Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism
Abstract
1. Introduction
2. Molecular Characteristics of MC5R
| Species | MC5R Expression in Different Tissues | Techniques |
|---|---|---|
| Human [51] | Present in brain, pancreas, lung, heart, testes, and fat tissues | RT-PCR |
| Mouse [41,78] | Abundant in the Harderian, lacrimal, and preputial glands; moderate in muscle and skin; low levels in adipose, spinal cord, and brain; absent in spleen, kidney, liver, heart, lung, and gonad | In situ hybridization |
| Rat [42] | Abundant in lacrimal, preputial, and Harderian glands; low levels in adrenal glands, pancreas, esophagus, and thymus; absent in thyroid gland, seminal vesicle, spleen, liver, and skeletal muscle | Western blot, In situ hybridization |
| Chicken [87] | Present in brain, kidney, liver, adrenals, ovary, testis, uropygial gland, and adipose tissue; absent in heart, spleen, and skeletal muscle | RT-PCR |
| Zebrafish [74] | Present in ovary, brain, gastrointestinal tract, and eye (mc5ra); present in ovary, brain, gastrointestinal tract, eye, and heart (mc5rb) | RT-PCR |
| Barfin flounder [88] | Present in pituitary, brain, eyeball, gill, atrium, ventricle, liver, head kidney, kidney, spleen, stomach, intestine, white muscle, inclinator muscle, testis, ovary, and skin | RT–PCR |
| Sea bass [62] | Present in retina, brain, liver, spleen, gill, testis, and dorsal skin; low levels in the pituitary, posterior kidney, fat tissue, intestine, red muscle, and ovary | RT–PCR |
| Goldfish [80] | Present in the kidney, spleen, skin, retina, and brain; low levels in the intestine, fat, muscle, gill, pituitary, and ovary | RT–PCR, Southern blot |
| Common carp [81] | Present in brain, skin, kidney, and pituitary; absent in thymus, spleen, head kidney, gut, gill, liver, heart, and muscle | RT–PCR |
| Blunt snout bream [37] | Present in brain, eyes, skin, testis, ovary, and gill; low levels in the muscle, intestine, kidney, head kidney, spleen, and liver | RT–PCR |
| Horn shark [71] | Present in brain, pituitary, skin, and liver | RT–PCR |
| Stingray [89] | Present in hypothalamus and inter-renal tissues | RT–PCR |
| Elephant shark [10] | Present in hypothalamus, pituitary, brain, and kidney | RT–PCR |
3. Pharmacology of MC5R
MC5R Ligands
4. The Effect of MRAPs on MC5R Pharmacology
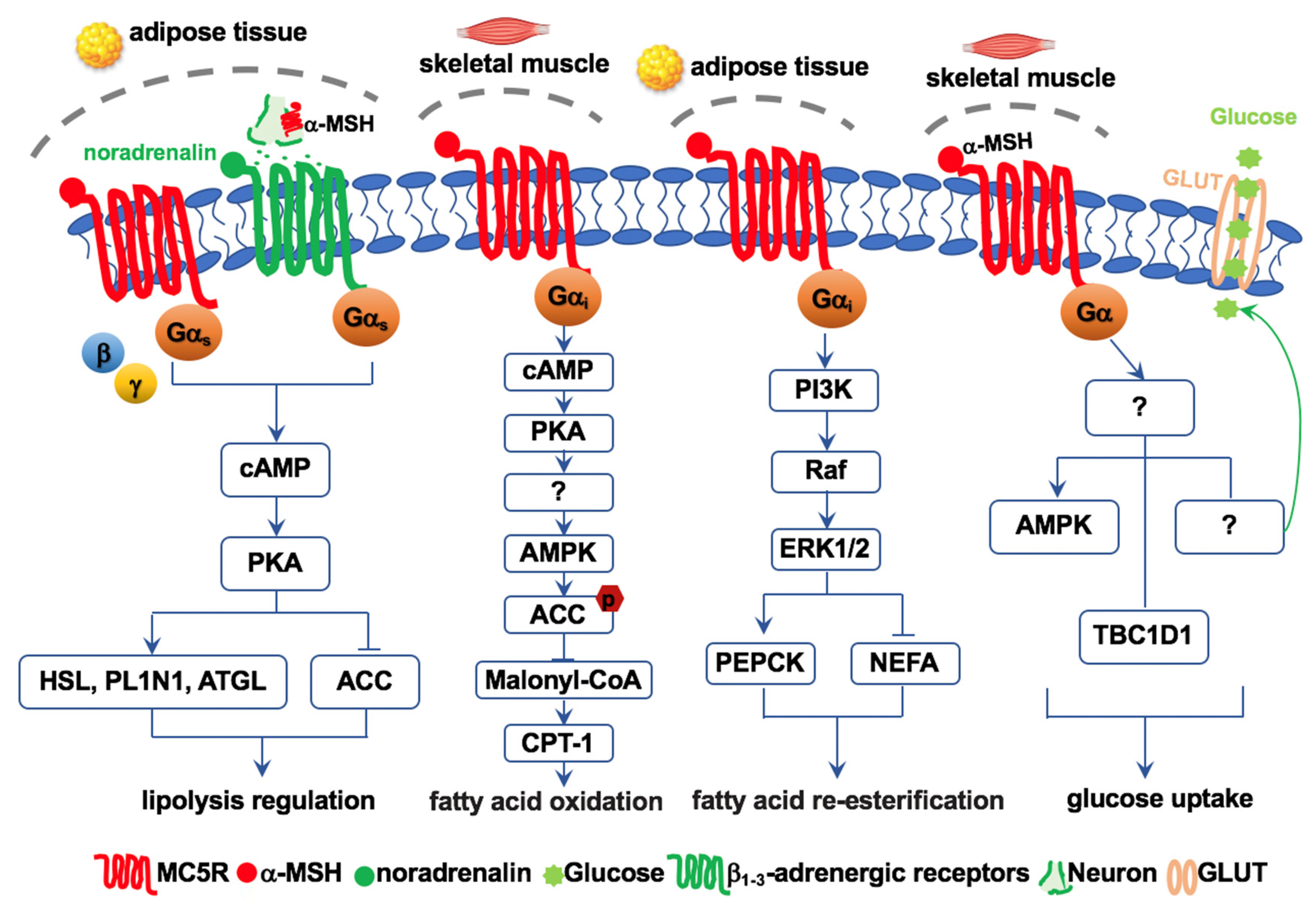
5. Functions of MC5R in Energy Metabolism
6. MC5R Regulates Lipolysis and Re-Esterification
7. MC5R Regulates Fatty Acid Oxidation
8. MC5R Regulates Glucose Homeostasis
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cone, R.D. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 2006, 27, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Melanocortin receptors. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt A, 2411–2413. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Shekar, S.N.; Cook, A.L.; Duffy, D.L.; Sturm, R.A. Red hair is the null phenotype of MC1R. Hum. Mutat. 2008, 29, E88–E94. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 receptor: Structure, function, and regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, D.Y.; Lin, Y.J.; Tao, Y.X. Melanocortin regulation of inflammation. Front. Endocrinol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.Q.; Rao, Y.Z.; Zhang, Y.; Chen, R.; Tao, Y.X. Regulation of melanocortin-1 receptor pharmacology by melanocortin receptor accessory protein 2 in orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2019, 285, 113291. [Google Scholar] [CrossRef]
- Ji, R.L.; Tao, Y.X. Melanocortin-1 receptor mutations and pigmentation: Insights from large animals. Prog. Mol. Biol. Transl. Sci. 2022, 189, 179–213. [Google Scholar] [PubMed]
- Chida, D.; Nakagawa, S.; Nagai, S.; Sagara, H.; Katsumata, H.; Imaki, T.; Suzuki, H.; Mitani, F.; Ogishima, T.; Shimizu, C. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18205–18210. [Google Scholar] [CrossRef]
- Dores, R.M. Observations on the evolution of the melanocortin receptor gene family: Distinctive features of the melanocortin-2 receptor. Front. Neurosci. 2013, 7, 28. [Google Scholar] [CrossRef]
- Barney, E.; Dores, M.R.; McAvoy, D.; Davis, P.; Racareanu, R.C.; Iki, A.; Hyodo, S.; Dores, R.M. Elephant shark melanocortin receptors: Novel interactions with MRAP1 and implication for the HPI axis. Gen. Comp. Endocrinol. 2019, 272, 42–51. [Google Scholar] [CrossRef]
- Dores, R.M.; Chapa, E. Hypothesis and Theory: Evaluating the co-evolution of the melanocortin-2 receptor and the accessory protein MRAP1. Front. Endocrinol. 2021, 12, 747843. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef]
- Tao, Y.X. The melanocortin-4 receptor: Physiology, pharmacology, and pathophysiology. Endocr. Rev. 2010, 31, 506–543. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Mutations in the melanocortin-3 receptor (MC3R) gene: Impact on human obesity or adiposity. Curr. Opin. Investig. Drugs 2010, 11, 1092–1096. [Google Scholar] [PubMed]
- Yang, Z.; Tao, Y.X. Mutations in melanocortin-3 receptor gene and human obesity. Prog. Mol. Biol. Transl. Sci. 2016, 140, 97–129. [Google Scholar] [CrossRef]
- You, P.; Hu, H.; Chen, Y.; Zhao, Y.; Yang, Y.; Wang, T.; Xing, R.; Shao, Y.; Zhang, W.; Li, D.; et al. Effects of melanocortin 3 and 4 receptor deficiency on energy homeostasis in rats. Sci. Rep. 2016, 6, 34938. [Google Scholar] [CrossRef]
- Lotta, L.A.; Mokrosiński, J.; de Oliveira, E.M.; Li, C.; Sharp, S.J.; Luan, J.; Brouwers, B.; Ayinampudi, V.; Bowker, N.; Kerrison, N.; et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 2019, 177, 597–607. [Google Scholar] [CrossRef]
- Liu, T.; Ji, R.L.; Tao, Y.X. Naturally occurring mutations in G protein-coupled receptors associated with obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2022, 234, 108044. [Google Scholar] [CrossRef]
- Cai, M.; Hruby, V.J. The melanocortin receptor system: A target for multiple degenerative diseases. Curr. Protein Pept. Sci. 2016, 17, 488–496. [Google Scholar] [CrossRef]
- Yuan, X.C.; Tao, Y.X. Fenoprofen—An old drug rediscovered as a biased allosteric enhancer for melanocortin receptors. ACS Chem. Neurosci. 2018, 10, 1066–1074. [Google Scholar] [CrossRef]
- Gantz, I.; Konda, Y.; Tashiro, T.; Shimoto, Y.; Miwa, H.; Munzert, G.; Watson, S.J.; DelValle, J.; Yamada, T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993, 268, 8246–8250. [Google Scholar] [CrossRef]
- Roselli-Rehfuss, L.; Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Low, M.J.; Tatro, J.B.; Entwistle, M.L.; Simerly, R.B.; Cone, R.D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. USA 1993, 90, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Gantz, I.; Miwa, H.; Konda, Y.; Shimoto, Y.; Tashiro, T.; Watson, S.J.; DelValle, J.; Yamada, T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993, 268, 15174–15179. [Google Scholar] [CrossRef]
- Mountjoy, K.G.; Mortrud, M.T.; Low, M.J.; Simerly, R.B.; Cone, R.D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 1994, 8, 1298–1308. [Google Scholar] [PubMed]
- Yang, L.K.; Tao, Y.X. Biased signaling at neural melanocortin receptors in regulation of energy homeostasis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt A, 2486–2495. [Google Scholar] [CrossRef]
- Chen, A.S.; Marsh, D.J.; Trumbauer, M.E.; Frazier, E.G.; Guan, X.M.; Yu, H.; Rosenblum, C.I.; Vongs, A.; Feng, Y.; Cao, L.; et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000, 26, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Kesterson, R.A.; Khong, K.; Cullen, M.J.; Pelleymounter, M.A.; Dekoning, J.; Baetscher, M.; Cone, R.D. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 2000, 141, 3518–3521. [Google Scholar] [CrossRef]
- Zhang, Y.; Kilroy, G.E.; Henagan, T.M.; Prpic-Uhing, V.; Richards, W.G.; Bannon, A.W.; Mynatt, R.L.; Gettys, T.W. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 2005, 19, 1482–1491. [Google Scholar] [CrossRef]
- Sutton, G.M.; Begriche, K.; Kumar, K.G.; Gimble, J.M.; Perez-Tilve, D.; Nogueiras, R.; McMillan, R.P.; Hulver, M.W.; Tschop, M.H.; Butler, A.A. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010, 24, 862–872. [Google Scholar] [CrossRef]
- Begriche, K.; Marston, O.J.; Rossi, J.; Burke, L.K.; McDonald, P.; Heisler, L.K.; Butler, A.A. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes Brain Behav. 2012, 11, 291–302. [Google Scholar] [CrossRef]
- Girardet, C.; Butler, A.A. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Patterson, C.M.; Sutton, A.K.; Burnett, K.H.; Myers, M.G., Jr.; Olson, D.P. Lateral hypothalamic Mc3r-expressing neurons modulate locomotor activity, energy expenditure, and adiposity in male mice. Endocrinology 2019, 160, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Balthasar, N.; Dalgaard, L.T.; Lee, C.E.; Yu, J.; Funahashi, H.; Williams, T.; Ferreira, M.; Tang, V.; McGovern, R.A.; Kenny, C.D.; et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005, 123, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Constitutive activity in melanocortin-4 receptor: Biased signaling of inverse agonists. Adv. Pharmacol. 2014, 70, 135–154. [Google Scholar] [CrossRef]
- Yang, Z.; Tao, Y.X. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and -4 receptors. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1485–1494. [Google Scholar] [CrossRef]
- Tao, Y.X. Mutations in melanocortin-4 receptor: From fish to men. Prog. Mol. Biol. Transl. Sci. 2022, 189, 215–257. [Google Scholar] [CrossRef]
- Liao, S.C.; Dong, J.J.; Xu, W.N.; Xi, B.W.; Tao, Y.X.; Liu, B.; Xie, J. Molecular cloning, tissue distribution, and pharmacological characterization of blunt snout bream (Megalobrama amblycephala) melanocortin-5 receptor. Fish Physiol. Biochem. 2019, 45, 311–321. [Google Scholar] [CrossRef]
- Zhou, Y.; Chawla, M.K.; Rios-Monterrosa, J.L.; Wang, L.; Zempare, M.A.; Hruby, V.J.; Barnes, C.A.; Cai, M. Aged brains express less melanocortin receptors, which correlates with age-related decline of cognitive functions. Molecules 2021, 26, 6266. [Google Scholar] [CrossRef]
- Simamura, E.; Shimada, H.; Shoji, H.; Otani, H.; Hatta, T. Effects of melanocortins on fetal development. Congenit. Anom. 2011, 51, 47–54. [Google Scholar] [CrossRef]
- Shukla, C.; Koch, L.G.; Britton, S.L.; Cai, M.; Hruby, V.J.; Bednarek, M.; Novak, C.M. Contribution of regional brain melanocortin receptor subtypes to elevated activity energy expenditure in lean, active rats. Neuroscience 2015, 310, 252–267. [Google Scholar] [CrossRef]
- Chen, W.; Kelly, M.A.; Opitz-Araya, X.; Thomas, R.E.; Low, M.J.; Cone, R.D. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91, 789–798. [Google Scholar] [CrossRef]
- Van der Kraan, M.; Adan, R.A.; Entwistle, M.L.; Gispen, W.H.; Burbach, J.P.; Tatro, J.B. Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology 1998, 139, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Caurnel, M.; Ruth, E.T.; Roger, D.C. Melanocortin-5 receptor deficiency promotes defensive behavior in male mice. Horm. Behav. 2004, 45, 58–63. [Google Scholar] [CrossRef]
- Morgan, C.; Cone, R.D. Melanocortin-5 receptor deficiency in mice blocks a novel pathway influencing pheromone-induced aggression. Behav. Genet. 2006, 36, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, M.; Saeed, M.; Xu, Y.; Ren, Q.; Sun, C. αMSH inhibits adipose inflammation via reducing FoxOs transcription and blocking Akt/JNK pathway in mice. Oncotarget 2017, 8, 47642–47654. [Google Scholar] [CrossRef]
- Webering, S.; Lunding, L.P.; Vock, C.; Schröder, A.; Gaede, K.I.; Herzmann, C.; Fehrenbach, H.; Wegmann, M. The alpha-melanocyte-stimulating hormone acts as a local immune homeostasis factor in experimental allergic asthma. Clin. Exp. Allergy 2019, 49, 1026–1039. [Google Scholar] [CrossRef]
- Taylor, A.W.; Kitaichi, N.; Biros, D. Melanocortin 5 receptor and ocular immunity. Cell. Mol. Biol. 2006, 52, 53–59. [Google Scholar]
- Lee, D.J.; Taylor, A.W. Both MC5R and A2AR are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J. Immunol. 2013, 191, 4103–4111. [Google Scholar] [CrossRef]
- Ng, T.F.; Manhapra, A.; Cluckey, D.; Choe, Y.; Vajram, S.; Taylor, A.W. Melanocortin 5 receptor expression and recovery of ocular immune privilege after uveitis. Ocul. Immunol. Inflamm. 2021, 1–11. [Google Scholar] [CrossRef]
- McDonald, T.; Muhammad, F.; Peters, K.; Lee, D.J. Combined deficiency of the melanocortin 5 receptor and adenosine 2a receptor unexpectedly provides resistance to autoimmune disease in a CD8+ T cell-dependent manner. Front. Immunol. 2021, 12, 742154. [Google Scholar] [CrossRef]
- Chagnon, Y.C.; Chen, W.J.; Pérusse, L.; Chagnon, M.; Nadeau, A.; Wilkison, W.O.; Bouchard, C. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Québec family study. Mol. Med. 1997, 3, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Shim, J.H.; Cho, M.C.; Choe, Y.K.; Hong, J.T.; Moon, D.C.; Kim, J.W.; Yoon, D.Y. Signaling pathways implicated in α-melanocyte stimulating hormone-induced lipolysis in 3T3-L1 adipocytes. J. Cell. Biochem. 2005, 96, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Valli-Jaakola, K.; Suviolahti, E.; Schalin-Jäntti, C.; Ripatti, S.; Silander, K.; Oksanen, L.; Salomaa, V.; Peltonen, L.; Kontula, K. Further evidence for the role of ENPP1 in obesity: Association with morbid obesity in Finns. Obesity 2008, 16, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Moller, C.L.; Pedersen, S.B.; Bjørn, R.; Conde-Frieboes, K.W.; Raun, K.; Grove, K.L.; Wulff, B.S. Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res. Notes 2015, 8, 559. [Google Scholar] [CrossRef]
- Bradley, R.L.; Mansfield, J.P.; Maratos-Flier, E. Neuropeptides, including neuropeptide Y and melanocortins, mediate lipolysis in murine adipocytes. Obes. Res. 2005, 13, 653–661. [Google Scholar] [CrossRef]
- An, J.J.; Rhee, Y.; Kim, S.H.; Kim, D.M.; Han, D.H.; Hwang, J.H.; Jin, Y.J.; Cha, B.S.; Baik, J.H.; Lee, W.T. Peripheral effect of α-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J. Biol. Chem. 2007, 282, 2862–2870. [Google Scholar] [CrossRef]
- Iwen, K.A.H.; Senyaman, O.; Schwartz, A.; Drenckhan, M.; Meier, B.; Hadaschik, D.; Klein, J. Melanocortin crosstalk with adipose functions: ACTH directly induces insulin resistance, promotes a pro-inflammatory adipokine profile and stimulates UCP-1 in adipocytes. J. Endocrinol. 2008, 196, 465–472. [Google Scholar] [CrossRef]
- Møller, C.L.; Raun, K.; Jacobsen, M.L.; Pedersen, T.Å.; Holst, B.; Conde-Frieboes, K.W.; Wulff, B.S. Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved. Mol. Cell. Endocrinol. 2011, 341, 9–17. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Chen, Y.; Luo, D.; Feng, F.; Liu, G.; Sun, C. α-MSH and Foxc2 promote fatty acid oxidation through C/EBPβ negative transcription in mice adipose tissue. Sci. Rep. 2016, 6, 36661. [Google Scholar] [CrossRef]
- Mller, C.L.; Kjobsted, R.; Enriori, P.J.; Jensen, T.E.; Garcia-Rudaz, C.; Litwak, S.A.; Raun, K.; Wojtaszewski, J.; Wulff, B.S.; Cowley, M.A. α-MSH stimulates glucose uptake in mouse muscle and phosphorylates Rab-GTPase-activating protein TBC1D1 independently of AMPK. PLoS ONE 2016, 11, e0157027. [Google Scholar] [CrossRef]
- Shipp, S.L.; Wang, G.; Cline, M.A.; Gilbert, E.R. Chick subcutaneous and abdominal adipose tissue depots respond differently in lipolytic and adipogenic activity to α-melanocyte stimulating hormone (α-MSH). Comp. Biochem. Physiol. A 2017, 209, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Rubio, V.C.; Cerdá-Reverter, J.M. Characterization of the sea bass melanocortin 5 receptor: A putative role in hepatic lipid metabolism. J. Exp. Biol. 2009, 212, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.H.; Anthonavage, M.; Pappas, A.; Rossetti, D.; Cavender, D.; Seiberg, M.; Eisinger, M. Melanocortin-5 receptor and sebogenesis. Eur. J. Pharmacol. 2011, 660, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Wang, D.; Montieth, A.; Lee, S.; Preble, J.; Foster, C.S.; Larson, T.A.; Ding, K.; Dvorak, J.D.; Lee, D.J. PD-1+ melanocortin receptor dependent-Treg cells prevent autoimmune disease. Sci. Rep. 2019, 9, 16941. [Google Scholar] [CrossRef]
- Shintani, A.; Sakata-Haga, H.; Moriguchi, K.; Tomosugi, M.; Sakai, D.; Tsukada, T.; Taniguchi, M.; Asano, M.; Shimada, H.; Otani, H.; et al. MC5R contributes to sensitivity to UVB waves and barrier function in mouse epidermis. JID Innov. 2021, 1, 100024. [Google Scholar] [CrossRef]
- Örenay, Ö.M.; Sarıfakıoğlu, E.; Gülekon, A. Evaluation of perilipin 2 and melanocortin 5 receptor serum levels with sebogenesis in acne vulgaris patients. Acta Dermatovenerol. Alp. Pannonica Adriat. 2021, 30, 7–9. [Google Scholar] [CrossRef]
- Gantz, I.; Shimoto, Y.; Konda, Y.; Miwa, H.; Dickinson, C.J.; Yamada, T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200, 1214–1220. [Google Scholar] [CrossRef]
- Chowdhary, B.P.; Gustavsson, I.; Wikberg, J.E.; Chhajlani, V. Localization of the human melanocortin-5 receptor gene (MC5R) to chromosome band 18p11.2 by fluorescence in situ hybridization. Cytogenet. Cell Genet. 1995, 68, 79–81. [Google Scholar] [CrossRef]
- Logan, D.W.; Bryson-Richardson, R.J.; Pagan, K.E.; Taylor, M.S.; Currie, P.D.; Jackson, I.J. The structure and evolution of the melanocortin and MCH receptors in fish and mammals. Genomics 2003, 81, 184–191. [Google Scholar] [CrossRef]
- Schiöth, H.B.; Raudsepp, T.; Ringholm, A.; Fredriksson, R.; Takeuchi, S.; Larhammar, D.; Chowdhary, B.P. Remarkable synteny conservation of melanocortin receptors in chicken, human, and other vertebrates. Genomics 2003, 81, 504–509. [Google Scholar] [CrossRef]
- Baron, A.; Veo, K.; Angleson, J.; Dores, R.M. Modeling the evolution of the MC2R and MC5R genes: Studies on the cartilaginous fish, Heterondotus francisci. Gen. Comp. Endocrinol. 2009, 161, 13–19. [Google Scholar] [CrossRef]
- Västermark, A.; Schiöth, H.B. The early origin of melanocortin receptors, agouti-related peptide, agouti signalling peptide, and melanocortin receptor-accessory proteins, with emphasis on pufferfishes, elephant shark, lampreys, and amphioxus. Eur. J. Pharmacol. 2011, 660, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cortes, R.; Navarro, S.; Agulleiro, M.J.; Guillot, R.; Garcia-Herranz, V.; Sanchez, E.; Cerdá-Reverter, J.M. Evolution of the melanocortin system. Gen. Comp. Endocrinol. 2014, 209, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ringholm, A.; Fredriksson, R.; Poliakova, N.; Yan, Y.L.; Postlethwait, J.H.; Larhammar, D.; Schioth, H.B. One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J. Neurochem. 2002, 82, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, M.; Chen, Y.J.; Zhang, C. Pharmacological modulation of two melanocortin-5 receptors by MRAP2 proteins in zebrafish. J. Mol. Endocrinol. 2019, 62, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.S.; Gatesy, J. Evolution of the MC5R gene in placental mammals with evidence for its inactivation in multiple lineages that lack sebaceous glands. Mol. Phylogenetics Evol. 2018, 120, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, M.; Liu, S.; Xue, J.; Chen, H.; Li, W.; Zhou, J.; Amanullah, A.; Guan, M.; Bao, J.; et al. Differential MC5R loss in whales and manatees reveals convergent evolution to the marine environment. Dev. Genes Evol. 2022, 232, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Labbe, O.; Desarnaud, F.; Eggerickx, D.; Vassart, G.; Parmentier, M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry 1994, 33, 4543–4549. [Google Scholar] [CrossRef]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef]
- Cerdá-Reverter, J.M.; Ling, M.K.; Schioth, H.B.; Peter, R.E. Molecular cloning, characterization and brain mapping of the melanocortin 5 receptor in the goldfish. J. Neurochem. 2003, 87, 1354–1367. [Google Scholar] [CrossRef]
- Metz, J.R.; Geven, E.J.; van den Burg, E.H.; Flik, G. ACTH, α-MSH, and control of cortisol release: Cloning, sequencing, and functional expression of the melanocortin-2 and melanocortin-5 receptor in Cyprinus carpio. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R814–R826. [Google Scholar] [CrossRef] [PubMed]
- Dores, R.M.; Scuba-Gray, M.; McNally, B.; Davis, P.; Takahashi, A. Evaluating the interactions between red stingray (Dasyatis akajei) melanocortin receptors and elephant shark (Callorhinchus milii) MRAP1 and MRAP2 following stimulation with either stingray ACTH(1-24) or stingray Des-Acetyl-αMSH: A pharmacological study in Chinese Hamster Ovary cells. Gen. Comp. Endocrinol. 2018, 265, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Dores, R.M.; Oberer, N.; Hoglin, B.; Thomas, A.; Faught, E.; Vijayan, M.V. Evaluating interactions between the melanocortin-5 receptor, MRAP1, and ACTH(1–24): A phylogenetic study. Gen. Comp. Endocrinol. 2020, 294, 113476. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yi, T.L.; Yang, D.Q.; Tao, Y.X. Regulation of melanocortin-5 receptor pharmacology by two isoforms of MRAP2 in ricefield eel (Monopterus albus). Gen. Comp. Endocrinol. 2021, 314, 113928. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Goytain, A.; Ng, T. NanoString nCounter technology: High-throughput RNA validation. Methods Mol. Biol. 2020, 2079, 125–139. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takahashi, S. Melanocortin receptor genes in the chicken-tissue distributions. Gen. Comp. Endocrinol. 1998, 112, 220–231. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tsuchiya, K.; Yamanome, T.; Schiöth, H.B.; Takahashi, A. Differential expressions of melanocortin receptor subtypes in melanophores and xanthophores of barfin flounder. Gen. Comp. Endocrinol. 2010, 168, 133–142. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hamamoto, A.; Takahashi, A.; Saito, Y. Dimerization of melanocortin receptor 1 (MC1R) and MC5R creates a ligand-dependent signal modulation: Potential participation in physiological color change in the flounder. Gen. Comp. Endocrinol. 2016, 230, 103–109. [Google Scholar] [CrossRef]
- Smith, A.I.; Funder, J.W. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr. Rev. 1988, 9, 159–179. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Verhaagen, J.; Edwards, P.M.; Schrama, L.H.; Burbach, J.P.; Gispen, W.H. Expression of the pro-opiomelanocortin gene in dorsal root ganglia, spinal cord and sciatic nerve after sciatic nerve crush in the rat. Brain Res. Mol. Brain Res. 1992, 16, 135–142. [Google Scholar] [CrossRef][Green Version]
- Van der Kraan, M.; Tatro, J.B.; Entwistle, M.L.; Brakkee, J.H.; Burbach, J.P.; Adan, R.A.; Gispen, W.H. Expression of melanocortin receptors and pro-opiomelanocortin in the rat spinal cord in relation to neurotrophic effects of melanocortins. Brain Res. Mol. Brain Res. 1999, 63, 276–286. [Google Scholar] [CrossRef]
- Cone, R.D.; Cowley, M.A.; Butler, A.A.; Fan, W.; Marks, D.L.; Low, M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001, 25, S63–S67. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Inoue, A.; Kita, T.; Nakamura, M.; Chang, A.C.; Cohen, S.N.; Numa, S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature 1979, 278, 423–427. [Google Scholar] [CrossRef]
- Gantz, I.; Fong, T.M. The melanocortin system. Am. J. Physiol. 2003, 284, E468–E474. [Google Scholar] [CrossRef]
- Cone, R.D.; Lu, D.; Koppula, S.; Vage, D.I.; Klungland, H.; Boston, B.; Chen, W.; Orth, D.N.; Pouton, C.; Kesterson, R.A. The melanocortin receptors: Agonists, antagonists, and the hormonal control of pigmentation. Recent Prog. Horm. Res. 1996, 51, 287–317. [Google Scholar]
- Takahashi, A.; Davis, P.; Reinick, C.; Mizusawa, K.; Sakamoto, T.; Dores, R.M. Characterization of melanocortin receptors from stingray Dasyatis akajei, a cartilaginous fish. Gen. Comp. Endocrinol. 2016, 232, 115–124. [Google Scholar] [CrossRef]
- Min, T.; Liu, M.; Zhang, H.; Liu, Y.; Wang, Z. Molecular and pharmacological characterization of poultry (Gallus gallus, Anas platyrhynchos, Anser cygnoides domesticus) and pig (Sus scrofa domestica) melanocortin-5 receptors and their mutants. Gen. Comp. Endocrinol. 2019, 283, 113233. [Google Scholar] [CrossRef]
- Xu, Y.H.; Guan, X.J.; Zhou, R.; Gong, R.J. Melanocortin 5 receptor signaling pathway in health and disease. Cell. Mol. Life Sci. 2020, 77, 3831–3840. [Google Scholar] [CrossRef]
- Bednarek, M.A.; MacNeil, T.; Tang, R.; Fong, T.M.; Cabello, M.A.; Maroto, M.; Teran, A. Potent and selective agonists of human melanocortin receptor 5: Cyclic analogues of α-melanocyte-stimulating hormone. J. Med. Chem. 2007, 50, 2520–2526. [Google Scholar] [CrossRef]
- Gimenez, L.E.; Noblin, T.A.; Williams, S.Y.; Mullick Bagchi, S.; Ji, R.L.; Tao, Y.X.; Jeppesen, C.B.; Conde-Frieboes, K.W.; Sawyer, T.K.; Grieco, P.; et al. Demonstration of a common DPhe7 to DNal(2′)7 peptide ligand antagonist switch for melanocortin-3 and melanocortin-4 receptors identifies the systematic mischaracterization of the pharmacological properties of melanocortin peptides. J. Med. Chem. 2022, 65, 5990–6000. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J.; Michaud, E.J.; Woychik, R.P. Molecular characterization of the mouse agouti locus. Cell 1992, 71, 1195–1204. [Google Scholar] [CrossRef]
- Miller, M.W.; Duhl, D.M.; Vrieling, H.; Cordes, S.P.; Ollmann, M.M.; Winkes, B.M.; Barsh, G.S. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 1993, 7, 454–467. [Google Scholar] [CrossRef]
- Ollmann, M.M.; Wilson, B.D.; Yang, Y.K.; Kerns, J.A.; Chen, Y.; Gantz, I.; Barsh, G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997, 278, 135–138. [Google Scholar] [CrossRef]
- Shutter, J.R.; Graham, M.; Kinsey, A.C.; Scully, S.; Luthy, R.; Stark, K.L. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997, 11, 593–602. [Google Scholar] [CrossRef]
- Fong, T.M.; Mao, C.; MacNeil, T.; Kalyani, R.; Smith, T.; Weinberg, D.; Tota, M.R.; Van der Ploeg, L.H. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem. Biophys. Res. Commun. 1997, 237, 629–631. [Google Scholar] [CrossRef]
- Koerperich, Z.M.; Ericson, M.D.; Freeman, K.T.; Speth, R.C.; Pogozheva, I.D.; Mosberg, H.I.; Haskell-Luevano, C. Incorporation of agouti-related protein (AgRP) human single nucleotide polymorphisms (SNPs) in the AgRP-derived macrocyclic scaffold c[Pro-Arg-Phe-Phe-Asn-Ala-Phe-dPro] decreases melanocortin-4 receptor antagonist potency and results in the discovery of melanocortin-5 receptor antagonists. J. Med. Chem. 2020, 63, 2194–2208. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; McGurk, S.; Smit, N.P.; Nibbering, P.H.; Ancans, J.; van der Laarse, A.; Thody, A.J. Ligand-dependent activation of the melanocortin 5 receptor: cAMP production and ryanodine receptor-dependent elevations of [Ca2+]i. Biochem. Biophys. Res. Commun. 2002, 290, 844–850. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Almeida, H.; Gouveia, A.M. Melanocortin 5 receptor signaling and internalization: Role of MAPK/ERK pathway and β-arrestins 1/2. Mol. Cell. Endocrinol. 2012, 361, 69–79. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Almeida, H.; Gouveia, A.M. α-MSH signalling via melanocortin 5 receptor promotes lipolysis and impairs re-esterification in adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1267–1275. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Pignatelli, D.; Almeida, H.; Gouveia, A.M. Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol. Cell. Endocrinol. 2009, 303, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Metherell, L.A.; Chapple, J.P.; Cooray, S.; David, A.; Becker, C.; Rüschendorf, F.; Naville, D.; Begeot, M.; Khoo, B.; Nürnberg, P. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 2005, 37, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rached, M.; Gallo-Payet, N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol. Endocrinol. 2007, 21, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J.A.; Hinkle, P.M. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc. Natl. Acad. Sci. USA 2007, 104, 20244–20249. [Google Scholar] [CrossRef]
- Gorrigan, R.J.; Guasti, L.; King, P.; Clark, A.J.; Chan, L.F. Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. J. Mol. Endocrinol. 2011, 46, 227–232. [Google Scholar] [CrossRef]
- Chan, L.F.; Webb, T.R.; Chung, T.T.; Meimaridou, E.; Cooray, S.N.; Guasti, L.; Chapple, J.P.; Egertova, M.; Elphick, M.R.; Cheetham, M.E.; et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc. Natl. Acad. Sci. USA 2009, 106, 6146–6151. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Berruien, N.N.A.; Smith, C.L. Emerging roles of melanocortin receptor accessory proteins (MRAP and MRAP2) in physiology and pathophysiology. Gene 2020, 757, 144949. [Google Scholar] [CrossRef]
- Sebag, J.A.; Hinkle, P.M. Opposite effects of the melanocortin-2 (MC2) receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J. Biol. Chem. 2009, 284, 22641–22648. [Google Scholar] [CrossRef]
- Wolverton, E.A.; Wong, M.K.-S.; Davis, P.E.; Hoglin, B.; Braasch, I.; Dores, R.M. Analyzing the signaling properties of gar (Lepisosteus oculatus) melanocortin receptors: Evaluating interactions with MRAP1 and MRAP2. Gen. Comp. Endocrinol. 2019, 282, 113215. [Google Scholar] [CrossRef]
- Hoglin, B.E.; Miner, M.; Dores, R.M. Pharmacological properties of whale shark (Rhincodon typus) melanocortin-2 receptor and melancortin-5 receptor: Interaction with MRAP1 and MRAP2. Gen. Comp. Endocrinol. 2020, 293, 113463. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.L.; Maekawa, F.; Kawashima, T.; Sakamoto, H.; Sakamoto, T.; Davis, P.; Dores, R.M. Analyzing the effects of co-expression of chick (Gallus gallus) melanocortin receptors with either chick MRAP1 or MRAP2 in CHO cells on sensitivity to ACTH(1–24) or ACTH(1–13)NH2: Implications for the avian HPA axis and avian melanocortin circuits in the hypothalamus. Gen. Comp. Endocrinol. 2018, 256, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Enriori, P.J.; Chen, W.; Garcia-Rudaz, M.C.; Grayson, B.E.; Evans, A.E.; Comstock, S.M.; Gebhardt, U.; Müller, H.L.; Reinehr, T.; Henry, B.A.; et al. α-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors. Mol. Metab. 2016, 5, 807–822. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Liu, J.M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. Corrigendum: MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 2017, 546, 440. [Google Scholar] [CrossRef]
- Butler, A.A.; Cone, R.D. Knockout models resulting in the development of obesity. Trends Genet. 2001, 17, S50–S54. [Google Scholar] [CrossRef]
- Chen, A.S.; Metzger, J.M.; Trumbauer, M.E.; Guan, X.M.; Yu, H.; Frazier, E.G.; Marsh, D.J.; Forrest, M.J.; Gopal-Truter, S.; Fisher, J.; et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000, 9, 145–154. [Google Scholar] [CrossRef]
- Renquist, B.J.; Murphy, J.G.; Larson, E.A.; Olsen, D.; Klein, R.F.; Ellacott, K.L.; Cone, R.D. Melanocortin-3 receptor regulates the normal fasting response. Proc. Natl. Acad. Sci. USA 2012, 109, E1489–E1498. [Google Scholar] [CrossRef]
- Sutton, G.M.; Trevaskis, J.L.; Hulver, M.W.; McMillan, R.P.; Markward, N.J.; Babin, M.J.; Meyer, E.A.; Butler, A.A. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 2006, 147, 2183–2196. [Google Scholar] [CrossRef]
- Butler, A.A.; Marks, D.L.; Fan, W.; Kuhn, C.M.; Bartolome, M.; Cone, R.D. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat. Neurosci. 2001, 4, 605–611. [Google Scholar] [CrossRef]
- Rowland, N.E.; Schaub, J.W.; Robertson, K.L.; Andreasen, A.; Haskell-Luevano, C. Effect of MTII on food intake and brain c-Fos in melanocortin-3, melanocortin-4, and double MC3 and MC4 receptor knockout mice. Peptides 2010, 31, 2314–2317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutton, A.K.; Goforth, P.B.; Gonzalez, I.E.; Dell’Orco, J.; Pei, H.; Myers, M.G., Jr.; Olson, D.P. Melanocortin 3 receptor-expressing neurons in the ventromedial hypothalamus promote glucose disposal. Proc. Natl. Acad. Sci. USA 2021, 118, e2103090118. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Liu, T.; Kong, X.; Sohn, J.W.; Vong, L.; Deng, Z.; Lee, C.E.; Lee, S.; Williams, K.W.; Olson, D.P.; et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat. Neurosci. 2014, 17, 911–913. [Google Scholar] [CrossRef] [PubMed]
- De Souza Cordeiro, L.M.; Elsheikh, A.; Devisetty, N.; Morgan, D.A.; Ebert, S.N.; Rahmouni, K.; Chhabra, K.H. Hypothalamic MC4R regulates glucose homeostasis through adrenaline-mediated control of glucose reabsorption via renal GLUT2 in mice. Diabetologia 2021, 64, 181–194. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Chaves, V.E.; Frasson, D.; Kawashita, N.H. Several agents and pathways regulate lipolysis in adipocytes. Biochimie 2011, 93, 1631–1640. [Google Scholar] [CrossRef]
- Kim, H.E.; Grant, A.R.; Simic, M.S.; Kohnz, R.A.; Nomura, D.K.; Durieux, J.; Riera, C.E.; Sanchez, M.; Kapernick, E.; Wolff, S.; et al. Lipid biosynthesis coordinates a mitochondrial-to-cytosolic stress response. Cell 2016, 166, 1539–1552. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wen, X. Jueming prescription and its ingredients, semen cassiae and Rhizoma Curcumae Longae, stimulate lipolysis and enhance the phosphorylation of hormone-sensitive lipase in cultured rat white adipose tissue. Mol. Med. Rep. 2017, 16, 6200–6207. [Google Scholar] [CrossRef][Green Version]
- Boston, B.A.; Cone, R.D. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology 1996, 137, 2043–2050. [Google Scholar] [CrossRef]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.; Isidori, A.M.; Frajese, V.; Caprio, M.; Chew, S.L.; Grossman, A.B.; Clark, A.J.; Michael Besser, G.; Fabbri, A. ACTH and alpha-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: Model for a central-peripheral melanocortin-leptin pathway. Mol. Cell. Endocrinol. 2003, 200, 99–109. [Google Scholar] [CrossRef]
- Ceddia, R.P.; Collins, S. A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin. Sci. 2020, 134, 473–512. [Google Scholar] [CrossRef]
- Resnyk, C.W.; Chen, C.; Huang, H.; Wu, C.H.; Simon, J.; Bihan-Duval, E.L.; Duclos, M.J.; Cogburn, L.A. RNA-Seq analysis of abdominal fat in genetically fat and lean chickens highlights a divergence in expression of genes controlling adiposity, hemostasis, and lipid metabolism. PLoS ONE 2015, 10, e0139549. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Segaloff, D.L. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology 2003, 144, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Donohoue, P.A.; Tao, Y.X.; Collins, M.; Yeo, G.S.H.; O’Rahilly, S.; Segaloff, D.L. Deletion of codons 88-92 of the melanocortin-4 receptor gene: A novel deleterious mutation in an obese female. J. Clin. Endocrinol. Metab. 2003, 88, 5841–5845. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Segaloff, D.L. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J. Clin. Endocrinol. Metab. 2004, 89, 3936–3942. [Google Scholar] [CrossRef][Green Version]
- Tao, Y.X.; Segaloff, D.L. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 5632–5638. [Google Scholar] [CrossRef]
- Rong, R.; Tao, Y.X.; Cheung, B.M.; Xu, A.; Cheung, G.C.; Lam, K.S. Identification and functional characterization of three novel human melanocortin-4 receptor gene variants in an obese Chinese population. Clin. Endocrinol. 2006, 65, 198–205. [Google Scholar] [CrossRef]
- Tao, Y.X. Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 1167–1174. [Google Scholar] [CrossRef]
- Fan, Z.C.; Tao, Y.X. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J. Cell. Mol. Med. 2009, 13, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Ludwig, M.; Woelfle, J.; Fan, Z.C.; Brumm, H.; Biebermann, H.; Tao, Y.X. A novel melanocortin-4 receptor gene mutation in a female patient with severe childhood obesity. Endocrine 2009, 36, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Tao, Y.X. Functional studies on twenty novel naturally occurring melanocortin-4 receptor mutations. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tao, Y.X. Functional characterization of nine novel naturally occurring human melanocortin-3 receptor mutations. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1752–1761. [Google Scholar] [CrossRef]
- He, S.; Tao, Y.X. Defect in MAPK signaling as a cause for monogenic obesity caused by inactivating mutations in the melanocortin-4 receptor gene. Int. J. Biol. Sci. 2014, 10, 1128–1137. [Google Scholar] [CrossRef]
- Hohenadel, M.G.; Thearle, M.S.; Grice, B.A.; Huang, H.; Dai, M.H.; Tao, Y.X.; Hunter, L.A.; Palaguachi, G.I.; Mou, Z.; Kim, R.C.; et al. Brain-derived neurotrophic factor in human subjects with function-altering melanocortin-4 receptor variants. Int. J. Obes. 2014, 38, 1068–1074. [Google Scholar] [CrossRef]
- Yang, F.; Huang, H.; Tao, Y.X. Biased signaling in naturally occurring mutations in human melanocortin-3 receptor gene. Int. J. Biol. Sci. 2015, 11, 423–433. [Google Scholar] [CrossRef]
- Yang, L.K.; Hou, Z.S.; Tao, Y.X. Biased signaling in naturally occurring mutations of G protein-coupled receptors associated with diverse human diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165973. [Google Scholar] [CrossRef]
- Huang, H.; Tao, Y.X. A small molecule agonist THIQ as a novel pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Int. J. Biol. Sci. 2014, 10, 817–824. [Google Scholar] [CrossRef]
- Tao, Y.X.; Huang, H. Ipsen 5i is a novel potent pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Front. Endocrinol. 2014, 5, 131. [Google Scholar] [CrossRef]
- Jiang, D.N.; Li, J.T.; Tao, Y.X.; Chen, H.P.; Deng, S.P.; Zhu, C.H.; Li, G.L. Effects of melanocortin-4 receptor agonists and antagonists on expression of genes related to reproduction in spotted scat, Scatophagus argus. J. Comp. Physiol. B 2017, 187, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Jain, S.S.; Rimbaud, S.; Dam, A.; Quadrilatero, J.; Ventura-Clapier, R.; Bonen, A.; Holloway, G.P. FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem. J. 2011, 437, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Hoy, A.J. Lipid metabolism in skeletal muscle: Generation of adaptive and maladaptive intracellular signals for cellular function. Am. J. Physiol. Endocrinol. Metab. 2011, 302, E1315–E1328. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of skeletal muscle in insulin resistance and glucose uptake. Comp. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Chen, S.; Murphy, J.; Toth, R.; Campbell, D.G.; Morrice, N.A.; Mackintosh, C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem. J. 2008, 409, 449–459. [Google Scholar] [CrossRef]
- Miinea, C.P.; Sano, H.; Kane, S.; Sano, E.; Fukuda, M.; Peranen, J.; Lane, W.S.; Lienhard, G.E. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 2005, 391, 87–93. [Google Scholar] [CrossRef]
- Roach, W.G.; Chavez, J.A.; Miinea, C.P.; Lienhard, G.E. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem. J. 2007, 403, 353–358. [Google Scholar] [CrossRef]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G., Jr.; Bevilacqua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef]
- Katsuki, A.; Sumida, Y.; Murashima, S.; Furuta, M.; Araki-Sasaki, R.; Tsuchihashi, K.; Hori, Y.; Yano, Y.; Adachi, Y. Elevated plasma levels of alpha-melanocyte stimulating hormone (alpha-MSH) are correlated with insulin resistance in obese men. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1260–1264. [Google Scholar] [CrossRef][Green Version]
- Hoggard, N.; Johnstone, A.M.; Faber, P.; Gibney, E.R.; Elia, M.; Lobley, G.; Rayner, V.; Horgan, G.; Hunter, L.; Bashir, S.; et al. Plasma concentrations of alpha-MSH, AgRP and leptin in lean and obese men and their relationship to differing states of energy balance perturbation. Clin. Endocrinol. 2004, 61, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Budry, L.; Lafont, C.; El Yandouzi, T.; Chauvet, N.; Conéjero, G.; Drouin, J.; Mollard, P. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc. Natl. Acad. Sci. USA 2011, 108, 12515–12520. [Google Scholar] [CrossRef] [PubMed]

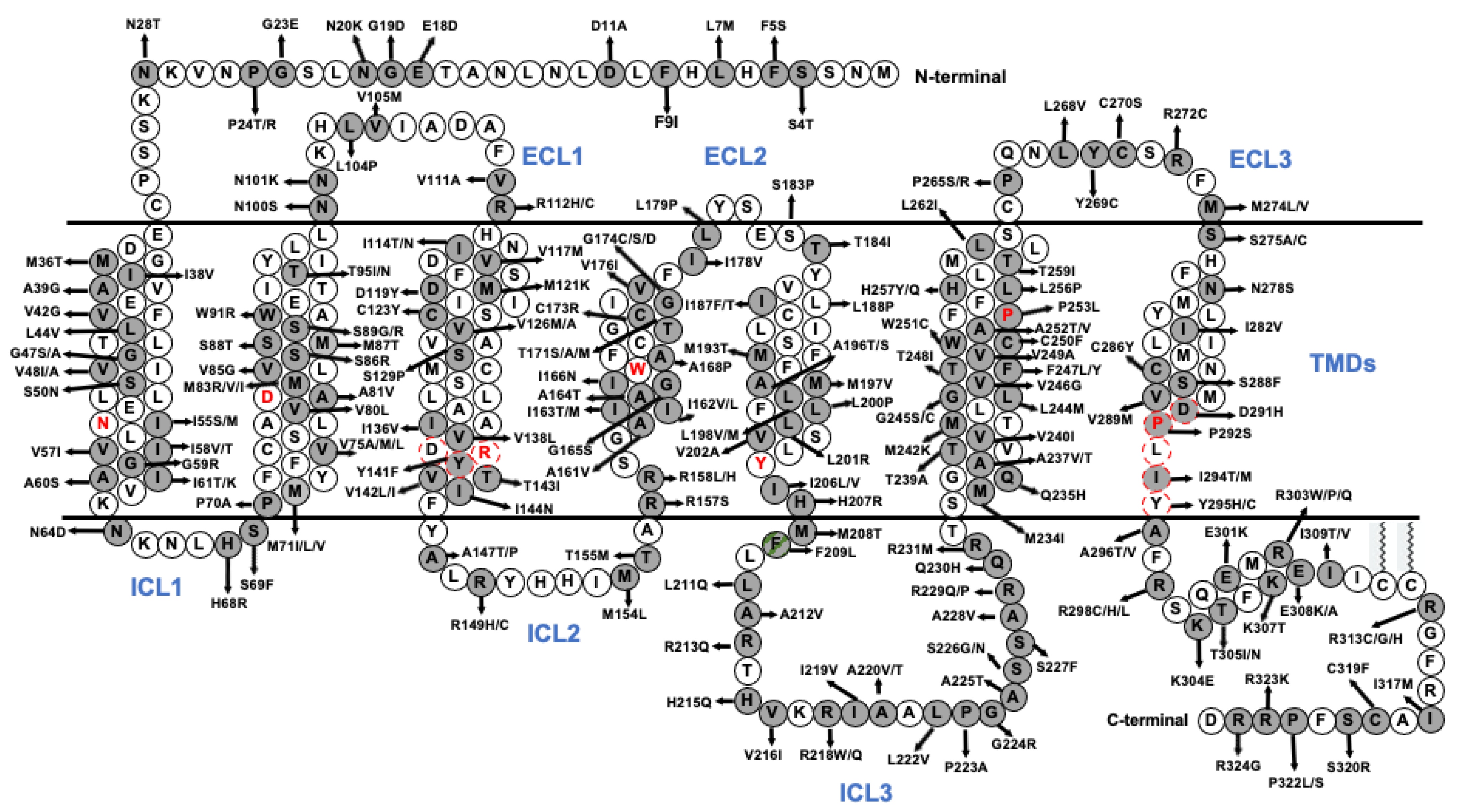
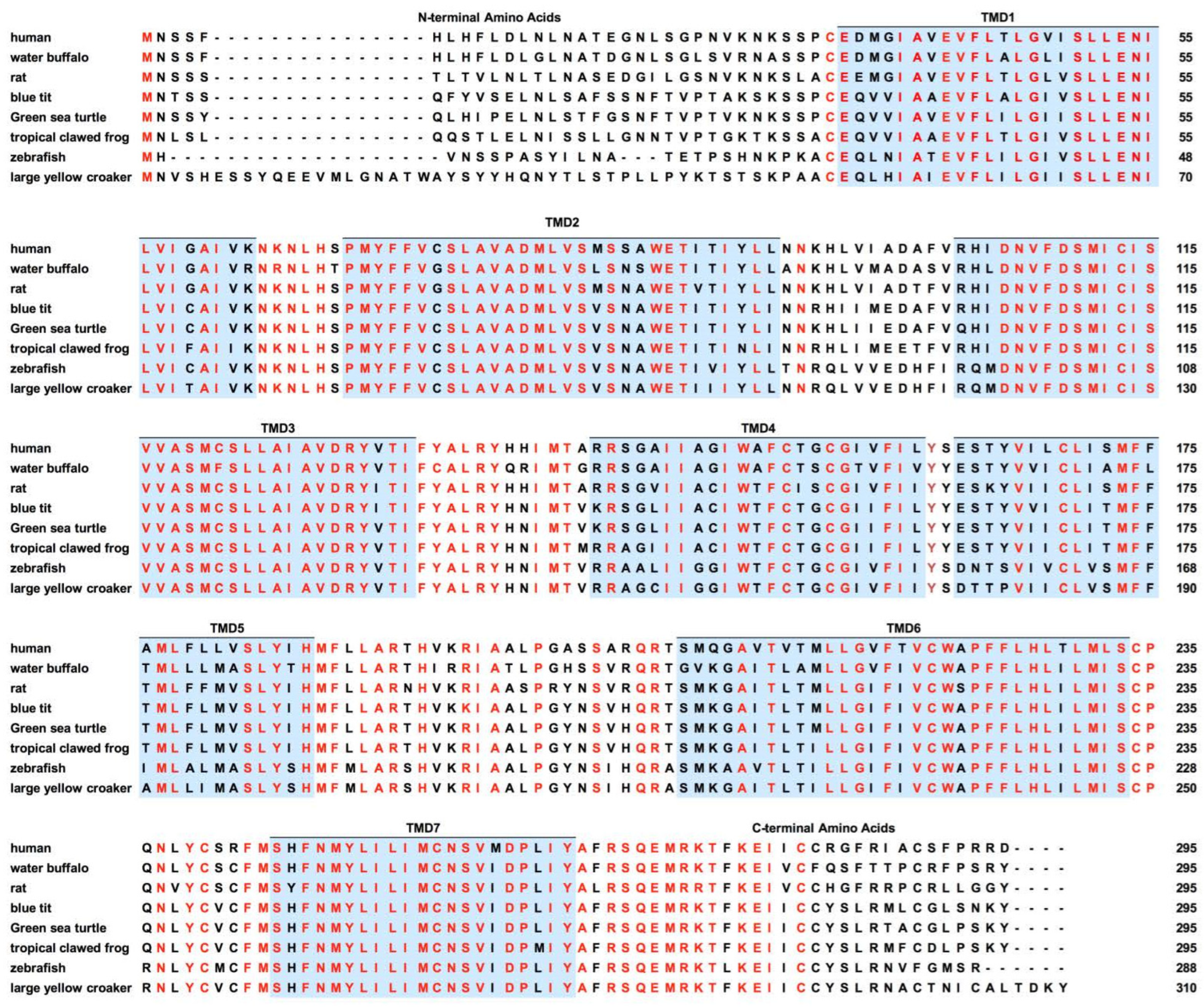
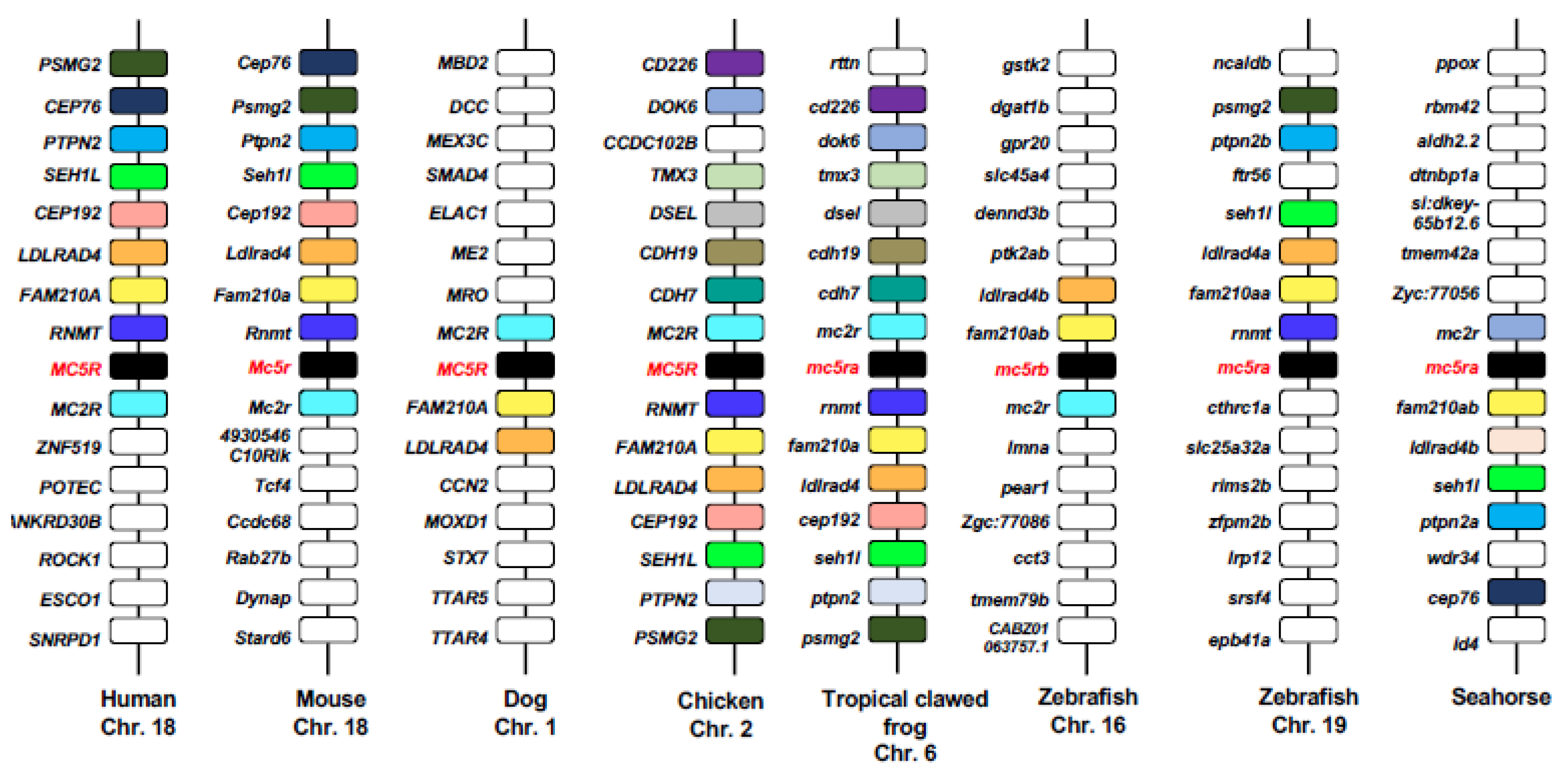
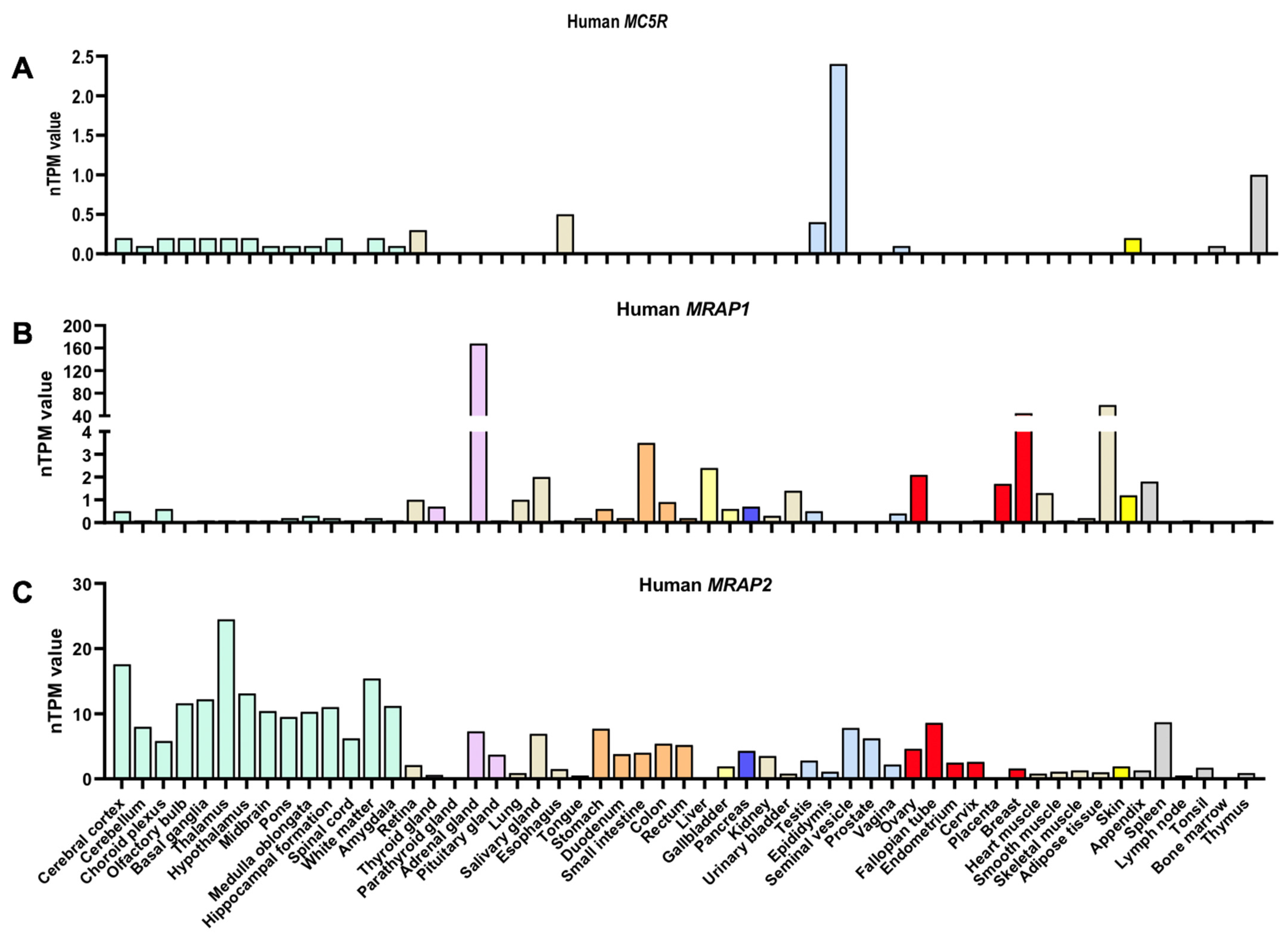
| Species | MRAPs | Effect of MRAPs on MC5R-Related Parameters | Cell Types | |
|---|---|---|---|---|
| MC5R Traffic to PM | MC5R Pharmacology | |||
| Human [116,119] | MRAP1, MRAP2 | Inhibition * | Inhibit its efficacy for NDP-MSH * | CHO HEK293T |
| Zebrafish [75] | MRAP2a | Inhibition | Inhibits the efficacy of both MC5Ra and MC5Rb with α-MSH and SHU9119 | CHO HEK293T |
| MRAP2b | NS | Inhibits MC5Ra but increases MC5Rb efficacy with α-MSH and SHU9119 | ||
| Mouse [75] | MRAP2 | NS | Inhibits efficacy with α-MSH and SHU9119 | CHO HEK293T |
| MRAP1 | — | — | — | |
| Elephant shark [10] | MRAP1 | NS | Increases sensitivity to ACTH but not Des-Acetyl-α-MSH | CHO |
| MRAP2 | NS | NS | ||
| Chicken [122] | MRAP1 | — | Increases sensitivity to ACTH | CHO |
| MRAP2 | — | No effect on responding to ACTH | ||
| Gar [120] | MRAP1 | Increase | Increases efficacy with NDP-MSH | CHO |
| MRAP2 | NS | Increases efficacy with ACTH | ||
| Whale shark [121] | MRAP1, MRAP2 | NS * | Increase sensitivity to ACTH but not des-acetyl-α-MSH * | CHO |
| Ricefield eel [84] | MRAP2X1 | NS | Increases maximal binding and inhibits efficacy with α-MSH and ACTH *; no influence on binding affinity to ACTH or α-MSH | HEK293T |
| MRAP2X2 | NS | Decreases binding affinity to ACTH but not a-MSH | ||
| Rainbow trout [83] | MRAP2 | NS | Increases sensitivity to ACTH | CHO |
| MRAP | — | — | — | |
| MC3R | MC4R | MC5R | |
|---|---|---|---|
| Energy-regulating tissues | Hypothalamus [22] | Hypothalamus, adipose, and skeletal tissue [13,26,27] | Liver, adipose, and skeletal tissue [53,54,62,110,124] |
| Feeding behavior | Feed efficiency, feeding rhythm, and energy expenditure [26,27,28,29,30] | Food intake and energy expenditure [13,25,125] | No report |
| Phenotype in knockout mouse | Moderate obesity, no hyperphagia, increased fat mass, and decreased lean mass [123,126] | severe obesity, hyperphagia, and hyperinsulinemia [13,27,123,127] | No visible phenotype, deficiency in exocrine gland secretion, and decreased glucose tolerance [41,124] |
| Lipid homeostasis | Triglyceride accumulation, lipolysis, and fatty acid oxidation [14,128,129] | Triglyceride synthesis, lipid mobilization, and fat accumulation [129,130,131] | Lipolysis, fatty acid oxidation, and fatty acid re-esterification [53,54,62,110] |
| Glucose homeostasis | Glucose uptake [14,132,133] | Glucose reabsorption, hyperglycemia, and hepatic glucose production [13,16,134] | Glucose uptake [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.-Q.; Hong, Y.; Tao, Y.-X. Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism. Int. J. Mol. Sci. 2022, 23, 8727. https://doi.org/10.3390/ijms23158727
Ji L-Q, Hong Y, Tao Y-X. Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism. International Journal of Molecular Sciences. 2022; 23(15):8727. https://doi.org/10.3390/ijms23158727
Chicago/Turabian StyleJi, Li-Qin, Ye Hong, and Ya-Xiong Tao. 2022. "Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism" International Journal of Molecular Sciences 23, no. 15: 8727. https://doi.org/10.3390/ijms23158727
APA StyleJi, L.-Q., Hong, Y., & Tao, Y.-X. (2022). Melanocortin-5 Receptor: Pharmacology and Its Regulation of Energy Metabolism. International Journal of Molecular Sciences, 23(15), 8727. https://doi.org/10.3390/ijms23158727







