchemical and Micromorphologic ESEM-EDX Analysis of Bone Mineralization at the Thread Interface in Human Dental Implants Retrieved for Mechanical Complications After 2 Months to 17 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Neri Basurto, R.; Solís Arrieta, L.; Villegas Castrejon, H. Study on the osseous consolidation in rat by environmental scanning electron microscopy. Vet. Mex. 2008, 39, 187–198. [Google Scholar]
- De Maté Sánchez Val, J.E.; Calvo Guirado, J.L.; Delgado Ruiz, R.A.; Gómez Moreno, G.; Ramírez Fernández, M.P.; Romanos, G.E. Bone neo-formation and mineral degradation of 4Bone.® Part I: Material characterization and SEM study in critical size defects in rabbits. Clin. Oral Implant. Res. 2015, 26, 1165–1169. [Google Scholar] [CrossRef]
- Zaichick, V.; Zaichick, S. The Ca, Cl, Mg, Na, and P mass fractions in human bone affected by Ewing’s sarcoma. Biol. Trace Elem. Res. 2014, 159, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Laval, J. Determination of Calcium/Phosphorus Atomic Ratio of Calcium Phosphate Apatites Using X-ray Diffractometry. J. Am. Ceram. Soc. 2001, 84, 359–366. [Google Scholar] [CrossRef]
- Henmi, A.; Okata, H.; Anada, T.; Yoshinari, M.; Mikami, Y.; Suzuki, O.; Sasano, Y. Bone matrix calcification during embryonic and postembryonic rat calvarial development assessed by SEM-EDX spectroscopy, XRD, and FTIR spectroscopy. J. Bone Miner. Metab. 2016, 34, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Okata, H.; Nakamura, M.; Henmi, A.; Yamaguchi, S.; Mikami, Y.; Shimauchi, H.; Sasano, Y. Calcification during bone healing in a standardised rat calvarial defect assessed by micro-CT and SEM-EDX. Oral Dis. 2015, 21, 74–82. [Google Scholar] [CrossRef]
- Akkus, O.; Adar, F.; Schaffler, M.B. Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone 2004, 34, 443–453. [Google Scholar] [CrossRef]
- Coyne, M.D.; Lobene, A.; Neumann, C.; Lachcik, P.; Weaver, C.M.; Nie, L.H. Determination of bone sodium (Na) and Na exchange in pig leg using in vivo neutron activation analysis (IVNAA). Physiol. Meas. 2019, 40, 075009. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.J.; Verbalis, J.G. Sodium homeostasis and bone. Curr. Opin. Nephrol. Hyperte. 2014, 23, 370–376. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apablaza, J.A.; Días, F.J.; Sánchez, K.G.; Navarro, P.; Venegas, C.; Fuentes, R. Analysis of the Chemical Composition and Morphological Characterization of Tissue Osseointegrated to a Dental Implant after 5 Years of Function. Int. J. Mol. Sci. 2022, 23, 8882. https://doi.org/10.3390/ijms23168882
Apablaza JA, Días FJ, Sánchez KG, Navarro P, Venegas C, Fuentes R. Analysis of the Chemical Composition and Morphological Characterization of Tissue Osseointegrated to a Dental Implant after 5 Years of Function. International Journal of Molecular Sciences. 2022; 23(16):8882. https://doi.org/10.3390/ijms23168882
Chicago/Turabian StyleApablaza, Josefa Alarcón, Fernando José Días, Karina Godoy Sánchez, Pablo Navarro, Camila Venegas, and Ramón Fuentes. 2022. "Analysis of the Chemical Composition and Morphological Characterization of Tissue Osseointegrated to a Dental Implant after 5 Years of Function" International Journal of Molecular Sciences 23, no. 16: 8882. https://doi.org/10.3390/ijms23168882
APA StyleApablaza, J. A., Días, F. J., Sánchez, K. G., Navarro, P., Venegas, C., & Fuentes, R. (2022). Analysis of the Chemical Composition and Morphological Characterization of Tissue Osseointegrated to a Dental Implant after 5 Years of Function. International Journal of Molecular Sciences, 23(16), 8882. https://doi.org/10.3390/ijms23168882






