The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond
Abstract
1. Introduction
2. Computational Details
3. Illustrative Crystal Systems
3.1. Diazanediium Dichloride
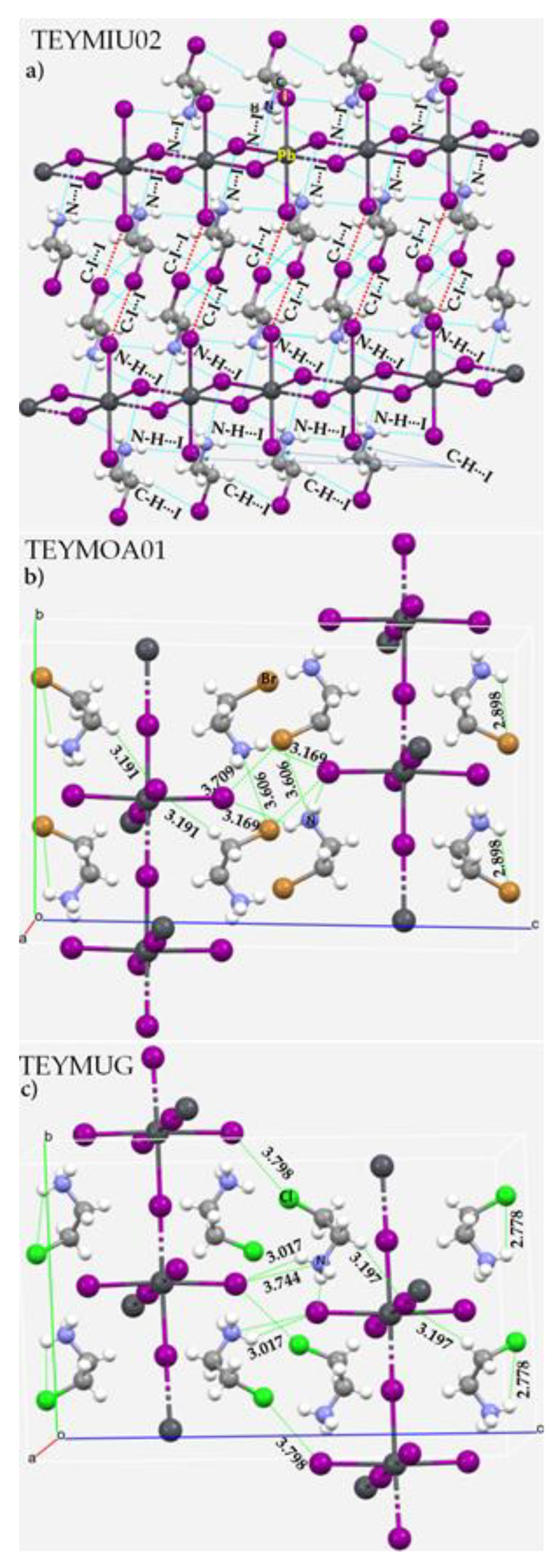
3.2. Methylammonium Lead Halide Perovskites
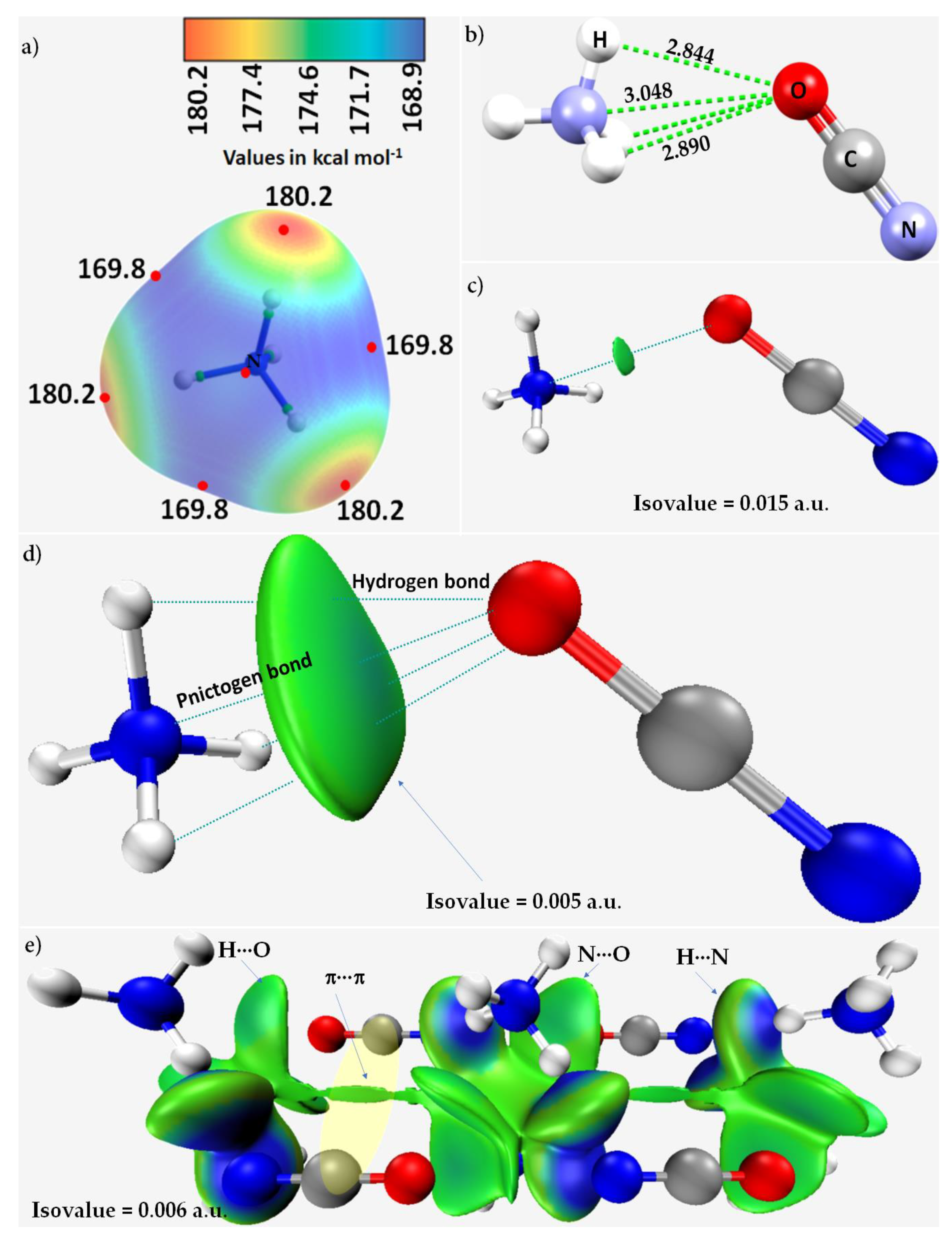
3.3. Methylammonium and Deutero-Methylammonium Halides
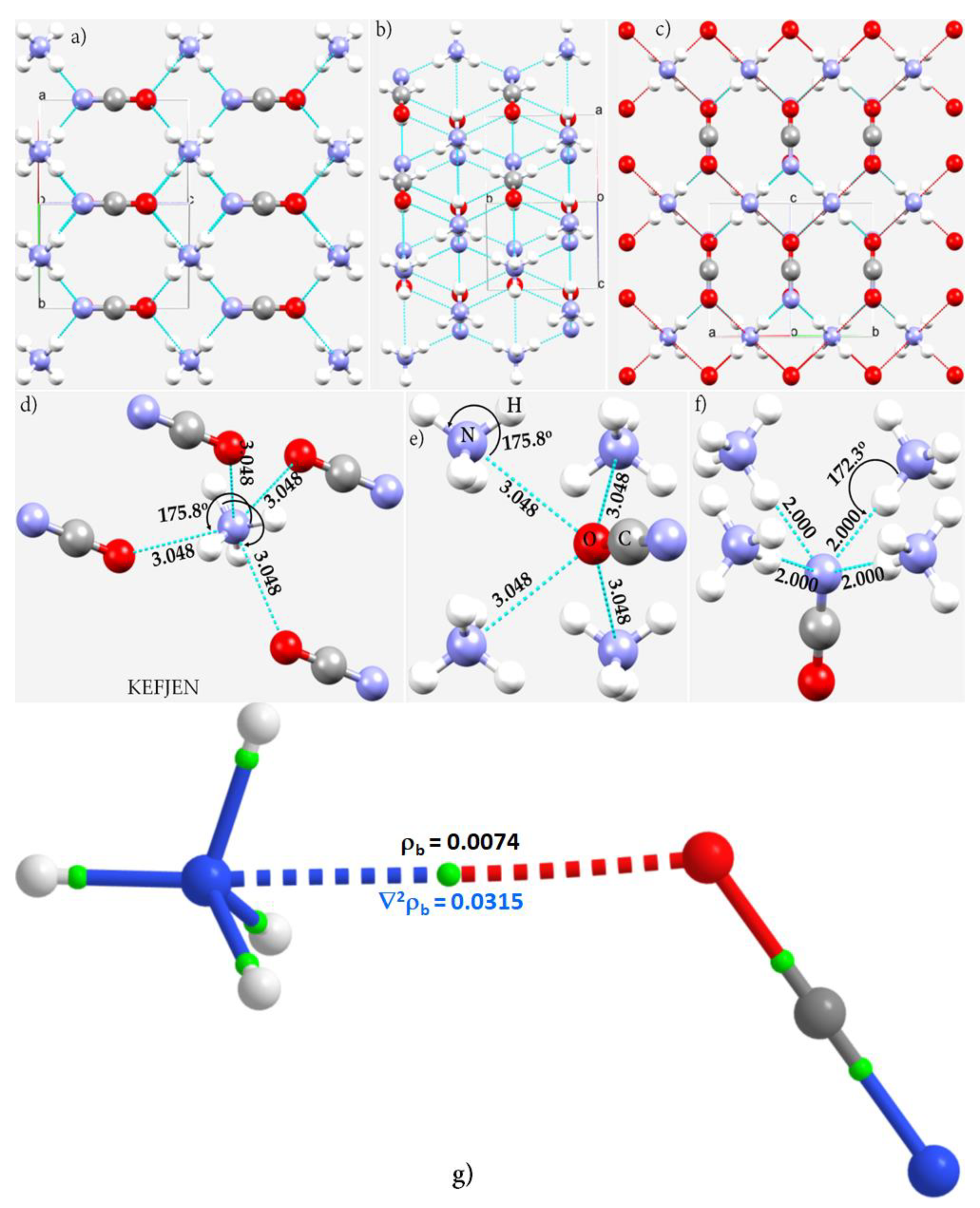
3.4. Ammonium Cyanate
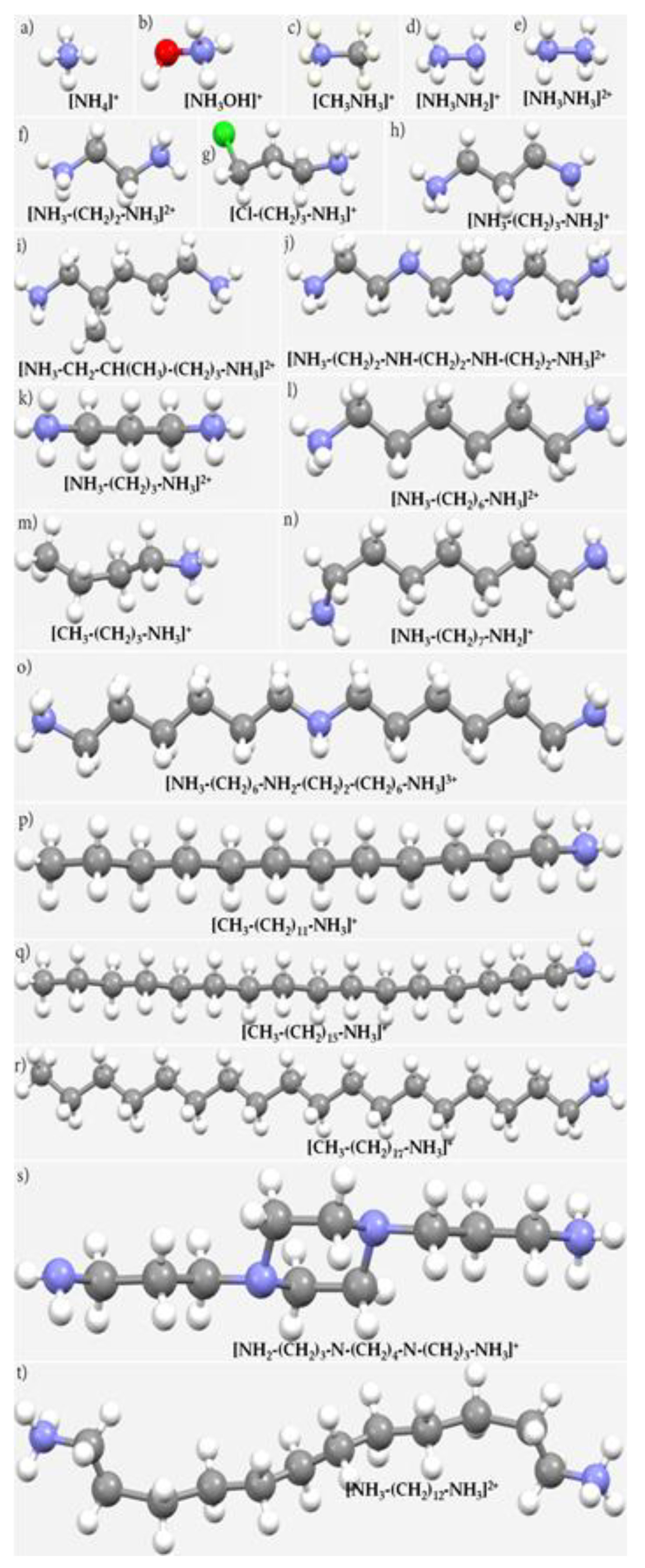
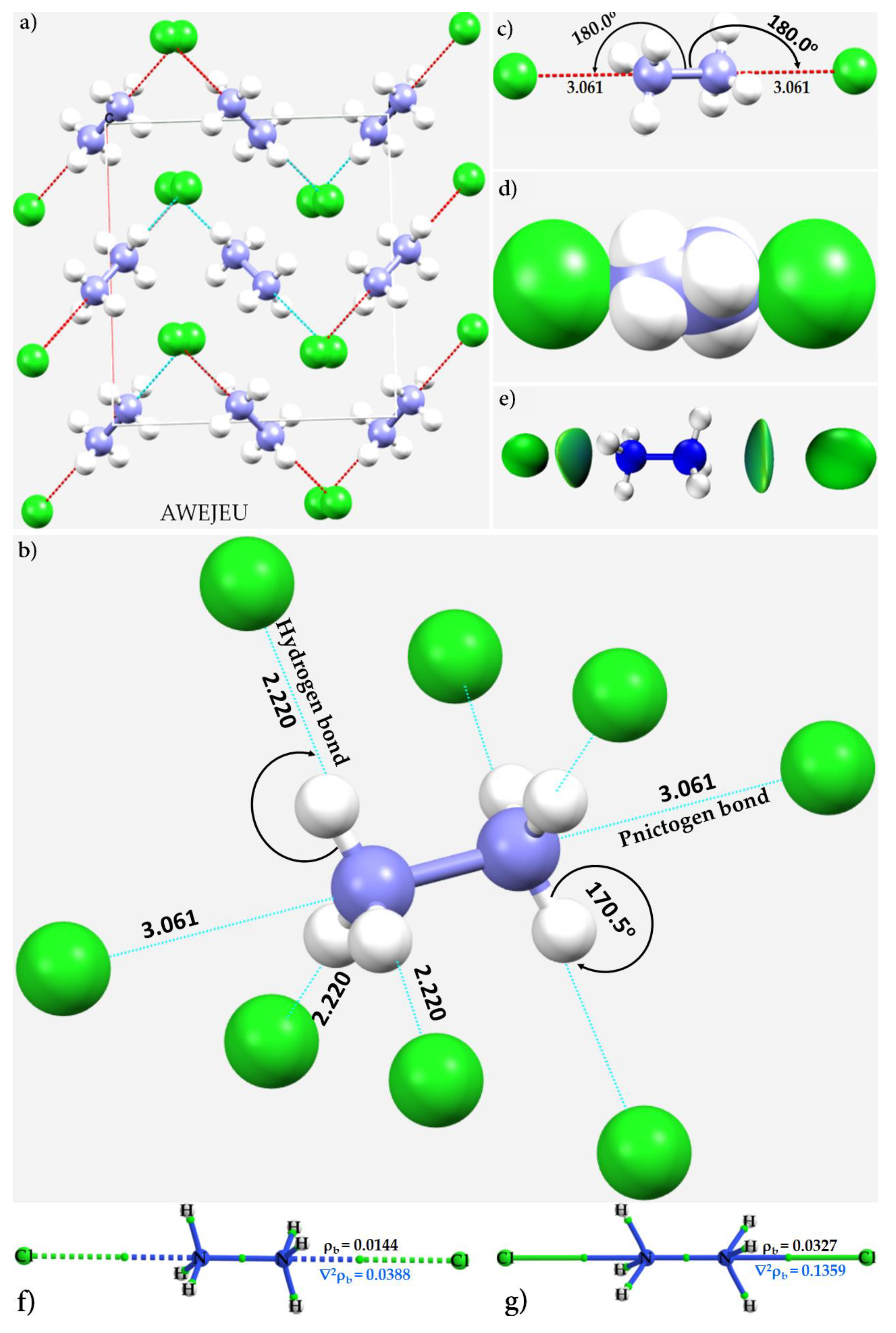
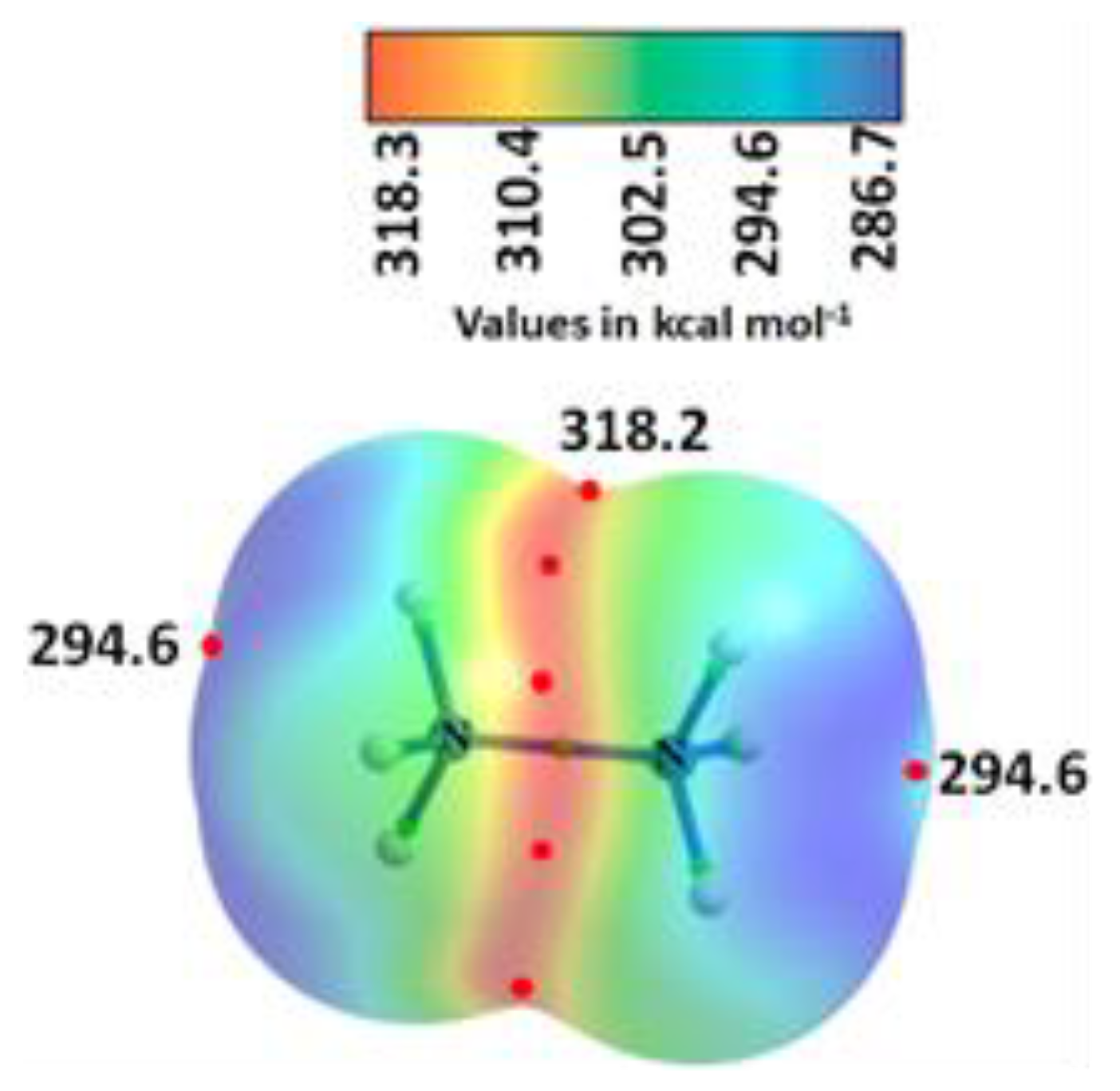
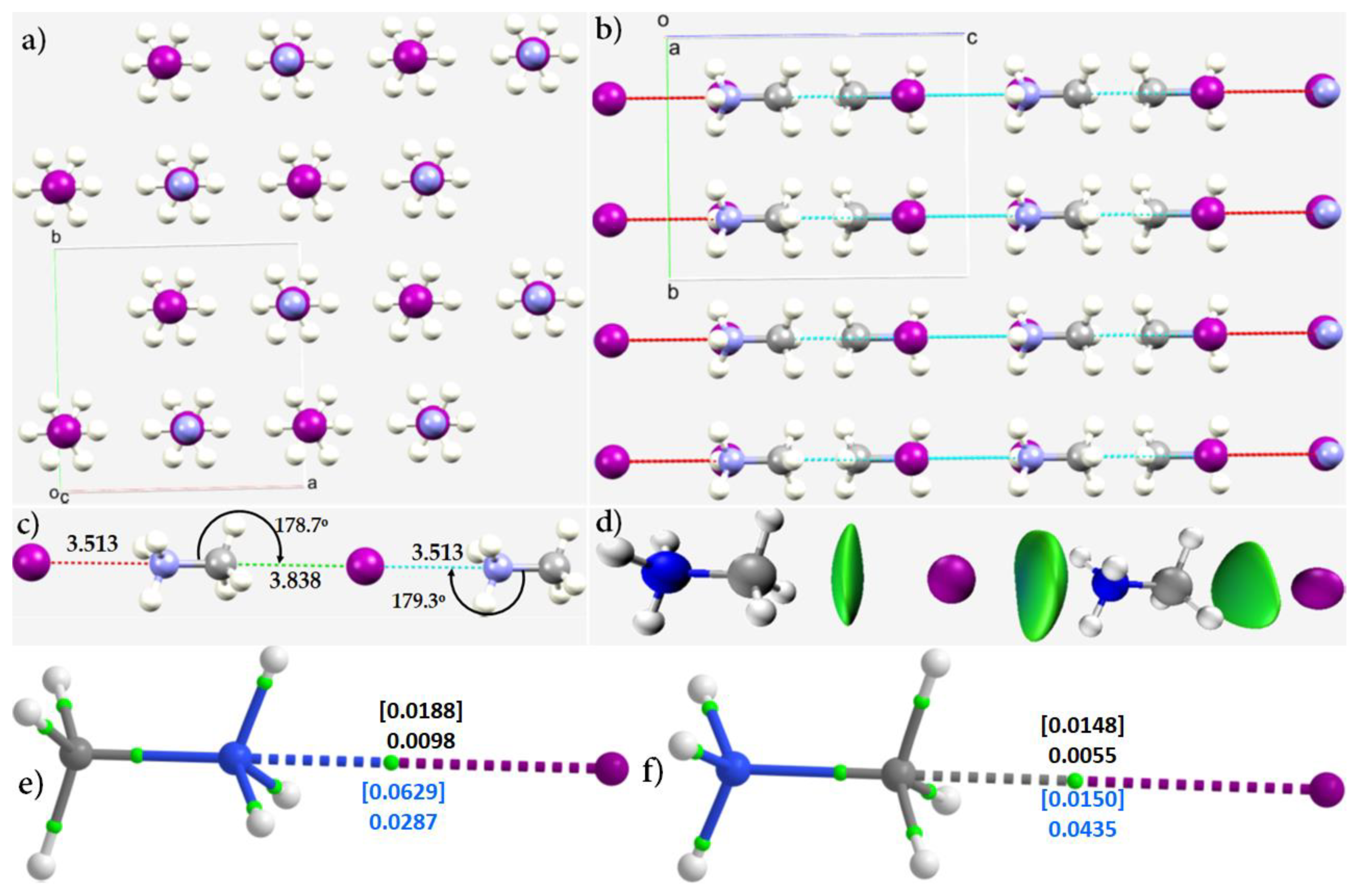
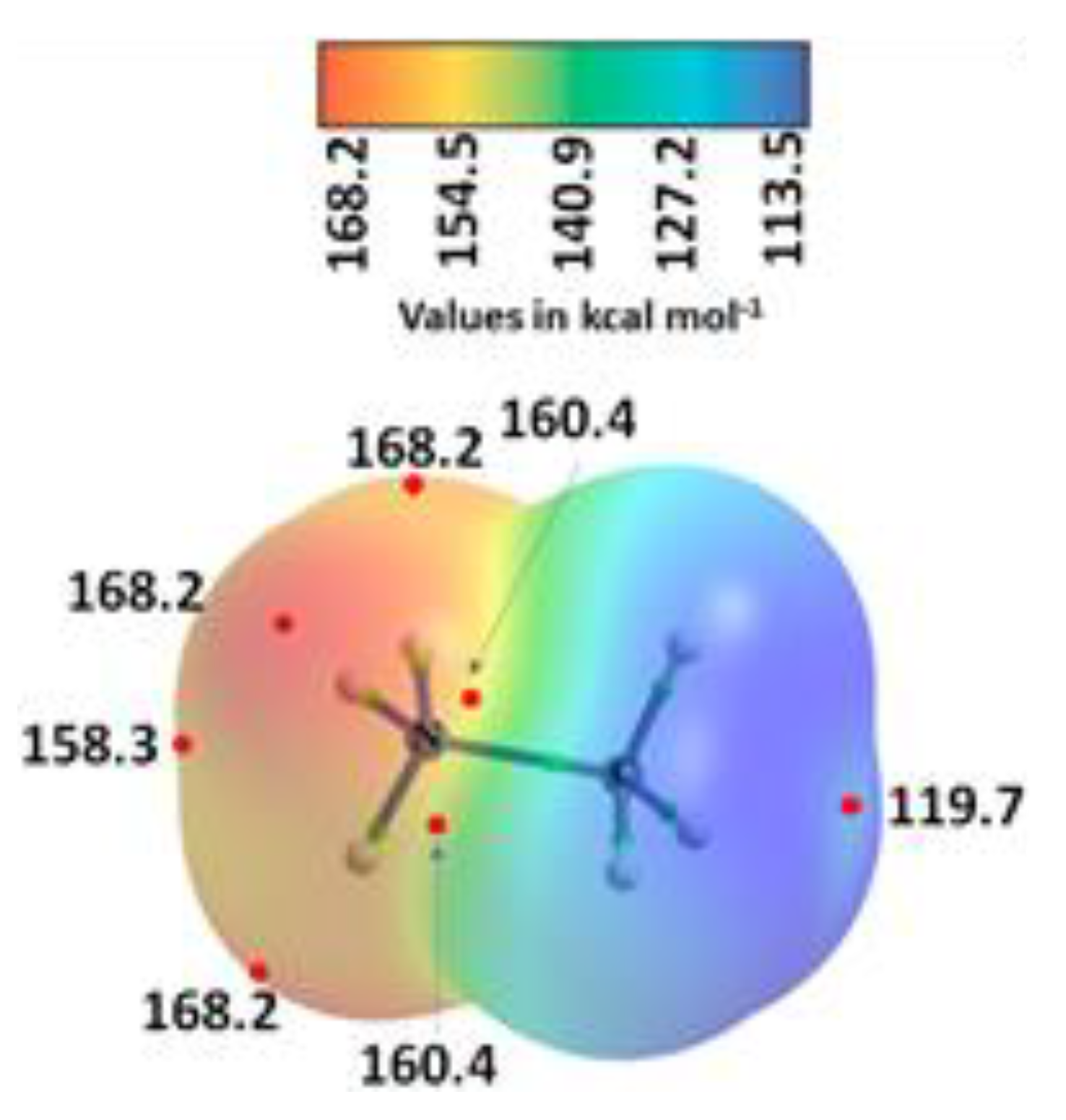
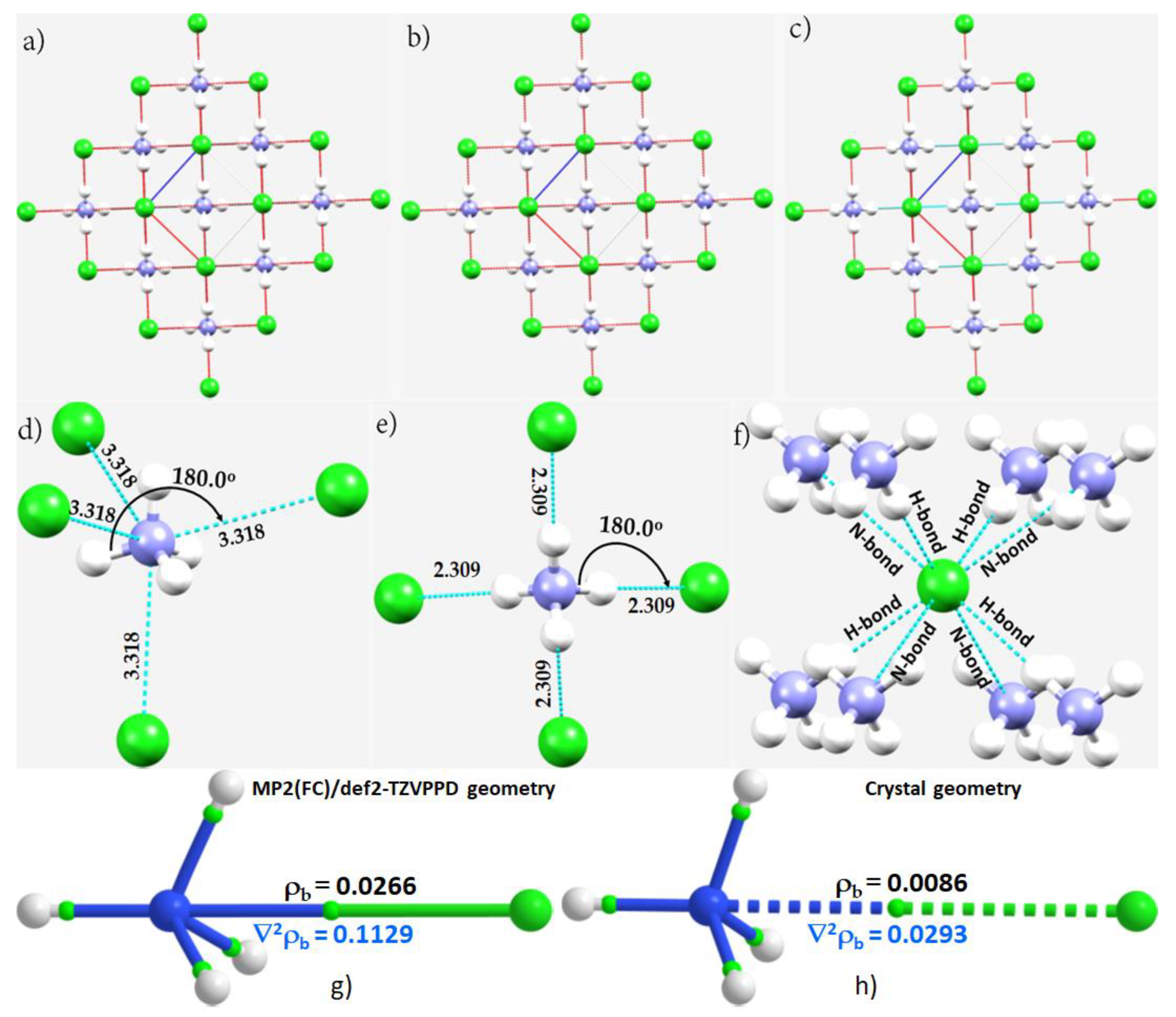
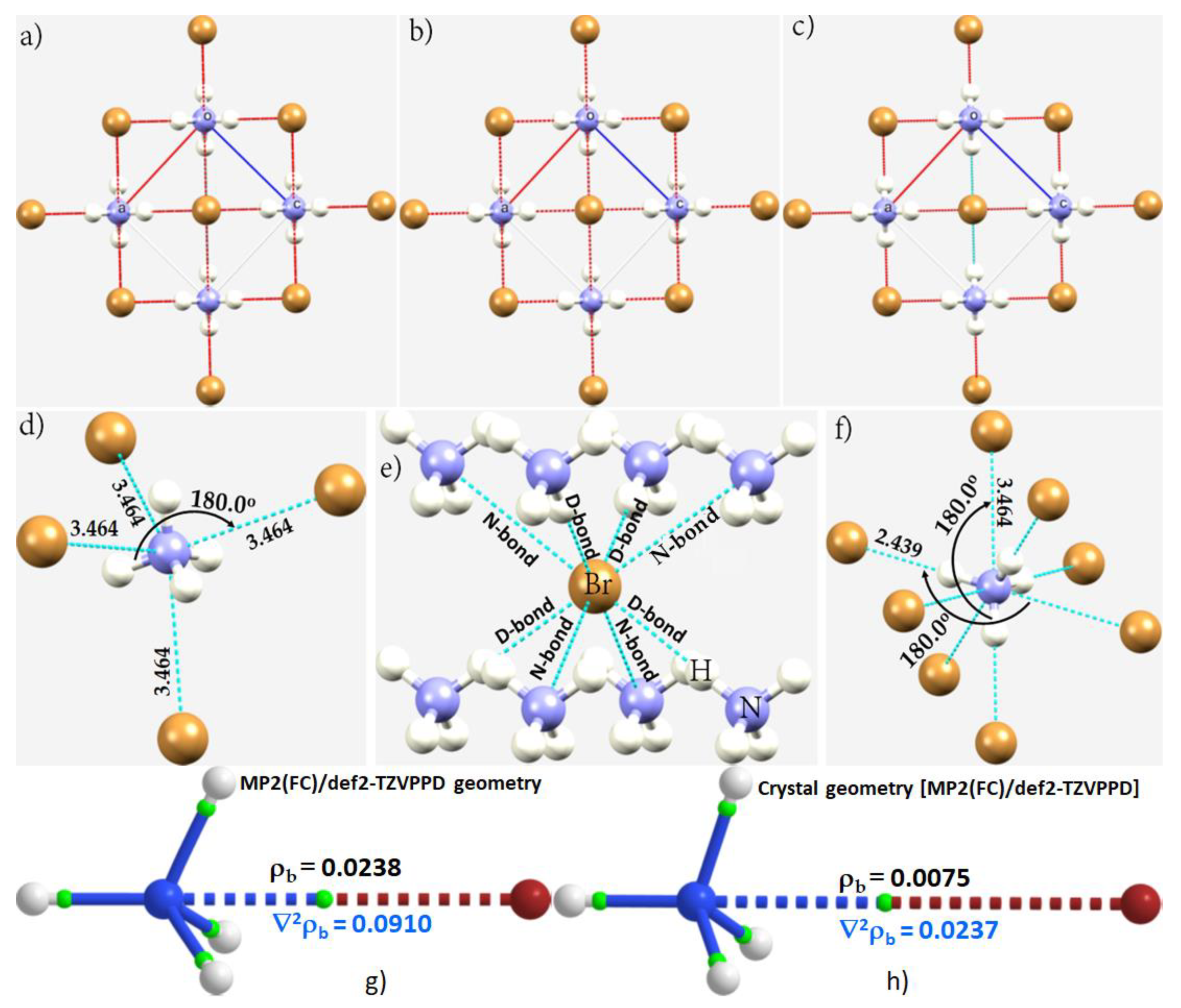
3.5. The Ammonium Halides
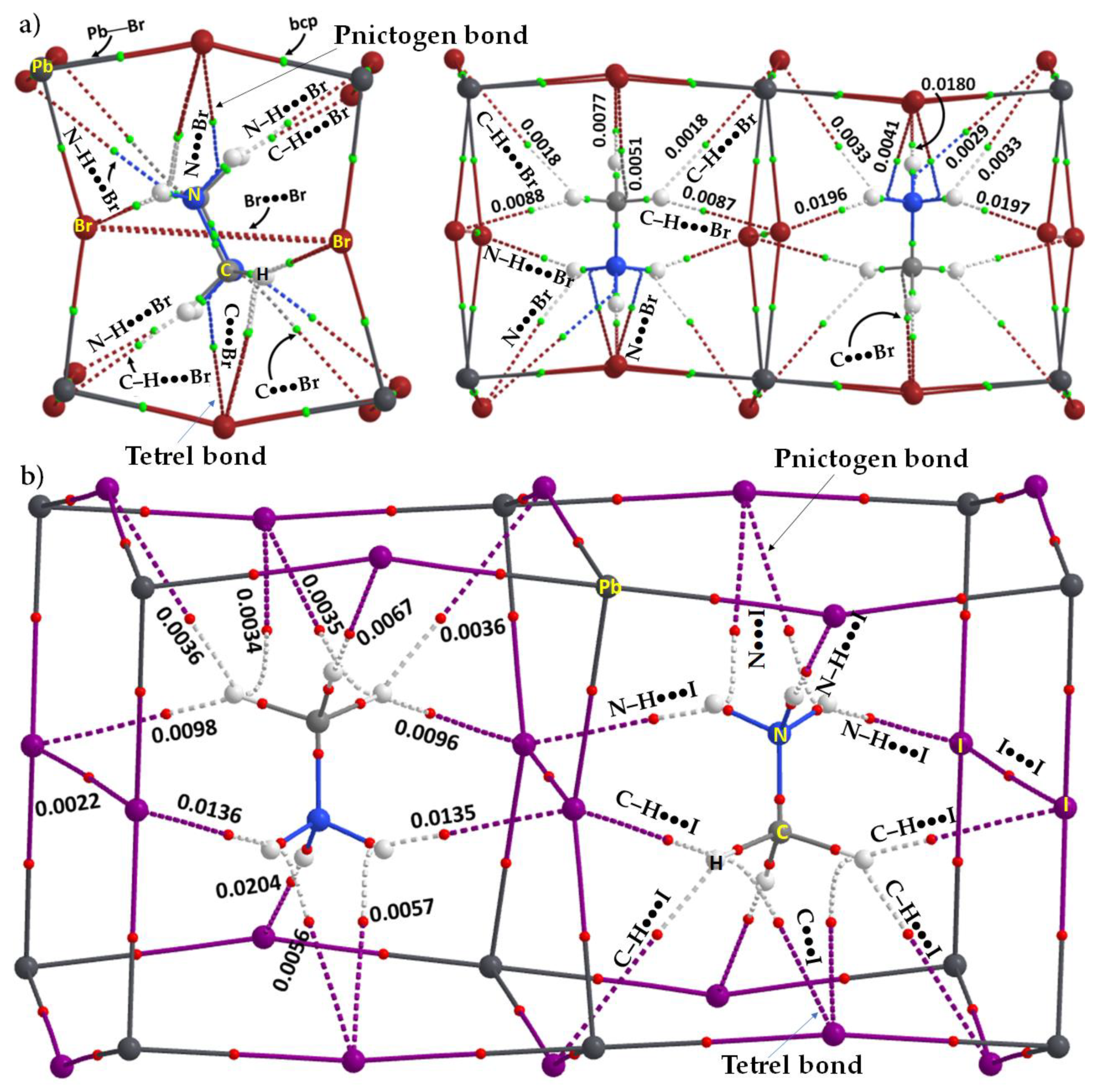
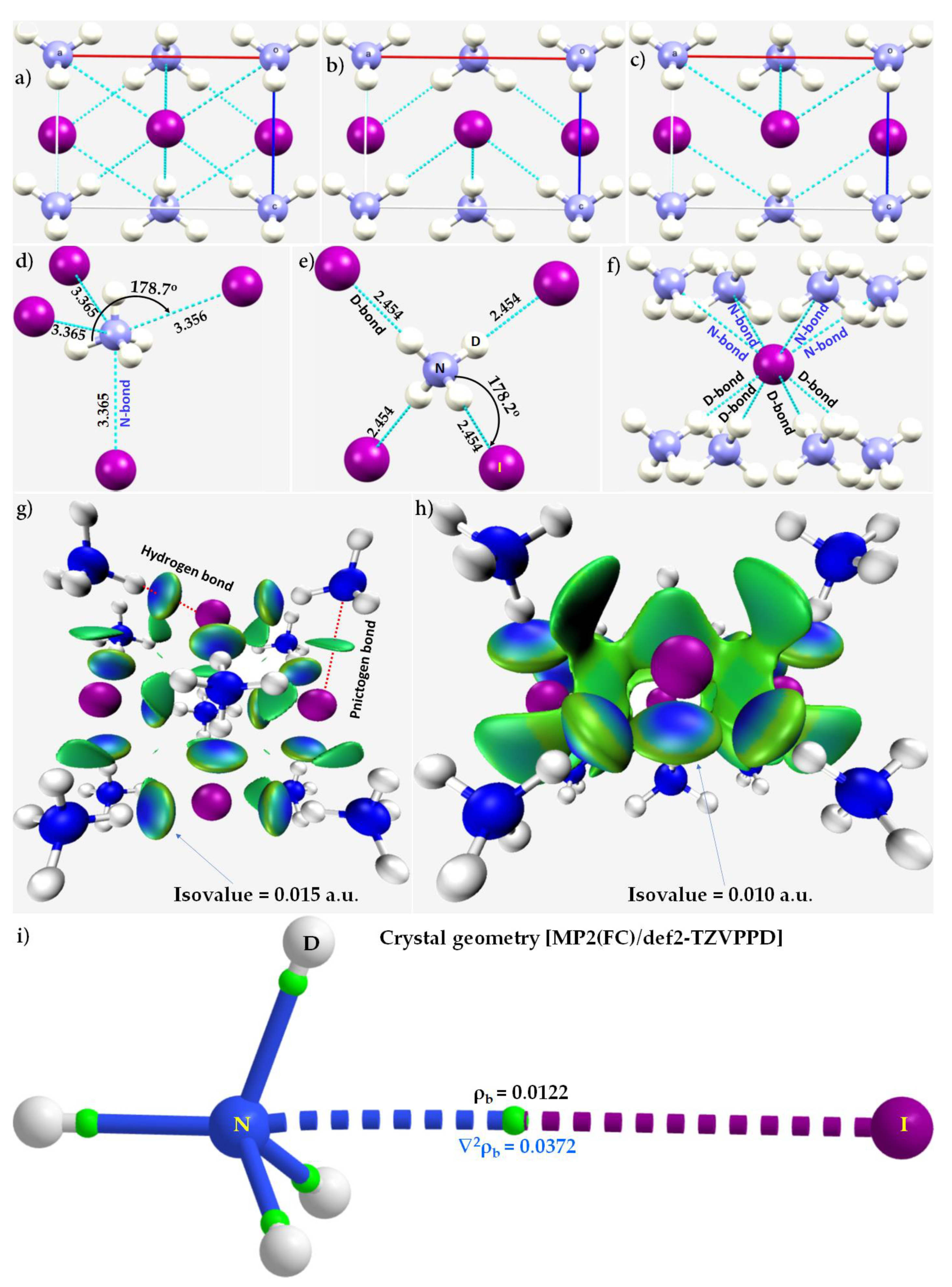
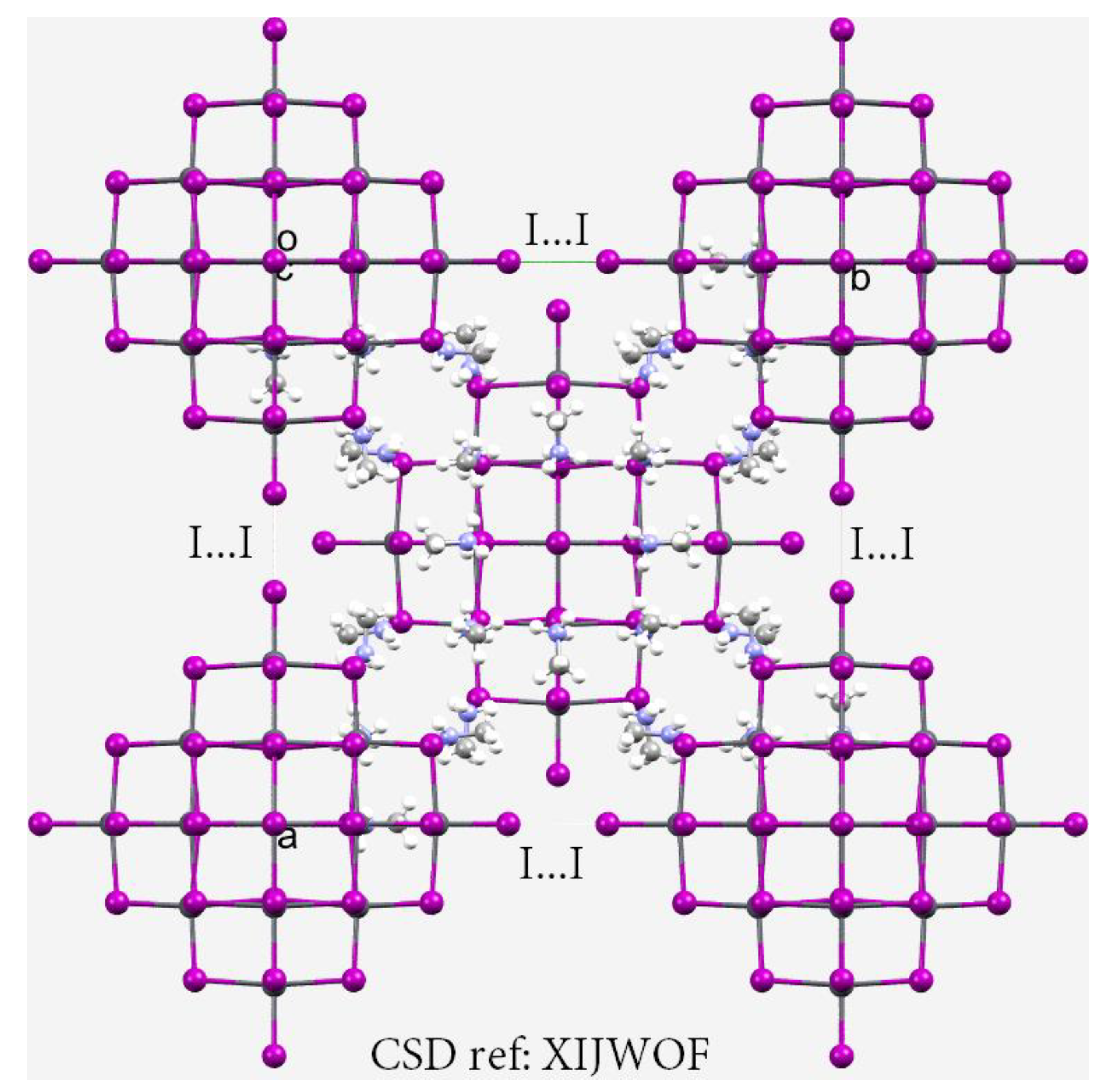
3.6. The Crystal Structure of ([I44Pb18][CH3NH3]
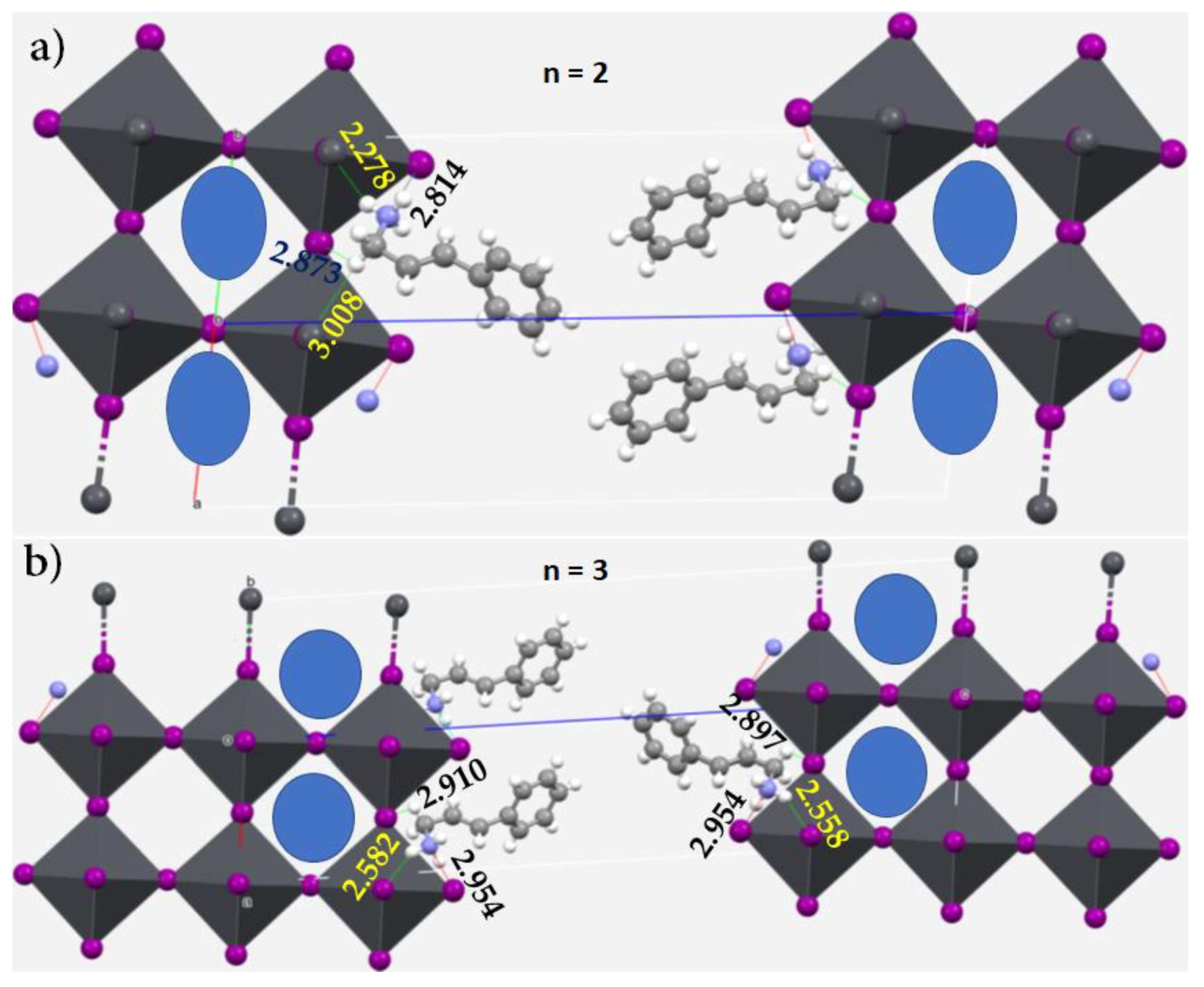
3.7. Pnictogen Bond in 2D Functional Crystals of Metal Halide Perovskites: Derivatives of Ammonium as Pnictogen Bond Donors
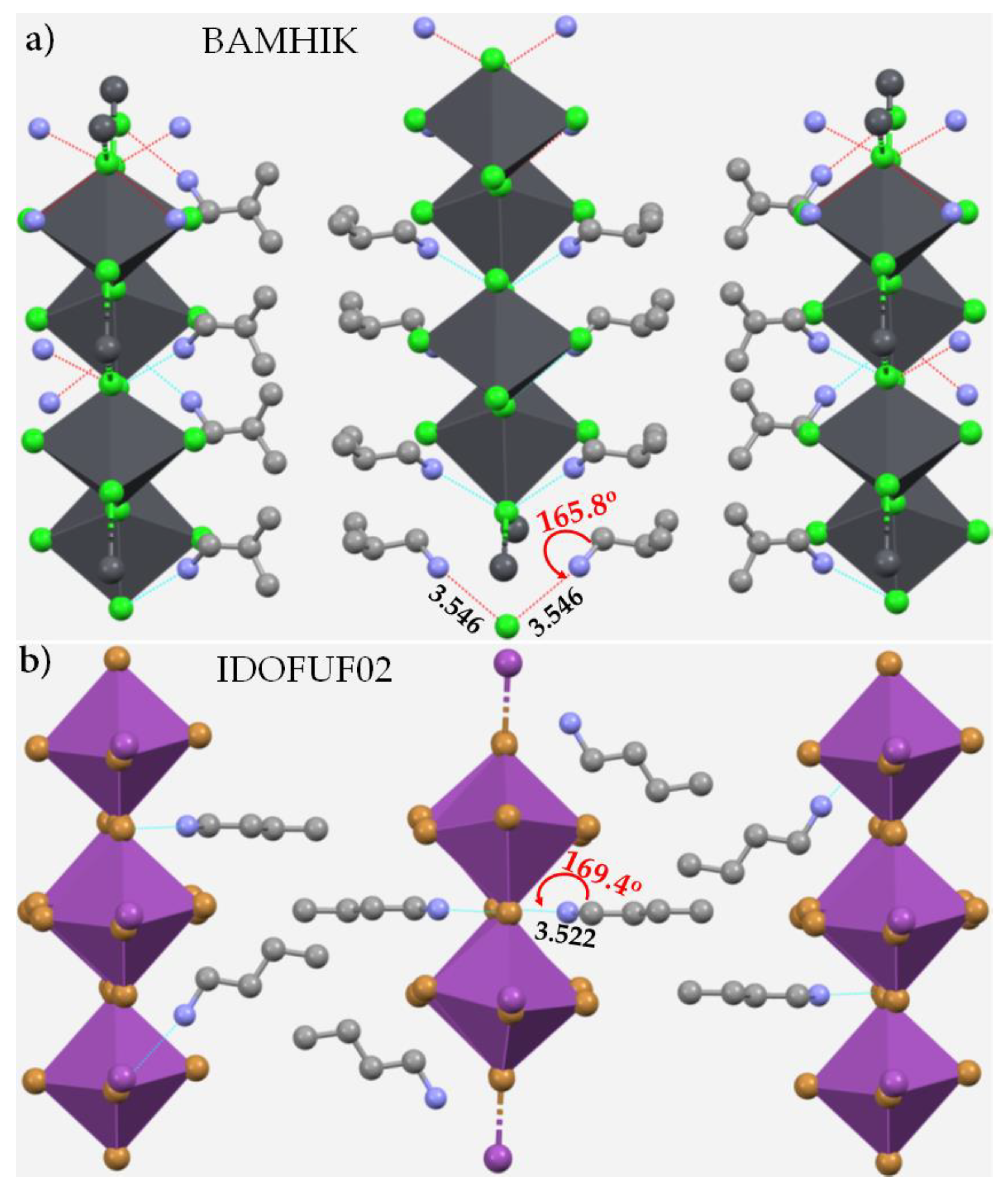
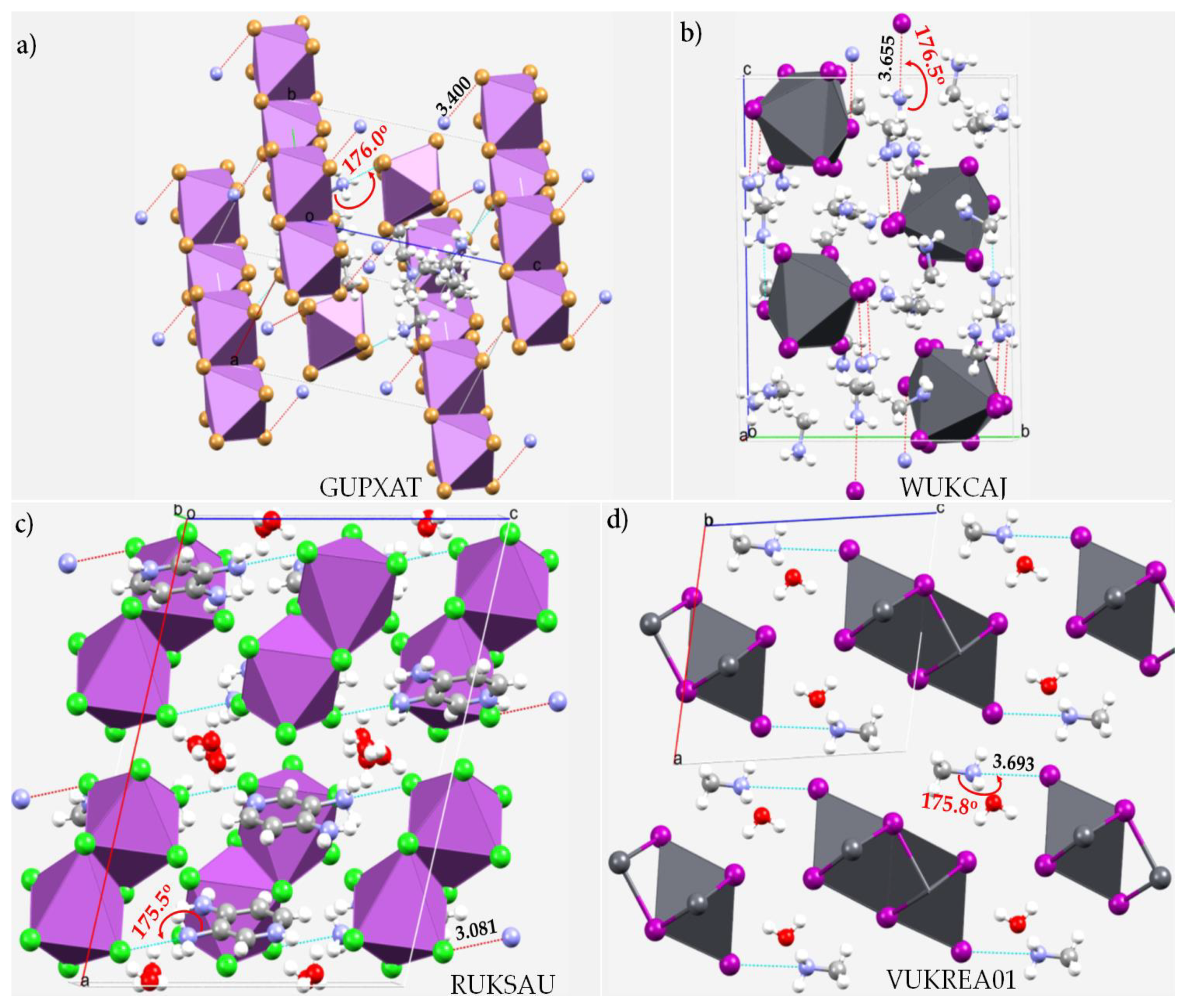
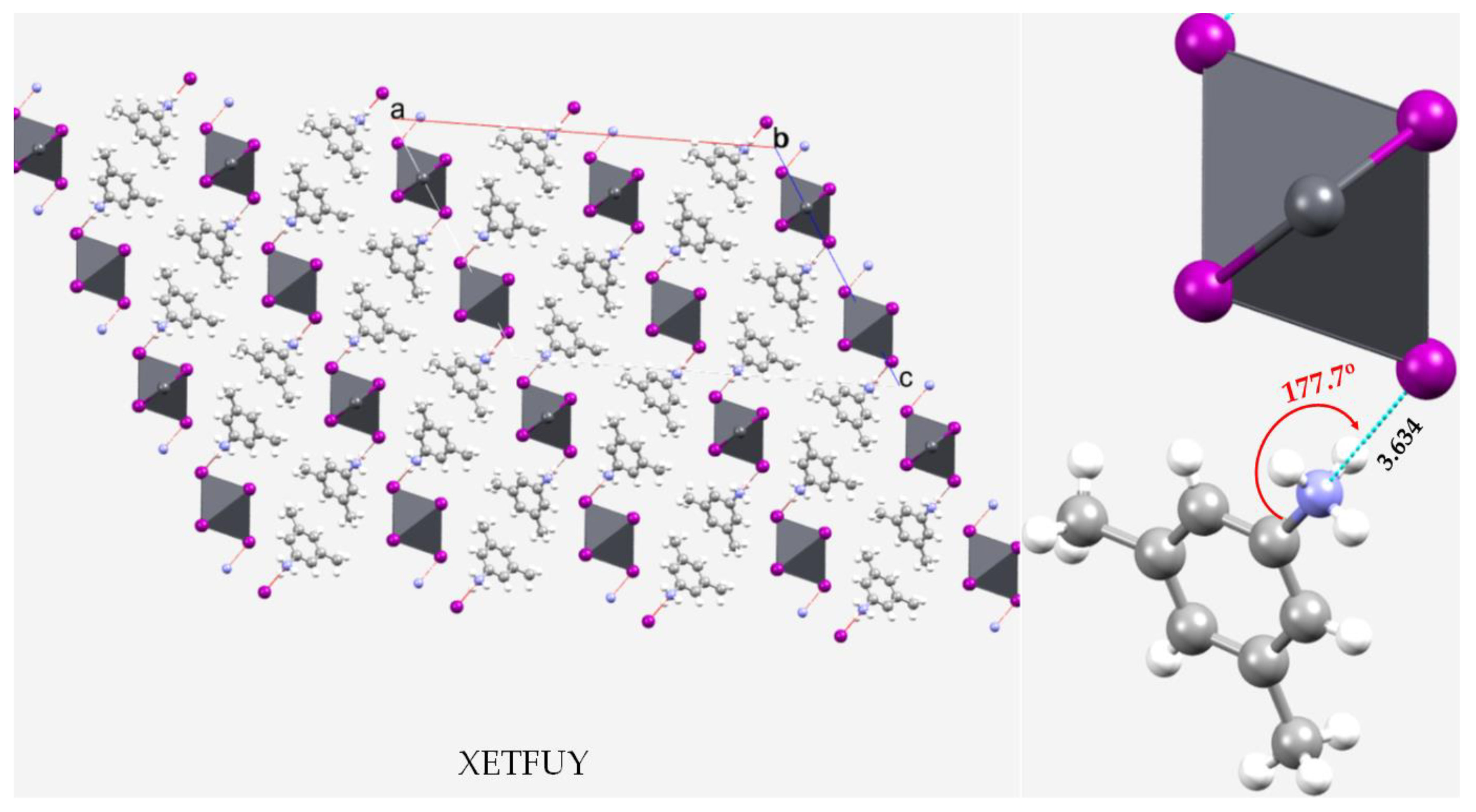
4. Pnictogen Bonding in 1D (One-Dimensional) Perovskite Systems
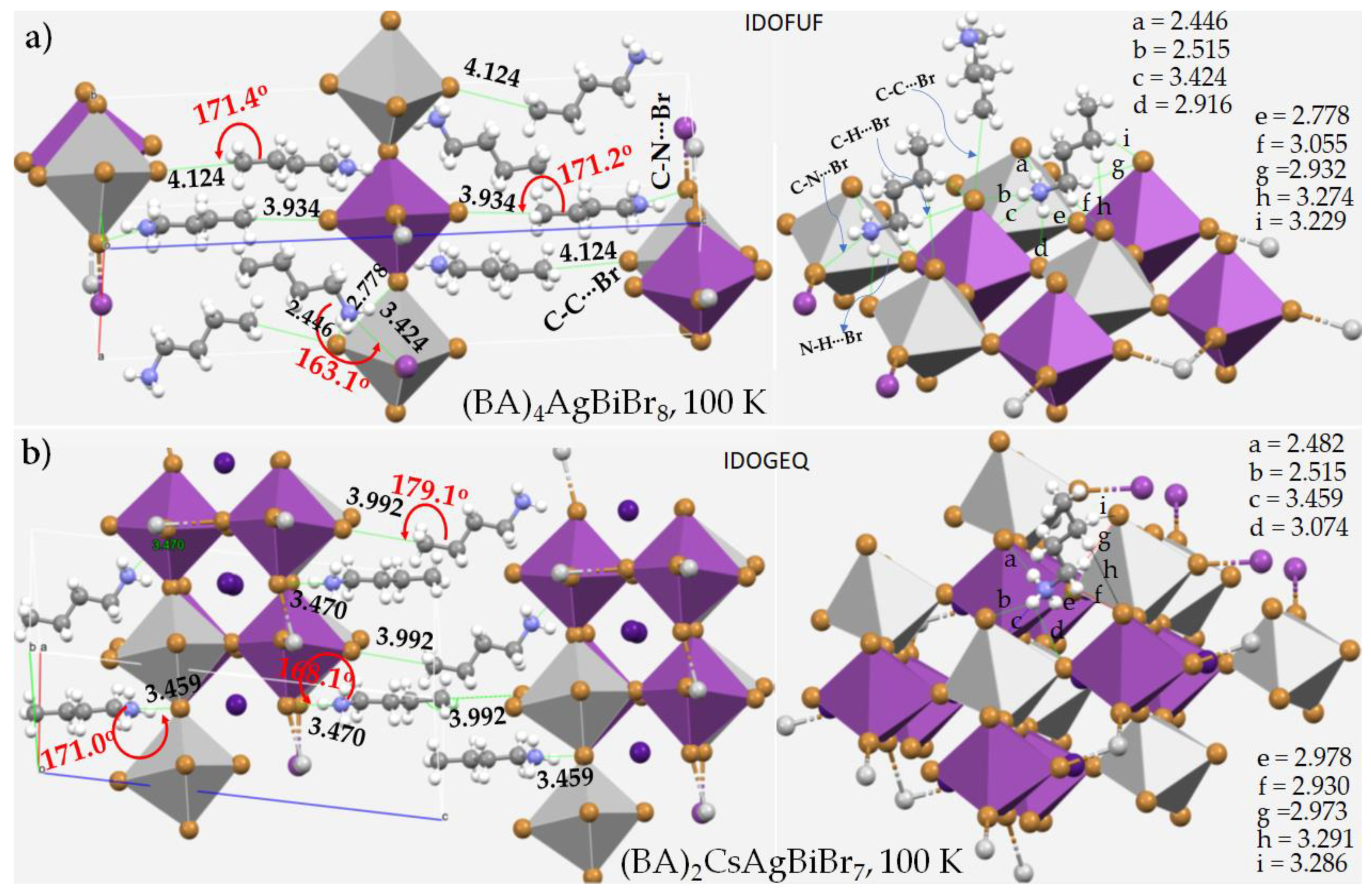
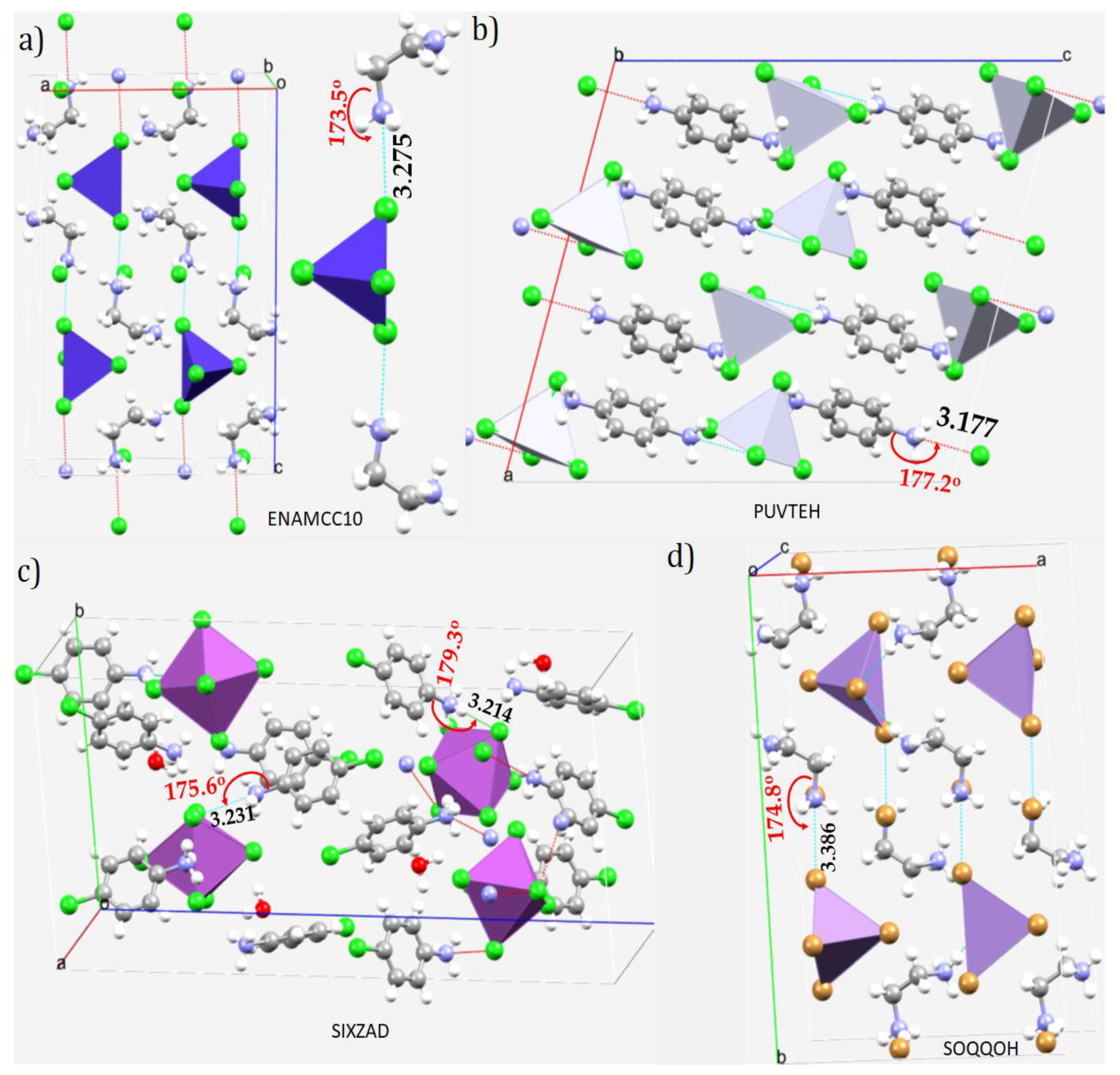
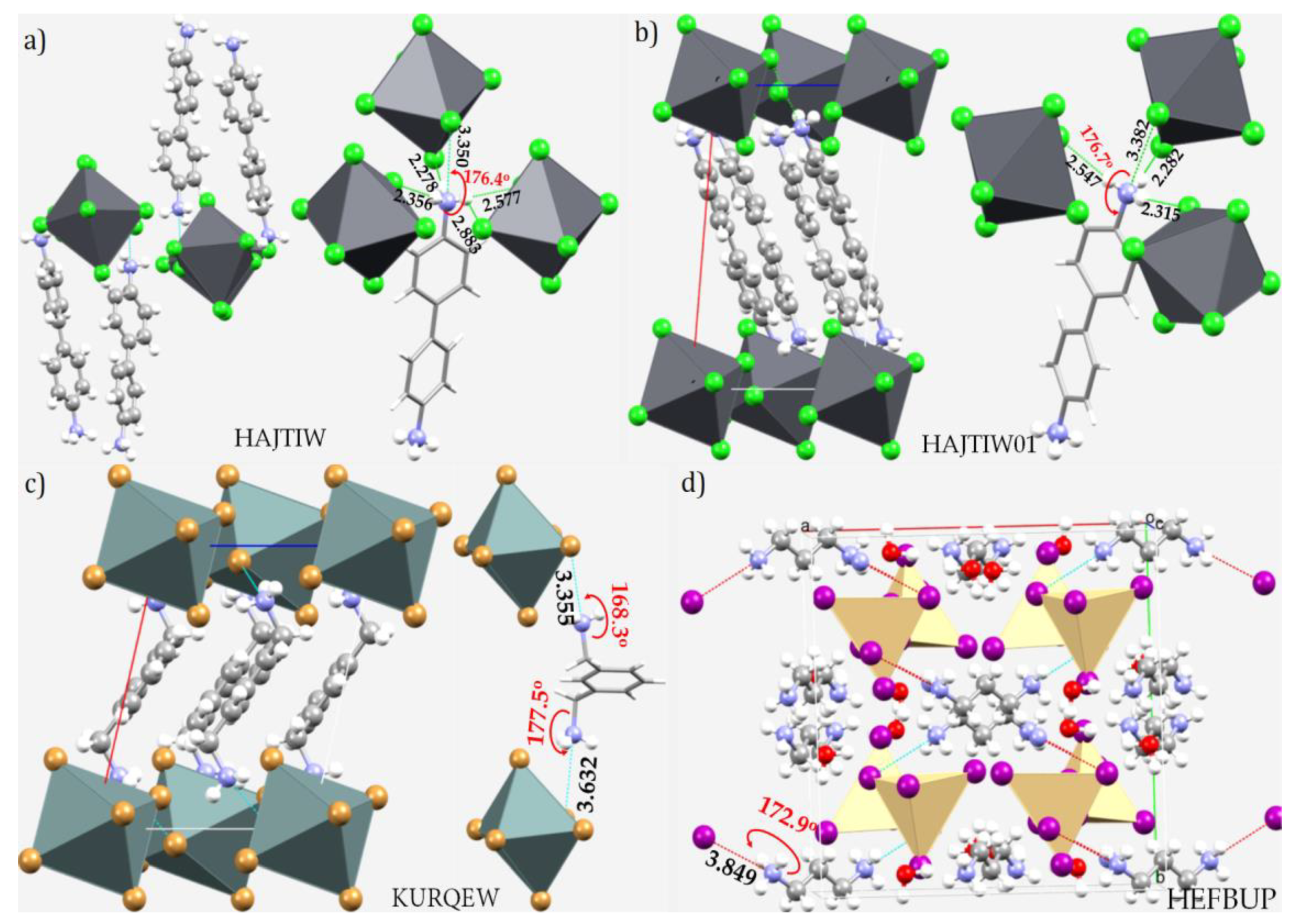
5. Pnictogen Bonding in Zero-Dimensional (0D) Crystal Systems
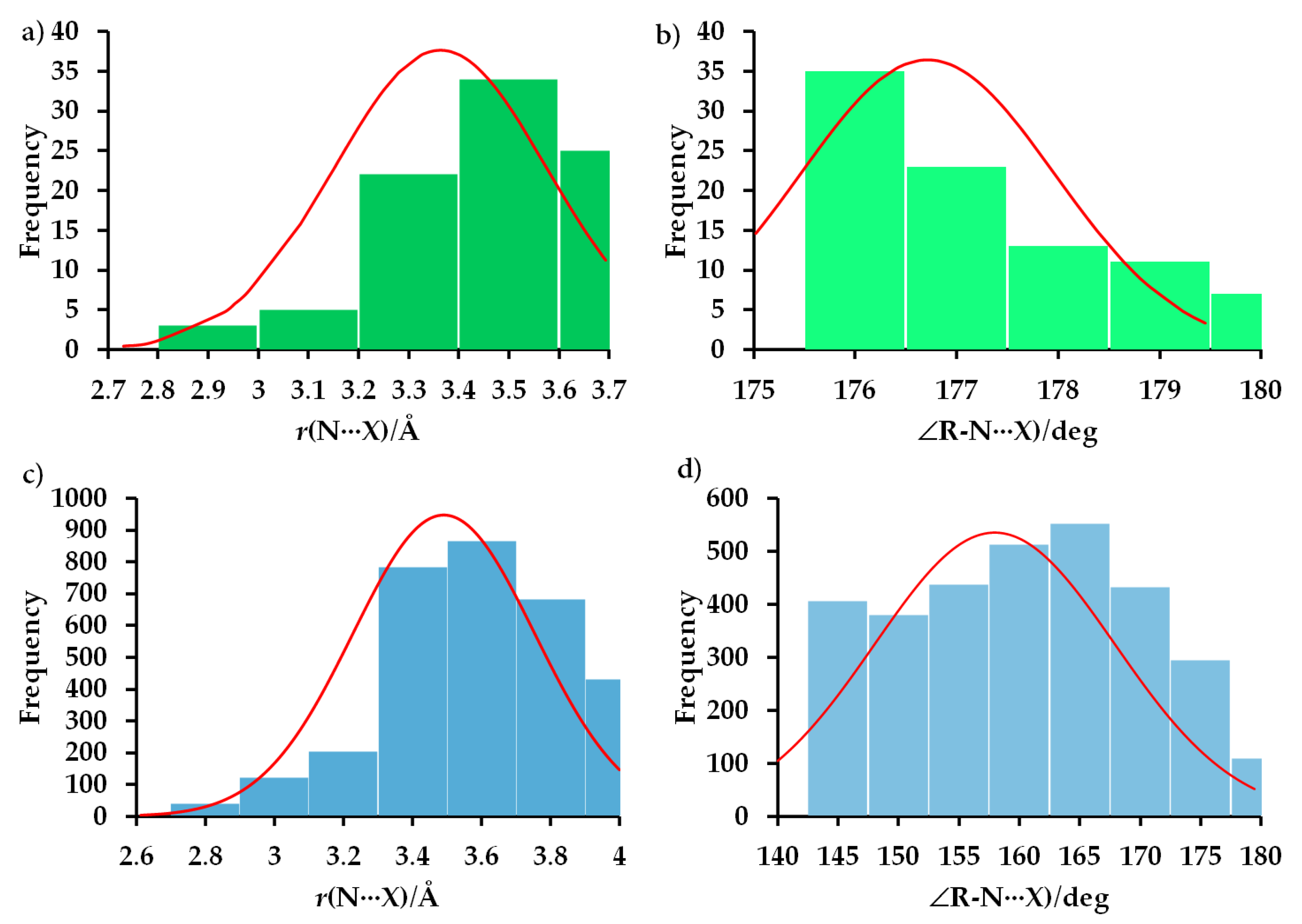
6. Statistical Analysis of N-Centered Pnictogen Bond Distances and Angles in Crystals
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Uyanik, M.; Kato, T.; Sahara, N.; Katade, O.; Ishihara, K. High-Performance Ammonium Hypoiodite/Oxone Catalysis for Enantioselective Oxidative Dearomatization of Arenols. ACS Catal. 2019, 9, 11619–11626. [Google Scholar] [CrossRef]
- Hinokuma, S.; Sato, K. Ammonia Combustion Catalysts. Chem. Lett. 2021, 50, 752–759. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries 2020; US Geological Survey: Reston, VA, USA, 2020. [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Ogasawara, K.; Nakao, T.; Kishida, K.; Ye, T.-N.; Lu, Y.; Abe, H.; Niwa, Y.; Sasase, M.; Kitano, M.; Hosono, H. Ammonia Decomposition over CaNH-Supported Ni Catalysts via an NH2−-Vacancy-Mediated Mars–van Krevelen Mechanism. ACS Catal. 2021, 11, 11005–11015. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B: Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Engelmann, Y.; van ‘t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-driven catalysis: Green ammonia synthesis with intermittent electricity. Green Chem. 2020, 22, 6258–6287. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. The Nitrogen Bond, or The Nitrogen-centered Pnictogen Bond: The Covalently Bound Nitrogen Atom in Molecular Entities and Crystals as a Pnictogen Bond Donor. Compounds 2022, 2, 80–110. [Google Scholar] [CrossRef]
- Reiss, G.J. UJONUD, dep. no. 790189. CSD Commun. 2010. [Google Scholar] [CrossRef]
- Partridge, H.; Bauschlicher, C.W. Calculation of magnesium (1+)-ligand relative binding energies. J. Phys. Chem. 1992, 96, 8827–8832. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, S.; Mhin, B.J.; Cho, S.J.; Kim, J. Structures and energetics of (Zn (NH3)n2+ (n = 4–6). Coordination number of Zn2+ by ammine. Chem. Phys. Lett. 1993, 216, 309–312. [Google Scholar] [CrossRef]
- Dudev, T.; Cowan, J.A.; Lim, C. Competitive binding in magnesium coordination chemistry: Water versus ligands of biological interest. J. Am. Chem. Soc. 1999, 121, 7665–7673. [Google Scholar] [CrossRef]
- Bérces, A.; Nukada, T.; Margl, P.; Ziegler, T. Solvation of Cu2+ in Water and Ammonia. Insight from Static and Dynamical Density Functional Theory. J. Phys. Chem. A 1999, 103, 9693–9701. [Google Scholar] [CrossRef]
- Pavelka, M.; Burda, J.V. Theoretical description of copper Cu (I)/Cu (II) complexes in mixed ammine-aqua environment. DFT and ab initio quantum chemical study. Chem. Phys. 2005, 312, 193–204. [Google Scholar] [CrossRef]
- Hancock, R.D.; Bartolotti, L.J. Density Functional Theory-Based Prediction of the Formation Constants of Complexes of Ammonia in Aqueous Solution: Indications of the Role of Relativistic Effects in the Solution Chemistry of Gold(I). Inorg. Chem. 2005, 44, 7175–7183. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Cukrowski, I.; Marques, H.M. DFT-X3LYP Studies on the Coordination Chemistry of Ni2+. Part 1: Six Coordinate [Ni(NH3)n(H2O)6-n]2+ Complexes. J. Phys. Chem. A 2008, 112, 10657–10666. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Cukrowski, I.; Marques, H.M. Low-spin complexes of Ni2+ with six NH3 and H2O ligands: A DFT–RX3LYP study. J. Mol. Str. (THEOCHEM) 2009, 915, 20–32. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Marques, H.M. The physical chemistry of coordinated aqua-, ammine-, and mixed-ligand Co2+ complexes: DFT studies on the structure, energetics, and topological properties of the electron density. Phys. Chem. Chem. Phys. 2010, 12, 2126–2138. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Jin, B.Y. Ligand(s)-to-metal charge transfer as a factor controlling the equilibrium constants of late first-row transition metal complexes: Revealing the Irving-Williams thermodynamical series. Phys. Chem. Chem. Phys. 2015, 17, 805–811. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- CSD 5.43; Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2022.
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. Halogen in Materials Design: Revealing the Nature of Hydrogen Bonding and Other Non-Covalent Interactions in the Polymorphic Transformations of Methylammonium Lead Tribromide Perovskite. Mater. Chem. Today 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. Significance of hydrogen bonding and other noncovalent interactions in determining octahedral tilting in the CH3NH3PbI3 hybrid organic-inorganic halide perovskite solar cell semiconductor. Sci. Rep. 2019, 9, 50. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Jin, B.Y. Significant evidence of C···O and C···C long-range contacts in several heterodimeric complexes of CO with CH3-X, should one refer to them as carbon and dicarbon bonds! Phys. Chem. Chem. Phys. 2014, 16, 17238–17252. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Carbon−carbon bonding between nitrogen heterocyclic carbenes and CO2. J. Phys. Chem. A 2017, 121, 8136–8146. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrandt, M. The Inorganic Crystal Structure Database (ICSD)—Present and Future. Crystallogr. Rev. 2004, 10, 17–22. [Google Scholar] [CrossRef]
- Belsky, A.; Hellenbrandt, M.; Karen, V.L.; Luksch, P. New developments in the Inorganic Crystal Structure Database (ICSD): Accessibility in support of materials research and design. Acta Crystallogr. B 2002, 58, 364–369. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Janjić, G.V.; Zarić, S.D. σ-Hole interactions of covalently-bonded nitrogen, phosphorus and arsenic: A survey of crystal structures. Crystals 2014, 4, 12–31. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Pnictogen Bond: The Covalently Bound Arsenic Atom in Molecular Entities in Crystals as a Pnictogen Bond Donor. Molecules 2022, 27, 3421. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. The Phosphorous Bond, or the Phosphorous-Centered Pnictogen Bond: The Covalently Bound Phosphorous Atom in Molecular Entities and Crystals as a Pnictogen Bond Donor. Molecules 2022, 27, 1487. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Stibium Bond or the Antimony-Centered Pnictogen Bond: The Covalently Bound Antimony Atom in Molecular Entities in Crystal Lattices as a Pnictogen Bond Donor. Int. J. Mol. Sci. 2022, 23, 4674. [Google Scholar] [CrossRef]
- Mokrai, R.; Barrett, J.; Apperley, D.C.; Batsanov, A.S.; Benkő, Z.; Heift, D. Weak Pnictogen Bond with Bismuth: Experimental Evidence Based on Bi−P Through-Space Coupling. Chem. Eur. J. 2019, 25, 4017–4024. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The use and misuse of van der Waals radii. Struct. Chem. 2021, 32, 623–629. [Google Scholar] [CrossRef]
- Schiemenz, G.P. The sum of van der Waals radii—A pitfall in the search for bonding. Z. Naturforsch. B 2007, 62, 235–243. [Google Scholar] [CrossRef]
- Dean, P.A.W. Facets of van der Waals Radii That Are Not Commonly Included in Undergraduate Textbooks. J. Chem. Ed. 2014, 91, 154–157. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. The Independent Gradient Model: A New Approach for Probing Strong and Weak Interactions in Molecules from Wave Function Calculations. ChemPhysChem 2018, 19, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. A direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Head-Gordon, T. Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer. Chem. Phys. Lett. 1994, 220, 122–128. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Wilson, A.K.; Woon, D.E.; Peterson, K.A.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. IX. The atoms gallium through krypton. J. Chem. Phys. 1999, 110, 7667–7676. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The s-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. The π-hole revisited. Phys. Chem. Chem. Phys. 2021, 23, 16458–16468. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Jin, B.-Y. Unusual bonding modes of perfluorobenzene in its polymeric (dimeric, trimeric and tetrameric) forms: Entirely negative fluorine interacting cooperatively with entirely negative fluorine. Phys. Chem. Chem. Phys. 2015, 17, 31624–31645. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. Can Combined Electrostatic and Polarization Effects Alone Explain the F···F Negative-Negative Bonding in Simple Fluoro-Substituted Benzene Derivatives? A First-Principles Perspective. Computation 2018, 6, 51. [Google Scholar] [CrossRef]
- Tschakert, J.; Zhong, Q.; Martin-Jimenez, D.; Carracedo-Cosme, J.; Romero-Muñiz, C.; Henkel, P.; Schlöder, T.; Ahles, S.; Mollenhauer, D.; Wegner, H.A.; et al. Surface-controlled reversal of the selectivity of halogen bonds. Nat. Commun. 2020, 11, 5630. [Google Scholar] [CrossRef]
- Riley, K.E.; Murray, J.S.; Fanfrlík, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability I: The effects of aromatic fluorine substitution on the strengths of halogen-bonding interactions involving chlorine, bromine, and iodine. J. Mol. Modeling 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R.; Jin, B.-Y. Can an entirely negative fluorine in a molecule, viz. perfluorobenzene, interact attractively with the entirely negative site (s) on another molecule (s)? Like liking like! RSC Adv. 2016, 6, 19098–19110. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K.; Pradeep, R.; Varadwaj, H.M.M.; Koichi, Y. Comment on “Extended Halogen Bonding between Fully Fluorinated Aromatic Molecules: Kawai et al., ACS Nano, 2015, 9, 2574”. arXiv 2017, arXiv:1802.09995. [Google Scholar]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Is the Fluorine in Molecules Dispersive? Is Molecular Electrostatic Potential a Valid Property to Explore Fluorine-Centered Non-Covalent Interactions? Molecules 2019, 24, 379. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Pearson Education: Harlow, UK, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J.T. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Love, I. An AIM analysis of S,O bonds. J. Phys. Chem. A 2009, 113, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally ob-served electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Kuznetsov, M.L. Relationships between Interaction Energy and Electron Density Properties for Homo Halogen Bonds of the [(A)nY–X···X–Z(B)m] Type (X = Cl, Br, I). Molecules 2019, 24, 2733. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Reed, A.R.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Weinhold, F.; Carpenter, J.E. The Structure of Small Molecules; Naaman, R., Vager, Z., Eds.; Plenum: New York, NY, USA, 1988; pp. 227–236. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (V. 19.10.12); TK Gristmill Software. Overland Park, KS, USA, 2019. Available online: http://aim.tkgristmill.com (accessed on 21 March 2022).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, P.R. Methylammonium Lead Trihalide Perovskite Solar Cell Semiconductors Are Not Organometallic: A Perspective. Helv. Chim. Acta 2017, 100, e1700090. [Google Scholar] [CrossRef]
- Bolte, M. AWEJEW, dep. no. 2090637. CSD Commun. 2021. [Google Scholar] [CrossRef]
- Qiu, H.; Li, F.; Jin, C.; Lu, J.; Yang, Z.; Pan, S.; Mutailipu, M. (N2H6)[HPO3F]2: Maximizing the optical anisotropy of deep-ultraviolet fluorophosphates. Chem. Commun. 2022, 58, 5594–5597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zuo, C.; Niu, M.; Zhou, C.; Bradley, S.J.; Hall, C.R.; Xu, W.; Wen, X.; Hao, X.; Gao, M.; et al. Revealing the Role of Methylammonium Chloride for Improving the Performance of 2D Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 25980–25990. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Revealing the Chemistry between Bandgap and Binding Energy for Pb/Sn-based Trihalide Perovskite Solar Cell Semiconductors. ChemSusChem 2018, 11, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Revealing the Cooperative Chemistry of the Organic Cation in the Methylammonium Lead Triiodide Perovskite Semiconductor System. ChemistrySelect 2018, 3, 7269–7282. [Google Scholar] [CrossRef]
- Chi, L.; Swainson, I.; Cranswick, L.; Her, J.-H.; Stephens, P.; Knop, O. The ordered phase of methylammonium lead chloride CH3ND3PbCl3. J. Solid State Chem. 2005, 178, 1376–1385. [Google Scholar] [CrossRef]
- Swainson, I.P.; Hammond, R.P.; Soullière, C.; Knop, O.; Massa, W. Phase transitions in the perovskite methylammonium lead bromide, CH3ND3PbBr3. J. Solid State Chem. 2003, 176, 97–104. [Google Scholar] [CrossRef]
- Whitfield, P.S.; Herron, N.; Guise, W.E.; Page, K.; Cheng, Y.Q.; Milas, I.; Crawford, M.K. Structures, Phase Transitions and Tricritical Behavior of the Hybrid Perovskite Methyl Ammonium Lead Iodide. Sci. Rep. 2016, 6, 35685. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Henry, P.F.; Di Pumpo, A.M.; Hansen, T.C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51, 4180–4183. [Google Scholar]
- Yamamuro, O.; Matsuo, T.; Suga, H.; David, W.I.F.; Ibberson, R.M.; Leadbetter, A.J. Neutron diffraction and calorimetric studies of methylammonium iodide. Acta Cryst. B 1992, 48, 329–336. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Chen, J.Y.; Yang, L.W.; Xu, G.; Jarvis, V.; Britten, J.F. KUBNOK02, dep. no. 1946189. CSD Commun. 2019. [Google Scholar] [CrossRef]
- Li, W.; Huang, D.; Lv, Y. Theoretical study on the mechanism and stereochemistry of the cinchona–thiourea organocatalytic hydrophosphonylation of an α-ketoester. Org. Biomol. Chem. 2013, 11, 7497–7506. [Google Scholar] [CrossRef]
- Sen, S.; Patwari, G.N. Electrostatics and Dispersion in X–H···Y (X = C, N, O; Y = N, O) Hydrogen Bonds and Their Role in X–H Vibrational Frequency Shifts. ACS Omega 2018, 3, 18518–18527. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Hybrid organic–inorganic CH3NH3PbI3 perovskite building blocks: Revealing ultra-strong hydrogen bonding and mulliken inner complexes and their implications in materials design. J. Comp. Chem. 2017, 38, 2802–2818. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.M.; Burns, L.A.; Parrish, R.M.; Ryno, A.G.; Sherrill, C.D. Levels of symmetry adapted perturbation theory (SAPT). I. Efficiency and performance for interaction energies. J. Chem. Phys. 2014, 140, 094106. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree–Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- MacLean, E.J.; Harris, K.D.M.; Kariuki, B.M.; Kitchin, S.J.; Tykwinski, R.R.; Swainson, I.P.; Dunitz, J.D. Ammonium Cyanate Shows N−H···N Hydrogen Bonding, Not N−H···O. J. Am. Chem. Soc. 2003, 125, 14449–14451. [Google Scholar] [CrossRef] [PubMed]
- Nelyubina, Y.V.; Antipin, M.Y.; Lyssenko, K.A. Are Halide···Halide Contacts a Feature of Rock-Salts Only? J. Phys. Chem. A 2007, 111, 1091–1095. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Korlyukov, A.A.; Lyssenko, K.A. Experimental Charge Density Evidence for Pnicogen Bonding in a Crystal of Ammonium Chloride. ChemPhysChem 2015, 16, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.A.; Peterson, S.W. Neutron Diffraction Determination of the Crystal Structure of Ammonium Bromide in Four Phases1. J. Am. Chem. Soc. 1953, 75, 1536–1542. [Google Scholar] [CrossRef]
- Kolomiichuk, V.N.; Dvoryankin, V.F. An electron diffraction determination of the positions of hydrogen atoms in NH4Br. Kristallografiya 1964, 9, 50–56. [Google Scholar]
- Kolomiichuk, V.N. An electron-diffraction study of a low-temperature ammonium bromide phase. Kristallografiya 1965, 10, 565–567. [Google Scholar]
- Balagurov, A.M.; Kozlenko, D.P.; Savenko, B.N.; Glazkov, V.P.; Somenkov, V.A.; Hull, S. Neutron diffraction study of structural changes in ammonium halides under high pressure. Phys. B Cond. Matter 1999, 265, 92–96. [Google Scholar] [CrossRef]
- Pistorius, C.W.F.T. Phase relations and structures of solids at high pressures. Prog. Solid State Chem. 1976, 11, 1–151. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Wang, L.; Wu, G.; Duan, D.; Bao, K.; Zhou, Q.; Liu, B.; Cui, T. Structural properties of ammonium iodide under high pressure. RSC Adv. 2015, 5, 40336–40340. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Khrustalev, V.N.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Solution Processing of Methylammonium Lead Iodide Perovskite from γ-Butyrolactone: Crystallization Mediated by Solvation Equilibrium. Chem. Mater. 2018, 30, 5237–5244. [Google Scholar] [CrossRef]
- Krautscheid, H.; Vielsack, F. [Pb18I44]8−—An Iodoplumbate with an Unusual Structure. Angew. Chem. Int. Ed. Engl. 1995, 34, 2035–2037. [Google Scholar] [CrossRef]
- Billing, D.G.; Lemmerer, A. Synthesis, characterization and phase transitions of the inorganic–organic layered perovskite-type hybrids [(CnH2n+1NH3)2PbI4] (n = 12, 14, 16 and 18). New J. Chem. 2008, 32, 1736–1746. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, X.; He, H.; Tong, G.; Hu, J.; Xiao, X.; Chen, J.; Li, M.; He, Y. Intermolecular Hydrogen-Bonding Correlated Structure Distortion and Broadband White-Light Emission in 5-Ammonium Valeric Acid Templated Lead Chloride Perovskites. Cryst. Growth Des. 2021, 21, 5731–5739. [Google Scholar] [CrossRef]
- Lemmerer, A.; Billing, D.G. Lead halide inorganic–organic hybrids incorporating diammonium cations. CrystEngComm 2012, 14, 1954–1966. [Google Scholar] [CrossRef]
- Lemmerer, A.; Billing, D.G. Synthesis, characterization and phase transitions of the inorganic–organic layered perovskite-type hybrids [(CnH2n+1NH3)2PbI4], n = 7, 8, 9 and 10. Dalton Trans. 2012, 41, 1146–1157. [Google Scholar] [CrossRef]
- Lee, J.-H.; Bristowe, N.C.; Lee, J.H.; Lee, S.-H.; Bristowe, P.D.; Cheetham, A.K.; Jang, H.M. Resolving the Physical Origin of Octahedral Tilting in Halide Perovskites. Chem. Mater. 2016, 28, 4259–4266. [Google Scholar] [CrossRef]
- Pradeesh, K.; Yadav, G.S.; Singh, M.; Vijaya Prakash, G. Synthesis, structure and optical studies of inorganic–organic hybrid semiconductor, NH3(CH2)12NH3PbI4. Mater. Chem. Phys. 2010, 124, 44–47. [Google Scholar] [CrossRef]
- Harchani, A.; Carpenter-Warren, C.L.; Slawin, A.M.Z.; Haddad, A. Structure and properties evolution with inorganic and organic acids of a new organo-chlorocadmate compound (C6H20N3)2[Cd2Cl10]: Theoretical approach. J. Mol. Str. 2019, 1192, 49–58. [Google Scholar] [CrossRef]
- Kefi, R.; Maher, E.G.; Zeller, M.; Lefebvre, F.; Ben Nasr, C. CCDC 851463: Experimental Crystal Structure Determination; University of Texas Arlington: Austin, TX, USA, 2012. [Google Scholar] [CrossRef]
- Song, N.; Chen, S.-P.; Fan, X.-W.; Tan, Y.-H.; Wei, W.-J.; Tang, Y.-Z. Regulating Reversible Phase Transition Behaviors by Poly-H/F Substitution in Hybrid Perovskite-Like 2[CH2FCH2NH3]·[CdCl4]. ACS Omega 2020, 5, 6773–6780. [Google Scholar] [CrossRef] [PubMed]
- Lartigue-Bourdeau, C.; Chanh, N.B.; Duplessix, R.; Gallois, B. Thermal study and crystal structure of a perovskite-type unsaturated molecular composite: Propargylamine and cadmium chloride complex salt. J. Phys. Chem. Solids 1993, 54, 349–356. [Google Scholar] [CrossRef]
- Bonamartini Corradi, A.; Cramarossa, M.R.; Manfredini, T.; Giusti, J.; Battaglia, L.P.; Saccani, A.; Sandrolini, F. Structural, Thermal, and Electrical Characterization of Bis(ethylethylene)diammonium Dichloride Tetrachlorocadmate(II) with Perovskite-like Structure. Chem. Mater. 1994, 6, 1499–1503. [Google Scholar] [CrossRef]
- Kind, R. Structural phase transitions in perovskite layer structures. Ferroelectrics 1980, 24, 81–88. [Google Scholar] [CrossRef]
- De Jongh, L.J.; Miedema, A.R. Experiments on simple magnetic model systems. Adv. Phys. 2001, 50, 947–1170. [Google Scholar] [CrossRef]
- Kind, R.; Plesko, S.; Arend, H.; Blinc, R.; Zeks, B.; Seliger, J.; Lozar, B.; Slak, J.; Levstik, A.; Filipic, C.; et al. Dynamics of the n-decylammonium chains in the perovskite-type layer structure compound (C10H21NH3)2CdCl4. J. Chem. Phys. 1979, 71, 2118–2130. [Google Scholar] [CrossRef]
- Depmeier, W. The uniqueness of the propyl compound in the series (CnH2n+1NH3)2MnCl4 with n = 1–10. J. Solid State Chem. 1979, 29, 15–26. [Google Scholar] [CrossRef]
- Mokhlisse, R.; Couzi, M.; Chanh, N.B.; Haget, Y.; Hauw, C.; Meresse, A. Raman scattering and X-ray diffraction study of structural phase transitions in the perovskite-type layer compound (C3H7NH3)2CdCl4. J. Phys. Chem. Solids 1985, 46, 187–195. [Google Scholar] [CrossRef]
- Chanh, N.B.; Hauw, C.; Meresse, A.; Rey-Lafon, M.; Ricard, L. X-ray ditffraction, differential scanning calorimetric and spectroscopic studies of phase transition in the bidimensional compound (C12H25NH3)2CdCl4. J. Phys. Chem. Solids 1985, 46, 1413–1420. [Google Scholar] [CrossRef]
- White, M.A. Energetics of long alkyl chains embedded in a crystalline matrix: (n-C18H37NH3)2CdCl4. J. Chem. Phys. 1984, 81, 6100–6105. [Google Scholar] [CrossRef]
- Couzi, M.; Chanh, N.B.; Meresse, A.; Negrier, P.; Papoular, R.J.; Millet, R. A neutron diffraction study of the high pressure structural phase transition in (CD3ND3)2MnCl4. Phase Trans. 1989, 14, 69–78. [Google Scholar] [CrossRef]
- Negrie, R.P.; Couzi, M.; Chanh, N.B.; Hauw, C.; Meresse, A. Structural phase transitions in the perovskite-type layer compound NH3(CH2)5NH3CdCl4. J. Phys. France 1989, 50, 405–430. [Google Scholar] [CrossRef]
- Chhor, K.; Abello, L.; Pommier, C.; Sourisseau, C. Reorientational motions in a perovskite-type layer compound [NH3(CH2)5NH3]MnCl4. A calorimetric study. J. Phys. Chem. Solids 1988, 49, 1079–1085. [Google Scholar] [CrossRef]
- Schenk, K.J.; Chapuis, G. Thermotropic phase transitions in bis(n-tetradecylammonium) tetrachlorocadmate(II) and some homologous compounds. J. Phys Chem. 1988, 92, 7141–7147. [Google Scholar] [CrossRef]
- Ishihara, T.; Takahashi, J.; Goto, T. Optical properties due to electronic transitions in two-dimensional semiconductors CnH2n+1NH3)2PbI4. Phys. Rev. B 1990, 42, 11099–11107. [Google Scholar] [CrossRef] [PubMed]
- Zangar, H.; Miane, J.L.; Courseille, C.; Chanh, N.B.; Couzi, M.; Mlik, Y. Structural phase transition in the perovskite-type layer compound (C3H7NH3)2PbCl4. Phys. Stat. Sol. 1989, 115, 107–118. [Google Scholar] [CrossRef]
- Robinson, S.W.; Mustoe, C.L.; White, N.G.; Brown, A.; Thompson, A.L.; Kennepohl, P.; Beer, P.D. Evidence for Halogen Bond Covalency in Acyclic and Interlocked Halogen-Bonding Receptor Anion Recognition. J. Am. Chem. Soc. 2015, 137, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kellett, C.W.; Kennepohl, P.; Berlinguette, C.P. π covalency in the halogen bond. Nature Commun. 2020, 11, 3310. [Google Scholar] [CrossRef]
- Anyfanti, G.; Bauzá, A.; Gentiluomo, L.; Rodrigues, J.; Portalone, G.; Frontera, A.; Rissanen, K.; Puttreddy, R. Short X···N Halogen Bonds With Hexamethylenetetraamine as the Acceptor. Front. Chem. 2021, 9, 623595. [Google Scholar] [CrossRef]
- Wolters, L.P.; Smits, N.W.G.; Guerra, C.F. Covalency in resonance-assisted halogen bonds demonstrated with cooperativity in N-halo-guanine quartets. Phys. Chem. Chem. Phys. 2015, 17, 1585–1592. [Google Scholar] [CrossRef]
- Lemmerer, A.; Billing, D.G. Effect of heteroatoms in the inorganic–organic layered perovskite-type hybrids [(ZCnH2nNH3)2PbI4], n = 2, 3, 4, 5, 6; Z = OH, Br and I; and [(H3NC2H4S2C2H4NH3)PbI4]. CrystEngComm 2010, 12, 1290–1301. [Google Scholar] [CrossRef]
- Xi, J.; Spanopoulos, I.; Bang, K.; Xu, J.; Dong, H.; Yang, Y.; Malliakas, C.D.; Hoffman, J.M.; Kanatzidis, M.G.; Wu, Z. Alternative Organic Spacers for More Efficient Perovskite Solar Cells Containing Ruddlesden–Popper Phases. J. Am. Chem. Soc. 2020, 142, 19705–19714. [Google Scholar] [CrossRef]
- Jana, M.K.; Song, R.; Liu, H.; Khanal, D.R.; Janke, S.M.; Zhao, R.; Liu, C.; Vardeny, Z.V.; Blum, V.; Mitzi, D.B. CCDC 2015614: Experimental Crystal Structure Determination; University of Texas Arlington: Austin, TX, USA, 2020. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Long, P.; Yao, Y.; Ji, C.; Li, L.; Sun, Z.; Hong, M.; Luo, J. A Multiaxial Layered Halide Double Perovskite Ferroelectric with Multiple Ferroic Orders. Chem. Mater. 2020, 32, 8965–8970. [Google Scholar] [CrossRef]
- Sourisseau, S.; Louvain, N.; Bi, W.; Mercier, N.; Rondeau, D.; Boucher, F.; Buzaré, J.-Y.; Legein, C. Reduced Band Gap Hybrid Perovskites Resulting from Combined Hydrogen and Halogen Bonding at the Organic−Inorganic Interface. Chem. Mater. 2007, 19, 600–607. [Google Scholar] [CrossRef]
- Connor, B.A.; Leppert, L.; Smith, M.D.; Neaton, J.B.; Karunadasa, H.I. Layered Halide Double Perovskites: Dimensional Reduction of Cs2AgBiBr6. J. Am. Chem. Soc. 2018, 140, 5235–5240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Guan, T.; Jiang, H.; Long, P.; Li, X.; Ji, C.; Chen, S.; Sun, Z.; Luo, J. Giant room temperature electrocaloric effect in a layered hybrid perovskite ferroelectric: [(CH3)2CHCH2NH3]2PbCl4. Nat. Commun. 2021, 12, 5502. [Google Scholar] [CrossRef] [PubMed]
- McClure, E.T.; McCormick, A.P.; Woodward, P.M. Four Lead-free Layered Double Perovskites with the n = 1 Ruddlesden–Popper Structure. Inorg. Chem. 2020, 59, 6010–6017. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Krummer, M.; Zimmermann, B.; Klingenberg, P.; Daub, M.; Hillebrecht, H. Perovskite-Related 2D Compounds in the System 5-Amino Valerian Acid Cation/MA/Pb/X (X = Cl, Br)—Synthesis, Crystal Structures, and Optical Properties. Eur. J. Inorg. Chem. 2020, 2020, 4581–4592. [Google Scholar] [CrossRef]
- Soe, C.M.M.; Nagabhushana, G.P.; Shivaramaiah, R.; Tsai, H.; Nie, W.; Blancon, J.-C.; Melkonyan, F.; Cao, D.H.; Traoré, B.; Pedesseau, L.; et al. Structural and thermodynamic limits of layer thickness in 2D halide perovskites. Proc. Nail. Acad. Sci. USA 2019, 116, 58–66. [Google Scholar] [CrossRef]
- Li, L. CCDC 1939731: Experimental Crystal Structure Determination; University of Texas Arlington: Austin, TX, USA, 2020. [Google Scholar] [CrossRef]
- Hillebrecht, H. CCDC 1999300: Experimental Crystal Structure Determination; University of Texas Arlington: Austin, TX, USA, 2020. [Google Scholar] [CrossRef]
- Feng, L.-J.; Zhao, Y.-Y.; Song, R.-Y.; Lei, X.-W. Three homologous 1D lead halide perovskites with broadband white-light emissions. Inorg. Chem. Commun. 2022, 136, 109146. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Che, X.; Sidhik, S.; Li, X.; Hadar, I.; Blancon, J.-C.; Yamaguchi, H.; Kepenekian, M.; Katan, C.; Even, J.; et al. From 2D to 1D Electronic Dimensionality in Halide Perovskites with Stepped and Flat Layers Using Propylammonium as a Spacer. J. Am. Chem. Soc. 2019, 141, 10661–10676. [Google Scholar] [CrossRef]
- Pipitone, C.; Boldrini, S.; Ferrario, A.; Garcìa-Espejo, G.; Guagliardi, A.; Masciocchi, N.; Martorana, A.; Giannici, F. Ultralow thermal conductivity in 1D and 2D imidazolium-based lead halide perovskites. Appl. Phys. Lett. 2021, 119, 101104. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, J.; Bu, X.-H. 1D Chiral Lead Halide Perovskites with Superior Second-Order Optical Nonlinearity. Adv. Opt. Mat. 2022, 10, 2101545. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, C.; Tian, Y.; Shu, Y.; Messier, J.; Wang, J.C.; van de Burgt, L.J.; Kountouriotis, K.; Xin, Y.; Holt, E.; et al. One-dimensional organic lead halide perovskites with efficient bluish white-light emission. Nature Commun. 2017, 8, 14051. [Google Scholar] [CrossRef] [PubMed]
- Essalhi, R.; Abdelbaky, M.S.M.; Elleuch, S.; Zouari, F.; García-Granda, S. Crystal structure, Hirschfield surface analysis, thermal and DFT investigation accomplished with photoluminescence study of bis(N, N-diethylethylendiammonium)decabromodiantimoinate(III). J. Mol. Str. 2020, 1221, 128828. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.-J.; Quasie, O.; Shan, S.-M.; Zhang, Y.-M.; Kong, L.-Y. Further C-15-acyl phragmalin derivatives from Chukrasia tabularis A. Juss. Phytochemistry 2015, 117, 410–416. [Google Scholar] [CrossRef]
- Mawhinney, T.P.; Li, Y.; Chance, D.L.; Kelley, S.P.; Mossine, V.V. Crystal structure of (R,S)-2-hydroxy-4-(methylsulfanyl)butanoic acid. Acta Crystallogr. E 2020, 76, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Senior, L.; Linden, A. Halobismuth(III) salts with substituted aminopyridinium cations. Acta Crystallogr. C 2020, 76, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Stoumpos, C.C.; Liu, Z.; Chang, R.P.H.; Kanatzidis, M.G. Controllable Perovskite Crystallization at a Gas–Solid Interface for Hole Conductor-Free Solar Cells with Steady Power Conversion Efficiency over 10%. J. Am. Chem. Soc. 2014, 136, 16411–16419. [Google Scholar] [CrossRef] [PubMed]
- Lemmerer, A.; Billing, D.G. Two packing motifs based upon chains of edge-sharing PbI6 octahedra. Acta Crystallogr. C 2006, 62, m597–m601. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Lin, Y.-H.; Zeng, G.-F.; Xi, S.-Q. Structure of 1,3-propanediammonium tetrachlorocobaltate(II). Acta Crystallogr. C 1992, 48, 542–543. [Google Scholar] [CrossRef]
- Wen, H.; Miller, S.E.; House, D.A.; McKee, V.; Robinson, W.T. The crystal structures of some chloromercury(II) anions with Co(III) complexes or protonated polyamines as cations. Inorg. Chim. Acta 1992, 193, 77–85. [Google Scholar] [CrossRef]
- Spengler, R.; Zouari, R.; Ben Salah, A.; Zimmermann, H.; Burzlaff, H. Redetermination of Bis(1,2-ethanediammonium) Dichloride Tetrachloromercurate(II). Acta Crystallogr. C 1998, 54, IUC9800034. [Google Scholar] [CrossRef]
- Smith, H.W.; Stratton, W.J. Preparation, properties, and crystal and molecular structure of ethylenediammonium tetrachlorocobaltate(II) chloride, (NH3CH2CH2NH3)2(CoCl4)Cl2. Inorg. Chem. 1977, 16, 1640–1645. [Google Scholar] [CrossRef]
- Kumar, M.; Verma, S.K.; Singh, B.; Thakur, A.; Kumar, A.; Jasrotia, D. 2D Interwoven Metal-Organic Framework in Tetrachloromercurate(II) based Hybrid Material. Chem. Sci. Trans. 2015, 4, 629–637. [Google Scholar]
- Ouerghi, Z.; Gornitzka, H.; Temel, E.; Dridi, I.; Kefi, R. A new non-centrosymmetric Chlorobismuthate(III) hybrid material: Crystal structure, optical properties and antibacterial study. J. Mol. Str. 2019, 1181, 338–347. [Google Scholar] [CrossRef]
- Mao, W.; Wang, J.; Hu, X.; Zhou, B.; Zheng, G.; Mo, S.; Li, S.; Long, F.; Zou, Z. Synthesis, crystal structure, photoluminescence properties of organic-inorganic hybrid materials based on ethylenediamine bromide. J. Saudi Chem. Soc. 2020, 24, 52–60. [Google Scholar] [CrossRef]
- Bourne, S.A.; Mangombo, Z. Phenylamines as building blocks to layered inorganic–organic structures. CrystEngComm 2004, 6, 438–442. [Google Scholar] [CrossRef]
- Dobrzycki, L.; Woźniak, K. 1D vs 2D crystal architecture of hybrid inorganic–organic structures with benzidine dication. J. Mol. Str. 2009, 921, 18–33. [Google Scholar] [CrossRef]
- Su, B.; Song, G.; Molokeev, M.S.; Lin, Z.; Xia, Z. Synthesis, Crystal Structure and Green Luminescence in Zero-Dimensional Tin Halide (C8H14N2)2SnBr6. Inorg. Chem. 2020, 59, 9962–9968. [Google Scholar] [CrossRef]
- Ishiharaa, H.; Horiuchib, K.; Douc, S.-q.; Gesingc, T.M.; Buhlc, J.-C.; Paulus, H.; Fuessd, H. Isolated versus Condensed Anion Structure IV: An NQR Study and X-ray Structure Analysis of [H3N(CH2)3NH3]CdI4·2H2O, [H3CNH2(CH2)3NH3]CdBr4, [(CH3)4N]2CdBr4, and [(CH3)3S]2CdBr4. Z. Naturforsch. A: Phys. Sci. 1998, 53, 717–724. [Google Scholar] [CrossRef]
- Makhinya, A.N.; Shusharina, E.A.; Baidina, I.A.; Il’in, M.A. Structure of the first nitrosoammine complexes of ruthenium with a coordinated sulfate ion: [Ru(NO)(NH3)4(SO4)](HSO4)·H2O and [Ru(NO)(NH3)3Cl(SO4)]·2H2O. J. Struct. Chem. 2011, 52, 946–953. [Google Scholar] [CrossRef]
- Willermann, M.; Mulcahy, C.; Sigel, R.K.O.; Cerdà, M.M.; Freisinger, E.; Sanz Miguel, P.J.; Roitzsch, M.; Lippert, B. Pyrazine as a Building Block for Molecular Architectures with PtII. Inorg. Chem. 2006, 45, 2093–2099. [Google Scholar] [CrossRef][Green Version]
- Liu, G.-N.; Zhao, R.-Y.; Xu, H.; Wang, Z.-H.; Liu, Q.-S.; Shahid, M.Z.; Miao, J.-L.; Chen, G.; Li, C. The structures, water stabilities and photoluminescence properties of two types of iodocuprate(i)-based hybrids. Dalton Trans. 2018, 47, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Siebel, S.; Dammann, C.; Sanz Miguel, P.J.; Drewello, T.; Kampf, G.; Teubner, N.; Bednarski, P.J.; Freisinger, E.; Lippert, B. Analogues of Cis- and Transplatin with a Rich Solution Chemistry: Cis-[PtCl2(NH3)(1-MeC-N3)] and trans-[PtI2(NH3)(1-MeC-N3)]. Chem. Eur. J. 2015, 21, 17827–17843. [Google Scholar] [CrossRef]
- Spackman, M.A. How Reliable Are Intermolecular Interaction Energies Estimated from Topological Analysis of Experimental Electron Densities? Cryst. Growth Des. 2015, 15, 5624–5628. [Google Scholar] [CrossRef]


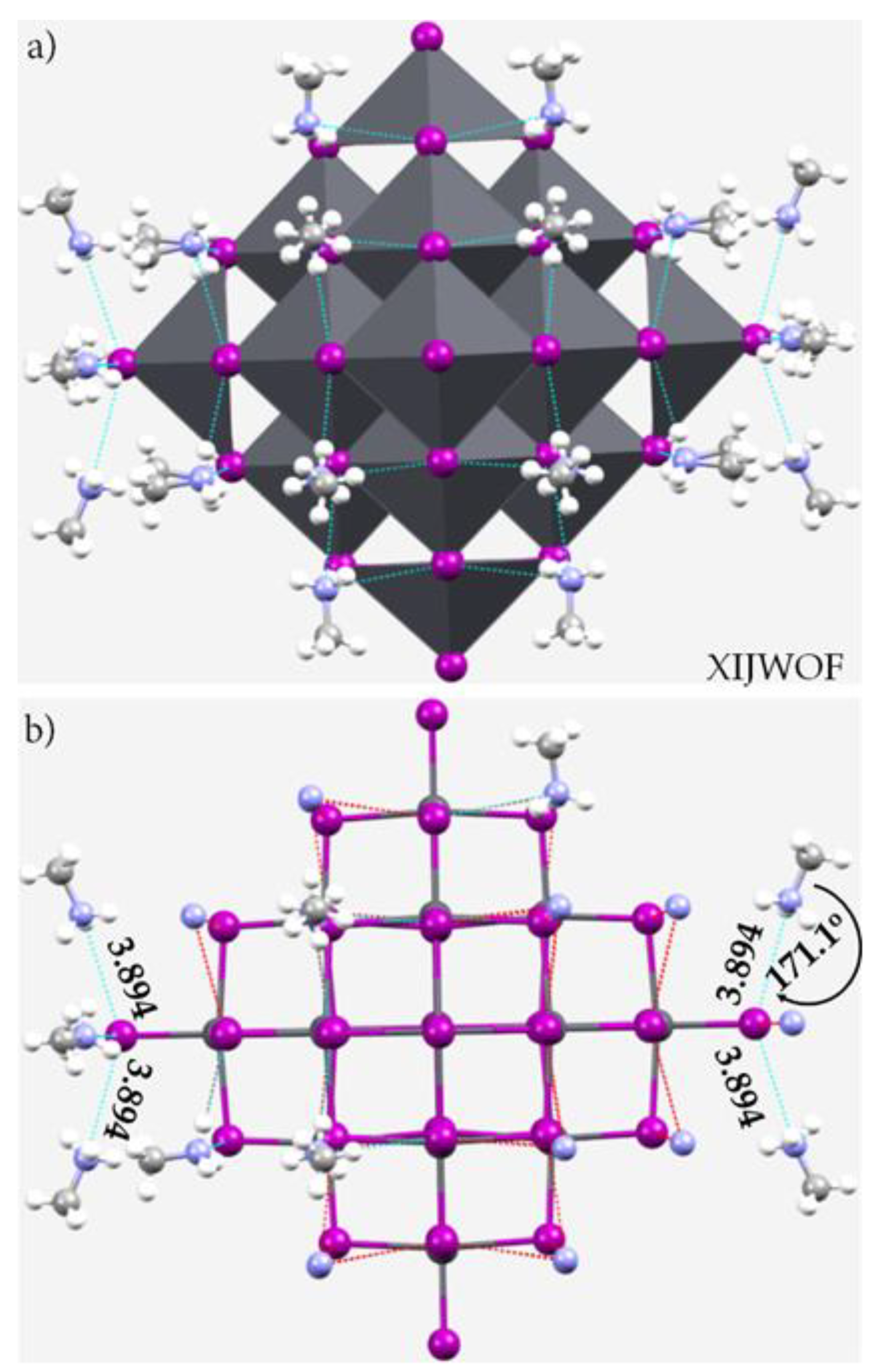
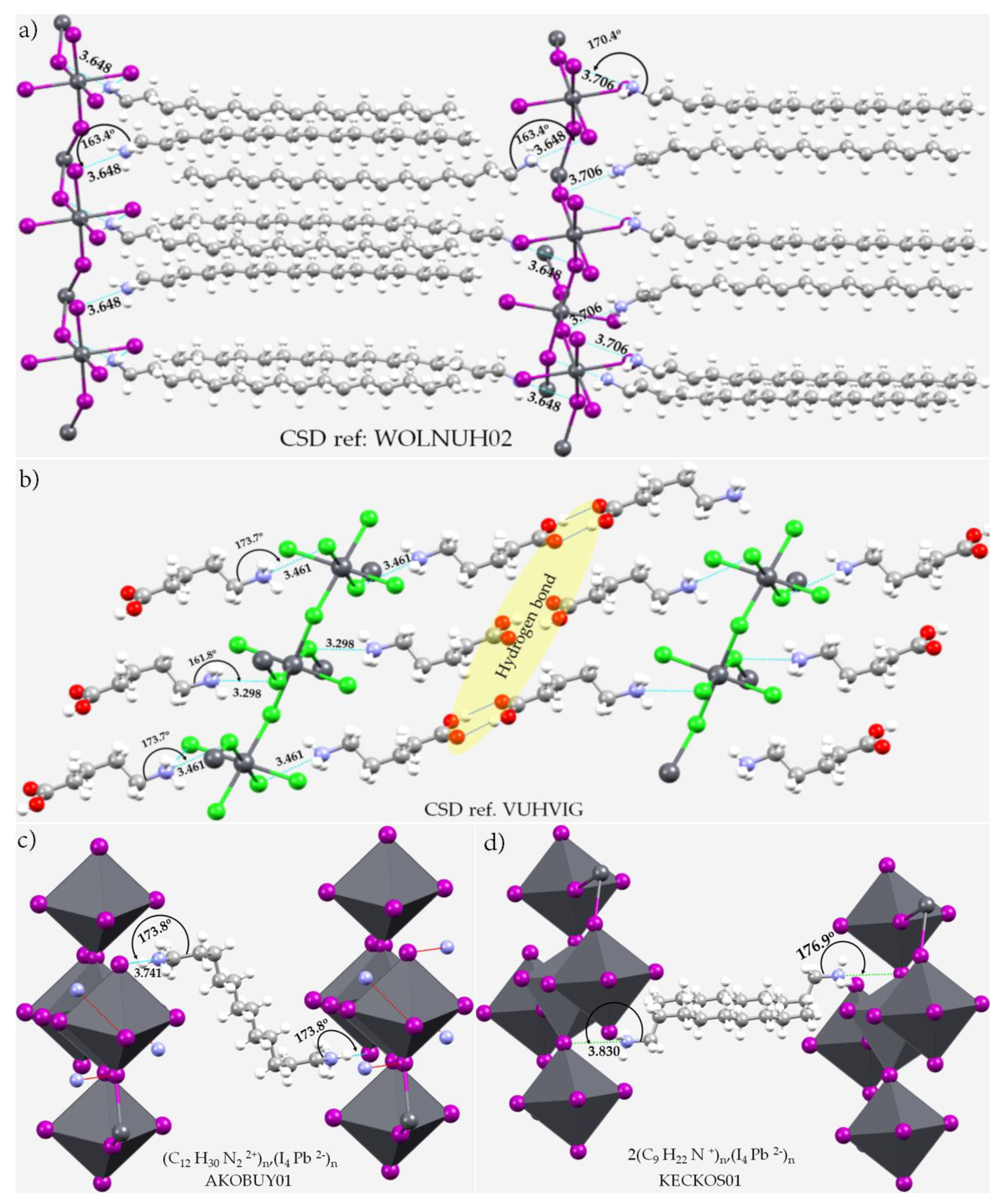
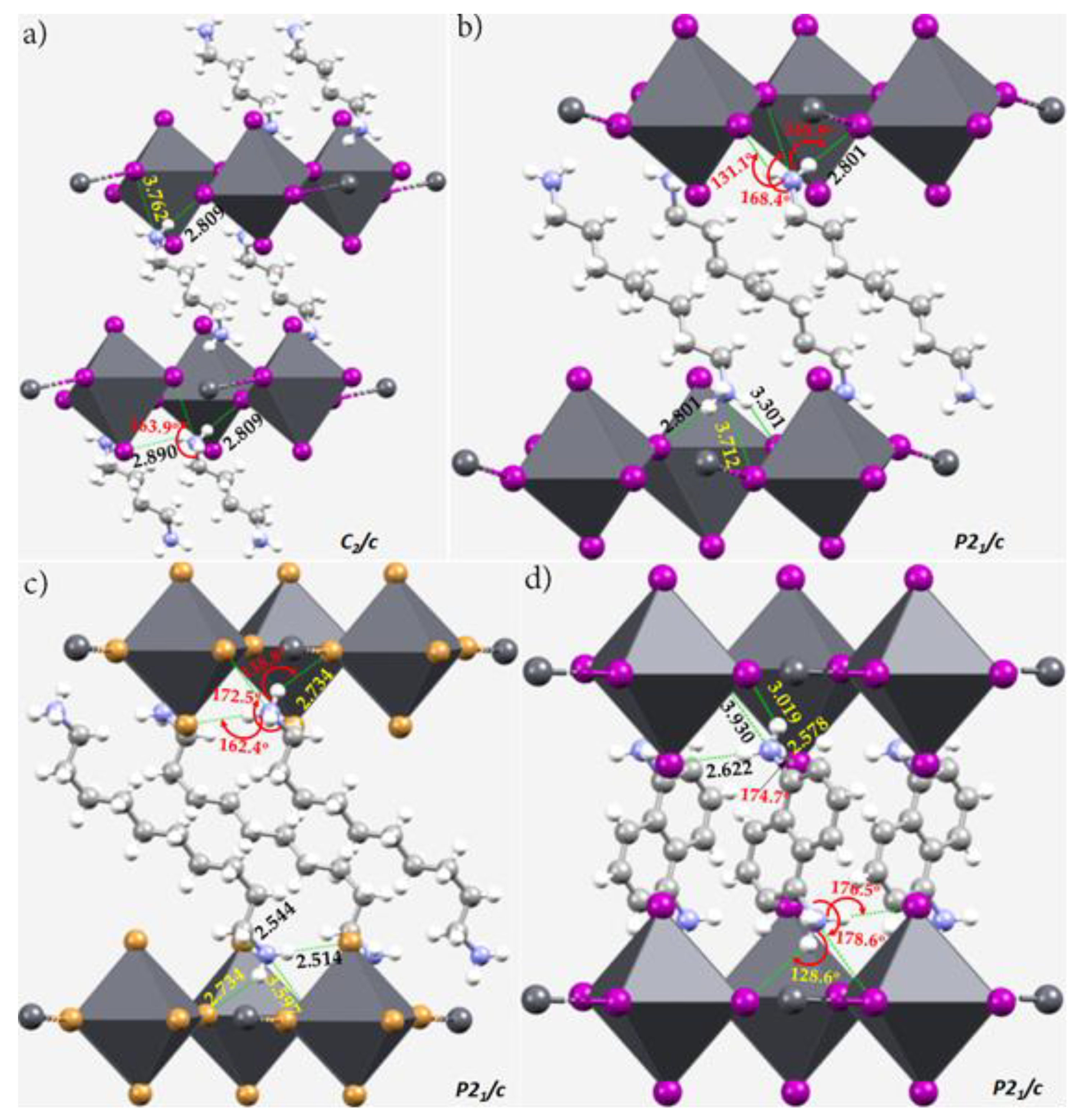

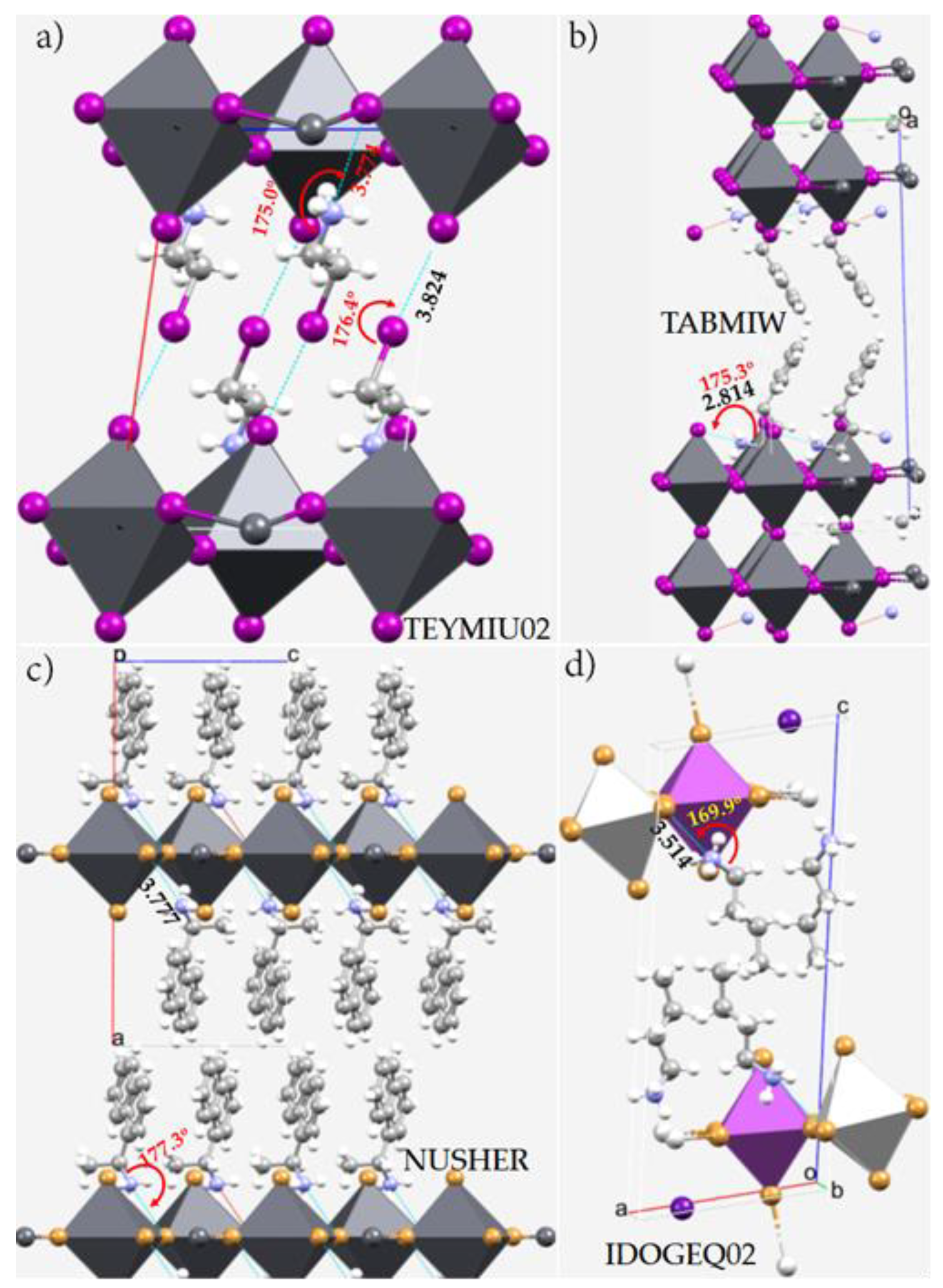


| System | (ωB97X-D)crystal geometry | (ωB97X-D)optimized geometry | [MP2(FC)]crystal geometry | [MP2(FC)]optimized geometry | ||||
|---|---|---|---|---|---|---|---|---|
| Eint | Eint(BSSE) | Eint | Eint(BSSE) | Eint | Eint(BSSE) | Eint | Eint(BSSE) | |
| H3C-H3N···I | −94.25 | −94.24 | −102.1 | −102.08 | −95.68 | −94.42 | −105.31 | −103.16 |
| H3N-H3C···I | −70.21 | −70.19 | −76.1 | −76.07 | −71.16 | −70.26 | −78.77 | −76.91 |
| Geometry (Complex Type) | Eels | Eexch | Eind | Edis | Eint(SAPT0) | Eint(SCF)(BSSE) a |
|---|---|---|---|---|---|---|
| Crystal geometry (H3C-H3N···I) | −91.00 | 9.32 | −8.87 | −2.87 | −93.44 | −94.24 |
| Crystal geometry (H3N-H3C···I) | −67.97 | 4.47 | −4.4 | −2.01 | −69.92 | −70.19 |
| ωB97X-D (H3C-H3N···I) | −108.8 | 26.84 | −14.12 | −5.93 | −102.00 | −102.08 |
| ωB97X-D (H3N-H3C···I) | −82.91 | 19.92 | −8.51 | −5.25 | −76.76 | −76.07 |
| MP2(FC)(H3C-H3N···I) | −111.74 | 31.04 | −14.95 | −6.55 | −102.2 | −105.31 |
| MP2(FC)(H3N-H3C···I) | −85.94 | 24.51 | −9.46 | −6.03 | −76.93 | −78.77 |
| Interaction Type | Donor NBO | Acceptor NBO | E(2)[MP2(FC] Geometry] | E(2)[ωB97X-D Geometry] | |
|---|---|---|---|---|---|
| (C)N···I | LP(4) I | → | RY*(1)N | 2.86 | 2.66 |
| (C)N···I | LP(4) I | → | BD*(1)N-C | 4.51 | 3.99 |
| N-H···I | LP(2)I | → | BD*(1)N-H | 0.62 | 0.51 |
| N-H···I | LP(2)I | → | BD*(1)N-H | 0.62 | 0.51 |
| N-H···I | LP(3)I | → | BD*(1)N-H | 0.83 | 0.68 |
| N-H···I | LP(4) I | → | BD*(1)N-H | 1.14 | 0.94 |
| N-H···I | LP(4) I | → | BD*(1)N-H | 1.14 | 0.94 |
| N-H···I | LP(4) I | → | BD*(1)N-H | 1.14 | 0.94 |
| (N)C···I | LP(4)I | → | RY*(1)(C) | 2.58 | 2.26 |
| (N)C···I | LP(1)I | → | BD*(1)C-N | 0.56 | 0.41 |
| (N)C···I | LP(4)I | → | BD*(C-N) | 10.22 | 8.14 |
| N-H···I | LP(2)I | → | BD*(1)C-H | 0.12 | 0.08 |
| N-H···I | LP(2)I | → | BD*(1)C-H | 0.12 | 0.08 |
| N-H···I | LP(3)I | → | BD*(1)C-H | 0.12 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond. Int. J. Mol. Sci. 2022, 23, 8816. https://doi.org/10.3390/ijms23158816
Varadwaj A, Varadwaj PR, Marques HM, Yamashita K. The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond. International Journal of Molecular Sciences. 2022; 23(15):8816. https://doi.org/10.3390/ijms23158816
Chicago/Turabian StyleVaradwaj, Arpita, Pradeep R. Varadwaj, Helder M. Marques, and Koichi Yamashita. 2022. "The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond" International Journal of Molecular Sciences 23, no. 15: 8816. https://doi.org/10.3390/ijms23158816
APA StyleVaradwaj, A., Varadwaj, P. R., Marques, H. M., & Yamashita, K. (2022). The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond. International Journal of Molecular Sciences, 23(15), 8816. https://doi.org/10.3390/ijms23158816









