Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System
Abstract
1. Introduction
2. Crosstalk between Neural Cells Regulates Cholesterol Homeostasis
2.1. Outline of the Brain Cholesterol Turnover
2.2. Brain Cholesterol Transporters
2.3. LDL Receptors
3. LDL Receptor Interactors in Normal and Pathological Brain Conditions
3.1. Reelin and F-Spondin
3.2. APP
3.3. Syntaxin 5 (Stx5), Selenoprotein P, Beclin 1 and NYGGF4
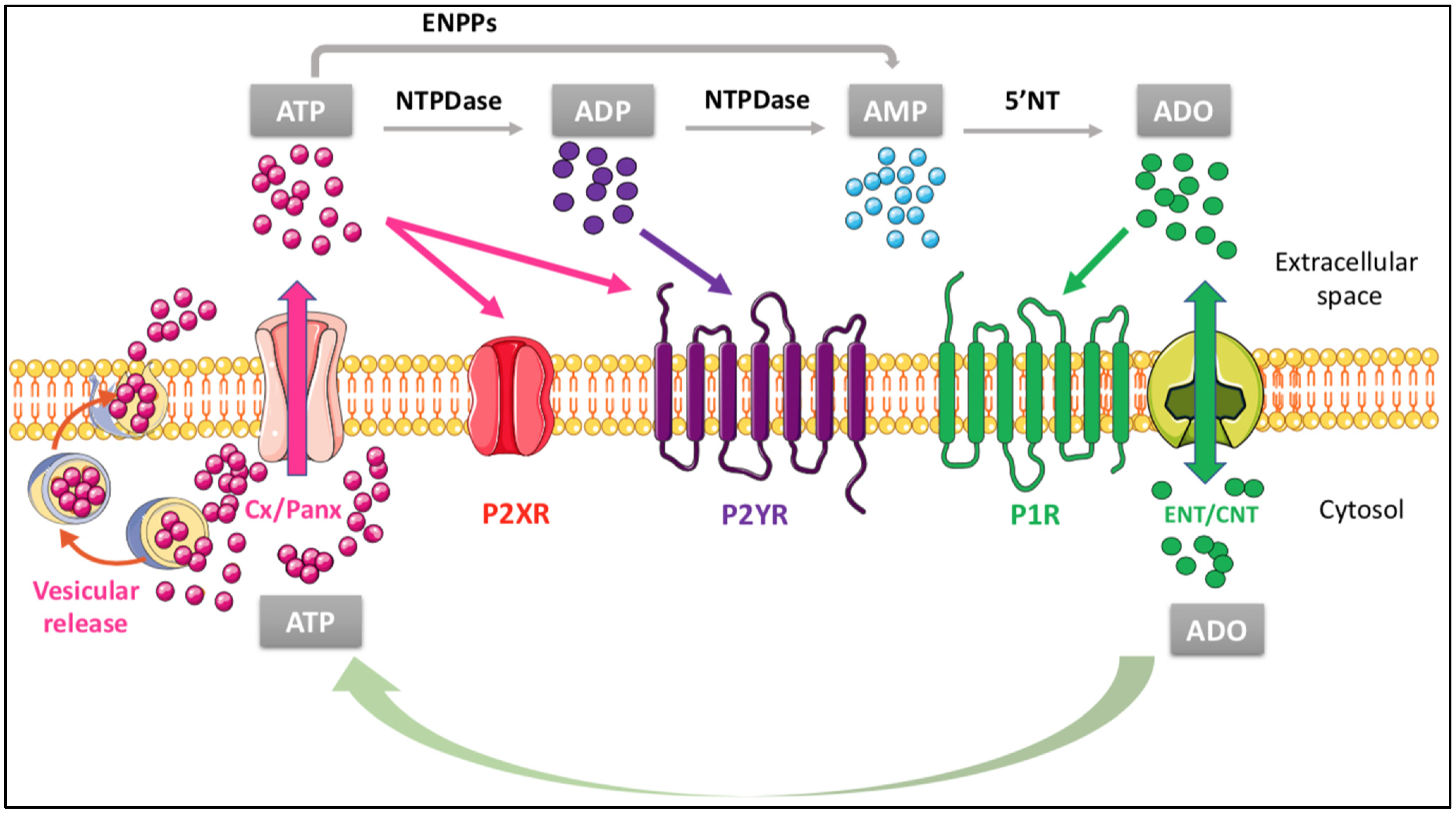
4. Influence of the Purinergic Signaling on the Main Functions of the Cholesterol Shuttle
4.1. Metabotropic P2 Receptors
4.2. Ionotropic P2 Receptors and Brain Cholesterol Turnover
4.3. Metabotropic P1 Receptors and Brain Cholesterol Turnover
4.4. Guanosine and Cholesterol Turnover
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preston, K.J.; Scalia, R.G.; Autieri, M.V. Adipocyte Phenotype Flexibility and Lipid Dysregulation. Cells 2022, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Kaptoge, S.; Wormser, D.; Willeit, P.; Butterworth, A.S.; Bansal, N.; O’Keeffe, L.M.; Gao, P.; Wood, A.M.; et al. Association of Cardiometabolic Multimorbidity With Mortality. JAMA 2015, 314, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Custers, E.E.M.; Kiliaan, A.J. Dietary lipids from body to brain. Prog. Lipid Res. 2022, 85, 101144. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.Y.; Li, M.; Han, L.; Tayie, F.; Yao, S.S.; Huang, Z.; Ai, P.; Liu, Y.Z.; Hu, Y.H.; Xu, B. Dietary Fat Intake and Cognitive Function among Older Populations: A Systematic Review and Meta-Analysis. J. Prev. Alzheimer’s Dis. 2019, 6, 204–211. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Pallottini, V.; Mazzoli, A.; Iannotta, L.; Tonini, C.; Morone, B.; Ståhlman, M.; Crescenzo, R.; Strazzullo, M.; Iossa, S.; et al. A Short-Term Western Diet Impairs Cholesterol Homeostasis and Key Players of Beta Amyloid Metabolism in Brain of Middle-Aged Rats. Mol. Nutr. Food Res. 2020, 64, e2000541. [Google Scholar] [CrossRef]
- Björkhem, I.; Meaney, S.; Fogelman, A.M. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Goritz, C.; Mauch, D.H.; Pfrieger, F.W. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol. Cell. Neurosci. 2005, 29, 190–201. [Google Scholar] [CrossRef]
- Fester, L.; Zhou, L.; Bütow, A.; Huber, C.; von Lossow, R.; Prange-Kiel, J.; Jarry, H.; Rune, G.M. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 2009, 19, 692–705. [Google Scholar] [CrossRef]
- de Chaves, E.I.; Rusiñol, A.E.; Vance, D.E.; Campenot, R.B.; Vance, J.E. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J. Biol. Chem. 1997, 272, 30766–30773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.Y.; He, X.Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J. Neuroendocrinol. 2016, 28, 12351. [Google Scholar] [CrossRef]
- Lu, F.; Ferriero, D.M.; Jiang, X. Cholesterol in Brain Development and Perinatal Brain Injury: More than a Building Block. Curr. Neuropharmacol. 2022, 20, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Madra, M.; Sturley, S.L. Niemann-Pick type C pathogenesis and treatment: From statins to sugars. Clin. Lipidol. 2010, 5, 387–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kacher, R.; Mounier, C.; Caboche, J.; Betuing, S. Altered Cholesterol Homeostasis in Huntington’s Disease. Front. Aging Neurosci. 2022, 14, 797220. [Google Scholar] [CrossRef]
- Vendruscolo, M. Lipid Homeostasis and Its Links with Protein Misfolding Diseases. Front. Mol. Neurosci. 2022, 15, 829291. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Kusumo, H.; Costa, L.G.; Guizzetti, M. Cholesterol efflux is differentially regulated in neurons and astrocytes: Implications for brain cholesterol homeostasis. Biochim. Biophys. Acta 2013, 1831, 263–275. [Google Scholar] [CrossRef]
- Koudinov, A.R.; Koudinova, N.V. Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. J. Neurol. Sci. 2005, 229–230, 233–240. [Google Scholar] [CrossRef]
- Camargo, N.; Goudriaan, A.; van Deijk, A.F.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, K.A.; Dijkhuizen, R.M.; Mansvelder, H.D.; et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; De Nuccio, C.; Visentin, S.; Martire, A.; Minghetti, L.; Popoli, P.; Ferrante, A. Myelin Defects in Niemann-Pick Type C Disease: Mechanisms and Possible Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 8858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, L. The Neuroprotective Effects of Moderate and Regular Caffeine Consumption in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 5568011. [Google Scholar] [CrossRef]
- Martin, M.; Dotti, C.G.; Ledesma, M.D. Brain cholesterol in normal and pathological aging. Biochim. Biophys. Acta 2010, 1801, 934–944. [Google Scholar] [CrossRef]
- Boyles, J.K.; Pitas, R.E.; Wilson, E.; Mahley, R.W.; Taylor, J.M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J. Clin. Investig. 1985, 76, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Rahman, S.; Ahmad, R.; Kumar, T.; Dutta, G.; Banerjee, S.; Abubakar, A.R.; Rowaiye, A.B.; Dhingra, S.; Ravichandiran, V.; et al. An evidence-based review of neuronal cholesterol role in dementia and statins as a pharmacotherapy in reducing risk of dementia. Expert Rev. Neurother. 2021, 21, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Westerterp, M. ABC Transporters, Cholesterol Efflux, and Implications for Cardiovascular Diseases. In Lipid Transfer in Lipoprotein Metabolism and Cardiovascular Disease; Advances in Experimental Medicine and Biology Series; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1276, pp. 67–83. [Google Scholar] [CrossRef]
- Khandker, L.; Jeffries, M.A.; Chang, Y.J.; Mather, M.L.; Evangelou, A.V.; Bourne, J.N.; Tafreshi, A.K.; Ornelas, I.M.; Bozdagi-Gunal, O.; Macklin, W.B.; et al. Cholesterol biosynthesis defines oligodendrocyte precursor heterogeneity between brain and spinal cord. Cell Rep. 2022, 38, 110423. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Jockusch, W.J.; Sivakumar, N.; Möbius, W.; Corthals, K.; Li, S.; Quintes, S.; Kim, Y.; Schaap, I.A.; Rhee, J.S.; et al. Critical time window of neuronal cholesterol synthesis during neurite outgrowth. J. Neurosci. 2012, 32, 7632–7645. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Li, D.; He, C.; He, K.; Xue, T.; Wan, L.; Zhang, C.; Liu, Q. Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory. Neuron 2021, 109, 957–970.e8. [Google Scholar] [CrossRef]
- Pikuleva, I.A. Cholesterol-metabolizing cytochromes P450. Drug Metab. Dispos. 2006, 34, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, Z.; Azizidoost, S.; Cheraghzadeh, M.; Mohammadi, A.; Kheirollah, A. Increased protein expression of ABCA1, HMG-CoA reductase, and CYP46A1 induced by garlic and allicin in the brain mouse and astrocytes-isolated from C57BL/6J. Avicenna J. Phytomed. 2021, 11, 73–483. [Google Scholar] [CrossRef]
- Rothblat, G.H.; de la Llera-Moya, M.; Atger, V.; Kellner-Weibel, G.; Williams, D.L.; Phillips, M.C. Cell cholesterol efflux: Integration of old and new observations provides new insights. J. Lipid Res. 1999, 40, 781–796. [Google Scholar] [CrossRef]
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome. Cell Rep. 2020, 32, 108132. [Google Scholar] [CrossRef]
- Villani, A.; Benjaminsen, J.; Moritz, C.; Henke, K.; Hartmann, J.; Norlin, N.; Richter, K.; Schieber, N.L.; Franke, T.; Schwab, Y.; et al. Clearance by Microglia Depends on Packaging of Phagosomes into a Unique Cellular Compartment. Dev. Cell 2019, 49, 77–88.e7. [Google Scholar] [CrossRef]
- Zareba, J.; Peri, F. Microglial ‘fat shaming’ in development and disease. Curr. Opin. Cell Biol. 2021, 73, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bossaerts, L.; Cacace, R.; Van Broeckhoven, C. The role of ATP-binding cassette subfamily A in the etiology of Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 31. [Google Scholar] [CrossRef]
- Okamoto, Y.; Tomioka, M.; Ogasawara, F.; Nagaiwa, K.; Kimura, Y.; Kioka, N.; Ueda, K. C-terminal of ABCA1 separately regulates cholesterol floppase activity and cholesterol efflux activity. Biosci. Biotechnol. Biochem. 2020, 84, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Costa, L.G.; Guizzetti, M. Retinoic acid isomers up-regulate ATP binding cassette A1 and G1 and cholesterol efflux in rat astrocytes: Implications for their therapeutic and teratogenic effects. J. Pharmacol. Exp. Ther. 2011, 338, 870–878. [Google Scholar] [CrossRef]
- Tomioka, M.; Toda, Y.; Mañucat, N.B.; Akatsu, H.; Fukumoto, M.; Kono, N.; Arai, H.; Kioka, N.; Ueda, K. Lysophosphatidylcholine export by human ABCA7. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 658–665. [Google Scholar] [CrossRef] [PubMed]
- DiBattista, A.M.; Dumanis, S.B.; Song, J.M.; Bu, G.; Weeber, E.; Rebeck, W.G.; Hoe, H.-S. Very low density lipoprotein receptor regulates dendritic spine formation in a RasGRF1/CaMKII dependent manner. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 904–917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trommsdorff, M.; Gotthardt, M.; Hiesberger, T.; Shelton, J.; Stockinger, W.; Nimpf, J.; Hammer, R.E.; Richardson, J.A.; Herz, J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 1999, 97, 689–701. [Google Scholar] [CrossRef]
- Hellwig, S.; Hack, I.; Zucker, B.; Brunne, B.; Junghans, D. Reelin together with ApoER2 regulates interneuron migration in the olfactory bulb. PLoS ONE 2012, 7, e50646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirota, Y.; Kubo, K.I.; Fujino, T.; Yamamoto, T.T.; Nakajima, K. ApoER2 Controls Not Only Neuronal Migration in the Intermediate Zone but Also Termination of Migration in the Developing Cerebral Cortex. Cereb. Cortex 2018, 28, 223–235. [Google Scholar] [CrossRef]
- May, P.; Rohlmann, A.; Bock, H.H.; Zurhove, K.; Marth, J.D.; Schomburg, E.D.; Noebels, J.L.; Beffert, U.; Sweatt, J.D.; Weeber, E.J.; et al. Neuronal LRP1 Functionally Associates with Postsynaptic Proteins and Is Required for Normal Motor Function in Mice. Mol. Cell. Biol. 2004, 24, 8872–8883. [Google Scholar] [CrossRef]
- Nakajima, C.; Kulik, A.; Frotscher, M.; Herz, J.; Schäfer, M.; Bock, H.H.; May, P. Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-D-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J. Biol. Chem. 2013, 288, 21909–21923. [Google Scholar] [CrossRef]
- Liu, Q.; Trotter, J.; Zhang, J.; Peters, M.M.; Cheng, H.; Bao, J.; Han, X.; Weeber, E.J.; Bu, G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive.; age-dependent synapse loss and neurodegeneration. J. Neurosci. 2010, 30, 17068–17078. [Google Scholar] [CrossRef]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef]
- Takahashi, S.; Suzuki, J.; Kohno, M.; Oida, K.; Tamai, T.; Miyabo, S.; Yamamoto, T.; Nakai, T. Enhancement of the binding of triglyceride-rich lipoproteins to the very lowdensity lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J. Biol. Chem. 1995, 270, 15747–15754. [Google Scholar] [CrossRef]
- Dlugosz, P.; Nimpf, J. The Reelin Receptors Apolipoprotein E receptor 2 (ApoER2) and VLDL Receptor. Int. J. Mol. Sci. 2018, 19, 3090. [Google Scholar] [CrossRef] [PubMed]
- Duit, S.; Mayer, H.; Blake, S.M.; Schneider, W.J.; Nimpf, J. Differential functions of ApoER2 and very low density lipoprotein receptor in Reelin signaling depend on differential sorting of the receptors. J. Biol. Chem. 2010, 285, 4896–4908. [Google Scholar] [CrossRef]
- Christie, R.H.; Chung, H.; Rebeck, W.; Strickland, D.; Hyman, B.T. Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor.; in the central nervous system and in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Beffert, U.; Morfini, G.; Bock, H.H.; Reyna, H.; Brady, S.T.; Herz, J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J. Biol. Chem. 2002, 277, 49958–49964. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Stockinger, W.; Christie, R.H.; Schneider, W.J.; Nimpf, J.; Hyman, B.T.; Rebeck, G.W. Expression and alternate splicing of apolipoprotein E receptor 2 in brain. Neuroscience 1999, 90, 903–911. [Google Scholar] [CrossRef]
- Weeber, E.J.; Beffert, U.; Jones, C.; Christian, J.M.; Forster, E.; Sweatt, J.D.; Herz, J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 2002, 277, 39944–39952. [Google Scholar] [CrossRef] [PubMed]
- Erbe, R.; Gore, J.; Gemmill, K.; Gaykalova, D.A.; Fertig, E.J. The use of machine learning to discover regulatory networks controlling biological systems. Mol. Cell 2022, 82, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Parets, S.; Fernández-Díaz, J.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Román, R.; Lladó, V.; Rosselló, C.A.; Fernández-García, P.; Escribá, P.V. Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes 2021, 11, 919. [Google Scholar] [CrossRef]
- D’Arcangelo, G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006, 8, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Nakajima, K. VLDLR is not essential for reelin-induced neuronal aggregation but suppresses neuronal invasion into the marginal zone. Development 2020, 147, dev189936. [Google Scholar] [CrossRef]
- Howell, B.W.; Gertler, F.B.; Cooper, J.A. Mouse disabled (mDab1): A Src binding protein implicated in neuronal development. EMBO J. 1997, 16, 121–132. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, G.; Homayouni, R.; Keshvara, L.; Rice, D.S.; Sheldon, M.; Curran, T. Reelin is a ligand for lipoprotein receptors. Neuron 1999, 24, 471–479. [Google Scholar] [CrossRef]
- Pramatarova, A.; Chen, K.; Howell, B.W. A genetic interaction between the APP and Dab1 genes influences brain development. Mol. Cell. Neurosci. 2008, 37, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Callahan, D.G.; Taylor, W.M.; Tilearcio, M.; Cavanaugh, T.; Selkoe, D.J.; Young-Pearse, T.L. Embryonic mosaic deletion of APP results in displaced Reelin-expressing cells in the cerebral cortex. Dev. Biol. 2017, 424, 138–146. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin Functions.; Mechanisms of Action and Signaling Pathways during Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Telese, F.; Ma, Q.; Perez, P.M.; Notani, D.; Oh, S.; Li, W.; Comoletti, D.; Ohgi, K.A.; Taylor, H.; Rosenfeld, M.G. LRP8-Reelin-Regulated Neuronal Enhancer Signature Underlying Learning and Memory Formation. Neuron 2015, 86, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.-S.; Pocivavsek, A.; Chakraborty, G.; Fu, Z.; Vicini, S.; Ehlers, M.D.; Rebeck, G.W. Apolipoprotein E Receptor 2 Interactions with the N-Methyl-D-aspartate Receptor. J. Biol. Chem. 2006, 281, 3425–3431. [Google Scholar] [CrossRef]
- Balmaceda, V.; Cuchillo-Ibáñez, I.; Pujadas, L.; García-Ayllón, M.-S.; Saura, C.A.; Nimpf, J.; Soriano, E.; Saez-Valero, J. ApoER2 processing by presenilin-1 modulates reelin expression. FASEB J. 2014, 28, 1543–1554. [Google Scholar] [CrossRef]
- Chen, Y.; Durakoglugil, M.S.; Xian, X.; Herz, J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. USA 2010, 107, 12011–12016. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, H.; Basak, J.; Kim, J. Apolipoprotein E in synaptic plasticity and Alzheimer’s disease: Potential cellular and molecular mechanisms. Mol. Cells 2014, 37, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J., 3rd; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef] [PubMed]
- De Gasperi, R.; Gama Sosa, M.A.; Wen, P.H.; Li, J.; Perez, G.M.; Curran, T.; Elder, G.A. Cortical development in the presenilin-1 null mutant mouse fails after splitting of the preplate and is not due to a failure of reelin-dependent signaling. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 2405–2414. [Google Scholar] [CrossRef]
- Wang, W.; Moerman-Herzog, A.M.; Slaton, A.; Barger, S.W. Presenilin 1 mutations influence processing and trafficking of the ApoE receptor apoER2. Neurobiol. Aging 2017, 49, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Südhof, T.C. Binding of F-spondin to amyloid-beta precursor protein: A candidate amyloid-beta precursor protein ligand that modulates amyloid-beta precursor protein cleavage. Proc. Natl. Acad. Sci. USA 2004, 101, 2548–2553. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.-S.; Wessner, D.; Beffert, U.; Becker, A.G.; Matsuoka, Y.; Rebeck, G.W. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 2005, 25, 9259–9268. [Google Scholar] [CrossRef]
- Rogers, J.T.; Zhao, L.; Trotter, J.H.; Rusiana, I.; Peters, M.M.; Li, Q.; Donaldson, E.; Banko, J.L.; Keenoy, K.E.; Rebeck, G.W.; et al. Reelin supplementation recovers sensorimotor gating, synaptic plasticity and associative learning deficits in the heterozygous reeler mouse. J. Psychopharmacol. 2013, 27, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Beffert, U.; Weeber, E.J.; Durudas, A.; Qiu, S.; Masiulis, I.; Sweatt, J.D.; Li, W.P.; Adelmann, G.; Frotscher, M.; Hammer, R.E.; et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 2005, 47, 567–579. [Google Scholar] [CrossRef]
- Chen, Y.; Beffert, U.; Ertunc, M.; Tang, T.S.; Kavalali, E.T.; Bezprozvanny, I.; Herz, J. Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 2005, 25, 8209–8216. [Google Scholar] [CrossRef]
- Ampuero, E.; Jury, N.; Härtel, S.; Marzolo, M.-P.; van Zundert, B. Interfering of the Reelin/ApoER2/PSD95 Signaling Axis Reactivates Dendritogenesis of Mature Hippocampal Neurons. J. Cell. Physiol. 2017, 232, 1187–1199. [Google Scholar] [CrossRef]
- Pohlkamp, T.; Wasser, C.R.; Herz, J. Functional Roles of the Interaction of APP and Lipoprotein Receptors. Front. Mol. Neurosci. 2017, 10, 54. [Google Scholar] [CrossRef]
- Hoe, H.S.; Rebeck, G.W. Regulated proteolysis of APP and ApoE receptors. Mol. Neurobiol. 2008, 37, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.; Marzolo, M.P. The functions of Reelin in membrane trafficking and cytoskeletal dynamics: Implications for neuronal migration.; polarization and differentiation. Biochem. J. 2017, 474, 3137–3165. [Google Scholar] [CrossRef] [PubMed]
- Tsuneura, Y.; Nakai, T.; Mizoguchi, H.; Yamada, K. New Strategies for the Treatment of Neuropsychiatric Disorders Based on Reelin Dysfunction. Int. J. Mol. Sci. 2022, 23, 1829. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, R.A.; Barría, M.I.; Lee, J.; Cam, J.; Araya, C.; Escudero, C.A.; Inestrosa, N.C.; Bronfman, F.C.; Bu, G.; Marzolo, M.-P. ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol. Neurodegener. 2007, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.N.; Cheung, H.N.; Li, W.; Lau, K.F. FE65: Roles beyond amyloid precursor protein processing. Cell. Mol. Biol. Lett. 2015, 20, 66–87. [Google Scholar] [CrossRef]
- Augustin, V.; Kins, S. Fe65: A Scaffolding Protein of Actin Regulators. Cells 2021, 10, 1599. [Google Scholar] [CrossRef]
- Stilling, R.M.; Rönicke, R.; Benito, E.; Urbanke, H.; Capece, V.; Burkhardt, S.; Bahari-Javan, S.; Barth, J.; Sananbenesi, F.; Schütz, A.L.; et al. K-Lysine acetyltransferase 2a regulates a hippocampal gene expression network linked to memory formation. EMBO J. 2014, 33, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Domingues, S.C.; Konietzko, U.; Henriques, A.G.; Rebelo, S.; Fardilha, M.; Nishitani, H.; Nitsch, R.M.; Da Cruz E. Silva, E.F.; da Cruz, E.; Silva, O.A. RanBP9 modulates AICD localization and transcriptional activity via direct interaction with Tip60. J. Alzheimer’s Dis. 2014, 42, 1415–1433. [Google Scholar] [CrossRef]
- Liu, Q.; Zerbinatti, C.V.; Zhang, J.; Hoe, H.-S.; Wang, B.; Cole, S.L.; Herz, J.; Mulia, L.; Bu, G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 2007, 56, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Dieckmann, M.; Jaeger, S.; Weggen, S.; Pietrzik, C.U. Stx5 is a novel interactor of VLDL-R to affect its intracellular trafficking and processing. Exp. Cell Res. 2013, 319, 1956–1972. [Google Scholar] [CrossRef]
- Jin, Y.; Chung, Y.W.; Jung, M.K.; Lee, J.H.; Ko, K.Y.; Jang, J.K.; Ham, M.; Kang, H.; Pack, C.G.; Mihara, H.; et al. Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes. Cell. Mol. Life Sci. 2020, 77, 2367–2386. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A.; Ojala, J.; Haapasalo, A.; Soininen, H.; Hiltunen, M. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog. Neurobiol. 2013, 106–107, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, T.; Liu, C.-C.; Shinohara, M.; Li, J.; Bu, G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-β. J. Neurosci. 2012, 32, 16458–16465. [Google Scholar] [CrossRef] [PubMed]
- Pflanzner, T.; Janko, M.C.; André-Dohmen, B.; Reuss, S.; Weggen, S.; Roebroek, A.J.M.; Kuhlman, C.R.W.; Pietrzik, C.U. LRP1 mediates bidirectional transcytosis of amyloid-β across the blood-brain barrier. Neurobiol. Aging 2011, 32, 2323.e1–2323.e11. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Araseki, M.; Nozawa, K.; Furukori, K.; Araki, Y.; Matsushima, T.; Nakaya, T.; Hata, S.; Saito, Y.; Uchida, S.; et al. Quantitative analysis of APP axonal transport in neurons: Role of JIP1 in enhanced APP anterograde transport. Mol. Biol. Cell 2014, 25, 3569–3580. [Google Scholar] [CrossRef]
- Lansbergen, G.; Akhmanova, A. Microtubule plus end: A hub of cellular activities. Traffic 2006, 7, 499–507. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Franciosi, S.; Takahashi, N.; Krug, L.; Schmeidler, J.; Taddei, K.; Haroutunian, V.; Fried, U.; Ehrlich, M.; Martins, R.N.; et al. Extensive proteomic screening identifies the obesity-related NYGGF4 protein as a novel LRP1-interactor, showing reduced expression in early Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Bres, E.E.; Faissner, A. Low Density Receptor-Related Protein 1 Interactions with the Extracellular Matrix: More Than Meets the Eye. Front. Cell Dev. Biol. 2019, 7, 31. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 2398212818817494. [Google Scholar] [CrossRef]
- Wright, N.J.; Lee, S.Y. Toward a Molecular Basis of Cellular Nucleoside Transport in Humans. Chem. Rev. 2021, 121, 5336–5358. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Di Liberto, V.; Mudò, G.; Garozzo, R.; Frinchi, M.; Fernandez-Dueñas, V.; Di Iorio, P.; Ciccarelli, R.; Caciagli, F.; Condorelli, D.F.; Ciruela, F.; et al. The Guanine-Based Purinergic System: The Tale of An Orphan Neuromodulation. Front. Pharmacol. 2016, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- IJzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine Receptors: A Further Update. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Butt, A.M. ATP: A ubiquitous gliotransmitter integrating neuron-glial networks. Semin. Cell Dev. Biol. 2011, 22, 205–213. [Google Scholar] [CrossRef]
- Delekate, A.; Füchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef]
- Reichenbach, N.; Delekate, A.; Breithausen, B.; Keppler, K.; Poll, S.; Schulte, T.; Peter, J.; Plescher, M.; Hansen, J.N.; Blank, N.; et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 2018, 215, 1649–1663. [Google Scholar] [CrossRef]
- Larramona-Arcas, R.; González-Arias, C.; Perea, G.; Gutiérrez, A.; Vitorica, J.; García-Barrera, T.; Gómez-Ariza, J.L.; Pascua-Maestro, R.; Ganfornina, M.D.; Kara, E.; et al. Sex-dependent calcium hyperactivity due to lysosomal-related dysfunction in astrocytes from APOE4 versus APOE3 gene targeted replacement mice. Mol. Neurodegener. 2020, 15, 35. [Google Scholar] [CrossRef]
- Dergunov, A.D.; Baserova, V.B. Different Pathways of Cellular Cholesterol Efflux. Cell Biochem. Biophys. 2022. [Google Scholar] [CrossRef]
- Martinez, L.O.; Najib, S.; Perret, B.; Cabou, C.; Lichtenstein, L. Ecto-F1-ATPase/P2Y pathways in metabolic and vascular functions of high-density lipoproteins. Atherosclerosis 2015, 238, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Malaval, C.; Martinez, L.O.; Sak, K.; Rolland, C.; Perez, C.; Nauze, M.; Champagne, E.; Tercé, F.; Gachet, C.; et al. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell. Mol. Life Sci. 2005, 62, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.C.; Vantourout, P.; Champagne, E.; Tercé, F.; Rolland, C.; Perret, B.; Collet, X.; Barbaras, R.; Martinez, L.O. Cell surface adenylate kinase activity regulates the F(1)-ATPase/P2Y (13)-mediated HDL endocytosis pathway on human hepatocytes. Cell. Mol. Life Sci. 2006, 63, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.C.; Malaval, C.; Ben Addi, A.; Verdier, C.; Pons, V.; Serhan, N.; Lichtenstein, L.; Combes, G.; Huby, T.; Briand, F.; et al. P2Y13 receptor is critical for reverse cholesterol transport. Hepatology 2010, 52, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Tran-Dinh, A.; Diallo, D.; Delbosc, S.; Varela-Perez, L.M.; Dang, Q.B.; Lapergue, B.; Burillo, E.; Michel, J.B.; Levoye, A.; Martin-Ventura, J.L.; et al. HDL and endothelial protection. Br. J. Pharmacol. 2013, 169, 493–511. [Google Scholar] [CrossRef]
- Yu, C.; Youmans, K.L.; LaDu, M.J. Proposed mechanism for lipoprotein remodelling in the brain. Biochim. Biophys. Acta 2010, 1801, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Lyly, A.; Marjavaara, S.K.; Kyttälä, A.; Uusi-Rauva, K.; Luiro, K.; Kopra, O.; Martinez, L.O.; Tanhuanpää, K.; Kalkkinen, N.; Suomalainen, A.; et al. Deficiency of the INCL protein Ppt1 results in changes in ectopic F1-ATP synthase and altered cholesterol metabolism. Hum. Mol. Genet. 2008, 17, 1406–1417. [Google Scholar] [CrossRef]
- Camden, J.M.; Schrader, A.M.; Camden, R.E.; González, F.A.; Erb, L.; Seye, C.I.; Weisman, G.A. P2Y2 nucleotide receptors enhance alpha-secretase-dependent amyloid precursor protein processing. J. Biol. Chem. 2005, 280, 18696–18702. [Google Scholar] [CrossRef]
- Norambuena, A.; Palma, F.; Poblete, M.I.; Donoso, M.V.; Pardo, E.; González, A.; Huidobro-Toro, J.P. UTP controls cell surface distribution and vasomotor activity of the human P2Y2 receptor through an epidermal growth factor receptor-transregulated mechanism. J. Biol. Chem. 2010, 285, 2940–2950. [Google Scholar] [CrossRef]
- Kong, Q.; Peterson, T.S.; Baker, O.; Stanley, E.; Camden, J.; Seye, C.I.; Erb, L.; Simonyi, A.; Wood, W.G.; Sun, G.Y.; et al. Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor. J. Neurochem. 2009, 109, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Obara, Y.; Sugama, J.; Kotani, A.; Koike, N.; Ohkubo, S.; Nakahata, N. P2Y2 receptor-Gq/11 signaling at lipid rafts is required for UTP-induced cell migration in NG 108-15 cells. J. Pharmacol. Exp. Ther. 2010, 334, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Sattler, C.; Benndorf, K. Enlightening activation gating in P2X receptors. Purinergic Signal. 2022, 18, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Murrell-Lagnado, R.D. The trafficking and targeting of P2X receptors. Front. Cell. Neurosci. 2013, 7, 233. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Lalo, U.; Evans, R.J. Lipid raft association and cholesterol sensitivity of P2X1-4 receptors for ATP: Chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors. J. Biol. Chem. 2010, 285, 32770–32777. [Google Scholar] [CrossRef]
- Masin, M.; Kerschensteiner, D.; Dumke, K.; Rubio, M.E.; Soto, F. Fe65 interacts with P2X2 subunits at excitatory synapses and modulates receptor function. J. Biol. Chem. 2006, 281, 4100–4108. [Google Scholar] [CrossRef]
- Murrell-Lagnado, R.D. Regulation of P2X Purinergic Receptor Signaling by Cholesterol. Curr. Top. Membr. 2017, 80, 211–232. [Google Scholar] [CrossRef]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Sebastián-Serrano, Á.; de Diego García, L.; Díaz-Hernández, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J. Neurosci. 2017, 37, 7063–7072. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, R.R.; Tao, Q.Q. The role of P2X7R in neuroinflammation and implications in Alzheimer’s disease. Life Sci. 2021, 271, 119187. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, J.H.; Lee, H.G.; Kim, S.U.; Lee, Y.B. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp. Mol. Med. 2007, 39, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F.; Tschirner, S.K.; Steiner, H. Secretases in Alzheimer’s disease: Novel insights into proteolysis of APP and TREM2. Curr. Opin. Neurobiol. 2022, 72, 101–110. [Google Scholar] [CrossRef] [PubMed]
- León-Otegui, M.; Gómez-Villafuertes, R.; Díaz-Hernández, J.I.; Díaz-Hernández, M.; Miras-Portugal, M.T.; Gualix, J. Opposite effects of P2X7 and P2Y2 nucleotide receptors on α-secretase-dependent APP processing in Neuro-2a cells. FEBS Lett. 2011, 585, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hernandez, J.I.; Gomez-Villafuertes, R.; León-Otegui, M.; Hontecillas-Prieto, L.; Del Puerto, A.; Trejo, J.L.; Lucas, J.J.; Garrido, J.J.; Gualix, J.; Miras-Portugal, M.T.; et al. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol. Aging 2012, 33, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Miras-Portugal, M.T.; Diaz-Hernandez, J.I.; Gomez-Villafuertes, R.; Diaz-Hernandez, M.; Artalejo, A.R.; Gualix, J. Role of P2X7 and P2Y2 receptors on α-secretase-dependent APP processing: Control of amyloid plaques formation “in vivo” by P2X7 receptor. Comput. Struct. Biotechnol. J. 2015, 13, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Poloni, T.E.; Terrazzan, A.; Moretti, E.; Gessi, S.; Ferrari, D. Alzheimer and Purinergic Signaling: Just a Matter of inflammation? Cells 2021, 10, 1267. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Rahman, A. The role of adenosine in Alzheimer’s disease. Curr. Neuropharmacol. 2009, 7, 207–216. [Google Scholar] [CrossRef]
- Boussadia, Z.; Chiodi, V.; Pazienti, A.; Martire, A. A major role for adenosine A2A receptor in the interaction between astrocytes and myelinated neurons: Possible implications for the therapy of neurodegenerative disorders. Purinergic Signal. 2022, 18, 5–7. [Google Scholar] [CrossRef]
- Tobias, F.; Pathmasiri, K.C.; Cologna, S.M. Mass spectrometry imaging reveals ganglioside and ceramide localization patterns during cerebellar degeneration in the Npc1-/- mouse model. Anal. Bioanal. Chem. 2019, 411, 5659–5668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xu, S.; Yan, Y.; Yu, H.; Ling, S.; Luo, J. Decreased Purinergic Inhibition of Synaptic Activity in a Mouse Model of Niemann-Pick Disease Type C. Hippocampus 2011, 21, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; De Nuccio, C.; Bernardo, A.; Pepponi, R.; Ferrante, A.; Minghetti, L.; Popoli, P. The Stimulation of Adenosine A2A Receptors Ameliorates the Pathological Phenotype of Fibroblasts from Niemann-Pick Type C Patients. J. Neurosci. 2013, 33, 15388–15393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrante, A.; De Nuccio, C.; Pepponi, R.; Visentin, S.; Martire, A.; Bernardo, A.; Minghetti, L.; Popoli, P. Stimulation of adenosine A2A receptors reduces intracellular cholesterol accumulation and rescues mitochondrial abnormalities in human neural cell models of Niemann-Pick C1. Neuropharmacology 2016, 103, 155–162. [Google Scholar] [CrossRef]
- Ferrante, A.; Pezzola, A.; Matteucci, A.; Di Biase, A.; Attorri, L.; Armida, M.; Martire, A.; Chern, Y.; Popoli, P. The adenosine A2A receptor agonist T1-11 ameliorates neurovisceral symptoms and extends the lifespan of a mouse model of Niemann-Pick type C disease. Neurobiol. Dis. 2018, 110, 1–11. [Google Scholar] [CrossRef]
- De Nuccio, C.; Bernardo, A.; Ferrante, A.; Pepponi, R.; Martire, A.; Falchi, M.; Visentin, S.; Popoli, P.; Minghetti, L. Adenosine A2A receptor stimulation restores cell functions and differentiation in Niemann-Pick type C-like oligodendrocytes. Sci. Rep. 2019, 9, 9782. [Google Scholar] [CrossRef]
- McGraw, C.; Yang, L.; Levental, I.; Lyman, E.; Robinson, A.S. Membrane cholesterol depletion reduces downstream signaling activity of the adenosine A2A receptor. Biochim. Biophys. Acta Biomembr. 2019, 1861, 760–767. [Google Scholar] [CrossRef]
- Lu, J.; Cui, J.; Li, X.; Wang, X.; Zhou, Y.; Yang, W.; Chen, M.; Zhao, J.; Pei, G. An Anti-Parkinson’s Disease Drug via Targeting Adenosine A2A Receptor Enhances Amyloid-β Generation and γ-Secretase Activity. PLoS ONE 2016, 11, e0166415. [Google Scholar] [CrossRef]
- Vuorimaa, A.; Rissanen, E.; Airas, L. In Vivo PET Imaging of Adenosine 2A Receptors in Neuroinflammatory and Neurodegenerative Disease. Contrast Media Mol. Imaging 2017, 2017, 6975841. [Google Scholar] [CrossRef]
- Zarrinmayeh, H.; Territo, P.R. Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and Their Applications. Mol. Imaging 2020, 19, 1536012120927609. [Google Scholar] [CrossRef]
- Massari, C.M.; Zuccarini, M.; Di Iorio, P.; Tasca, C.I. Guanosine Mechanisms of Action: Toward Molecular Targets. Front. Pharmacol. 2021, 12, 653146. [Google Scholar] [CrossRef]
- Di Iorio, P.; Beggiato, S.; Ronci, M.; Nedel, C.B.; Tasca, C.I.; Zuccarini, M. Unfolding New Roles for Guanine-Based Purines and Their Metabolizing Enzymes in Cancer and Aging Disorders. Front. Pharmacol. 2021, 12, 653549. [Google Scholar] [CrossRef]
- Ballerini, P.; Ciccarelli, R.; Di Iorio, P.; Buccella, S.; D’Alimonte, I.; Giuliani, P.; Masciulli, A.; Nargi, E.; Beraudi, A.; Rathbone, M.P.; et al. Guanosine effect on cholesterol efflux and apolipoprotein E expression in astrocytes. Purinergic Signal. 2006, 2, 637–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ballerini, P.; Di Iorio, P.; Caciagli, F.; Rathbone, M.P.; Jiang, S.; Nargi, E.; Buccella, S.; Giuliani, P.; D’Alimonte, I.; Fischione, G.; et al. P2Y2 receptor up-regulation induced by guanosine or UTP in rat brain cultured astrocytes. Int. J. Immunopathol. Pharmacol. 2006, 19, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Kasimov, M.R.; Zefirov, A.L. Brain Cholesterol Metabolism and Its Defects: Linkage to Neurodegenerative Diseases and Synaptic Dysfunction. Acta Nat. 2016, 8, 58–73. [Google Scholar] [CrossRef]
- Dai, L.; Zou, L.; Meng, L.; Qiang, G.; Yan, M.; Zhang, Z. Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets. Mol. Neurobiol. 2021, 58, 2183–2201. [Google Scholar] [CrossRef]
| Purine Receptors | |||
|---|---|---|---|
| P1 Receptors | P2 Receptors | ||
| Receptor subtypes | Metabotropic receptors | Metabotropic P2Y receptors including | Ionotropic P2X receptors including |
| A1, A2A, A2B, A3 | P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y14, P2Y14 | P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, P2X7 | |
| P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y14, P2Y14 | |||
| Ligand(s) | Adenosine (ADO) | ATP, ADP, UTP, UDP | ATP |
| Downstream effectors | Coupling to different types of G proteins and molecular pathways | Coupling to different types of G proteins and molecular pathways | Ion channels whose activation allows cation entry |
| (A) Activities of purinergic receptors in relation to the brain “cholesterol shuttle” | |||
|---|---|---|---|
| Purine Signal | Cell Type | Activity on Cholesterol Shuttle and Related Dysregulation | Ref. |
| P2Y1R | Immortalized APOE4 astrocytes | Increased Ca2+ excitability coupled to an altered lipidome pattern and intracellular cholesterol accumulation, possibly related to receptor hyperactivity. | [111] |
| P2Y1R in cooperation with other P2YR (P2Y12R or P2Y13R) | Brain neural cells | To be explored in relation to a possible increase in the activity of neuronal ecto-F1-ATPase and ApoA-I uptake, similar to that observed in the liver or endothelial cells to assure the process known as “brain reverse cholesterol transport” (BRCT). | [118,119] |
| P2Y2R | Human 1321N1 astrocytoma cells | Increased release of sAPPα deriving from the alpha- secretase activity. It should be investigated if this effect may provoke P2Y2R redistribution/internalization, as observed in peripheral cells. | [120,121] |
| Rat cortical neurons upon IL1β stimulus | Receptor up-regulation coupled to an increased production of the protective sAPP alpha. | [122] | |
| Rat cultured astrocytes | Receptor expression increased by cell stimulation with GUO, which also enhanced the UTP release from these cells, thus, contributing to the protective activity of astrocyte UTP/P2Y2R against AD risk. | [155] | |
| P2X2R | CA1 hippocampal pyramidal cell/Schaffer collateral synapses | Interaction of the beta-amyloid precursor protein-binding protein Fe65 with the receptor at postsynaptic excitatory synapses, which resulted in receptor activity inhibition. | [127] |
| P2X7R | Neural cells | Altered membrane lipid raft composition consequent to receptor stimulation. | [125] |
| Increased ROS formation, which in turn, triggered Aβ peptide formation. | [131] | ||
| Microglia | ATP release induced by Aβ1-42 peptide, which in turn, stimulated P2X7R -related ROS production. | [132] | |
| Neural cells | P2X7R inhibition increased alpha-secretase activity, diminishing the number of amyloid plaques. | [134,135,136] | |
| AD | Receptor polymorphisms. | [137] | |
| A2AR | Fibroblasts from NPC1 patients as well as in human neuronal and oligoglial cell lines, in which NPC1 phenotype had been induced by siRNA | Receptor stimulation reduced the harmful intracellular accumulation of cholesterol as well as mitochondrial damage. | [144,145] |
| Primary culture of oligodendrocyte progenitors | Receptor stimulation counteracted the cell maturation arrest induced by the inhibition of cholesterol transport and restored cell morphology. | [147] | |
| Primary neuronal cells from AD mouse model | Istradefylline, a receptor antagonist, increased Aβ generation as well as A2AR KO-potentiated Aβ generation and gamma-secretase activity | [149] | |
| (B) Influence of cholesterol turnover modifications on purinergic signals | |||
| Cholesterol turnover alterations | Cell type | Modification of the purinergic signal | Ref. |
| Membrane cholesterol depletion | Neural cells | Reduction of calcium currents induced by P2X2,4R stimulation, likely related to the expression of these receptors within membrane lipid rafts. | [125,126,127] |
| Potentiated receptor activity caused by membrane cholesterol depletion as well as P2X7R stimulation. | [128] | ||
| Reduction in membrane cholesterol levels | NCP1 cells | Impairment of normal A2AR activity with a decrease in cyclic adenosine monophosphate (cAMP) production. | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passarella, D.; Ronci, M.; Di Liberto, V.; Zuccarini, M.; Mudò, G.; Porcile, C.; Frinchi, M.; Di Iorio, P.; Ulrich, H.; Russo, C. Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 8683. https://doi.org/10.3390/ijms23158683
Passarella D, Ronci M, Di Liberto V, Zuccarini M, Mudò G, Porcile C, Frinchi M, Di Iorio P, Ulrich H, Russo C. Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System. International Journal of Molecular Sciences. 2022; 23(15):8683. https://doi.org/10.3390/ijms23158683
Chicago/Turabian StylePassarella, Daniela, Maurizio Ronci, Valentina Di Liberto, Mariachiara Zuccarini, Giuseppa Mudò, Carola Porcile, Monica Frinchi, Patrizia Di Iorio, Henning Ulrich, and Claudio Russo. 2022. "Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System" International Journal of Molecular Sciences 23, no. 15: 8683. https://doi.org/10.3390/ijms23158683
APA StylePassarella, D., Ronci, M., Di Liberto, V., Zuccarini, M., Mudò, G., Porcile, C., Frinchi, M., Di Iorio, P., Ulrich, H., & Russo, C. (2022). Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System. International Journal of Molecular Sciences, 23(15), 8683. https://doi.org/10.3390/ijms23158683









