Abstract
Bromocriptine-QR is a sympatholytic dopamine D2 agonist for the treatment of type 2 diabetes that has demonstrated rapid (within 1 year) substantial reductions in adverse cardiovascular events in this population by as yet incompletely delineated mechanisms. However, a chronic state of elevated sympathetic nervous system activity and central hypodopaminergic function has been demonstrated to potentiate an immune system pro-oxidative/pro-inflammatory condition and this immune phenotype is known to contribute significantly to the advancement of cardiovascular disease (CVD). Therefore, the possibility exists that bromocriptine-QR therapy may reduce adverse cardiovascular events in type 2 diabetes subjects via attenuation of this underlying chronic pro-oxidative/pro-inflammatory state. The present study was undertaken to assess the impact of bromocriptine-QR on a wide range of immune pro-oxidative/pro-inflammatory biochemical pathways and genes known to be operative in the genesis and progression of CVD. Inflammatory peripheral blood mononuclear cell biology is both a significant contributor to cardiovascular disease and also a marker of the body’s systemic pro-inflammatory status. Therefore, this study investigated the effects of 4-month circadian-timed (within 2 h of waking in the morning) bromocriptine-QR therapy (3.2 mg/day) in type 2 diabetes subjects whose glycemia was not optimally controlled on the glucagon-like peptide 1 receptor agonist on (i) gene expression status (via qPCR) of a wide array of mononuclear cell pro-oxidative/pro-inflammatory genes known to participate in the genesis and progression of CVD (OXR1, NRF2, NQO1, SOD1, SOD2, CAT, GSR, GPX1, GPX4, GCH1, HMOX1, BiP, EIF2α, ATF4, PERK, XBP1, ATF6, CHOP, GSK3β, NFkB, TXNIP, PIN1, BECN1, TLR2, TLR4, TLR10, MAPK8, NLRP3, CCR2, GCR, L-selectin, VCAM1, ICAM1) and (ii) humoral measures of sympathetic tone (norepinephrine and normetanephrine), whole-body oxidative stress (nitrotyrosine, TBARS), and pro-inflammatory factors (IL-1β, IL-6, IL-18, MCP-1, prolactin, C-reactive protein [CRP]). Relative to pre-treatment status, 4 months of bromocriptine-QR therapy resulted in significant reductions of mRNA levels in PBMC endoplasmic reticulum stress-unfolded protein response effectors [GRP78/BiP (34%), EIF2α (32%), ATF4 (29%), XBP1 (25%), PIN1 (14%), BECN1 (23%)], oxidative stress response proteins [OXR1 (31%), NRF2 (32%), NQO1 (39%), SOD1 (52%), CAT (26%), GPX1 (33%), GPX4 (31%), GCH1 (30%), HMOX1 (40%)], mRNA levels of TLR pro-inflammatory pathway proteins [TLR2 (46%), TLR4 (20%), GSK3β (19%), NFkB (33%), TXNIP (18%), NLRP3 (32%), CCR2 (24%), GCR (28%)], mRNA levels of pro-inflammatory cellular receptor proteins CCR2 and GCR by 24% and 28%, and adhesion molecule proteins L-selectin (35%) and VCAM1 (24%). Relative to baseline, bromocriptine-QR therapy also significantly reduced plasma levels of norepinephrine and normetanephrine by 33% and 22%, respectively, plasma pro-oxidative markers nitrotyrosine and TBARS by 13% and 10%, respectively, and pro-inflammatory factors IL-18, MCP1, IL-1β, prolactin, and CRP by 21%,13%, 12%, 42%, and 45%, respectively. These findings suggest a unique role for circadian-timed bromocriptine-QR sympatholytic dopamine agonist therapy in reducing systemic low-grade sterile inflammation to thereby reduce cardiovascular disease risk.
1. Introduction
Bromocriptine-QR is a unique formulation of micronized bromocriptine mesylate, a potent sympatholytic, dopamine D2 receptor agonist that is approved for the treatment of type 2 diabetes (T2D) by the U.S. Food and Drug Administration [1,2]. In a large, randomized trial of T2D subjects, the majority (75%) of whom were without pre-existing cardiovascular disease (CVD), the circadian-timed (within 2 h of morning waking) administration of this therapy significantly reduced cardiovascular events by 40–55% over a single year [3,4,5,6]. Because these protective cardiovascular (CV) effects could not be explained by major changes in plasma lipids, HbA1c, or blood pressure, a plausible explanation for the beneficial CV effect may be related to the reduced chronic overactivation of sympathetic tone [7,8,9,10,11], a pathophysiologic abnormality that both causes and exacerbates the metabolic syndrome (MS), T2D and CVD [12,13,14,15,16,17,18]. Although poorly appreciated, the scientific literature is replete with studies that corroborate a prominent role of the chronic elevation of sympathetic nervous system (SNS) tone in the genesis and amplification of cellular/tissue oxidative stress and the resultant inflammation, particularly within the immune system [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], which promotes insulin resistance and CVD [14,30,31,32,33,34,37,38,39,40,41,42,43,44,45,46,47,48,49]. Likewise, low central dopaminergic activity, particularly at the D2 receptor, is associated with a pro-oxidative/pro-inflammatory (PO/PI) state in multiple tissues including the immune system [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. This hypo-dopaminergic PO/PI state can result from a loss of dopaminergic inhibition of central sympathetic outflow, as well as from a direct reduction of D2 receptor activation in specific brain tissues and peripheral sites. Conversely, bromocriptine has been shown to elicit anti-inflammatory responses in the treatment of lupus, rheumatoid arthritis, uveitis, and postpartum cardiomyopathy [67,68,69,70,71,72,73,74,75], suggesting that its sympatholytic, dopaminergic actions might promote anti-inflammatory effects in T2D. Multiple studies have highlighted the critical role of the daily circadian rhythm of the central nervous system (CNS)’s dopaminergic activity in the maintenance of metabolic health (reviewed in ref [1]). Therefore, the present investigation was undertaken to assess the impact of circadian-timed bromocriptine-QR on the indicators of sympathetic tone, gene expression in peripheral blood mononuclear cells (PBMC), and plasma factors that are indicative of a PO/PI state in T2D subjects. We hypothesized, based on the basic scientific and clinical observations described above, that bromocriptine-QR treatment would reduce plasma norepinephrine/normetanephrine levels and reduce plasma and peripheral blood mononuclear cells’ (PBMC) biochemical elements involved in the generation of a pro-oxidative/pro-inflammatory state, explaining at least in part, the observed cardioprotective effect of the drug [3,4,5,6].
Although increased SNS and decreased dopaminergic tone can induce an immune and metabolic tissue PO/PI state that potentiates MS and CVD, it is also true that MS components (including hyperglycemia, insulin resistance, dyslipidemia, elevated plasma FFA levels, increased renin–angiotensin system activity, hypertension, elevated plasma thrombin, endothelin 1, and angiotensin 2 levels, and obesity) feedback to potentiate the PO/PI state within both immune and non-immune cell types. This reverberating circuit in turn can contribute to the onset and exacerbation of MS, T2D, and CVD [14,37,38,40,41,42,44,46,47,48,49,76,77,78,79,80,81,82,83,84,85,86]. Thus, a systemic chronic increase in the immune PO/PI state (initiated via an autonomic imbalance of elevated SNS tone and/or metabolic influences) fuels a whole-body shift in cardiometabolic status from health toward MS, T2D, and CVD, setting up a self-sustaining cycle of whole-body cardiometabolic pathology [84,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. Studies of MS subjects with and without T2D have provided evidence that the PO/PI state of PBMCs contributes to the development of MS and CVD [77,78,79,80,81,84,86,101,102,103]. Two major cellular dysfunctions that interact to contribute to an immunocyte (e.g., PBMCs) PO/PI state are endoplasmic reticulum (ER) stress and toll-like receptor (TLR) pro-inflammatory pathway overactivation.
In a recent study, we demonstrated that bromocriptine-QR treatment in inadequately controlled T2D subjects treated with a glucagon-like peptide 1 receptor agonist (GLP-1RA) improved endothelial dysfunction, reduced blood pressure, and enhanced suppression of post-meal hepatic glucose production without increasing plasma insulin-effects suggestive of reduced SNS tone [104]. The present investigation was undertaken with this same study population to assess the impact of this 4-month treatment with bromocriptine-QR on PBMC PO/PI status by evaluating the mRNA expression of a series of genes involved in or counter-responsive to a PO/PI state. These genes include those coding for endoplasmic reticulum (ER) stress response proteins, proteins responsive to a pro-oxidative environment (i.e., antioxidant gene expression), TLR cascade activity proteins, pro-inflammatory protein inducers, and pro-inflammatory adhesion molecules. Additionally, we investigated the impact of bromocriptine-QR on the plasma biomarkers of systemic pro-oxidative stress (thiobarbituric acid reactive substances (TBARS), nitrotyrosine), pro-inflammatory factors (IL-18, IL-6, MCP1, IL-1β, prolactin, CRP) and sympathetic tone (norepinephrine, normetanephrine) to complement these PBMC gene expression studies in order to gain a more complete picture of the whole-body PO/PI mechanisms that could explain the CVD protective effect of this anti-diabetes therapy. A more detailed explanation of the rationale of the specific PBMC mRNAs of the ER stress and TLR activation pathways and plasma factors examined in this study are provided in the Methods section.
2. Results
2.1. Baseline Characteristics of Study Subjects
The baseline demographic and metabolic characteristics of the study subjects are presented in Table 1.

Table 1.
Baseline characteristics.
The study utilized open recruitment for the T2D subjects but due to the high Hispanic population in the vicinity of the study site (San Antonio, TX, USA) and the high rate of T2D among Hispanics, the enrollment population resulted in being all Hispanic.
2.2. Bromocriptine-QR Effect on Plasma Levels of SNS Tone Markers, Pro-Oxidative Stress Markers, and Pro-Inflammatory Factors
Following 4 months of timed (within 2 h of waking in the morning) daily bromocriptine-QR therapy, the fasting plasma levels of norepinephrine decreased by 33% (from 499 to 336 pg/mL, p < 0.001), and those of normetanephrine decreased by 22% (from 56.5 to 44.3 pg/mL, p < 0.02). Such treatment reduced the plasma systemic oxidative stress marker nitrotyrosine by 13% (from 214 to 187 nmol/L, p < 0.03) and its related downstream oxidative stress marker TBARS by 10% (from 10.2 to 9.2 μM/L MDA, p < 0.05 1-tailed) (Figure 1).
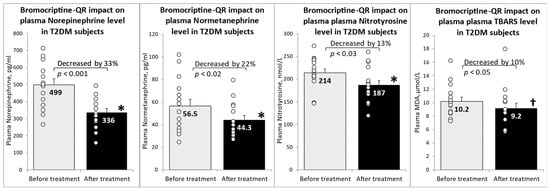
Figure 1.
Effect (change from baseline) of 4 months of bromocriptine-QR treatment on plasma markers of elevated sympathetic tone and oxidative stress. * Significant, 2-tailed t-test; † Significant, 1-tailed t-test.
Furthermore, bromocriptine-QR therapy reduced the plasma levels of the pro-inflammatory factors MCP1 (by 13% from 284 to 246 pg/mL, p < 0.05), IL-1β (by 12% from 141 to 124 pg/mL, p < 0.04), prolactin (by 42% from 13.0 to 4.6 ng/mL, p < 0.02), IL-18 (by 21% from 343 to 270 pg/mL, p < 0.03), and CRP (by 45% from 5.2 to 2.9 mg/L). There was a non-significant trend toward a reduction in plasma IL-6 levels following bromocriptine-QR treatment (by 27% from 8.5 to 6.2 pg/mL, NS). However, among subjects with an elevated baseline lL-6 level (>6 pg/mL), the plasma IL-6 level decreased by 44% (from 11.1 to 6.2 pg/mL, p < 0.02) (Figure 2).
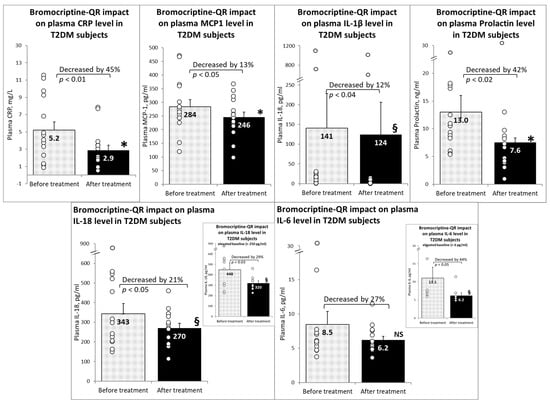
Figure 2.
Effect (change from baseline) of 4 months of bromocriptine-QR treatment on plasma inflammatory markers. * Significant, 2-tailed t-test; § Significant, Wilcoxon signed rank test; NS, Not Significant.
2.3. Effects of Bromocriptine-QR Therapy on mRNA Expression of PBMC ER Stress-Associated Unfolded Protein Response Genes
Bromocriptine-QR therapy reduced the expression of GRP78/BiP, which acts as the initiation protein for the ER-unfolded protein response via the release and thus activation of its tethered stress sensor proteins PERK, IER1, and ATF6 by 34% (p < 0.002), as well as the gene expression levels of the major downstream signal transduction amplifiers of the ER stress response responsible for maintaining cell viability: EIF2α, ATF4, and XBP1 by 32% (p < 0.01), 29% (p < 0.01), and 25% (p < 0.04), respectively. Similarly, the ER stress response survival protein BECN1, responsible for ER stress-induced autophagy activation, and PIN1, responsible for DNA repair and inflammation activation, were reduced by bromocriptine-QR by 23% (p < 0.04) and 14% (p < 0.005), respectively. However, such treatment was without an effect on the levels of the GRP78-tethered activators PERK (4% decrease, NS) or ATF6 (11% decrease, NS) or their downstream target transcription factors MAPK8/JNK (1% decrease, NS) and CHOP (+4%, NS)- pathways largely responsible for the initiation of cellular apoptosis (Figure 3).
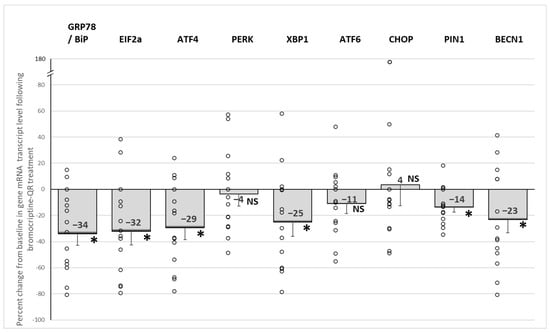
Figure 3.
Effect (change from baseline) of 4 months of bromocriptine-QR treatment on PBMC ER stress-associated unfolded protein response gene mRNA level. * Significant, 2-tailed t-test; NS, Not Significant.
2.4. Effect of Bromocriptine-QR Therapy on mRNA Expression of PBMC Oxidative Stress Response (Anti-Oxidant) Genes
Bromocriptine-QR therapy reduced the mRNA expression of the master oxidative stress response (antioxidant) gene regulators, NRF2, OXR1, and NQO1 by 32% (p < 0.005), 31% (p < 0.006), and 39% (p < 0.02), respectively. Likewise, such treatment also reduced the target oxidative stress response genes of these master antioxidant gene regulators, including SOD1 (by 52%, p < 0.0001), CAT (by 26%, p < 0.02), GPX1 (by 33%, p < 0.01), and GPX4 (by 31%, p < 0.01), without an effect on either SOD2 (9% decrease, NS) or GSR (9% decrease, NS). Consistent with this drug-induced reduction in the oxidative stress response gene expression, the expression of the two major oxidative stress response genes, GCH1 (by 30%, p < 0.01) and HMOX1, were also reduced (by 40%, p < 0.001) (Figure 4).
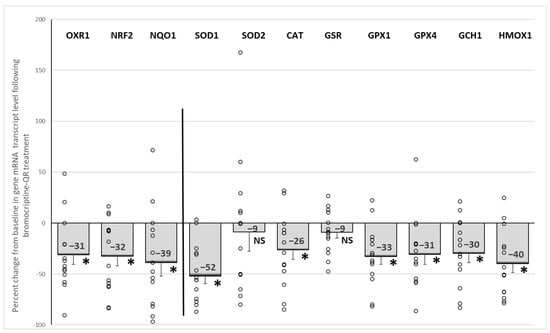
Figure 4.
Effect (change from baseline) of 4 months of bromocriptine-QR treatment on the PBMC oxidative stress response (antioxidant) gene mRNA level. * Significant, 2-tailed t-test; NS, Not Significant.
2.5. Effect of Bromocriptine-QR Therapy on mRNA Expression of PBMC Pro-Inflammatory Genes
Bromocriptine-QR therapy reduced the mRNA expression of the damage-associated molecular pattern (DAMP)-activated pro-inflammatory pathway initiators TLR2 (by 46%, p < 0.001) and TLR4 (by 20%, p < 0.05), without an effect on TLR10 (5% decrease, NS). The treatment also reduced the mRNA expression of the TLR downstream pro-inflammatory gene signaling pathway proteins GSK3β (by 19%, p < 0.03), NFkB (by 33%, p < 0.03), TXNIP (by 18%, p < 0.05, 1-tailed), and NLRP3 (by 32%, p < 0.003), as well as the expression of the PBMC pro-inflammatory initiation receptors, CCR2 (by 24%, p < 0.02) and GCR (by 28%, p < 0.01) (Figure 5).
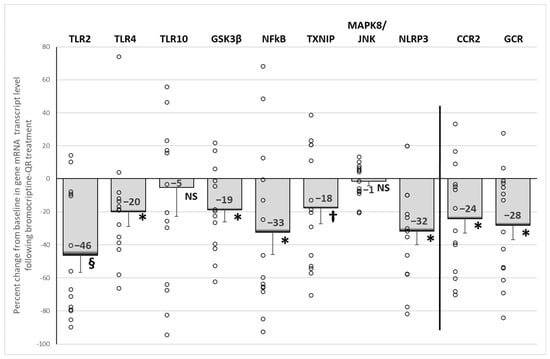
Figure 5.
Effect (change from baseline) of 4 months of bromocriptine-QR treatment on PBMC Proinflammatory gene mRNA level. * Significant, 2-tailed t-test; † Significant, 1-tailed t-test; § Significant, Wilcoxon signed rank test; NS, Not Significant.
2.6. Effect of Bromocriptine-QR Therapy on mRNA Expression of PBMC Secretory Adhesion Molecule Genes
Bromocriptine-QR therapy reduced the mRNA expression of the secretory adhesion molecule genes: L-selectin (SELL, by 35%, p < 0.02) and vascular cell adhesion molecule 1 (vCAM, by 24%, p < 0.02), without an effect on intercellular adhesion molecule 1 (iCAM, 3% decrease, NS), Figure 6.
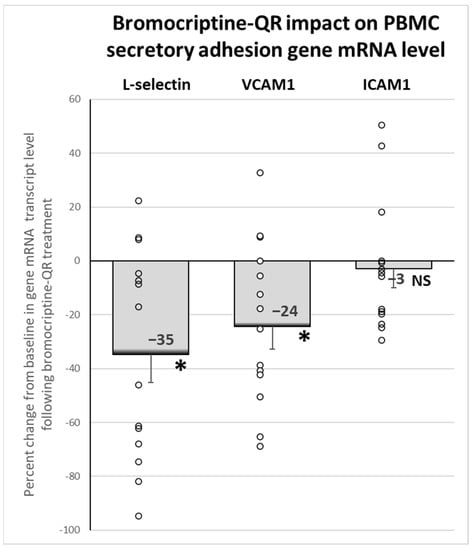
Figure 6.
Effect (change from baseline) of 4 months of bromocriptine-QR treatment on PBMC Secretory Adhesion Molecule gene mRNA level. * Significant, 2-tailed t-test; NS, Not Significant.
There was a significant positive correlation between the change in the plasma total measured catecholamines (norepinephrine plus normetanephrine) level and the change in the PBMC NLRP3 expression (Pearson’s r = 0.75, p = 0.002) and the change in the EIF2α expression (Pearson’s r = 0.56, p = 0.047) from baseline to week 24 of bromocriptine-QR therapy. However, there was no significant such correlation between the change in the plasma norepinephrine plus normetanephrine level and the PBMC NRF2 expression. There was a negative correlation between the change from baseline in fasting RHI and the change in HbA1c (Kendall’s tau-b, τb = −0.42) (i.e., reduction in HbA1c correlates with a reduction in endothelial dysfunction), which was statistically significant (p = 0.041).
2.7. Adverse Effects
The adverse effects were as previously described in [104]. Cycloset was generally well tolerated. The Cycloset dose was decreased to 2.4 mg/day for symptoms of either orthostatic hypotension, lightheadedness, nausea, or vomiting in three patients (20% of the study population).
3. Discussion
This study is the first to demonstrate that bromocriptine-QR treatment of T2D subjects is associated with a widespread reduction in the expression of PBMC genes associated with an active systemic pro-oxidative/pro-inflammatory state. Bromocriptine-QR treatment for 4 months caused a simultaneous reduction in the ER stress genes, oxidative stress response genes, and TLR pathway proinflammatory genes, concurrent with a reduction in the plasma biomarkers of oxidative stress and inflammation and sympatholytic effects to reduce plasma norepinephrine and normetanephrine levels in T2D subjects. The effect was observed in a mixed-study population of T2D males and females (including post-menopausal females). To our knowledge, this is the first demonstration of these cumulative effects in response to any anti-diabetes agent.
Bromocriptine-QR therapy reduced the gene expression of several PBMC PO/PI signature genes including the ER stress-response proteins GRP78, XBP1, EIF2α, and ATF4 that function as part of the unfolded protein response (UPR) to alleviate ER (oxidative) stress [89,102,105,106,107]. However, such treatment did not influence the PERK→CHOP, ATF6→CHOP, or ER stress→JNK gene expression levels that couple to apoptosis [105], suggesting the presence of a chronic low-grade, pro-inflammatory, cellular/ER stress response that was attenuated by the bromocriptine-QR therapy. It should also be noted that sustained low-grade ER stress activates not only the pro-inflammatory pathway [108] but also autophagy to compensate for the increased misfolded protein degradation exemplified by the increased BECN1 gene expression. BECN1 is a major autophagy activator gene whose increased expression is associated with the ER stress response [109,110,111,112] and its expression was also reduced by bromocriptine-QR therapy. Sustained low-grade cellular oxidative stress can lead to genomic damage that induces increased expression of PIN1 to initiate DNA repair and a pro-inflammatory response in immunocytes [113]; PIN1 expression was also reduced by bromocriptine-QR therapy. Activation of the ER UPR also induces the expression of antioxidant genes to assist with the refolding of unfolded/misfolded proteins and manage the increase in cellular ROS. Bromocriptine-QR treatment also reduced the mRNA expression of both the master antioxidant gene regulators, OXR1, NRF2, and NQO1, as well as multiple down-stream antioxidant target genes including SOD1, CAT, GPX1, GPX4, GCH1 (that act collectively to combat cellular elevations in ROS), and HMOX1 that are elevated in metabolic syndrome conditions [114,115,116,117,118,119,120,121,122,123,124,125,126]. HMOX1 is an antioxidant that is induced by ROS and exerts protective effects against PBMC inflammation, macrophage foam cell formation, vascular inflammation, and dendritic cell-facilitated atherogenesis [123,124]. Collectively, the bromocriptine-QR-induced reductions in these antioxidant gene expressions indicate that bromocriptine-QR alleviates an elevated oxidative stress environment known to exist in cardiometabolic diseases such as T2D [76,77,80,81,82,83,84,85,86,89,101,102,105,106,107,127,128].
In addition to the reduction in chronic PBMC cellular and ER oxidative stress states, bromocriptine-QR also decreased both ER stress-associated and TLR-induced pro-inflammatory pathway proteins. As discussed above and in the Methods section, ER stress UPR proteins in PBMC can induce and activate the pro-inflammatory pathway proteins including TLR2, TLR4, GSK3β, NFkB, TXNIP, NLPR3, BECN1, PIN1, CCR2, and GCR. These same pro-inflammatory pathway proteins also are induced via TLR activation by DAMPs, which originate from the oxidation of cellular and extracellular matrix proteins. DAMPs stimulate the plasma membrane TLR 2 and 4 receptors that, via multiple parallel pathways including the activation of GSK3β, induce NFkB leading to increased expression of TXNIP and NLRP3, whose interaction allows the construction of the NLRP3 inflammasome that increases the production of several inflammatory cytokines (e.g., IL-1B and IL-18) and the secretion of extracellular adhesion molecules (L-selectin, vCAM, iCAM, and others) that stimulate monocyte trans-endothelial migration and promote tissue damage [37,43,82,108,127,129,130,131,132,133,134,135]. Importantly, the downstream molecular intermediates of the ER stress UPR that activate these pro-inflammatory proteins (e.g., GSK3β, NFKB, TXNIP, and NLRP3) also cross-activate these same pro-inflammatory targets within the TLR pathway (e.g., ATF4 and XBP-1-mediated) [136,137] (see Figure 7).
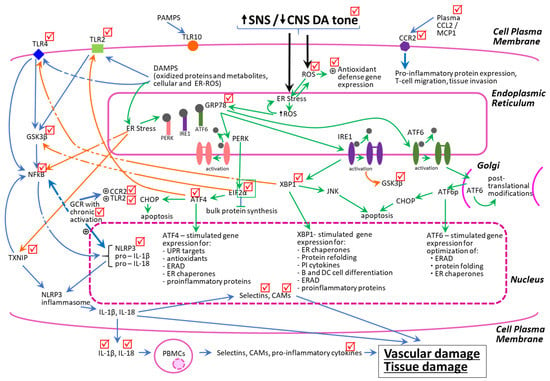
Figure 7.
Schematic representation of the interacting dynamics of PBMC pro-oxidative and pro-inflammatory-state biochemical pathways. The check marks represent molecular sites of bromocriptine-QR action to reduce gene expression or plasma levels of pro-oxidative/pro-inflammatory factors. The green arrows represent ER stress-related actions, the blue arrows represent TLR-initiated pro-inflammatory pathways, and the orange arrows represent crosstalk activities between the ER stress and TLR-activated pathways leading to potentiation of vascular disease. All arrows indicate stimulation of gene expression and/or protein synthesis/activation of the target.
Thus, under the conditions of chronic ROS stress to the PBMCs (resulting from elevated SNS tone, diminished CNS dopaminergic activity, the MS milieu, and diabetes), a positive reverberating feedback loop is created between the ER stress UPR and TLR inflammation pathway, which produces and maintains a low-grade systemic inflammatory state leading to vascular and tissue damage. Remarkably, bromocriptine-QR therapy simultaneously reduced the PBMC mRNA expression levels, not only of the above-mentioned ER stress proteins and oxidative stress response proteins, but also of the pro-inflammatory pathway proteins, TLR2, TLR4, GSK3β, NFkB, TXNIP, NLPR3, BECN1, and PIN1, and the adhesion molecules, L-selectin and vCAM, that these pathways induce. Bromocriptine-QR therapy did not, however, reduce TLR10 expression, which is an orphan receptor that is activated by pathogen-associated molecular patterns (PAMPs) and assists in the innate immune response to infection. Lastly, the stimulation of the PBMC CCR2 receptor by circulating/paracrine/autocrine CCL2 (MCP-1) is a potent stimulus for the PBMC pro-inflammatory activation and induction of adhesion molecule synthesis and secretion, whereas the nuclear GC receptor (GCR) is upregulated in response to the chronic pro-inflammatory state [129,130,131,132,133]. Moreover, in a chronic pro-inflammatory condition, GCR activation by corticosteroids potentiates further pro-inflammatory processes [129,130,131,132]. Bromocriptine-QR treatment reduced the mRNA expression of both of these signature pro-inflammatory receptors (CCR2 and GCR), as well as the above-mentioned PBMC adhesion molecules (Figure 7).
Bromocriptine-QR induced widespread decreases in PBMC PO/PI gene expression, which were coupled with reductions in plasma levels of the pro-inflammatory chemokine MCP-1/CCL2, cytokines IL-1B and IL-18, and the pro-inflammatory hormone prolactin, elevations of which are associated with accelerated cardiometabolic disease [47,138,139,140,141,142,143,144,145,146,147,148,149,150]. Consistent with these treatment-induced reductions in PBMC and plasma pro-inflammatory genes and factors, respectively, bromocriptine-QR therapy also reduced the plasma CRP level by 45%, an indicator of low-grade systemic inflammation and a potential predictor of CVD, especially in T2D patients [151,152,153]. Also, bromocriptine-QR treatment reduced the plasma markers of the systemic oxidative stress- nitrotyrosine and TBARS. In metabolic syndrome, MCP-1, IL-18, and IL-1B are elevated and stimulate pro-inflammatory actions of tissue neutrophils and macrophages in vascular endothelial and smooth muscle cells. Bromocriptine-QR treatment reduced both the plasma MCP-1/CCL2 level and the expression of its PBMC receptor, CCR2, suggesting a dual inhibitory action on this inflammatory pathway. Although these plasma factors are influenced by several environmental conditions (such as diet, psychosocial stress, circadian phase—time of day, and others) that can cause within-group variation [154,155,156], bromocriptine-QR treatment nonetheless caused a significant reduction in their plasma levels.
As described above, numerous clinical and preclinical studies have documented that a PBMC and systemic pro-oxidative/pro-inflammatory condition typifies the T2D state and contributes to the cardiometabolic pathology of the disease [84,87,88,89,90,91,92,93,94,95,96,97,98,99,100,103,157,158,159,160,161,162,163,164]. The chronic metabolic syndrome milieu of T2D, including glucolipotoxicity, insulin resistance, and dyslipidemia, in turn, feeds back to potentiate the immune system’s PO/PI state, and, as the cycle churns, it precipitates damage to organs and the cardiovascular system (endothelial dysfunction, arteriosclerosis, atherosclerosis, fibrosis, myocardial infarction, heart failure) [14,37,38,40,41,42,44,46,47,48,49,76,77,78,79,80,81,82,83,84,85,86]. An external engine that can drive the rate of this cycle is an altered autonomic regulation typified by a simultaneous elevation of sympathetic tone and a CNS hypodopaminergic state. As discussed above, the increased SNS/decreased CNS dopamine activity stimulates the PO/PI state [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] and aggravates the MS milieu [1,165] increasing the PO/PI—MS—CVD cycle rate (see Figure 8).
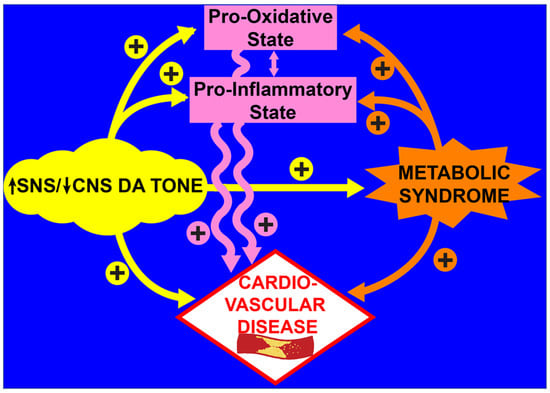
Figure 8.
Schematic of interacting, parallel, and reinforcing pathways of increased SNS and decreased CNS dopaminergic tone in the development of cardiovascular disease.
It should be emphasized that these observed effects of elevated SNS/hypodopaminergic CNS activity can be manifested via alterations of the direct binding of norepinephrine and dopamine to the norepinephrine and dopamine receptors on PBMCs, as well as via their indirect CNS effects on the components of the (circadian) neuroendocrine axis. Circadian-timed treatment with the sympatholytic dopamine D2 receptor agonist, bromocriptine-QR, simultaneously decreased plasma norepinephrine and normetanephrine levels in association with declines in plasma pro-inflammatory factors, biomarkers of systemic oxidative stress, PBMC ER stress, the oxidative stress response, and inflammatory gene expression. This response to sympatholytic, dopamine agonist therapy in T2D subjects is not surprising given that previous studies have identified elevated sympathetic tone and decreased central nervous system dopaminergic activity as potent inducers of systemic immune inflammation at multiple tissue sites including the liver, kidney, vasculature, and heart [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. In fact, the current study findings indicate a significant positive correlation between the bromocriptine-QR-induced reduction in plasma total measured catecholamines (norepinephrine plus normetanephrine) (in agreement with its sympatholytic activity) and its reduction in the PBMC gene expression of the ER stress-response gene, EIF2α, and also of the penultimate TLR and ER stress-responsive initiation factor for pro-inflammatory biochemical events- NLRP3 in these study subjects. This effect was independent of weight or BMI changes, neither of which were altered by treatment in these study subjects [104].
Although the elevated sympathetic tone and decreased CNS dopaminergic activity are both drivers of hypertension, insulin resistance, postprandial hyperglycemia, and hypertriglyceridemia [1,165], which can contribute to increased CVD risk, available evidence indicates a substantial impact of the pro-oxidative/pro-inflammatory state in the development of CVD as described above. It follows, therefore, that the anti-inflammatory effect of bromocriptine-QR in T2D is likely to be a major contributor to the reduction in CV events observed with this therapy in T2D subjects [3,4,5,6]. Vascular inflammation contributes to endothelial dysfunction and is an established pathophysiologic abnormality that plays an important role in the development of CVD. In a separate study with this current subject group, bromocriptine-QR therapy did, in fact, improve endothelial dysfunction [104]. Additionally, immune inflammation is a significant contributor to heart failure, ischemic reperfusion injury, and peripartum cardiomyopathy, each of which has been shown to be improved by bromocriptine treatment [73,74,75,135,166,167,168,169,170,171,172,173,174].
Importantly, in preclinical studies of vascular disease, circadian-timed bromocriptine administration to reinstate the natural circadian peak in CNS dopaminergic activity at waking from daily sleep, which is reduced in insulin-resistant states (and causative in its genesis) [1,175], reduced aortic enzymes that potentiate the cellular generation of ROS and endothelial NOS uncoupling, two vascular pathophysiological events induced by a local pro-inflammatory environment and that contribute to endothelial dysfunction and CVD [11]. However, this bromocriptine effect was absent when administered outside of the natural CNS dopaminergic activity circadian peak window [11]. Preclinical studies have located a major aspect of the biological clock for the circadian rhythm of CNS dopaminergic activity to reside in dopaminergic neurons projecting from the supramammillary nucleus to the suprachiasmatic nucleus (SCN) [11,175,176,177,178,179,180,181]. The loss of the natural circadian peak of CNS dopaminergic activity at the SCN clock via direct neuronal neurotoxin micro-administration to the SCN area or high-fat feeding results in an elevated sympathetic tone that causes marked insulin resistance, glucose intolerance, and loss of hypothalamic glucose sensing, which is necessary for appropriate postprandial glucose disposal [178,180]. Further, reinstatement of this circadian peak of CNS dopaminergic activity in high-fat-fed animals by either appropriately circadian-timed direct dopamine administration to the SCN area or systemic bromocriptine administration restores normal glucose metabolism and sympathetic tone in these insulin-resistant animals but not when such administrations are made outside of the natural CNS dopaminergic circadian peak activity window [178,180]. Likewise, bromocriptine administration to obese insulin-resistant humans in the morning (time of the natural circadian peak of CNS dopaminergic activity at waking) improves postprandial insulin resistance but not so when it is administered in the evening [182]. Multiple studies have identified alterations in whole-body circadian rhythmicity associated with (and that may drive) CVD [183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199]. It may be that the “resetting” towards normal of CNS circadian dopaminergic aberrations that via the neuroendocrine axis resultantly reduces elevated SNS tone and improves metabolism [11,175,181,200,201,202] also resultantly resets towards normal those aberrant circadian immune activities that potentiate the PO/PI state. Consequently, both neurometabolic and neuroimmune responses to circadian timed dopaminergic stimulation may work in concert to drive/participate in the improvement in the immune PO/PI state and its cardiometabolic sequelae in T2D. This postulate is worthy of further investigation. In this regard, it should be noted that the present study’s effects of circadian-timed bromocriptine-QR therapy may be the consequence of an influence (via the circadian neuroendocrine axis) that directly reduced the PBMC PO/PI gene expression at the time of cell harvest (in the morning after an overnight fast) or an influence of a phase shift of the circadian rhythm of PBMC PO/PI gene expressions so that the trough (or otherwise lower) level in these daily gene expressions now occurred at this time of harvest (i.e., the treatment moved the PBMC PO/PI response rhythm out of phase with the neuroendocrine stimulus rhythms for inflammation to reduce the PO/PI gene expressions at the morning time of the cell harvest). It is also possible that, given the established circadian rhythm of PBMC gene expressions and activities [203,204,205,206], the anti-inflammatory bromocriptine-QR effect delineated herein underestimated its true effect as a consequence of circadian PBMC harvest time (i.e., harvest was not at the daily peak of the PO/PI gene expressions prior to treatment). Further studies are required and warranted for establishing the relationships between the circadian time of bromocriptine-QR administration and the drug’s influence on the circadian rhythms of PBMC PO/PI gene expressions and pro-inflammatory phenotypes.
The present study has several limitations including the relatively small sample size, absence of a placebo-treated T2D group, and lack of an NGT group with which to compare the baseline values. Moreover, the study population was entirely of Hispanic ethnicity, a group known to have increased rates of both T2D and vascular complications associated with T2D relative to the Caucasian population [207], and this may have affected the magnitude of the bromocriptine-QR response on the PO/PI state. However, it is well documented that T2D is a PO/PI state that potentiates CVD across ethnic populations. Further, even with the modest sample size, bromocriptine-QR caused significant reductions in a wide range of bona fide pro-oxidative/pro-inflammatory parameters and functions that were associated with a significant improvement in endothelial dysfunction previously reported in the same study subjects [104]. In as much as the present study findings are consistent with numerous previous studies reporting a pro-inflammatory role for elevated sympathetic tone and decreased CNS dopaminergic activity, the present results suggest that a larger, controlled study of the involvement of anti-oxidative/anti-inflammatory actions of circadian-timed bromocriptine-QR in improving the cardiometabolic status of T2D subjects across ethnic populations is warranted.
4. Materials and Methods
4.1. Study Design and Therapeutic Intervention Regimen
Description of the T2D study patients and study design of the therapeutic intervention with bromocriptine-QR has been previously described in detail [104]. Briefly, 15 Hispanic (11 females [8 of whom were post-menopausal at baseline]; 4 males) T2D subjects whose glycemia was poorly controlled (HbA1c > 7.5%) with a stable dose of liraglutide (1.2–1.8 mg/d; n = 15) plus metformin (n = 12) or low-dose glargine insulin (n = 3) comprised the study population. Subjects had a mean (±SEM) age = 57 ± 9 years, BMI = 33.4 ± 4.4 kg/m2, A1c = 8.3 ± 0.5%, and diabetes duration = 10.2 ± 5.6 years. Body weight (88 ± 13 kg) was stable (±3 lbs) for the 3 months prior to the study. Only subjects with a daytime feeding/night-time sleeping schedule were included. All subjects followed a standard ADA-recommended diet for diabetes, did not participate in any excessively heavy exercise programs, and were not taking any medications known to alter glucose metabolism (with the exception of metformin and insulin). Individuals with evidence of major organ system disease, diabetic proliferative retinopathy, and symptomatic neuropathy, as determined by physical examination, history, and screening laboratory tests, were excluded. Subjects were not taking any medications that would alter neurosynaptic function. Major exclusion criteria included individuals with evidence of major organ system disease, diabetic proliferative retinopathy, and symptomatic neuropathy. Subjects continued to take their normal pre-study dose of liraglutide plus either metformin or insulin throughout the study. Subjects were instructed to start Cycloset at a dose of 0.8 mg/day in the morning within two hours of waking. After 1 week, the daily Cycloset dose was increased by 0.8 mg in each subsequent week until the maximum dose of 3.2 mg once daily was reached after 4 weeks. In three patients, the final Cycloset dose was decreased to 2.4 mg/day for the symptoms of orthostatic hypotension, lightheadedness, nausea, and vomiting. Baseline blood samples for plasma analyses and PBMC isolation/analyses were taken in the morning after an overnight fast before and 4 months after initiation of bromocriptine-QR therapy. The 4-month sample size was obtained in the morning before that day’s dose of bromocriptine-QR was ingested. The study was approved by the University of Texas Health Science Center at San Antonio (UTHSCSA) IRB and informed written consent was obtained from all participants prior to the study. All clinical study procedures were conducted at the Clinical Research Center at the Texas Diabetes Institute (TDI), University Hospital System.
4.2. Rationale for PBMC Analyses of Specific Gene mRNA Expression Levels as Assessment of the PO/PI State in T2D
The ER is a complex organelle that is involved in protein biogenesis, appropriate tertiary structure acquisition (folding), and trafficking within the cell (proteostasis). ER proteins and small molecules function in concert, in large part via oxidation-reduction reactions, to maintain an ER environment that is favorable for the execution of its proteostasis functions. However, pathophysiological stresses present in individuals with MS (including hyperglycemia, elevated plasma FFA levels, oxidized LDL, triglycerides, and other biomolecules), as well as increased intracellular reactive oxygen species (ROS) from chronically elevated SNS stimulation, mitochondrial dysfunction, and an overactive ER, disrupt the ER machinery and cause ER stress. This ER stress is characterized by the accumulation of unfolded and misfolded proteins within the ER that trigger the canonical unfolded protein response (UPR) [89,102,105,106,107]. The UPR functions to slow protein synthesis, increase unfolded/misfolded protein degradation, and correct misfolded protein structure. However, if the UPR cannot keep pace with the level of protein misfolding, an ER redox imbalance-driven process ensues and stimulates the expression of pro-inflammatory genes, accelerates autophagy, and causes cellular apoptosis. Since the UPR itself is ROS-generating, as it escalates in the presence of the metabolic syndrome milieu or direct SNS noradrenergic stimulation, a vicious self-sustaining cycle of ER stress and pro-inflammatory protein secretion is manifested [89,102,105,106,107]. The ER stress within PBMCs contributes to their PO/PI phenotype that potentiates local and systemic vascular disease and CVD [166,167,208,209,210,211,212,213] and insulin resistance [76,102,214,215,216].
Overactivation of certain cellular toll-like receptors (TLRs) can potentiate the PBMC PO/PI phenotype. TLRs are a family (TLRs 1–10) of the plasma membrane and endosomal pattern recognition receptors that on immune cells activate several arms of the innate immune defense response against pathogens [217,218]. TLR overactivation by PAMPs, such as bacterial or viral particles [217,218,219,220], or by endogenous damage/danger-associated molecular pattern (DAMP) molecules (heat-shock proteins, fatty acids, oxidized phospholipids, oxidized LDL, extracellular matrix breakdown products, nucleic acid fragments, intracellular oxidized molecules, ROS) [80,221,222] activate the TLR signaling cascade to express and activate downstream potent pro-inflammatory effectors including glycogen synthase kinase 3 beta (GSK3β), nuclear factor kappa-B p65 subunit (NFkB), thioredoxin interacting protein (TXNIP), and the NLR family pyrin domain-containing protein 3 (NLRP3) inflammasome (collectively termed sterile inflammation). Chronic activation of these TLR response pathways results in inflammatory cytokine and adhesion molecule production and secretion to potentiate vascular and tissue inflammation, promoting CVD [80,217,218,219,220,221,222,223,224]. Such chronic DAMP stimulations of PBMC TLR 2 and 4 can also potentiate the metabolic syndrome itself [80,221,222]. Importantly, there is extensive crosstalk between the ER stress-response and TLR activation pathways such that the transcription factors and enzymes of each of these pro-inflammatory pathways can cross over and stimulate the other pro-inflammatory pathways at various points to amplify the overall inflammatory response to accelerate cardiovascular disease [136,137].
The PBMC analyses in the present study [104] were performed on quickly frozen (in liquid nitrogen) cell samples. In vivo, the activation state of the enzymes and redox fluxes of biomolecules in their cellular micro-environments are rapidly changing and difficult to quantitate [225,226] and such measurements from harvested, processed, and frozen tissues are inappropriate. The PBMC transcriptome reflects the system-wide inflammatory state, including the effect of changes in response to pharmaceutical intervention [227,228,229]. Consequently, a realistic evaluation of the sustained changes in the redox status in frozen cell samples is best obtained from the measures of the gene expression levels for long-lived proteins, which participate in or are responsive to a sustained PO/PI cellular status, including ER stress proteins and ROS-responsive anti-oxidative proteins, which, in composite, can provide an overview of the impact of drug interventions toward or away from a chronic pro-oxidative/pro-inflammatory pathophysiological condition [77,80,84,85,164,230,231,232,233]. Cellular inflammatory responses to chronic oxidative stress that potentiate CVD are long-lived and can be assessed by measures of PBMC pro-inflammatory protein gene expression [76,77,78,80,82,101,136,137,222]. Several critical elements of this cellular PO/PI gene response system have been delineated and the present study evaluates the PBMC expression level of select mRNAs for several proteins established to be critically involved in intersecting the pathways of the cellular redox balance and inflammatory status. These proteins include several ER stress-response proteins (GRP78, EIF2α, ATF4, XBP1, ATF6, PERK, CHOP, BECN1, PIN1), master regulators of oxidative stress-response proteins (NRF2, OXR1, NQO1), downstream key oxidative stress-response [antioxidant] enzymes (SOD1, SOD2, GSR, NOS2, CAT, GPX1, GPX4, GCH1, HMOX1), protein activators of the TLR inflammatory pathway (cell membrane and nuclear receptor activators of inflammation, master pro-inflammasome transcription factors, and inflammatory cytokine transcription factors including TLR2, TLR4, TLR10, GSK3β, NFkB, MAPK8/JNK, TXNIP, NLRP3, CCR2, GCR), and pro-inflammatory cellular/tissue adhesion molecules (SELL, VCAM1, ICAM1) to derive a composite fingerprint of the cellular redox/inflammatory state before and after 4 months of bromocriptine-QR therapy. Under the conditions of chronic low-grade inflammation from a systemic oxidative stress environment, PBMCs exhibit ER stress, which is exemplified by an increase in the stress sensor, chaperone protein GRP78, that upon increased cellular redox stress releases the three bound protein activators of the ER stress response—PERK, IRE1, and ATF6. PERK phosphorylates EIF2α, which in turn (i) induces ER actions to handle the increased workload by slowing bulk protein synthesis and (ii) activates ATF4 to initiate ER-associated misfolded protein degradation activity, autophagy, antioxidant gene expression, and UPR gene expression all to potentiate cell survival. The IRE-1 protein functions via the induction and activation of XBP1 to upregulate mRNAs of the UPR, stimulate autophagy, and activate the inflammatory response to ER stress. The ATF6 protein is functionally associated with improved protein folding, post-translational modifications, and translocation to and from Golgi, but also activates XBP1 resulting in pro-inflammatory gene expression [89,105,106,107,108,230,234,235]. ER stress elements, including EIF2α, ATF4, and XBP1 (and TLR activation cascade pathways) can induce and activate GSK3β, which acts as a positive regulator of the inflammatory process by inducing multiple transcription factors (e.g., mitogen-activated protein kinase 14, p38, JNK1/2, c-Jun, ATF-2, and/or NFkB) and increasing the production of pro-inflammatory cytokines including IL-1B and IL-18 [105,136,137,236,237]. In response to sustained ER stress, beclin 1 (BCN1) protein is overexpressed and augments autophagy to alleviate the buildup of misfolded proteins [109,110,111,112] in order to potentiate cell survival. Also, upon sustained ER stress in immunocytes, PIN1, a proline-associated isomerase whose over-expression is associated with a range of diseases, is upregulated and promotes inflammatory cytokine expression and apoptosis (reviewed in ref [113]). Although increased local and intracellular ROS can initiate cellular ER stress, the ER stress response also generates ROS. Thus, under the conditions of sustained low-grade local ROS increase, PBMCs respond by upregulating antioxidant pathways [89,102,105,106,107]. NRF2 (including its immediate target NQO1) and OXR1 act as master transcription factors for the downstream induction of multiple antioxidant genes in response to ROS increases and ER stress [114,115,116,117,118,119]. The downstream ROS-response antioxidant gene whose expression is increased are (i) SOD1 and 2 as well as CAT that decrease ROS and the long-lived pro-oxidant H2O2, respectively, and (ii) the glutathione-dependent-reducing enzymes GSR, GPX1, and GPX4 that reduce elevated levels of H2O2, ROS, and lipid peroxidation levels and thus combat ROS-induced inflammation and apoptosis [120,121,122]. HMOX1 is another potent antioxidant enzyme that is induced by ER stress and catalyzes the metabolism of plasma-free heme, a potent oxidizing agent that induces ER stress, to generate cytoprotective biliverdin, carbon monoxide, and ferrous ion. HMOX1 is upregulated by NRF2 in response to cellular (ER) oxidative stress and has potent anti-inflammatory and anti-apoptotic activities (reviewed in ref [123,124]). GCH1 functions as the rate-limiting enzyme in tetrahydrobiopterin synthesis and its expression is induced in response to the redox stress of elevated H2O2 to generate tetrahydrobiopterin, a cofactor for iNOS (NOS2) (and endothelial NOS) and subsequent NO production to inhibit an existing inflammatory condition [125,126].
The cellular PBMC sterile pro-inflammatory state (inflammation not induced by microbes) is driven largely via TLRs’ activation by DAMPs (damaged intracellular proteins, phospholipids, and extracellular matrix components), which are generated by local and intracellular oxidative stress reactions, including those emanating from the ER. TLRs can also be activated by dysregulated metabolites such as saturated fatty acids, oxidized LDL, oxidized phospholipids, homocysteine, and glucose [80,217,218,220,221,222]. Chronic activation of TLR2 and/or TLR4 by DAMPs initiates a cascade of parallel signal transduction reactions leading to the induction and activation of GSK3β and its downstream targets NFkB and NLRP3, which associate with TXNIP (induced by ER stress and increased cellular ROS) to form the NLRP3 inflammasome enzyme complex that generates pro-inflammatory cytokines including IL-1β and IL-18 [83,238,239,240,241,242,243,244]. Chronic elevation of these two cytokines stimulates the synthesis of the vascular immunocyte adhesion molecules (e.g., L-selectin, vCAM, iCAM), which potentiate immunocyte tissue invasion and migration [242,245,246,247,248,249]. The cell surface receptor CCR2, when activated by binding to its circulating ligand, CCL2/MCP-1, initiates PBMC pro-inflammatory cytokine production, migration, and tissue invasion, which leads to vascular and organ inflammatory damage (e.g., atherosclerosis, fibrosis, apoptosis, necrosis) [138,144,250,251,252,253,254]. The nuclear receptor GCR, when stimulated in response to acute stress, acts as a potent anti-inflammatory signal transducer to reduce inflammatory cytokine production. However, if overactivated by its ligand corticosteroid hormones, GCR can increase CCR2, TLR2, and NLRP3 expression to support a pro-inflammatory phenotype under chronic stress conditions such as MS and T2D [129,130,131,132,133]. In total, such vascular inflammation activation from systemic/PBMC pro-oxidative stress augments vascular endothelial dysfunction, smooth muscle cell proliferation, arteriosclerosis, and cardiomyocyte damage/death [37,43,81,82,86,101,134,135,144,166,167,208,209,210,211,212,213,214,215,216,241,242,250,251,252,253,254]. Most importantly, the members of the UPR pathways of ER stress can activate the TLR pro-inflammatory cascade and vice-versa creating a self-sustaining pro-oxidative/pro-inflammatory condition, ultimately producing organ damage [136,137]. In insulin-resistant states such as T2D, the above PBMC ER stress, ROS-responsive antioxidant and pro-inflammatory pathways, and operative protein levels are elevated [37,38,39,40,41,76,77,78,79,80,81,84,85,98,102,103,115,134,144,157,158,159,160,161,162,163,164,214,215,216,222,230,231,239,241]. Figure 7 presents a schematic representation of the interacting dynamics of these PBMC pro-oxidative and pro-inflammatory pathways and activities and the impact of bromocriptine-QR thereupon.
4.3. Biochemical Assays of Blood Samples
Blood samples were collected in EDTA-preconditioned tubes for the isolation of plasma, which was used for analyses of all humoral analytes.
4.3.1. Assessment of SNS Tone Markers (Norepinephrine, Normetanephrine)
Plasma norepinephrine was determined with an ELISA kit (Cat# 17-NORHU-E01-RES, ALPCO, Salem, NH), and plasma normetanephrine was determined with an ELISA kit (Cat# NMN31-K02 Eagle Bio, Nashua, NH).
4.3.2. Assessment of Plasma Oxidative Stress and Pro-Inflammatory Markers
Plasma Thiobarbituric Acid Reactive Substances (TBARS) were determined with a TBARS assay kit (Cat# 700870, Cayman Chemicals, Ann Arbor, MI, USA). Plasma nitrotyrosine was determined with a nitrotyrosine assay kit (Cat# STA-305, Cell Biolabs, San Diego, CA, USA). Plasma IL-1b was determined with a ProQuantum immunoassay (Cat# A35574, ThermoFisher, Waltham, MA, USA). Plasma IL-6 and IL-18 were determined with a Legendplex immunoassay (Cat# 740118, BioLegend, San Diego, CA, USA). Plasma prolactin was determined with an ELISA kit (Cat# DPRL00, R&D Systems, Minneapolis, MN, USA). Plasma CRP level was assayed with an ELISA kit (Cat # DCRP00, R&D Systems, Minneapolis, MN, USA).
4.4. Assessment of PBMC Pro-Oxidative/Pro-Inflammatory Status
4.4.1. PBMC Isolation
Blood samples were collected from study subjects after an overnight fast at baseline and after 4 months of bromocriptine-QR therapy as described above. PBMN cells were isolated from 5 mL of EDTA-treated blood within two hours with Polymorphprep (Axis-Shield, Oslo, Norway) solution containing sodium diatrizoate 13.8% (w/v) and Dextran 500 8.0% (w/v) by centrifuging the samples layered over Polymorphprep at 450–500× g for 30–35 min in a swing-out rotor at 18–22 °C per the manufacturers’ instructions. PBMN cells were collected, washed in phosphate-buffered saline (PBS), and stored in liquid nitrogen at −196 °C before analysis.
4.4.2. PBMC Gene Expression Analysis by Real-Time qPCR—mRNA Extraction and PCR Methods including Primers
Total RNA was isolated from PBMN cells with Trizol Reagent (ThermoFisher, Waltham, MA, USA Cat# 15596026). One mL of Trizol Reagent was added to frozen PBMN cells, cells were homogenized, and total RNA was isolated per the manufacturer’s instructions and resuspended in RNAse-free water. Total RNA quantity and purity were determined by UV spectroscopy, and then genomic DNA was digested with a double-strand specific thermolabile DNase, and cDNA was synthesized using SuperScript IV VILO MasterMix with ezDNAse (ThermoFisher, Waltham, MA, USA Cat# 11766050) using 2 ug of total RNA per sample in a 20 μL reaction. Real-time qPCR was performed with a Taqman fast advance master mix (ThermoFisher, Waltham, MA, USA Cat# 4444964) on an AriaMX (Agilent, Santa Clara, CA, USA) qPCR instrument with the following primer/probe sets (ThermoFisher, Waltham, MA, USA):
ER Stress-Associated Unfolded Protein Response Genes
ATF4 (Activating Transcription Factor 4) Cat# Hs00909569_g1
ATF6 (Activating Transcription Factor 6) Cat# Hs00232586_m1
BECN1 (Beclin 1) Cat# Hs01007018_m1
BiP/GRP78 (Heat-Shock Protein Family A (Hsp70) Member 5) Cat# Hs99999174_m1
CHOP (DNA Damage Inducible Transcript 3) Cat# Hs01090850_m1
EIF2α (Eukaryotic Translation Initiation Factor 2, Subunit 1 Alpha) Cat# Hs00187953_m1
GSK3β (Glycogen Synthase Kinase 3 Beta) Cat# Hs01047719_m1
NFkB (NF-kappa-B p65 subunit) Cat# Hs01042014_m1
PERK (Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3) Cat# Hs00984003_m1
PIN1 (Peptidylprolyl Cis/Trans Isomerase, NIMA-Interacting 1) Cat# Hs01598309_m1
TXNIP (Thioredoxin Interacting Protein) Cat# Hs01006897_g1
XBP1 (X-Box Binding Protein 1 functional variant) Cat# Hs03929085_g1
Oxidative Stress Response (Antioxidant) Genes
CAT (Catalase) Cat# Hs00156308_m1
GCH1 (GTP Cyclohydrolase 1) Cat# Hs00609198_m1
OXR1 (Oxidation resistance gene 1) Cat# Hs00757847_g1
GPX1 (Glutathione peroxidase 1) Cat# Hs01028922_g1
GPX4 (Glutathione peroxidase 4) Cat# Hs00157812
GSR (Glutathione–Disulfide Reductase) Cat# Hs00167317
HMOX1 (Heme Oxygenase 1) Cat# Hs01110250_m1
NQO1 (NAD(P)H Quinone Dehydrogenase 1) Cat# Hs01045993_g1
NRF2 (Nuclear factor erythroid 2-related factor 2) Cat# Hs00975961_g1
SOD1 (Superoxide dismutase 1, cytoplasmic) Cat# Hs00533490_m1
SOD2 (Superoxide dismutase 2, mitochondrial) Cat# Hs00167309_m1
Pro-Inflammatory Genes
CCR2 (C-C Motif Chemokine Receptor 2) Cat# Hs00356601_m1
GCR (Glucocorticoid receptor) Cat# Hs00353740_m1
GSK3β (Glycogen Synthase Kinase 3 Beta) Cat# Hs01047719_m1
MAPK8/JNK (Mitogen-Activated Protein Kinase 8) Cat# Hs01548508_m1
NFkB (NF-kappa-B p65 subunit) Cat# Hs01042014_m1
NLRP3 (NLR Family Pyrin Domain-Containing Protein 3) Cat# Hs00918082_m1
TLR2 (Toll-like receptor 2) Cat# Hs00610101_m1
TLR4 (Toll-like receptor 4) Cat# Hs01060206_m1
TLR10 (Toll-like Receptor 10) Cat# Hs01675179_m1
TXNIP (Thioredoxin Interacting Protein) Cat# Hs01006897_g1
Secretory Adhesion Genes
ICAM1 (Intercellular Adhesion Molecule 1) Cat# Hs00164932_m1
SELL (L-selectin) Cat# Hs00174151_m1
VCAM1 (Vascular Cell Adhesion Molecule 1) Cat# Hs01003372_m1
Housekeeping Gene
RPS17 (Ribosomal Protein S17) Cat# Hs00734303_g1
Cq values were calculated with Agilent Aria software version 1.5. The relative expression of genes was calculated using the 2−ΔΔCq method as previously described [255]. Ribosomal Protein S17 (RPS17) has been shown to be a good reference gene for normalizing gene expression in PBMCs [256] and was used as the internal reference. Relative gene expression was calculated as a fold-change from the control samples.
4.5. Statistical Analyses
Statistical differences in the changes from the baseline of the PBMN gene expression and plasma biochemical parameters were first analyzed for normality with the Shapiro–Wilk test and then evaluated using a Student’s 2-tailed paired t-test for normally distributed data or Wilcoxon signed rank test for non-normal data unless noted otherwise in the Results section. A p value < 0.05 was accepted as statistically significant; results that were not statistically significant are labeled as not significant (NS). In certain instances, wherein a downstream target of a significantly affected gene or biochemical parameter was examined, a Student’s 1-tailed test was additionally employed to test for the significance of the same directional change. All data are expressed as the mean ± SEM of the study population. Statistical analysis was performed with SigmaPlot Version 14.5 (Systat, San Jose, CA, USA).
Correlation analyses were performed to evaluate the relationship between the change from baseline to week 24 of bromocriptine-QR treatment in the plasma total measured catecholamines (norepinephrine plus normetanephrine) level with such treatment changes in the expression of select PBMC genes representing key focal activation points for (a) the effector response to endoplasmic reticulum stress—(EIF2α), (b) the oxidative stress defense (master antioxidant gene transcription factor—NRF2), and (c) the TLR and ER stress-responsive penultimate initiation factor required for pro-inflammatory biochemical events (NLRP3). Also, the relationship between the changes from baseline to week 24 of bromocriptine-QR treatment in the fasting reactive hyperemia index (RHI) with such treatment changes in HbA1c was evaluated. Pearson’s correlation analyses were employed for normally distributed variables and non-parametric Kendal’s Tau-b analyses were employed for non-normally distributed data (change in HbA1c).
5. Conclusions
In conclusion, the present study findings of a circadian-timed bromocriptine-QR simultaneous reduction in sympathetic tone and an immune and systemic pro-inflammatory/pro-oxidative state provide potential biological mechanisms that can contribute to the cardioprotective effects of bromocriptine-QR therapy observed in T2D subjects [3,4,5,6].
Author Contributions
Conceptualization, A.H.C., E.C., R.C., B.C. and R.A.D.; methodology, A.H.C., E.C., R.A.D., M.E. and B.C.; investigation, M.A., M.E., C.A., J.A., C.T., E.C. and N.C.; formal analysis, A.H.C., E.C., M.E. and B.C.; data curation and validation, E.C., M.E. and A.H.C.; writing—original draft preparation, A.H.C., E.C. and R.A.D.; writing—review and editing, A.H.C., R.A.D. and M.E.; supervision, A.H.C., E.C. and R.A.D.; project administration, R.A.D. and A.H.C.; funding acquisition, A.H.C. and R.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by S2 Therapeutics, Inc. and VeroScience, LLC (Investigator-Initiated Grant to RAD).
Institutional Review Board Statement
The study was approved by the University of Texas Health Science Center at San Antonio (UTHSCSA) IRB.
Informed Consent Statement
Informed written consent was obtained from all participants prior to the study.
Data Availability Statement
The data presented in this study will be made available on request to the corresponding author.
Acknowledgments
The author thanks the Texas Diabetes Institute of the University Health System (technicians and laboratory equipment and supplies), and the Division of Diabetes, University of Texas Health Science Center at San Antonio (nursing staff, faculty time and effort, additional laboratory technicians, equipment, materials, and supplies).
Conflicts of Interest
AHC, ME., BC, and NC are all employees of VeroScience, a company that developed and via partnership markets Cycloset, the active agent of which is bromocriptine, for the treatment of type 2 diabetes in the U.S. AHC serves as the President and Chief Scientific Officer and is a shareholder of VeroScience. VeroScience holds patents relating to the metabolic effects of bromocriptine. RAD is a member of the advisory boards of Astra Zeneca, Janssen, Lexicon, Boehringer-Ingelheim Lilly Alliance and Novo Nordisk. RAD is a member of the speakers’ bureau of Novo Nordisk, Merck, and AstraZeneca. RAD has grant support from AstraZeneca and Janssen. EC is a member of the advisory boards of Boehringer-Ingelheim, Lilly Alliance, and Sanofi. EC is a member of the speaker bureau of AstraZeneca, Janssen and Boehringer-Ingelheim Lilly Alliance. EC has grant support from AstraZeneca and Janssen. MA, CA, JA, RC, and CT have no conflicts of interest to declare.
Abbreviations
| Abbreviations used in Figure 7 | |
| CNS | Central nervous system |
| DA | Dopaminergic |
| DAMP | Damage-associated molecular pattern |
| ER | Endoplasmic reticulum |
| ERAD | ER-associated degradation |
| PBMC | Peripheral blood mononuclear cells |
| PI | Pro-inflammatory |
| ROS | Reactive oxygen species |
| SNS | Sympathetic nervous system |
| UPR | Unfolded protein response |
| Genes mentioned in Figure 7 | |
| ATF4 | Activating Transcription Factor 4 |
| ATF6 | Activating Transcription Factor 6 |
| BiP/GRP78 | Heat-Shock Protein Family A (Hsp70) Member 5 |
| CCR2 | C-C Motif Chemokine Receptor 2 |
| CHOP | DNA Damage Inducible Transcript 3 |
| EIF2α | Eukaryotic Translation Initiation Factor 2, Subunit 1 Alpha |
| GCR | Glucocorticoid receptor |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IRE1 | Endoplasmic Reticulum to Nucleus Signaling 1 |
| MAPK8/JNK | Mitogen-Activated Protein Kinase 8 |
| MCP1/CCL2 | Monocyte Chemoattractant Protein 1 |
| NFkB | NF-kappa-B p65 subunit |
| NLRP3 | NLR Family Pyrin Domain-Containing Protein 3 |
| PERK | Eukaryotic Translation Initiation Factor 2 Alpha Kinase 3 |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| TLR10 | Toll-like Receptor 10 |
| TXNIP | Thioredoxin Interacting Protein |
| VCAM1 | Vascular Cell Adhesion Molecule 1 |
| XBP1 | X-Box Binding Protein 1 functional variant |
References
- Raskin, A.; Cincotta, A.H. Bromocriptine-QR therapy for the management of type 2 diabetes mellitus: Developmental basis and therapeutic profile summary. Expert Rev. Endocrinol. Metab. 2016, 11, 113–148. [Google Scholar] [CrossRef] [PubMed]
- Cycloset [Package Insert]; VeroScience: Tiverton, RI, USA, 2020.
- Gaziano, J.M.; Cincotta, A.H.; O’Connor, C.M.; Ezrokhi, M.; Rutty, D.; Ma, Z.J.; Scranton, R.E. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010, 33, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Cincotta, A.H.; Vinik, A.; Blonde, L.; Bohannon, N.; Scranton, R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J. Am. Heart Assoc. 2012, 1, e002279. [Google Scholar] [CrossRef] [PubMed]
- Chamarthi, B.; Gaziano, J.M.; Blonde, L.; Vinik, A.; Scranton, R.E.; Ezrokhi, M.; Rutty, D.; Cincotta, A.H. Timed Bromocriptine-QR Therapy Reduces Progression of Cardiovascular Disease and Dysglycemia in Subjects with Well-Controlled Type 2 Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 157698. [Google Scholar] [CrossRef]
- Chamarthi, B.; Ezrokhi, M.; Rutty, D.; Cincotta, A.H. Impact of bromocriptine-QR therapy on cardiovascular outcomes in type 2 diabetes mellitus subjects on metformin. Postgrad. Med. 2016, 128, 761–769. [Google Scholar] [CrossRef]
- Franchi, F.; Lazzeri, C.; Barletta, G.; Ianni, L.; Mannelli, M. Centrally mediated effects of bromocriptine on cardiac sympathovagal balance. Hypertension 2001, 38, 123–129. [Google Scholar] [CrossRef]
- Mannelli, M.; Delitala, G.; De Feo, M.L.; Maggi, M.; Cuomo, S.; Piazzini, M.; Guazzelli, R.; Serio, M. Effects of different dopaminergic antagonists on bromocriptine-induced inhibition of norepinephrine release. J. Clin. Endocrinol. Metab. 1984, 59, 74–78. [Google Scholar] [CrossRef]
- Sowers, J.R. Dopaminergic control of circadian norepinephrine levels in patients with essential hypertension. J. Clin. Endocrinol. Metab. 1981, 53, 1133–1137. [Google Scholar] [CrossRef]
- Sowers, J.R.; Stern, N.; Nyby, M.D.; Jasberg, K.A. Dopaminergic regulation of circadian rhythms of blood pressure, renin and aldosterone in essential hypertension. Cardiovasc. Res. 1982, 16, 317–323. [Google Scholar] [CrossRef]
- Ezrokhi, M.; Zhang, Y.; Luo, S.; Cincotta, A.H. Time-of-Day-Dependent Effects of Bromocriptine to Ameliorate Vascular Pathology and Metabolic Syndrome in SHR Rats Held on High Fat Diet. Int. J. Mol. Sci. 2021, 22, 6142. [Google Scholar] [CrossRef]
- Huggett, R.J.; Burns, J.; Mackintosh, A.F.; Mary, D.A. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 2004, 44, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Huggett, R.J.; Hogarth, A.J.; Mackintosh, A.F.; Mary, D.A. Sympathetic nerve hyperactivity in non-diabetic offspring of patients with type 2 diabetes mellitus. Diabetologia 2006, 49, 2741–2744. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Adithan, C.; Ananthanarayanan, P.H.; Pal, P.; Nanda, N.; Durgadevi, T.; Lalitha, V.; Syamsunder, A.N.; Dutta, T.K. Sympathovagal imbalance contributes to prehypertension status and cardiovascular risks attributed by insulin resistance, inflammation, dyslipidemia and oxidative stress in first degree relatives of type 2 diabetics. PLoS ONE 2013, 8, e78072. [Google Scholar] [CrossRef]
- Wulsin, L.R.; Horn, P.S.; Perry, J.L.; Massaro, J.M.; D’Agostino, R.B. Autonomic Imbalance as a Predictor of Metabolic Risks, Cardiovascular Disease, Diabetes, and Mortality. J. Clin. Endocrinol. Metab. 2015, 100, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Sympathetic Nervous System, Hypertension, Obesity and Metabolic Syndrome. High Blood Press. Cardiovasc. Prev. 2016, 23, 175–179. [Google Scholar] [CrossRef]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Golden, S.H.; Folsom, A.R.; Haskell, W.; Liao, D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: The Atherosclerosis Risk in Communities study, 1987–1998. Circulation 2003, 107, 2190–2195. [Google Scholar] [CrossRef]
- Hu, A.; Jiao, X.; Gao, E.; Koch, W.J.; Sharifi-Azad, S.; Grunwald, Z.; Ma, X.L.; Sun, J.Z. Chronic beta-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J. Pharmacol. Exp. Ther. 2006, 318, 469–475. [Google Scholar] [CrossRef]
- Bleeke, T.; Zhang, H.; Madamanchi, N.; Patterson, C.; Faber, J.E. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ. Res. 2004, 94, 37–45. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.Y.; Kim, K.; Darvas, M.; Davila, M.L.; Riggins, G.J.; Rothman, P.B.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature 2018, 564, 273–277. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Lorton, D. Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)? Int. J. Mol. Sci. 2018, 19, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ahmari, N.; Santisteban, M.M.; Miller, D.R.; Geis, N.M.; Larkin, R.; Redler, T.; Denson, H.; Khoshbouei, H.; Baekey, D.M.; Raizada, M.K.; et al. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H279–H289. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B. Chemical sympathectomy attenuates lipopolysaccharide-induced increase of plasma cytokine levels in rats pretreated by ACTH. J. Neuroimmunol. 2019, 337, 577086. [Google Scholar] [CrossRef] [PubMed]
- Fonkoue, I.T.; Marvar, P.J.; Norrholm, S.; Li, Y.; Kankam, M.L.; Jones, T.N.; Vemulapalli, M.; Rothbaum, B.; Bremner, J.D.; Le, N.A.; et al. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain Behav. Immun. 2020, 83, 260–269. [Google Scholar] [CrossRef]
- Mishra, I.; Pullum, K.B.; Thayer, D.C.; Plummer, E.R.; Conkright, B.W.; Morris, A.J.; O’Hara, B.F.; Demas, G.E.; Ashley, N.T. Chemical sympathectomy reduces peripheral inflammatory responses to acute and chronic sleep fragmentation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R781–R789. [Google Scholar] [CrossRef]
- Kavelaars, A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav. Immun. 2002, 16, 799–807. [Google Scholar] [CrossRef]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar]
- Abboud, F.M.; Singh, M.V. Autonomic regulation of the immune system in cardiovascular diseases. Adv. Physiol. Educ. 2017, 41, 578–593. [Google Scholar] [CrossRef]
- Moreira, H.G.; Lage, R.L.; Martinez, D.G.; Ferreira-Santos, L.; Rondon, M.; Negrao, C.E.; Nicolau, J.C. Sympathetic nervous activity in patients with acute coronary syndrome: A comparative study of inflammatory biomarkers. Clin. Sci. 2017, 131, 883–895. [Google Scholar] [CrossRef]
- Tian, X.; Guo, R.; Zhang, Y.; Xu, L.; Liu, X.; Hou, Y. Effects of the Sympathetic Nervous System on Regulatory T Cell and T Helper 1 Chemokine Expression in Patients with Acute Coronary Syndrome. Neuroimmunomodulation 2016, 23, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Fonkoue, I.T.; Le, N.A.; Kankam, M.L.; DaCosta, D.; Jones, T.N.; Marvar, P.J.; Park, J. Sympathoexcitation and impaired arterial baroreflex sensitivity are linked to vascular inflammation in individuals with elevated resting blood pressure. Physiol. Rep. 2019, 7, e14057. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.Z.; Wu, J.M.; Hu, G.M.; Gu, H.J.; Feng, Y.N.; Wang, S.X.; Cong, W.W.; Li, M.Z.; Xu, W.L.; Song, Y.; et al. alpha1-AR overactivation induces cardiac inflammation through NLRP3 inflammasome activation. Acta Pharmacol. Sin. 2020, 41, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.H.; Jenkins, N.T.; Padilla, J.; Parrish, A.R.; Fadel, P.J. Norepinephrine increases NADPH oxidase-derived superoxide in human peripheral blood mononuclear cells via alpha-adrenergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1124–R1132. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Millar, B.A.; Perez, S.; Carter, J.; Wood, C.; ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sympathetic modulation of immunity: Relevance to disease. Cell Immunol. 2008, 252, 27–56. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Souza-Neto, F.V.; Jimenez-Gonzalez, S.; Cachofeiro, V. Oxidative Stress and Vascular Damage in the Context of Obesity: The Hidden Guest. Antioxidants 2021, 10, 406. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Katsiki, N. Oxidative Stress and Inflammation: Their Role in the Pathogenesis of Peripheral Artery Disease with or Without Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2018, 16, 547–554. [Google Scholar] [CrossRef]
- Donath, M.Y. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 193–196. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Lv, S.; Wang, H.; Li, X. The Role of the Interplay between Autophagy and NLRP3 Inflammasome in Metabolic Disorders. Front. Cell Dev. Biol. 2021, 9, 634118. [Google Scholar] [CrossRef]
- Colak, Y.; Hasan, B.; Erkalma, B.; Tandon, K.; Zervos, X.; Menzo, E.L.; Erim, T. Pathogenetic mechanisms of nonalcoholic fatty liver disease and inhibition of the inflammasome as a new therapeutic target. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101710. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroma, E.; Abbate, A.; Toldo, S. NLRP3 Inflammasome Inhibitors in Cardiovascular Diseases. Molecules 2021, 26, 976. [Google Scholar] [CrossRef] [PubMed]
- Tsiotra, P.C.; Tsigos, C. Stress, the endoplasmic reticulum, and insulin resistance. Ann. N. Y. Acad. Sci. 2006, 1083, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Panzhinskiy, E.; Ren, J.; Nair, S. Protein tyrosine phosphatase 1B and insulin resistance: Role of endoplasmic reticulum stress/reactive oxygen species/nuclear factor kappa B axis. PLoS ONE 2013, 8, e77228. [Google Scholar] [CrossRef]
- Arnold, N.; Gori, T.; Schnabel, R.B.; Schulz, A.; Prochaska, J.H.; Zeller, T.; Binder, H.; Pfeiffer, N.; Beutel, M.; Espinola-Klein, C.; et al. Relation between Arterial Stiffness and Markers of Inflammation and Hemostasis—Data from the Population-based Gutenberg Health Study. Sci. Rep. 2017, 7, 6346. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Fatehi-Hassanabad, Z.; Chan, C.B.; Furman, B.L. Reactive oxygen species and endothelial function in diabetes. Eur. J. Pharmacol. 2010, 636, 8–17. [Google Scholar] [CrossRef]
- Alicka, M.; Marycz, K. The Effect of Chronic Inflammation and Oxidative and Endoplasmic Reticulum Stress in the Course of Metabolic Syndrome and Its Therapy. Stem. Cells Int. 2018, 2018, 4274361. [Google Scholar] [CrossRef]
- Toth, B.E.; Vecsernyes, M.; Zelles, T.; Kardar, K.; Nagy, G. Role of Peripheral and Brain-Derived Dopamine (DA) in Immune Regulation. Adv. Neuroimmune Biol. 2012, 3, 111–155. [Google Scholar] [CrossRef]
- Levite, M. Dopamine and T cells: Dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. 2016, 216, 42–89. [Google Scholar] [CrossRef]
- Felger, J.C.; Treadway, M.T. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 2017, 42, 216–241. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. Curr. Top Behav. Neurosci. 2017, 31, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Li, Z.; Haroon, E.; Woolwine, B.J.; Jung, M.Y.; Hu, X.; Miller, A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 2016, 21, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.; Ribeiro, L. Dopaminergic Pathways in Obesity-Associated Inflammation. J. Neuroimmune. Pharmacol. 2020, 15, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.; Lima, M.; Marino, F.; Cosentino, M.; Ribeiro, L. Dopaminergic Receptors and Tyrosine Hydroxylase Expression in Peripheral Blood Mononuclear Cells: A Distinct Pattern in Central Obesity. PLoS ONE 2016, 11, e0147483. [Google Scholar] [CrossRef]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef]
- Han, X.; Li, B.; Ye, X.; Mulatibieke, T.; Wu, J.; Dai, J.; Wu, D.; Ni, J.; Zhang, R.; Xue, J.; et al. Dopamine D2 receptor signalling controls inflammation in acute pancreatitis via a PP2A-dependent Akt/NF-kappaB signalling pathway. Br. J. Pharmacol. 2017, 174, 4751–4770. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wu, J.; Manaenko, A.; Yang, P.; Tang, J.; Fu, W.; Zhang, J.H. Activation of Dopamine D2 Receptor Suppresses Neuroinflammation through alphaB-Crystalline by Inhibition of NF-kappaB Nuclear Translocation in Experimental ICH Mice Model. Stroke 2015, 46, 2637–2646. [Google Scholar] [CrossRef]
- Balkowiec-Iskra, E.; Kurkowska-Jastrzebska, I.; Joniec, I.; Ciesielska, A.; Muszynska, A.; Przybylkowski, A.; Czlonkowska, A.; Czlonkowski, A. MPTP-induced central dopamine depletion exacerbates experimental autoimmune encephalomyelitis (EAE) in C57BL mice. Inflamm. Res. 2007, 56, 311–317. [Google Scholar] [CrossRef]
- Kawano, M.; Takagi, R.; Saika, K.; Matsui, M.; Matsushita, S. Dopamine regulates cytokine secretion during innate and adaptive immune responses. Int. Immunol. 2018, 30, 591–606. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Sugino, Y.; Shibagaki, F.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Dopamine attenuates lipopolysaccharide-induced expression of proinflammatory cytokines by inhibiting the nuclear translocation of NF-kappaB p65 through the formation of dopamine quinone in microglia. Eur. J. Pharmacol. 2020, 866, 172826. [Google Scholar] [CrossRef]
- Liu, A.; Ding, S. Anti-inflammatory Effects of Dopamine in Lipopolysaccharide (LPS)-stimulated RAW264.7 Cells via Inhibiting NLRP3 Inflammasome Activation. Ann. Clin. Lab. Sci. 2019, 49, 353–360. [Google Scholar] [PubMed]
- Shimojo, G.; Joseph, B.; Shah, R.; Consolim-Colombo, F.M.; De Angelis, K.; Ulloa, L. Exercise activates vagal induction of dopamine and attenuates systemic inflammation. Brain Behav. Immun. 2019, 75, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Romero, M.C.; Delgado-Cortes, M.J.; Sarmiento, M.; de Pablos, R.M.; Espinosa-Oliva, A.M.; Arguelles, S.; Bandez, M.J.; Villaran, R.F.; Maurino, R.; Santiago, M.; et al. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology 2012, 33, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhao, H.; Zhang, W.; Liu, B.; Liu, Y.; Guo, Y.; Nie, L. Overexpression of conserved dopamine neurotrophic factor (CDNF) in astrocytes alleviates endoplasmic reticulum stress-induced cell damage and inflammatory cytokine secretion. Biochem. Biophys. Res. Commun. 2013, 435, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.E. Bromocriptine treatment of systemic lupus erythematosus. Lupus 2001, 10, 762–768. [Google Scholar] [CrossRef]
- Alvarez-Nemegyei, J.; Cobarrubias-Cobos, A.; Escalante-Triay, F.; Sosa-Munoz, J.; Miranda, J.M.; Jara, L.J. Bromocriptine in systemic lupus erythematosus: A double-blind, randomized, placebo-controlled study. Lupus 1998, 7, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Liuqin, L.; Hao, L.; Shiwen, Y.; Zhongping, Z.; Dongying, C.; Fan, L.; Hanshi, X.; Xiuyan, Y.; Yujin, Y. The effects of bromocriptine on preventing postpartum flare in systemic lupus erythematosus patients from South China. J. Immunol. Res. 2015, 2015, 316965. [Google Scholar] [CrossRef]
- Jara, L.J.; Cruz-Cruz, P.; Saavedra, M.A.; Medina, G.; Garcia-Flores, A.; Angeles, U.; Miranda-Limon, J.M. Bromocriptine during pregnancy in systemic lupus erythematosus: A pilot clinical trial. Ann. N. Y. Acad. Sci. 2007, 1110, 297–304. [Google Scholar] [CrossRef]
- Palestine, A.G.; Muellenberg-Coulombre, C.G.; Kim, M.K.; Gelato, M.C.; Nussenblatt, R.B. Bromocriptine and low dose cyclosporine in the treatment of experimental autoimmune uveitis in the rat. J. Clin. Investig. 1987, 79, 1078–1081. [Google Scholar] [CrossRef]
- Figueroa, F.; Carrion, F.; Martinez, M.E.; Rivero, S.; Mamani, I.; Gonzalez, G. Effects of bromocriptine in patients with active rheumatoid arthritis. Rev. Med. Chil. 1998, 126, 33–41. [Google Scholar] [PubMed]
- Hilfiker-Kleiner, D.; Struman, I.; Hoch, M.; Podewski, E.; Sliwa, K. 16-kDa prolactin and bromocriptine in postpartum cardiomyopathy. Curr. Heart Fail Rep. 2012, 9, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Haghikia, A.; Berliner, D.; Vogel-Claussen, J.; Schwab, J.; Franke, A.; Schwarzkopf, M.; Ehlermann, P.; Pfister, R.; Michels, G.; et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur. Heart J. 2017, 38, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Schwab, J.; Vogel-Claussen, J.; Berliner, D.; Pfeffer, T.; Konig, T.; Zwadlo, C.; Moulig, V.A.; Franke, A.; Schwarzkopf, M.; et al. Bromocriptine treatment in patients with peripartum cardiomyopathy and right ventricular dysfunction. Clin. Res. Cardiol. 2019, 108, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Abad-Jimenez, Z.; Maranon, A.M.; Iannantuoni, F.; Escribano-Lopez, I.; Lopez-Domenech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Szpigel, A.; Hainault, I.; Carlier, A.; Venteclef, N.; Batto, A.F.; Hajduch, E.; Bernard, C.; Ktorza, A.; Gautier, J.F.; Ferre, P.; et al. Lipid environment induces ER stress, TXNIP expression and inflammation in immune cells of individuals with type 2 diabetes. Diabetologia 2018, 61, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xia, C.Q.; Butfiloski, E.; Clare-Salzler, M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: Implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clin. Immunol. 2018, 195, 139–148. [Google Scholar] [CrossRef]
- Li, H.; Peng, W.; Jian, W.; Li, Y.; Li, Q.; Li, W.; Xu, Y. ROCK inhibitor fasudil attenuated high glucose-induced MCP-1 and VCAM-1 expression and monocyte-endothelial cell adhesion. Cardiovasc. Diabetol. 2012, 11, 65. [Google Scholar] [CrossRef]
- Akhter, N.; Madhoun, A.; Arefanian, H.; Wilson, A.; Kochumon, S.; Thomas, R.; Shenouda, S.; Al-Mulla, F.; Ahmad, R.; Sindhu, S. Oxidative Stress Induces Expression of the Toll-Like Receptors (TLRs) 2 and 4 in the Human Peripheral Blood Mononuclear Cells: Implications for Metabolic Inflammation. Cell Physiol. Biochem. 2019, 53, 1–18. [Google Scholar] [CrossRef]
- Yasunari, K.; Watanabe, T.; Nakamura, M. Reactive oxygen species formation by polymorphonuclear cells and mononuclear cells as a risk factor of cardiovascular diseases. Curr. Pharm. Biotechnol. 2006, 7, 73–80. [Google Scholar] [CrossRef]
- Takahashi, M. Cell-Specific Roles of NLRP3 Inflammasome in Myocardial Infarction. J. Cardiovasc. Pharmacol. 2019, 74, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Komura, T.; Sakai, Y.; Honda, M.; Takamura, T.; Matsushima, K.; Kaneko, S. CD14+ monocytes are vulnerable and functionally impaired under endoplasmic reticulum stress in patients with type 2 diabetes. Diabetes 2010, 59, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Lopez, I.; de Maranon, A.M.; Iannantuoni, F.; Lopez-Domenech, S.; Abad-Jimenez, Z.; Diaz, P.; Sola, E.; Apostolova, N.; Rocha, M.; Victor, V.M. The Mitochondrial Antioxidant SS-31 Modulates Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy in Type 2 Diabetes. J. Clin. Med. 2019, 8, 1322. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Forte, M.; Raffa, S. Circulating Leukocytes and Oxidative Stress in Cardiovascular Diseases: A State of the Art. Oxid. Med. Cell Longev. 2019, 2019, 2650429. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 2010, 16, 396–399. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Hummasti, S.; Hotamisligil, G.S. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 2010, 107, 579–591. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Flamment, M.; Hajduch, E.; Ferre, P.; Foufelle, F. New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 2012, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Targeting endoplasmic reticulum stress in metabolic disease. Expert. Opin. Ther. Targets 2013, 17, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kouri, G.; Wollheim, C.B. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J. Cell Sci. 2005, 118, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.D.; Alejandro, E.U.; Luciani, D.S.; Kalynyak, T.B.; Hu, X.; Li, H.; Lin, Y.; Townsend, R.R.; Polonsky, K.S.; Johnson, J.D. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 8452–8457. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Bowes, A.J.; Werstuck, G.H. Diabetes, hyperglycemia and accelerated atherosclerosis: Evidence supporting a role for endoplasmic reticulum (ER) stress signaling. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 151–157. [Google Scholar] [CrossRef]
- Mozzini, C.; Garbin, U.; Stranieri, C.; Pasini, A.; Solani, E.; Tinelli, I.A.; Cominacini, L.; Fratta Pasini, A.M. Endoplasmic reticulum stress and Nrf2 repression in circulating cells of type 2 diabetic patients without the recommended glycemic goals. Free Radic. Res. 2015, 49, 244–252. [Google Scholar] [CrossRef]
- Rajan, S.; Shankar, K.; Beg, M.; Varshney, S.; Gupta, A.; Srivastava, A.; Kumar, D.; Mishra, R.K.; Hussain, Z.; Gayen, J.R.; et al. Chronic hyperinsulinemia reduces insulin sensitivity and metabolic functions of brown adipocyte. J. Endocrinol. 2016, 230, 275–290. [Google Scholar] [CrossRef]
- Tampakakis, E.; Tabit, C.E.; Holbrook, M.; Linder, E.A.; Berk, B.D.; Frame, A.A.; Breton-Romero, R.; Fetterman, J.L.; Gokce, N.; Vita, J.A.; et al. Intravenous Lipid Infusion Induces Endoplasmic Reticulum Stress in Endothelial Cells and Blood Mononuclear Cells of Healthy Adults. J. Am. Heart Assoc. 2016, 5, e002574. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Lenin, R.; Sankaramoorthy, A.; Mohan, V.; Balasubramanyam, M. Altered immunometabolism at the interface of increased endoplasmic reticulum (ER) stress in patients with type 2 diabetes. J. Leukoc. Biol. 2015, 98, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.T.; Holtby-Ottenhof, S.; Shi, Y.; Damjanovic, S.; Sharma, A.M.; Werstuck, G.H. Metabolic syndrome and acute hyperglycemia are associated with endoplasmic reticulum stress in human mononuclear cells. Obes. (Silver Spring) 2012, 20, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Alatrach, M.; Agyin, C.; Adams, J.; Chilton, R.; Triplitt, C.; DeFronzo, R.A.; Cersosimo, E. Glucose lowering and vascular protective effects of cycloset added to GLP-1 receptor agonists in patients with type 2 diabetes. Endocrinol. Diabetes Metab. 2018, 1, e00034. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.; Sarada, D.; Ramkumar, K.M. Crosstalk between endoplasmic reticulum stress and oxidative stress: Focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1. Eur. J. Pharmacol. 2021, 892, 173749. [Google Scholar] [CrossRef]
- Delaunay-Moisan, A.; Appenzeller-Herzog, C. The antioxidant machinery of the endoplasmic reticulum: Protection and signaling. Free Radic. Biol. Med. 2015, 83, 341–351. [Google Scholar] [CrossRef]
- Todd, D.J.; Lee, A.H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674. [Google Scholar] [CrossRef]
- Miani, M.; Colli, M.L.; Ladriere, L.; Cnop, M.; Eizirik, D.L. Mild endoplasmic reticulum stress augments the proinflammatory effect of IL-1beta in pancreatic rat beta-cells via the IRE1alpha/XBP1s pathway. Endocrinology 2012, 153, 3017–3028. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef]
- Boyce, M.; Py, B.F.; Ryazanov, A.G.; Minden, J.S.; Long, K.; Ma, D.; Yuan, J. A pharmacoproteomic approach implicates eukaryotic elongation factor 2 kinase in ER stress-induced cell death. Cell Death Differ. 2008, 15, 589–599. [Google Scholar] [CrossRef]
- Kong, F.J.; Ma, L.L.; Guo, J.J.; Xu, L.H.; Li, Y.; Qu, S. Endoplasmic reticulum stress/autophagy pathway is involved in diabetes-induced neuronal apoptosis and cognitive decline in mice. Clin. Sci. 2018, 132, 111–125. [Google Scholar] [CrossRef]
- Ning, B.; Zhang, Q.; Wang, N.; Deng, M.; Fang, Y. beta-Asarone Regulates ER Stress and Autophagy via Inhibition of the PERK/CHOP/Bcl-2/Beclin-1 Pathway in 6-OHDA-Induced Parkinsonian Rats. Neurochem. Res. 2019, 44, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Shen, Z.J.; Malter, J.S. Pinning down signaling in the immune system: The role of the peptidyl-prolyl isomerase Pin1 in immune cell function. Crit. Rev. Immunol. 2008, 28, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B. Role of the Nrf2 signaling system in health and disease. Clin. Genet. 2014, 86, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Fratta Pasini, A.M.; Ferrari, M.; Stranieri, C.; Vallerio, P.; Mozzini, C.; Garbin, U.; Zambon, G.; Cominacini, L. Nrf2 expression is increased in peripheral blood mononuclear cells derived from mild-moderate ex-smoker COPD patients with persistent oxidative stress. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1733–1743. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Volkert, M.R.; Crowley, D.J. Preventing Neurodegeneration by Controlling Oxidative Stress: The Role of OXR1. Front. Neurosci. 2020, 14, 611904. [Google Scholar] [CrossRef]
- Rebrin, I.; Sohal, R.S. Pro-oxidant shift in glutathione redox state during aging. Adv. Drug Deliv. Rev. 2008, 60, 1545–1552. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Jimenez, L.A.; Torres, M.; Forman, H.J. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox Signal 2005, 7, 42–59. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Gall, T.; Balla, G.; Balla, J. Heme, Heme Oxygenase, and Endoplasmic Reticulum Stress—A New Insight into the Pathophysiology of Vascular Diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Upmacis, R.K.; Crabtree, M.J.; Deeb, R.S.; Shen, H.; Lane, P.B.; Benguigui, L.E.; Maeda, N.; Hajjar, D.P.; Gross, S.S. Profound biopterin oxidation and protein tyrosine nitration in tissues of ApoE-null mice on an atherogenic diet: Contribution of inducible nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2878–H2887. [Google Scholar] [CrossRef]
- Ishii, M.; Shimizu, S.; Wajima, T.; Hagiwara, T.; Negoro, T.; Miyazaki, A.; Tobe, T.; Kiuchi, Y. Reduction of GTP cyclohydrolase I feedback regulating protein expression by hydrogen peroxide in vascular endothelial cells. J. Pharmacol. Sci. 2005, 97, 299–302. [Google Scholar] [CrossRef]
- Rocha, M.; Diaz-Morales, N.; Rovira-Llopis, S.; Escribano-Lopez, I.; Banuls, C.; Hernandez-Mijares, A.; Diamanti-Kandarakis, E.; Victor, V.M. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Diabetes. Curr. Pharm. Des. 2016, 22, 2640–2649. [Google Scholar] [CrossRef]
- Liu, H.; Cao, M.M.; Wang, Y.; Li, L.C.; Zhu, L.B.; Xie, G.Y.; Li, Y.B. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen. Comp. Endocrinol. 2015, 210, 124–129. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Yang, T.; Song, M.; Wang, C.; Yao, Y.; Fan, H. Glucocorticoid-Driven NLRP3 Inflammasome Activation in Hippocampal Microglia Mediates Chronic Stress-Induced Depressive-Like Behaviors. Front. Mol. Neurosci. 2019, 12, 210. [Google Scholar] [CrossRef]
- Busillo, J.M.; Azzam, K.M.; Cidlowski, J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef]
- Yeager, M.P.; Pioli, P.A.; Collins, J.; Barr, F.; Metzler, S.; Sites, B.D.; Guyre, P.M. Glucocorticoids enhance the in vivo migratory response of human monocytes. Brain Behav. Immun. 2016, 54, 86–94. [Google Scholar] [CrossRef]
- Penton-Rol, G.; Cota, M.; Polentarutti, N.; Luini, W.; Bernasconi, S.; Borsatti, A.; Sica, A.; LaRosa, G.J.; Sozzani, S.; Poli, G.; et al. Up-regulation of CCR2 chemokine receptor expression and increased susceptibility to the multitropic HIV strain 89.6 in monocytes exposed to glucocorticoid hormones. J. Immunol. 1999, 163, 3524–3529. [Google Scholar]
- Lv, S.; Li, X.; Wang, H. The Role of the Effects of Endoplasmic Reticulum Stress on NLRP3 Inflammasome in Diabetes. Front. Cell Dev. Biol. 2021, 9, 663528. [Google Scholar] [CrossRef]
- Ruan, Y.; Zeng, J.; Jin, Q.; Chu, M.; Ji, K.; Wang, Z.; Li, L. Endoplasmic reticulum stress serves an important role in cardiac ischemia/reperfusion injury (Review). Exp. Ther. Med. 2020, 20, 268. [Google Scholar] [CrossRef]
- Kim, S.; Joe, Y.; Surh, Y.J.; Chung, H.T. Differential Regulation of Toll-Like Receptor-Mediated Cytokine Production by Unfolded Protein Response. Oxid. Med. Cell Longev. 2018, 2018, 9827312. [Google Scholar] [CrossRef]
- Kim, S.; Joe, Y.; Kim, H.J.; Kim, Y.S.; Jeong, S.O.; Pae, H.O.; Ryter, S.W.; Surh, Y.J.; Chung, H.T. Endoplasmic reticulum stress-induced IRE1alpha activation mediates cross-talk of GSK-3beta and XBP-1 to regulate inflammatory cytokine production. J. Immunol. 2015, 194, 4498–4506. [Google Scholar] [CrossRef]
- Dragomir, E.; Simionescu, M. Monocyte chemoattractant protein-1—A major contributor to the inflammatory process associated with diabetes. Arch Physiol. Biochem. 2006, 112, 239–244. [Google Scholar] [CrossRef]
- Troseid, M.; Seljeflot, I.; Arnesen, H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc. Diabetol. 2010, 9, 11. [Google Scholar] [CrossRef]
- Hung, J.; McQuillan, B.M.; Chapman, C.M.; Thompson, P.L.; Beilby, J.P. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1268–1273. [Google Scholar] [CrossRef]
- Santalahti, K.; Maksimow, M.; Airola, A.; Pahikkala, T.; Hutri-Kahonen, N.; Jalkanen, S.; Raitakari, O.T.; Salmi, M. Circulating Cytokines Predict the Development of Insulin Resistance in a Prospective Finnish Population Cohort. J. Clin. Endocrinol. Metab. 2016, 101, 3361–3369. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin. Nephrol. 2007, 27, 98–114. [Google Scholar] [CrossRef]
- Niu, J.; Kolattukudy, P.E. Role of MCP-1 in cardiovascular disease: Molecular mechanisms and clinical implications. Clin. Sci. 2009, 117, 95–109. [Google Scholar] [CrossRef]
- Franca, C.N.; Izar, M.C.O.; Hortencio, M.N.S.; do Amaral, J.B.; Ferreira, C.E.S.; Tuleta, I.D.; Fonseca, F.A.H. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef]
- Xia, Y.; Frangogiannis, N.G. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm. Allergy Drug Targets 2007, 6, 101–107. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Peiro, C.; Lorenzo, O.; Carraro, R.; Sanchez-Ferrer, C.F. IL-1beta Inhibition in Cardiovascular Complications Associated to Diabetes Mellitus. Front. Pharmacol. 2017, 8, 363. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol. Ther. Exp. (Warsz.) 2009, 57, 165–176. [Google Scholar] [CrossRef]
- Dourado, M.; Cavalcanti, F.; Vilar, L.; Cantilino, A. Relationship between Prolactin, Chronic Kidney Disease, and Cardiovascular Risk. Int. J. Endocrinol. 2020, 2020, 9524839. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, Y.; Liu, E. C-reactive protein and cardiovascular disease: From animal studies to the clinic (Review). Exp. Ther. Med. 2020, 20, 1211–1219. [Google Scholar] [CrossRef]
- Xu, Y.; Whitmer, K. C-reactive protein and cardiovascular disease in people with diabetes: High-sensitivity CRP testing can help assess risk for future cardiovascular disease events in this population. Am. J. Nurs. 2006, 106, 66–72. [Google Scholar] [CrossRef]
- Parrinello, C.M.; Lutsey, P.L.; Ballantyne, C.M.; Folsom, A.R.; Pankow, J.S.; Selvin, E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am. Heart J. 2015, 170, 380–389. [Google Scholar] [CrossRef]
- Mallard, A.R.; Hollekim-Strand, S.M.; Ingul, C.B.; Coombes, J.S. High day-to-day and diurnal variability of oxidative stress and inflammation biomarkers in people with type 2 diabetes mellitus and healthy individuals. Redox. Rep. 2020, 25, 64–69. [Google Scholar] [CrossRef]
- Kanabrocki, E.L.; Murray, D.; Hermida, R.C.; Scott, G.S.; Bremner, W.F.; Ryan, M.D.; Ayala, D.E.; Third, J.L.; Shirazi, P.; Nemchausky, B.A.; et al. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol. Int. 2002, 19, 423–439. [Google Scholar] [CrossRef]
- Maio, R.; Perticone, M.; Sciacqua, A.; Tassone, E.J.; Naccarato, P.; Bagnato, C.; Iannopollo, G.; Sesti, G.; Perticone, F. Oxidative stress impairs endothelial function in nondipper hypertensive patients. Cardiovasc. Ther. 2012, 30, 85–92. [Google Scholar] [CrossRef]
- Avogaro, A.; Pagnin, E.; Calo, L. Monocyte NADPH oxidase subunit p22(phox) and inducible hemeoxygenase-1 gene expressions are increased in type II diabetic patients: Relationship with oxidative stress. J. Clin. Endocrinol. Metab. 2003, 88, 1753–1759. [Google Scholar] [CrossRef][Green Version]
- Farah, R.; Shurtz-Swirski, R.; Lapin, O. Intensification of oxidative stress and inflammation in type 2 diabetes despite antihyperglycemic treatment. Cardiovasc. Diabetol. 2008, 7, 20. [Google Scholar] [CrossRef]
- Huang, X.; Sun, M.; Li, D.; Liu, J.; Guo, H.; Dong, Y.; Jiang, L.; Pan, Q.; Man, Y.; Wang, S.; et al. Augmented NADPH oxidase activity and p22phox expression in monocytes underlie oxidative stress of patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 91, 371–380. [Google Scholar] [CrossRef]
- Cortez-Espinosa, N.; Garcia-Hernandez, M.H.; Reynaga-Hernandez, E.; Cortes-Garcia, J.D.; Corral-Fernandez, N.E.; Rodriguez-Rivera, J.G.; Bravo-Ramirez, A.; Gonzalez-Amaro, R.; Portales-Perez, D.P. Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (HbA1c > 8%). Metabolism 2012, 61, 1538–1546. [Google Scholar] [CrossRef]
- He, L.; Wong, C.K.; Cheung, K.K.; Yau, H.C.; Fu, A.; Zhao, H.L.; Leung, K.M.; Kong, A.P.; Wong, G.W.; Chan, P.K.; et al. Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1 analog, on human peripheral lymphocytes in patients with type 2 diabetes. J. Diabetes Investig. 2013, 4, 382–392. [Google Scholar] [CrossRef]
- Mandal, L.K.; Choudhuri, S.; Dutta, D.; Mitra, B.; Kundu, S.; Chowdhury, I.H.; Sen, A.; Chatterjee, M.; Bhattacharya, B. Oxidative stress-associated neuroretinal dysfunction and nitrosative stress in diabetic retinopathy. Can. J. Diabetes 2013, 37, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Costantino, S.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Scavone, G.; Villano, A.; Pitocco, D.; Lanza, G.; et al. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ. Cardiovasc. Genet. 2015, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lenin, R.; Maria, M.S.; Agrawal, M.; Balasubramanyam, J.; Mohan, V.; Balasubramanyam, M. Amelioration of glucolipotoxicity-induced endoplasmic reticulum stress by a “chemical chaperone” in human THP-1 monocytes. Exp. Diabetes Res. 2012, 2012, 356487. [Google Scholar] [CrossRef] [PubMed]
- Chamarthi, B.; Vinik, A.; Ezrokhi, M.; Cincotta, A.H. Circadian-timed quick-release bromocriptine lowers elevated resting heart rate in patients with type 2 diabetes mellitus. Endocrinol. Diabetes Metab. 2020, 3, e00101. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.N.; Kvakan, H.; Luft, F.C. Immune-related effects in hypertension and target-organ damage. Curr. Opin. Nephrol. Hypertens. 2011, 20, 113–117. [Google Scholar] [CrossRef]
- Wrigley, B.J.; Lip, G.Y.; Shantsila, E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur. J. Heart Fail. 2011, 13, 1161–1171. [Google Scholar] [CrossRef]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid. Med. Cell Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, S.; Chen, X.; Ye, R.; Zhu, W.; Liu, X. NLRP3 Is Involved in Ischemia/Reperfusion Injury. CNS Neurol. Disord. Drug Targets 2016, 15, 699–712. [Google Scholar] [CrossRef]
- Gao, J.; Guo, J.; Li, H.; Bai, S.; Li, H.; Wu, B.; Wang, L.; Xi, Y.; Tian, Y.; Yang, G.; et al. Involvement of dopamine D2 receptors activation in ischemic post-conditioning-induced cardioprotection through promoting PKC-epsilon particulate translocation in isolated rat hearts. Mol. Cell Biochem. 2013, 379, 267–276. [Google Scholar] [CrossRef]
- Li, H.; Wei, C.; Gao, J.; Bai, S.; Li, H.; Zhao, Y.; Li, H.; Han, L.; Tian, Y.; Yang, G.; et al. Mediation of dopamine D2 receptors activation in post-conditioning-attenuated cardiomyocyte apoptosis. Exp. Cell Res. 2014, 323, 118–130. [Google Scholar] [CrossRef]
- Li, H.Z.; Guo, J.; Gao, J.; Han, L.P.; Jiang, C.M.; Li, H.X.; Bai, S.Z.; Zhang, W.H.; Li, G.W.; Wang, L.N.; et al. Role of dopamine D2 receptors in ischemia/reperfusion induced apoptosis of cultured neonatal rat cardiomyocytes. J. Biomed. Sci. 2011, 18, 18. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Hicks, C.A.; Ward, M.A.; Cardwell, G.P.; Reymann, J.M.; Allain, H.; Bentue-Ferrer, D. Dopamine D2 receptor agonists protect against ischaemia-induced hippocampal neurodegeneration in global cerebral ischaemia. Eur. J. Pharmacol. 1998, 352, 37–46. [Google Scholar] [CrossRef]
- Narkar, V.; Kunduzova, O.; Hussain, T.; Cambon, C.; Parini, A.; Lokhandwala, M. Dopamine D2-like receptor agonist bromocriptine protects against ischemia/reperfusion injury in rat kidney. Kidney Int. 2004, 66, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, Y.; Ezrokhi, M.; Li, Y.; Tsai, T.H.; Cincotta, A.H. Circadian peak dopaminergic activity response at the biological clock pacemaker (suprachiasmatic nucleus) area mediates the metabolic responsiveness to a high-fat diet. J. Neuroendocrinol. 2018, 30, e12563. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Luo, J.; Cincotta, A.H. Suprachiasmatic nuclei monoamine metabolism of glucose tolerant versus intolerant hamsters. Neuroreport 1999, 10, 2073–2077. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Luo, J.; Meier, A.H.; Cincotta, A.H. Dopaminergic neurotoxin administration to the area of the suprachiasmatic nuclei induces insulin resistance. Neuroreport 1997, 8, 3495–3499. [Google Scholar] [CrossRef]
- Luo, S.; Ezrokhi, M.; Cominos, N.; Tsai, T.H.; Stoelzel, C.R.; Trubitsyna, Y.; Cincotta, A.H. Experimental dopaminergic neuron lesion at the area of the biological clock pacemaker, suprachiasmatic nuclei (SCN) induces metabolic syndrome in rats. Diabetol. Metab. Syndr. 2021, 13, 11. [Google Scholar] [CrossRef]
- Luo, S.; Liang, Y.; Cincotta, A.H. Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose-intolerant state in hamsters. Neuroendocrinology 1999, 69, 160–166. [Google Scholar] [CrossRef]
- Stoelzel, C.R.; Zhang, Y.; Cincotta, A.H. Circadian-timed dopamine agonist treatment reverses high-fat diet-induced diabetogenic shift in ventromedial hypothalamic glucose sensing. Endocrinol. Diabetes Metab. 2020, 3, e00139. [Google Scholar] [CrossRef]
- Ezrokhi, M.; Luo, S.; Trubitsyna, Y.; Cincotta, A.H. Neuroendocrine and metabolic components of dopamine agonist amelioration of metabolic syndrome in SHR rats. Diabetol. Metab. Syndr. 2014, 6, 104. [Google Scholar] [CrossRef]
- Bahler, L.; Verberne, H.J.; Brakema, E.; Tepaske, R.; Booij, J.; Hoekstra, J.B.; Holleman, F. Bromocriptine and insulin sensitivity in lean and obese subjects. Endocr. Connect. 2016, 5, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.S.; McMahon, A.M.; Friedman, D.; Rabinovich-Nikitin, I.; Kirshenbaum, L.A.; Martino, T.A. Circadian influence on inflammatory response during cardiovascular disease. Curr. Opin. Pharmacol. 2020, 57, 60–70. [Google Scholar] [CrossRef]
- Denis, R.G.; Joly-Amado, A.; Cansell, C.; Castel, J.; Martinez, S.; Delbes, A.S.; Luquet, S. Central orchestration of peripheral nutrient partitioning and substrate utilization: Implications for the metabolic syndrome. Diabetes Metab. 2014, 40, 191–197. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ravussin, E.; Johannsen, D.L.; Stull, A.J.; Cefalu, W.T.; Johnson, W.D. Endothelial Dysfunction: An Early Cardiovascular Risk Marker in Asymptomatic Obese Individuals with Prediabetes. Br. J. Med. Med. Res. 2012, 2, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.A.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms. 2006, 21, 458–469. [Google Scholar] [CrossRef]
- Kudo, T.; Horikawa, K.; Shibata, S. Circadian rhythms in the CNS and peripheral clock disorders: The circadian clock and hyperlipidemia. J. Pharmacol. Sci. 2007, 103, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Holmback, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef]
- Maemura, K.; Takeda, N.; Nagai, R. Circadian rhythms in the CNS and peripheral clock disorders: Role of the biological clock in cardiovascular diseases. J. Pharmacol. Sci. 2007, 103, 134–138. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. [Google Scholar] [CrossRef]
- Manfredini, R.; Fabbian, F.; Manfredini, F.; Salmi, R.; Gallerani, M.; Bossone, E. Chronobiology in aortic diseases—“Is this really a random phenomenon?”. Prog. Cardiovasc. Dis. 2013, 56, 116–124. [Google Scholar] [CrossRef]
- Maywood, E.S.; O’Neill, J.; Wong, G.K.; Reddy, A.B.; Hastings, M.H. Circadian timing in health and disease. Prog. Brain Res. 2006, 153, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, I.; Manzano-Pech, L.; Rubio-Ruiz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef] [PubMed]
- Prasai, M.J.; George, J.T.; Scott, E.M. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab. Vasc. Dis. Res. 2008, 5, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.F.; Westgate, E.J.; FitzGerald, G.A. Peripheral circadian clocks in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Shimokawa, H. Reactive oxygen species in cardiovascular health and disease: Special references to nitric oxide, hydrogen peroxide, and Rho-kinase. J. Clin. Biochem. Nutr. 2020, 66, 83–91. [Google Scholar] [CrossRef]
- Shinozaki, K.; Ayajiki, K.; Kashiwagi, A.; Masada, M.; Okamura, T. Malfunction of vascular control in lifestyle-related diseases: Mechanisms underlying endothelial dysfunction in the insulin-resistant state. J. Pharmacol. Sci. 2004, 96, 401–405. [Google Scholar] [CrossRef]
- Young, M.E.; Bray, M.S. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 2007, 8, 656–667. [Google Scholar] [CrossRef]
- Cincotta, A. Hypothalamic role in the insulin resistance syndrome. In Insulin Resistance and Insulin Resistance Syndrome; Hansen, B., Shafrir, E., Eds.; Taylor and Francis: London, UK, 2002; pp. 271–312. [Google Scholar]
- Liang, Y.; Lubkin, M.; Sheng, H.; Scislowski, P.W.; Cincotta, A.H. Dopamine agonist treatment ameliorates hyperglycemia, hyperlipidemia, and the elevated basal insulin release from islets of ob/ob mice. Biochim. Biophys. Acta 1998, 1405, 1–13. [Google Scholar] [CrossRef]
- Cincotta, A.H.; MacEachern, T.A.; Meier, A.H. Bromocriptine redirects metabolism and prevents seasonal onset of obese hyperinsulinemic state in Syrian hamsters. Am. J. Physiol. 1993, 264, E285–E293. [Google Scholar] [CrossRef]
- Lech, K.; Ackermann, K.; Revell, V.L.; Lao, O.; Skene, D.J.; Kayser, M. Dissecting Daily and Circadian Expression Rhythms of Clock-Controlled Genes in Human Blood. J. Biol. Rhythms. 2016, 31, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Schutte, H.; Doevendans, P.A.; Sluijter, J.P.G.; van Laake, L.W. Minimally Invasive Ways of Determining Circadian Rhythms in Humans. Physiology (Bethesda) 2021, 36, 7–20. [Google Scholar] [CrossRef] [PubMed]
- James, F.O.; Cermakian, N.; Boivin, D.B. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep 2007, 30, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; James, F.O.; Wu, A.; Cho-Park, P.F.; Xiong, H.; Sun, Z.S. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 2003, 102, 4143–4145. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Gonzalez, A.; Umpierrez, D.; Pimentel, D. Diabetes mellitus in the Hispanic/Latino population: An increasing health care challenge in the United States. Am. J. Med. Sci. 2007, 334, 274–282. [Google Scholar] [CrossRef]
- Wenzel, P. Monocytes as immune targets in arterial hypertension. Br. J. Pharmacol. 2019, 176, 1966–1977. [Google Scholar] [CrossRef]
- Frodermann, V.; Nahrendorf, M. Macrophages and Cardiovascular Health. Physiol. Rev. 2018, 98, 2523–2569. [Google Scholar] [CrossRef]
- Hilgendorf, I.; Swirski, F.K.; Robbins, C.S. Monocyte fate in atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 272–279. [Google Scholar] [CrossRef]
- Luft, F.C.; Dechend, R.; Muller, D.N. Immune mechanisms in angiotensin II-induced target-organ damage. Ann. Med. 2012, 44 (Suppl. 1), S49–S54. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bezsonov, E.E.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Immunity in Atherosclerosis: Focusing on T and B Cells. Int. J. Mol. Sci. 2021, 22, 8379. [Google Scholar] [CrossRef]
- Flores-Gomez, D.; Bekkering, S.; Netea, M.G.; Riksen, N.P. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 62–69. [Google Scholar] [CrossRef]
- Su, J.; Zhou, L.; Kong, X.; Yang, X.; Xiang, X.; Zhang, Y.; Li, X.; Sun, L. Endoplasmic reticulum is at the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in the pathogenesis of diabetes mellitus. J. Diabetes Res. 2013, 2013, 193461. [Google Scholar] [CrossRef]
- Salvado, L.; Palomer, X.; Barroso, E.; Vazquez-Carrera, M. Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol. Metab. 2015, 26, 438–448. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. 2008, 32 (Suppl. 7), S52–S54. [Google Scholar] [CrossRef]
- Sabroe, I.; Parker, L.C.; Dower, S.K.; Whyte, M.K. The role of TLR activation in inflammation. J. Pathol. 2008, 214, 126–135. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. TLRs and chronic inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef]
- Said, E.A.; Tremblay, N.; Al-Balushi, M.S.; Al-Jabri, A.A.; Lamarre, D. Viruses Seen by Our Cells: The Role of Viral RNA Sensors. J. Immunol. Res. 2018, 2018, 9480497. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Toll-like Receptor as a Molecular Link between Metabolic Syndrome and Inflammation: A Review. Curr. Drug Targets 2019, 20, 1264–1280. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Liu, C.; Youn, J.Y.; Cai, H. Toll-Like Receptor 2 (TLR2) Knockout Abrogates Diabetic and Obese Phenotypes While Restoring Endothelial Function via Inhibition of NOX1. Diabetes 2021, 70, 2107–2119. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, J.S.Y.; Stefanovic, N.; Al-Sharea, A.; Simpson, D.S.; Mukhamedova, N.; Jandeleit-Dahm, K.; Murphy, A.J.; Sviridov, D.; Vince, J.E.; et al. Specific NLRP3 Inhibition Protects Against Diabetes-Associated Atherosclerosis. Diabetes 2021, 70, 772–787. [Google Scholar] [CrossRef]
- Holley, A.E.; Cheeseman, K.H. Measuring free radical reactions in vivo. Br. Med. Bull. 1993, 49, 494–505. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Boss, A.; Kao, C.H.; Murray, P.M.; Marlow, G.; Barnett, M.P.; Ferguson, L.R. Human Intervention Study to Assess the Effects of Supplementation with Olive Leaf Extract on Peripheral Blood Mononuclear Cell Gene Expression. Int. J. Mol. Sci. 2016, 17, 2019. [Google Scholar] [CrossRef]
- Liew, C.C.; Ma, J.; Tang, H.C.; Zheng, R.; Dempsey, A.A. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef]
- Olsen, K.S.; Skeie, G.; Lund, E. Whole-Blood Gene Expression Profiles in Large-Scale Epidemiological Studies: What Do They Tell? Curr. Nutr. Rep. 2015, 4, 377–386. [Google Scholar] [CrossRef]
- Restaino, R.M.; Deo, S.H.; Parrish, A.R.; Fadel, P.J.; Padilla, J. Increased monocyte-derived reactive oxygen species in type 2 diabetes: Role of endoplasmic reticulum stress. Exp. Physiol. 2017, 102, 139–153. [Google Scholar] [CrossRef]
- Degasperi, G.R.; Denis, R.G.; Morari, J.; Solon, C.; Geloneze, B.; Stabe, C.; Pareja, J.C.; Vercesi, A.E.; Velloso, L.A. Reactive oxygen species production is increased in the peripheral blood monocytes of obese patients. Metabolism 2009, 58, 1087–1095. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Adams-Huet, B.; Chen, X.; Kaur, H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E1844–E1850. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, M.; Garcia-Perez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.; Lee, S.Y. Glycogen synthase kinase 3beta in Toll-like receptor signaling. BMB Rep. 2016, 49, 305–310. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Martin, M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 2011, 53, 130–140. [Google Scholar] [CrossRef]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Therapeutic regulation of the NLRP3 inflammasome in chronic inflammatory diseases. Arch. Pharm. Res. 2021, 44, 16–35. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Padron, J.G.; Saito Reis, C.A.; Kendal-Wright, C.E. The Role of Danger Associated Molecular Patterns in Human Fetal Membrane Weakening. Front. Physiol. 2020, 11, 602. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Ji, N.; Qi, Z.; Wang, Y.; Yang, X.; Yan, Z.; Li, M.; Ge, Q.; Zhang, J. Pyroptosis: A New Regulating Mechanism in Cardiovascular Disease. J. Inflamm. Res. 2021, 14, 2647–2666. [Google Scholar] [CrossRef]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006, 83, 447S–455S. [Google Scholar] [CrossRef] [PubMed]
- Gracie, J.A.; Robertson, S.E.; McInnes, I.B. Interleukin-18. J. Leukoc. Biol. 2003, 73, 213–224. [Google Scholar] [CrossRef]
- Dhawan, V.; Mahajan, N.; Jain, S. Role of C-C chemokines in Takayasu’s arteritis disease. Int. J. Cardiol. 2006, 112, 105–111. [Google Scholar] [CrossRef]
- Morel, J.C.; Park, C.C.; Woods, J.M.; Koch, A.E. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J. Biol. Chem. 2001, 276, 37069–37075. [Google Scholar] [CrossRef]
- Khan, A.I.; Landis, R.C.; Malhotra, R. L-Selectin ligands in lymphoid tissues and models of inflammation. Inflammation 2003, 27, 265–280. [Google Scholar] [CrossRef]
- Gautier, E.L.; Jakubzick, C.; Randolph, G.J. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1412–1418. [Google Scholar] [CrossRef]
- Barlic, J.; Murphy, P.M. Chemokine regulation of atherosclerosis. J. Leukoc. Biol. 2007, 82, 226–236. [Google Scholar] [CrossRef]
- O’Connor, T.; Borsig, L.; Heikenwalder, M. CCL2-CCR2 Signaling in Disease Pathogenesis. Endocr. Metab. Immune. Disord. Drug Targets 2015, 15, 105–118. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Grechko, A.V.; Myasoedova, V.A.; Orekhov, A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp. Mol. Pathol. 2018, 104, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Schober, A. Chemokines in vascular dysfunction and remodeling. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Usarek, E.; Baranczyk-Kuzma, A.; Kazmierczak, B.; Gajewska, B.; Kuzma-Kozakiewicz, M. Validation of qPCR reference genes in lymphocytes from patients with amyotrophic lateral sclerosis. PLoS ONE 2017, 12, e0174317. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).