Rheological and Film-Forming Properties of Chitosan Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Steady Shear Rheological Studies
2.2. Mechanical Studies
2.3. Swelling Behavior
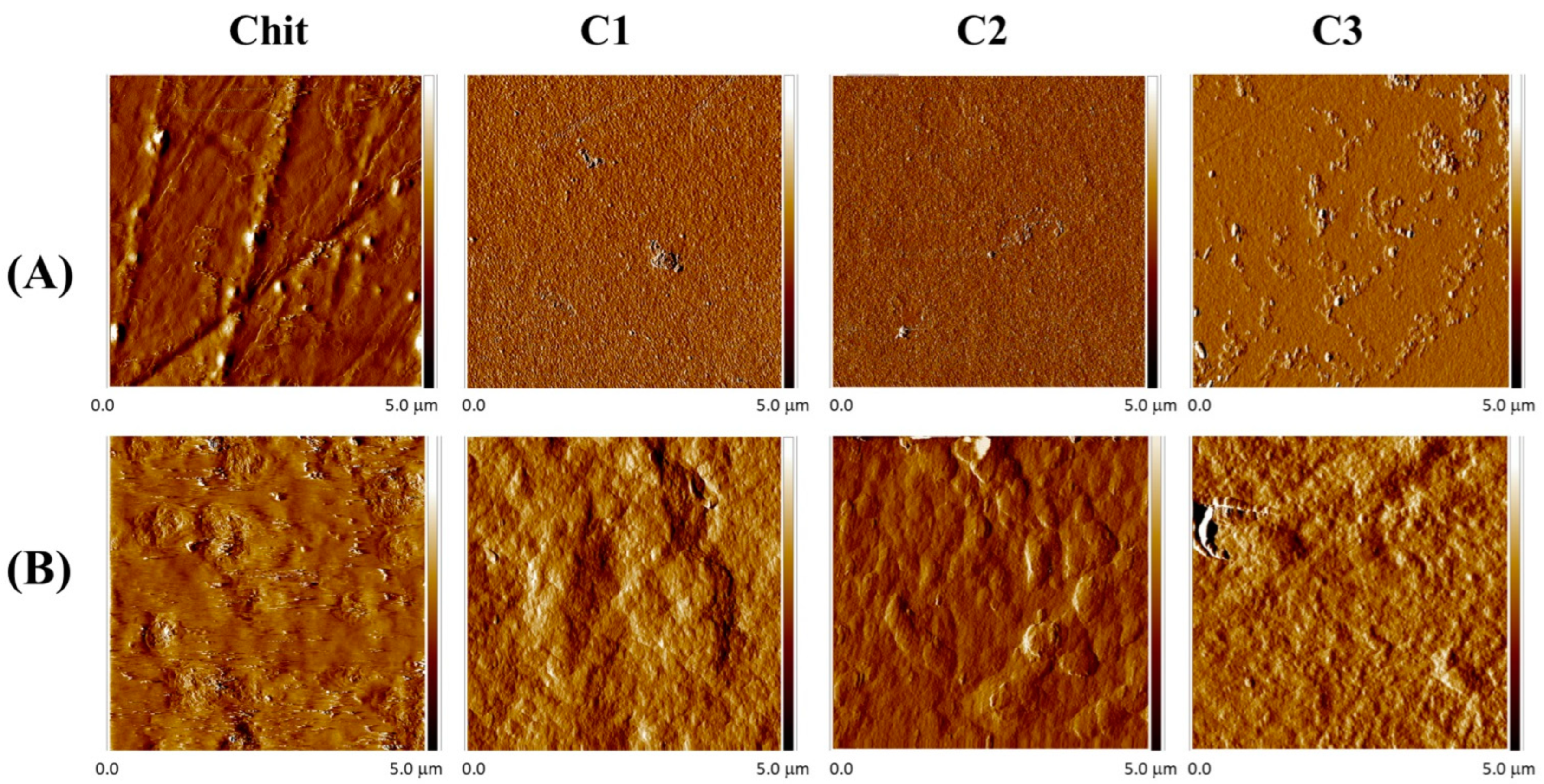
2.4. Morphological Properties
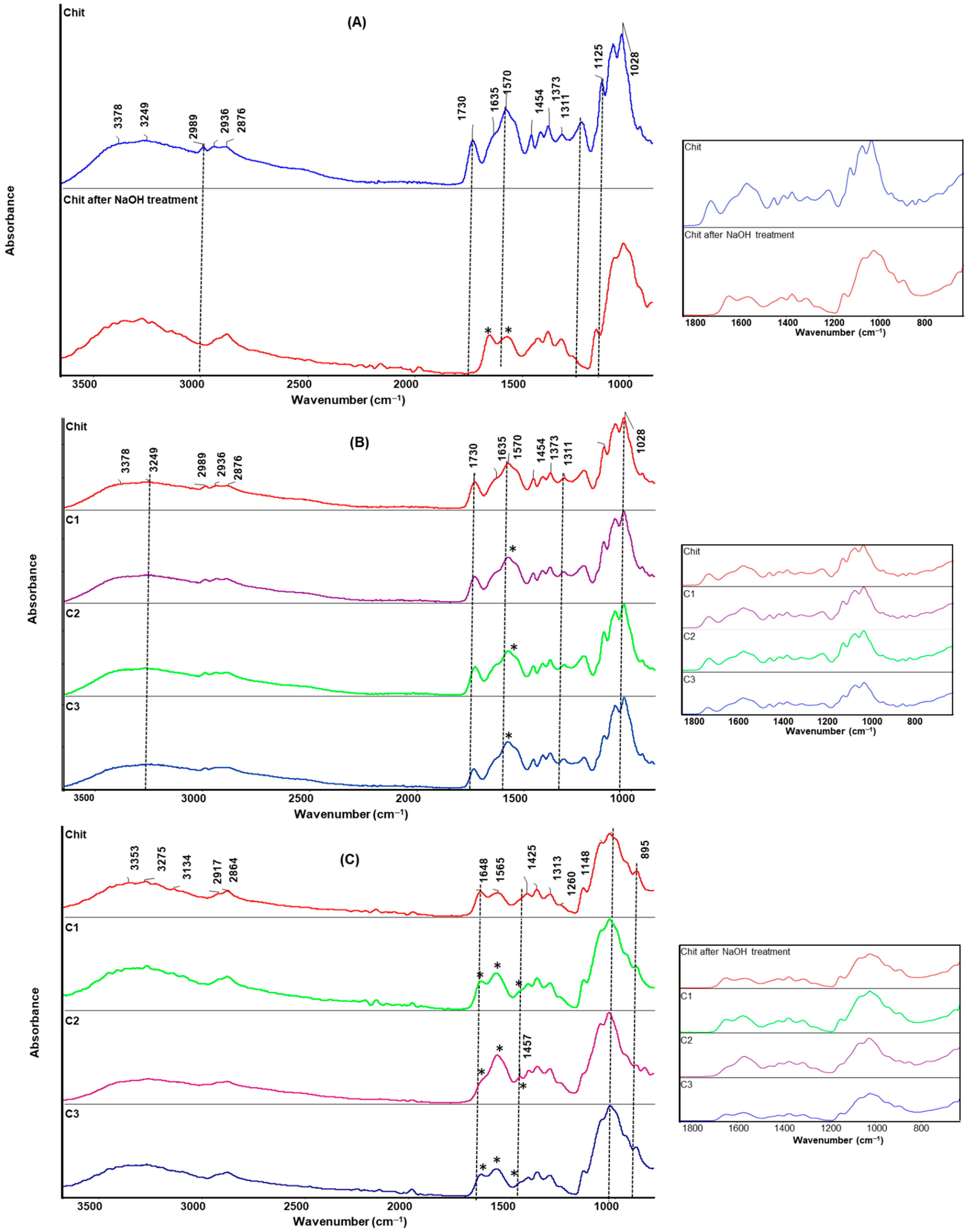
2.5. Infrared Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Solutions and Films Preparation
3.3. Steady Shear Tests
3.4. Mechanical Test
3.5. Swelling Behavior
3.6. Scanning Electron Microscopy (SEM)
3.7. Atomic Force Microscopy (AFM)
3.8. Infrared Spectroscopy (ATR-FTIR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Barbosa de Lima, M.A.; de Oliveira Franco, L.; de Campos-Takaki, G.M. Review: Seafood waste as attractive source of chitin and chitosan production and applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Raghavendra, N.N. Review: Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wang, B.-J.; Weng, Y.-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT—Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films. Effect of the type of solvent acid. LWT—Food Sci. Technol. 2021, 135, 109984. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, M.; Jiang, Q. Chitosan membranes from acetic acid and imidazolium ionic liquids: Effect of imidazolium structure on membrane properties. J. Mol. Liq. 2021, 340, 117209. [Google Scholar] [CrossRef]
- Zhuang, P.-Y.; Li, Y.-L.; Fan, L.; Lin, J.; Hu, Q.-L. Modification of chitosan membrane with poly(vinyl alcohol) and biocompatibility evaluation. Int. J. Biol. Macromol. 2012, 50, 658–663. [Google Scholar] [CrossRef]
- Valachova, K.; Soltes, L. Review: Versatile use of chitosan and hyaluronan in medicine. Molecules 2021, 26, 1195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, N.; Kaur, R.; Kaur, R. A review on valorization of chitinous waste. J. Polym. Res. 2021, 28, 406. [Google Scholar] [CrossRef]
- Boesel, L.F. Effect of plasticizers on the barrier and mechanical properties of biomimetic composites of chitosan and clay. Carbohydr. Polym. 2015, 115, 356–363. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, J.; Shehzad, H.; Le, Z.; Li, Z.; Adesina, A. Adsorption of U(VI) onto the carboxymethylated chitosan/Na-bentonite membrances: Kinetic, isothermic and thermodynamic studies. J. Radioanal. Nucl. Chem. 2018, 317, 1377–1385. [Google Scholar] [CrossRef]
- Lewandowska, K.; Szulc, M. Rheological and mechanical studies of chitosan blends with the addition of an ionic liquid. Prog. Chem. Appl. Chitin Deriv. 2019, 24, 119–126. [Google Scholar] [CrossRef]
- Kumar, R.; Mishra, I.; Kumar, G. Synthesis and evaluation of mechanical property of chitosan/PVP blends through nanoindentation—A nanoscale study. J. Polym. Environ. 2021, 29, 3770–3778. [Google Scholar] [CrossRef]
- Sari, M.; Tamrin; Kaban, J.; Alfian, Z. A novel composites membrane pectin from Cyclea Barbata Miers blend with chitosan for accelerated wound healing. Polym. Test. 2021, 99, 107207. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. A review on the preparation and characterization of chitosan-clay nanocomposite films and coatings for food packaging applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100102. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomarcomolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Electrospun fibers of chitosan-grafted polycaprolactone/poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) blends. J. Mater. Chem. B 2016, 4, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.B. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Bhowmick, A.; Banerjee, S.L.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocompostes with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 2018, 106, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical and barrier properties investigation of chitosan–clay nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ren, X.; Hanna, M.A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006, 99, 1684–1691. [Google Scholar] [CrossRef]
- Günister, E.; Pestreli, D.; Űnlü, C.H.; Atici, O.; Güngör, N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carbohydr. Polym. 2007, 67, 358–365. [Google Scholar] [CrossRef]
- Kabiri, K.; Mirzadeh, H.; Zohuriaan-Mehr, M.J.; Darliri, M. Chitosan-modified nanoclay-poly(AMPS) nanocomposite hydrogels with improved gel strength. Polym. Int. 2009, 58, 1252–1259. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Averous, L. Progress in nano-biocomposites based on polysaccharides and nanoclay. Mater. Sci. Eng. R Rep. 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Mondal, D.; Bhowmick, B.; Mollick, M.M.R.; Maity, D.; Saha, N.R.; Rangarajan, V.; Rana, D.; Sen, R.; Chattopadhyay, D. Antimicrobial activity and biodegradation behaviour of poly(butylene adipate-co-terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40079. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Characterization of chitosan composites with various clays. Int. J. Biol. Macromol. 2014, 65, 534–541. [Google Scholar] [CrossRef]
- Lewandowska, K. Characterization of chitosan composites with synthetic polymers and inorganic additives. Int. J. Biol. Macromol. 2015, 81, 159–164. [Google Scholar] [CrossRef]
- Lewandowska, K. Surface properties of chitosan composites with poly(N-vinylpyrrolidone) and montmorillonite. Polym. Sci. Ser. A 2017, 59, 215–222. [Google Scholar] [CrossRef]
- Lewandowska, K.; Furtos, G. Study of apatite layer formation on SBF-treated chitosan composite thin films. Polym. Test. 2018, 71, 173–181. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; Gonzalez, J.M.D.; Converti, A.; de Souza Olivera, P.R. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Mucha, M. Rheological properties of chitosan blends with poly(ethylene oxide) and poly(vinyl alcohol) in solution. React. Funct. Polym. 1998, 38, 19–25. [Google Scholar] [CrossRef]
- Wanchoo, R.K.; Thakur, A.; Sweta, A. Viscometric and rheological behaviour of chitosan-hydrophilic polymer blends. Chem. Biochem. Eng. Q. 2008, 22, 15–24. [Google Scholar]
- Bertolo, M.R.V.; Martins, V.C.A.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M.G. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, L.; Guo, J.; Zhao, J.; Tang, H. Intrinsic viscosity and rheological properties of natural and substituted guar gums in seawater. Int. J. Biol. Macromol. 2015, 76, 262–268. [Google Scholar] [CrossRef]
- Park, S.Y.; Jun, S.T.; Marsh, K.S. Physical properties of PVOH/chitosan-blended films cast from different solvents. Food Hydrocoll. 2001, 15, 499–502. [Google Scholar] [CrossRef]
- Ghosh, A.; Ali, M.A. Studies on physicochemical characteristics of chitosan derivatives with dicarboxylic acids. J. Mater. Sci. 2012, 47, 1196–1204. [Google Scholar] [CrossRef]
- Melro, E.; Antunes, F.E.; da Silva, G.J.; Cruz, I.; Ramos, P.E.; Carvalho, F.; Alves, I. Chitosan films in food applications. Tuning film properties by changing acidic dissolution conditions. Polymers 2021, 13, 1. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Santarén, J.; Rasteiro, M.G.; Gamelas, J.A.F. Improving colloidal stability of sepiolite suspensions: Effect of the mechanical disperser and chemical dispersant. Minerals 2020, 10, 779. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Saldanha, T.D.P.; Navarro, M.V.M.; Medeiros, A.C.; Ginani, M.F.; Raffin, F.N. Mechanical properties and release studies of chitosan films impregnated with silver sulfadiazine. J. Appl. Polym. Sci. 2006, 102, 3462–3470. [Google Scholar] [CrossRef]
- Baskar, D.; Sampath Kumar, T.S. Effect of deacetylation time on the preparation, properties and swelling behaviour of chitosan films. Carbohydr. Polym. 2009, 78, 767–772. [Google Scholar] [CrossRef]
- He, Q.; Ao, Q.; Gong, Y.; Zhang, X. Preparation of chitosan films using different neutralizing solutions to improve endothelial cell compatibility. J. Mater. Sci. 2011, 22, 2791–2802. [Google Scholar] [CrossRef]
- Takara, E.A.; Marchese, J.; Ochoa, N.A. NaOH treatment of chitosan films: Impact on macromolecular structure and film properties. Carbohydr. Polym. 2015, 132, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Son, J.H.S.; Kim, S.-K. Properties of chitosan films as a function of pH and solvent type. J. Food Sci. E 2006, 71, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Li, X.; Wu, Q.; Duan, L.; Zhou, S.; Zhang, Y. Effect of lactic acid and mixed acid aqueous solutions on the preparation, structure and properties of thermoplastic chitosan. Eur. Polym. J. 2020, 134, 109850. [Google Scholar] [CrossRef]
- Park, S.Y.; Marsh, K.S.; Rhim, J.W. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. J. Food Sci. 2002, 67, 194–197. [Google Scholar] [CrossRef]
- Ritthidej, G.C.; Phaechamud, T.; Koizumi, T. Moist heat treatment on physicochemical change of chitosan salt films. Int. J. Pharm. 2002, 232, 11–22. [Google Scholar] [CrossRef]
- Nataraj, D.; Sakkara, S.; Meghwal, M.; Reddy, N. Crosslinked chitosan films with controllable properties for commercial applications. Int. J. Biol. Macromol. 2018, 120, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Bohdanecký, M.; Kovář, I. Viscosity of Polymer Solution; Jenkins, A.D., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1982; Volume 2, pp. 167–186. [Google Scholar]
- Roberts, G.A.F.; Domoszy, J.G. Determination of viscometric constants for chitosan. Int. J. Biol. Macromol. 1982, 4, 374–377. [Google Scholar] [CrossRef]
- Broussignac, P. Chitosan: A natural polymer not well known by the industry. Chem. Ind. Genie Chim. 1968, 99, 1241–1247. [Google Scholar]
- Lei, M.; Huang, W.; Sun, J.; Shao, J.; Duan, W.; Wu, T.; Wang, Y. Synthesis, characterization, and performance of carboxymethyl chitosam with different molecular weight as additive in water-based drilling fluid. J. Mol. Liq. 2020, 310, 113135. [Google Scholar] [CrossRef]
- Cai, J.; Fei, P.; Xiong, Z.; Shi, Y.; Yan, K.; Xiong, H. Surface acetylation of bamboo cellulose: Preparation and rheological properties. Carbohydr. Polym. 2013, 92, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chalah, K.; Benmounah, A.; Mahdad, M.; Kheribet, R. Rheological study of sodium carboxymethylcellulose: Effect of concentration and molecular weight. Mater. Today Proc. 2022, 53, 185–190. [Google Scholar] [CrossRef]
- Romain, S.; Le, K.A.; Ries, M.E.; Budtova, T. Viscosity of cellulose-imidazolium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 7222–7228. [Google Scholar]
- Polish Norm PN-81/C-89034 (ISO 527-1 and 527-2); International Standard: Plastic—Determination of Tensile Properties—Second Edition. ISO: Geneva, Switzerland, 2012.
- Xianmiao, C.; Yubao, L.; Yi, Z.; Li, Z.; Jidong, L.; Huanan, W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater. Sci. Eng. C 2009, 29, 29–35. [Google Scholar] [CrossRef]
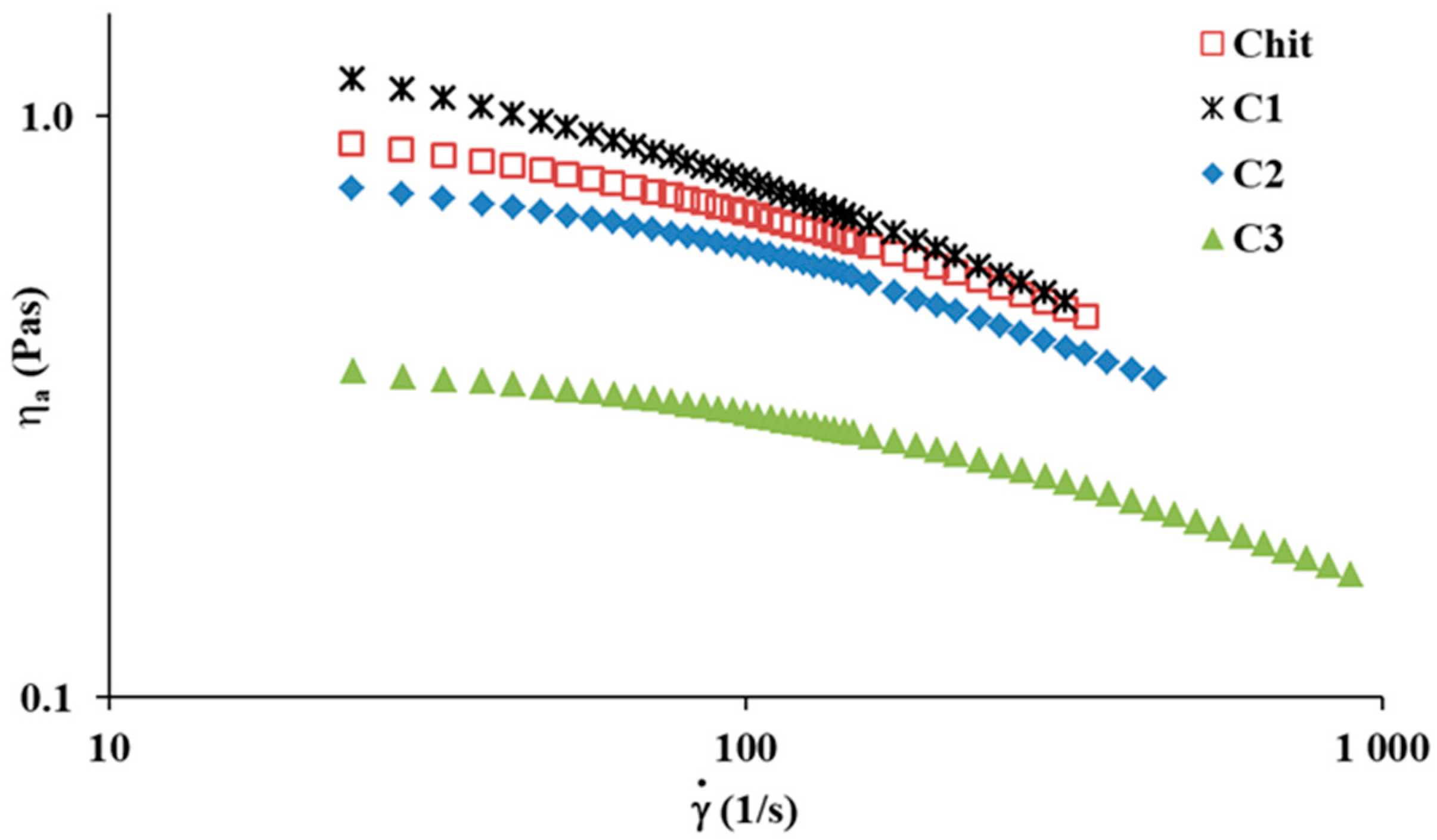
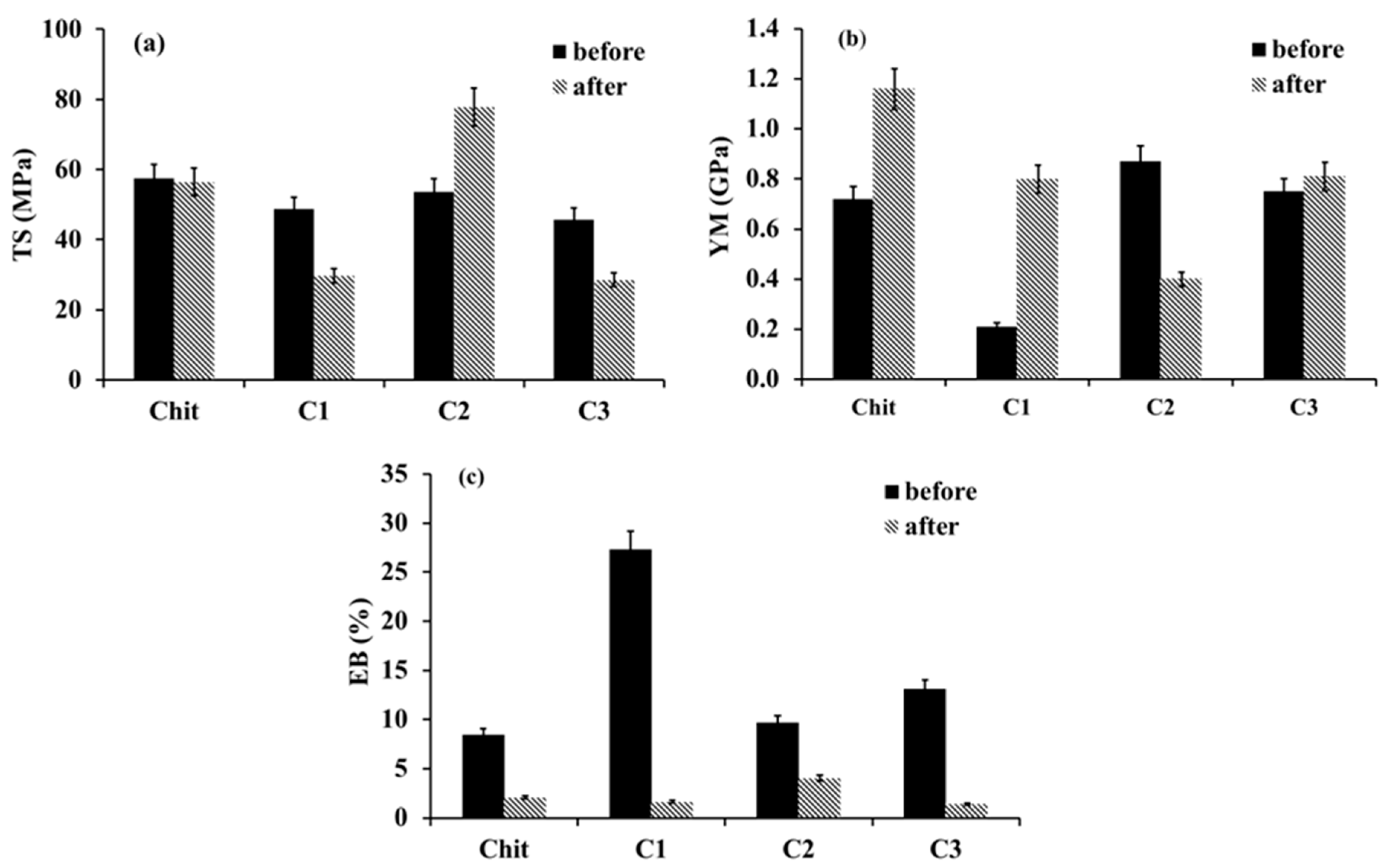
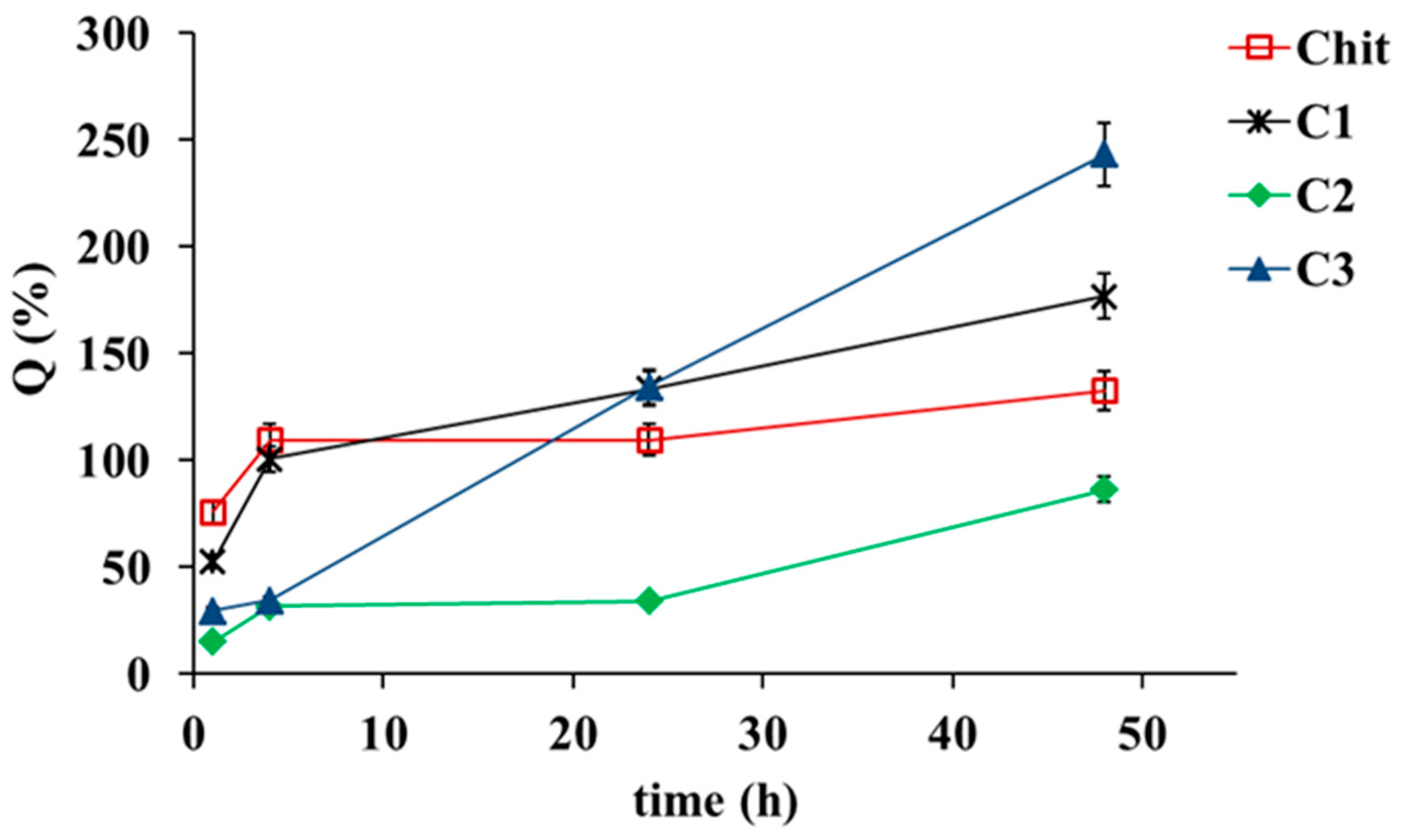
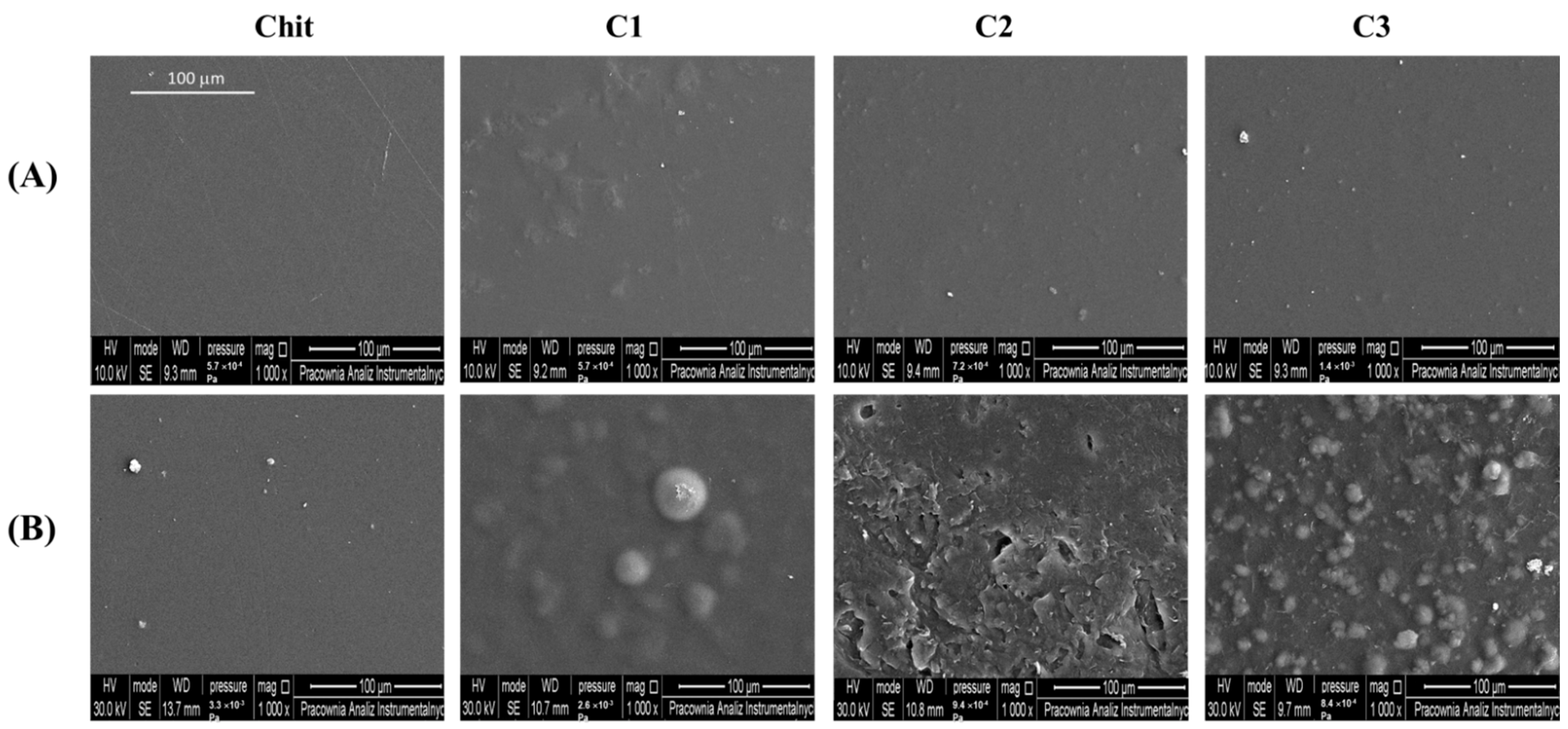
| Power Law Model | Cross Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | n | k (Pas)n | R2 | η0 (Pas) | η∞ (Pas) | λ (1/s) | m | R2 |
| Chit | 0.73 | 2.35 | 0.999 | 1.08 | 0.0863 | 0.0196 | 0.77 | 1.00 |
| C1 | 0.64 | 3.93 | 0.998 | 1.59 | 0.0878 | 0.0377 | 0.75 | 1.00 |
| C2 | 0.72 | 2.07 | 0.997 | 0.80 | 0.197 | 2.78 × 10−3 | 1.14 | 1.00 |
| C3 | 0.76 | 0.89 | 0.998 | 0.40 | 0.0497 | 7.79 × 10−3 | 0.82 | 1.00 |
| Sample | Ea (kJ/mol) | |||
|---|---|---|---|---|
| 0 s−1 | R2 | 247 s−1 | R2 | |
| Chit | 38.62 | 0.993 | 33.39 | 0.995 |
| C1 | 32.92 | 0.969 | 25.27 | 1 |
| C2 | 35.21 | 0.997 | 30.49 | 1 |
| C3 | 37.13 | 0.993 | 29.36 | 1 |
| Sample | Before | After |
|---|---|---|
| Rq (nm) | Rq (nm) | |
| Chit | 3.75 | 6.77 |
| C1 | 1.07 | 64.4 |
| C2 | 0.529 | 47.0 |
| C3 | 3.08 | 32.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, K.; Szulc, M. Rheological and Film-Forming Properties of Chitosan Composites. Int. J. Mol. Sci. 2022, 23, 8763. https://doi.org/10.3390/ijms23158763
Lewandowska K, Szulc M. Rheological and Film-Forming Properties of Chitosan Composites. International Journal of Molecular Sciences. 2022; 23(15):8763. https://doi.org/10.3390/ijms23158763
Chicago/Turabian StyleLewandowska, Katarzyna, and Marta Szulc. 2022. "Rheological and Film-Forming Properties of Chitosan Composites" International Journal of Molecular Sciences 23, no. 15: 8763. https://doi.org/10.3390/ijms23158763
APA StyleLewandowska, K., & Szulc, M. (2022). Rheological and Film-Forming Properties of Chitosan Composites. International Journal of Molecular Sciences, 23(15), 8763. https://doi.org/10.3390/ijms23158763







