Geometrical Properties of the Nucleus and Chromosome Intermingling Are Possible Major Parameters of Chromosome Aberration Formation
Abstract
:1. Introduction
2. Results
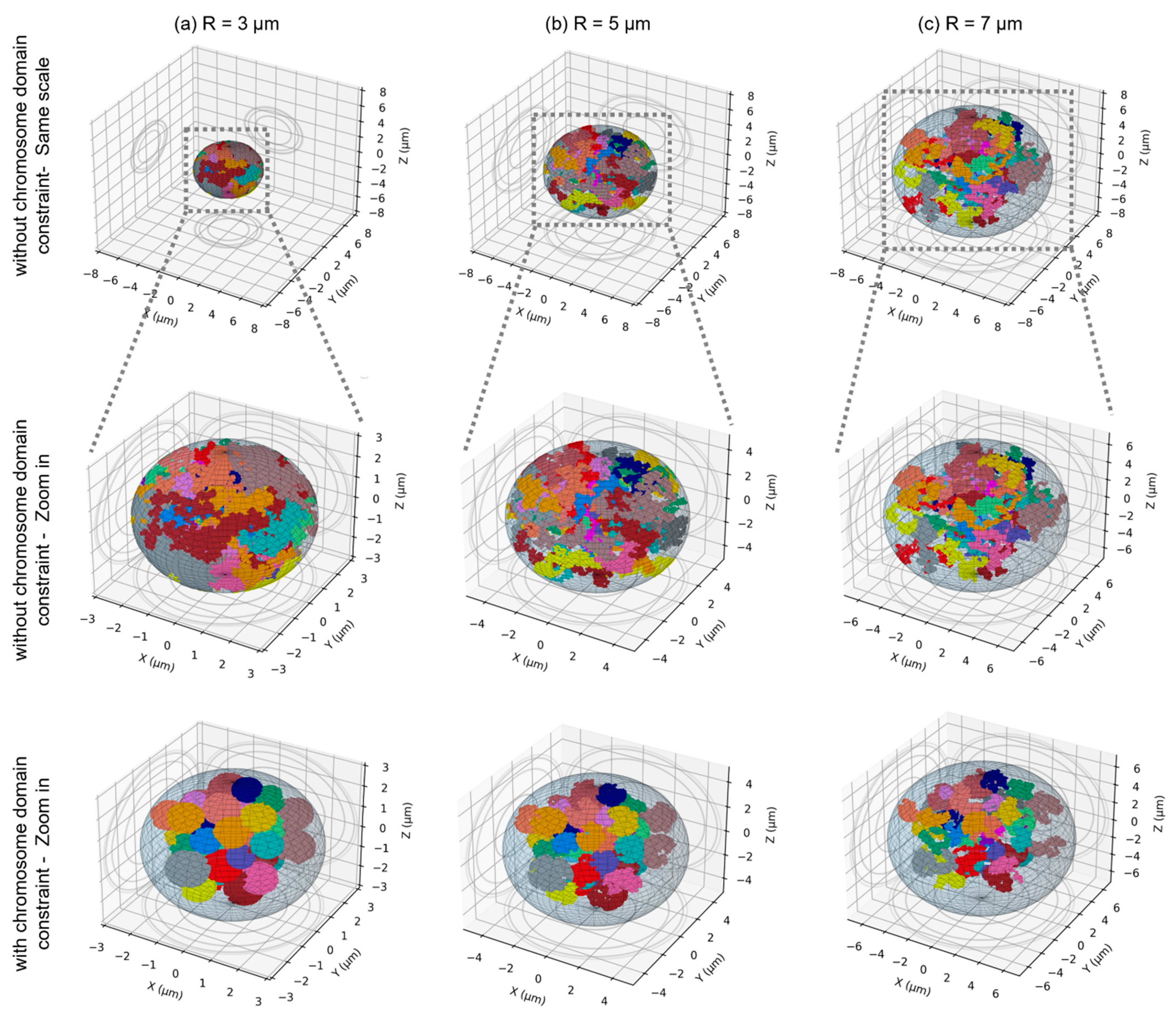
2.1. Nuclear Geometries Considered for This Work
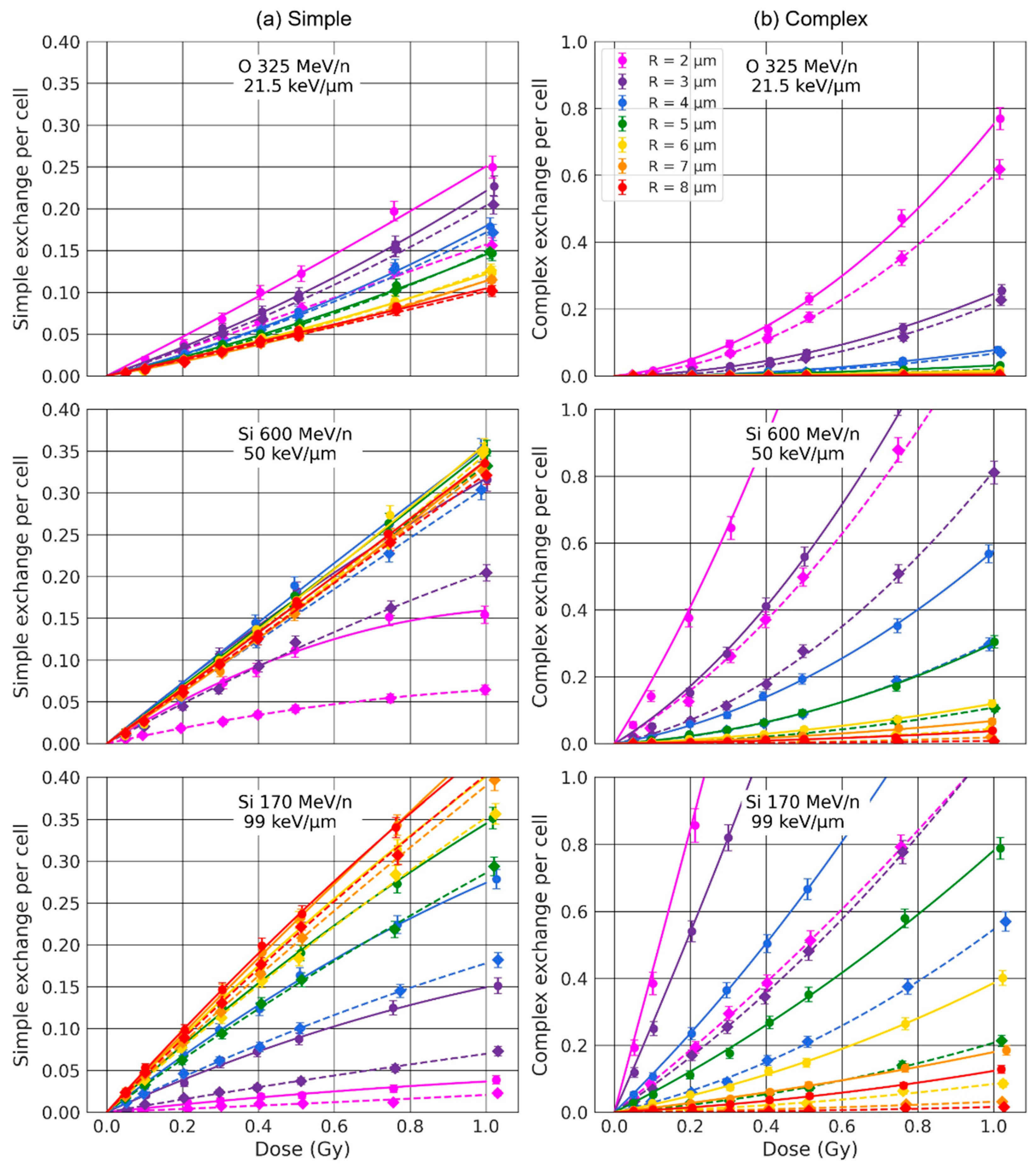
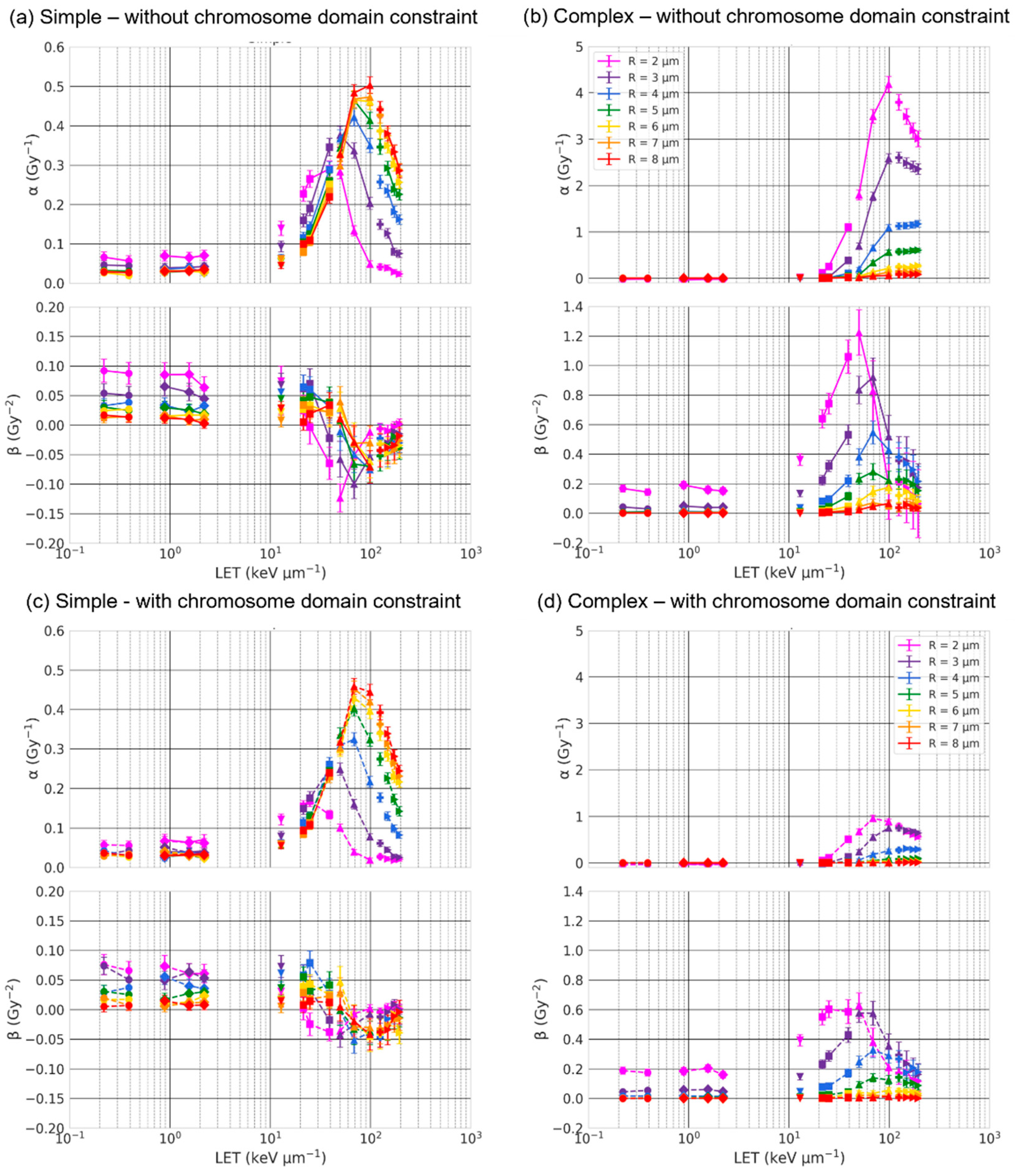
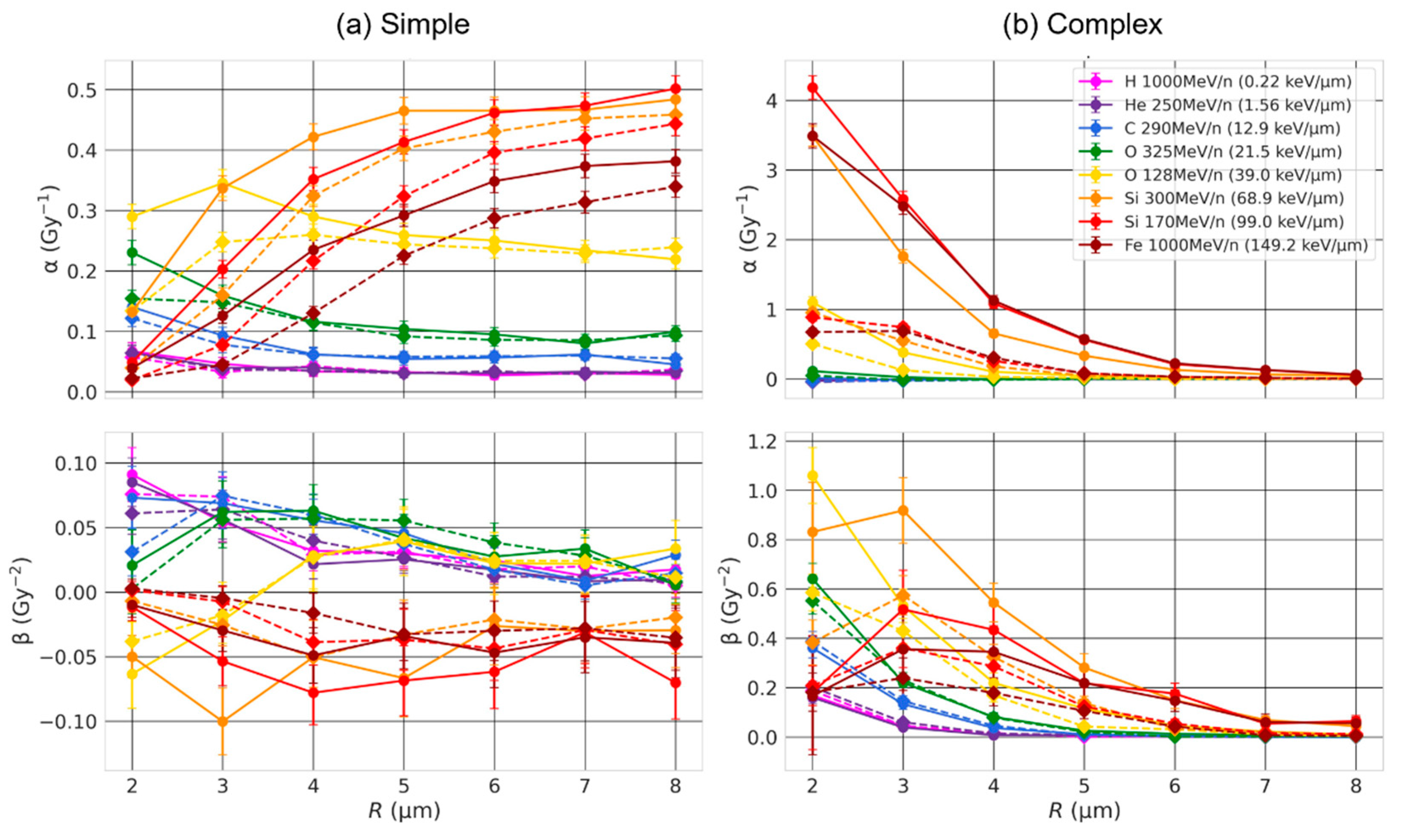
2.2. Effect of the Nuclear Volume
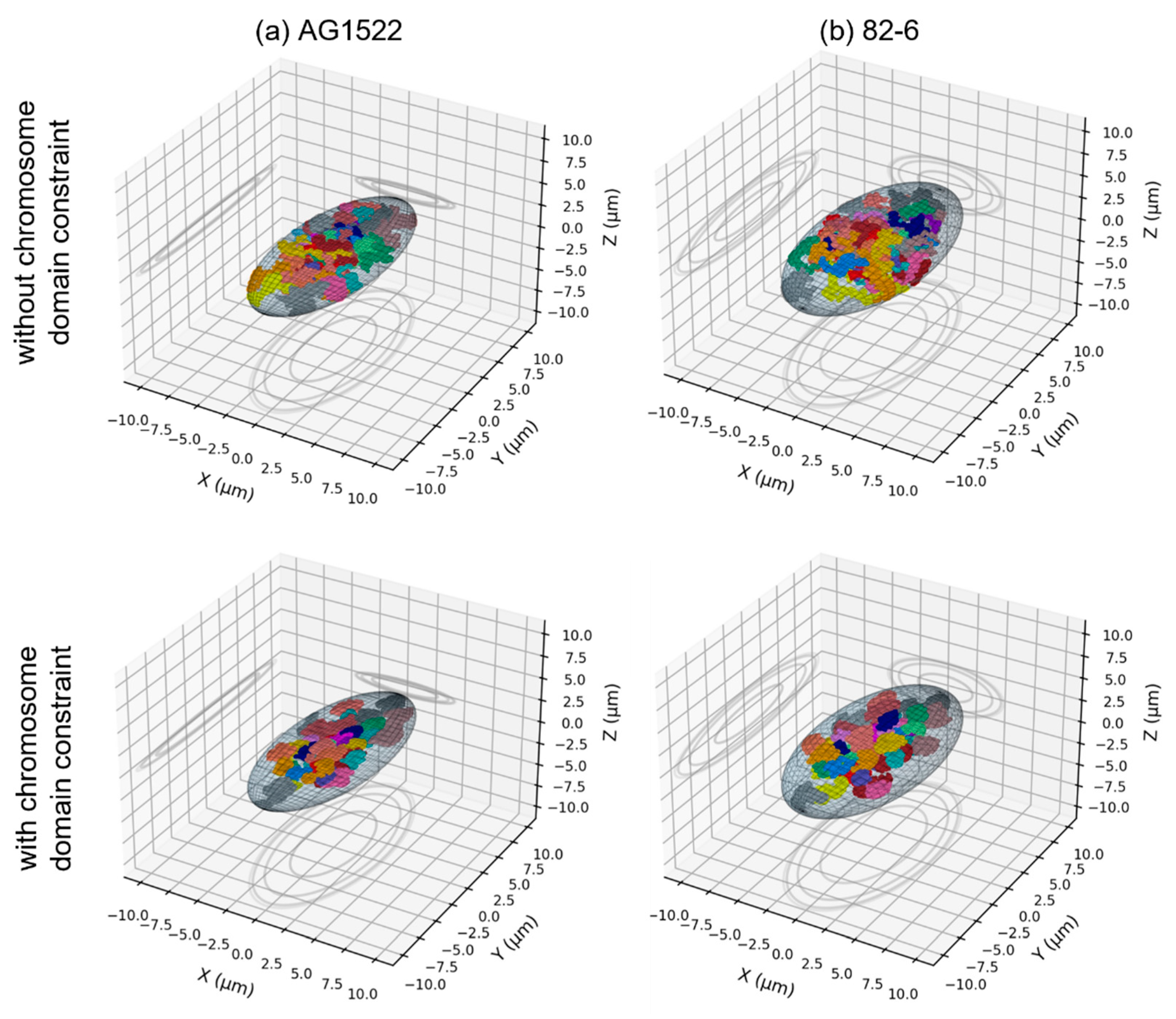
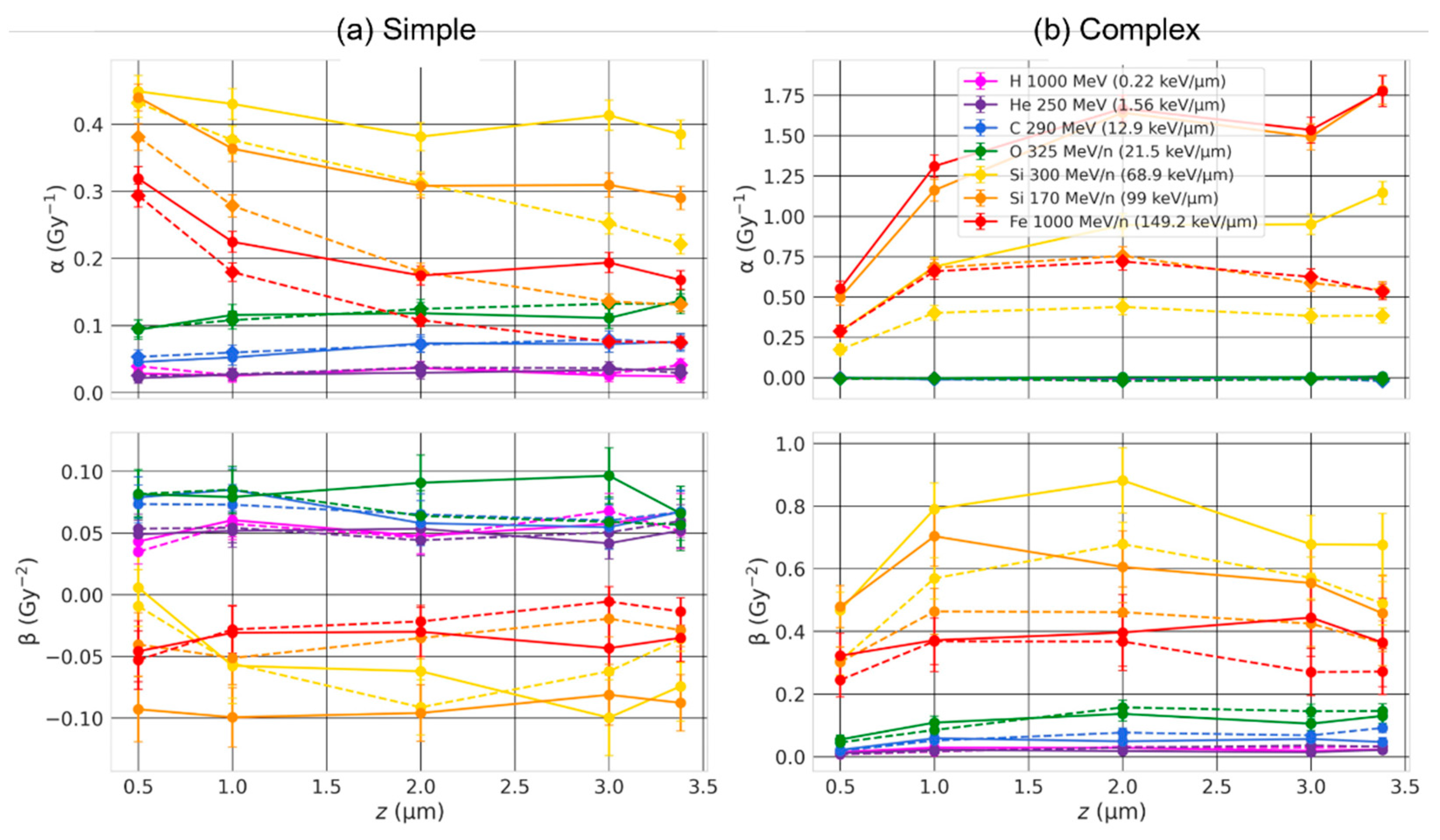
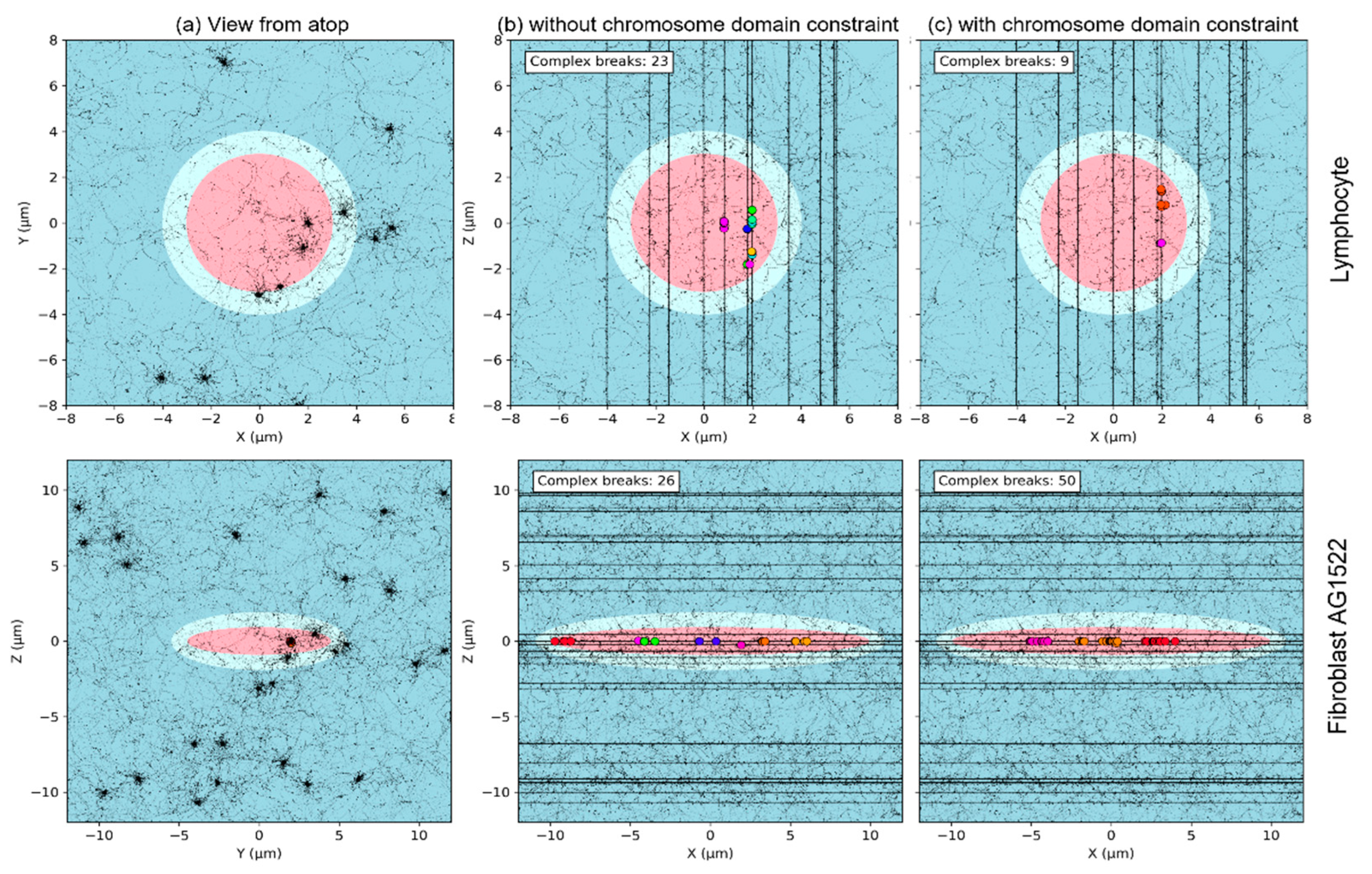
2.3. Effect of the Nuclear Shape
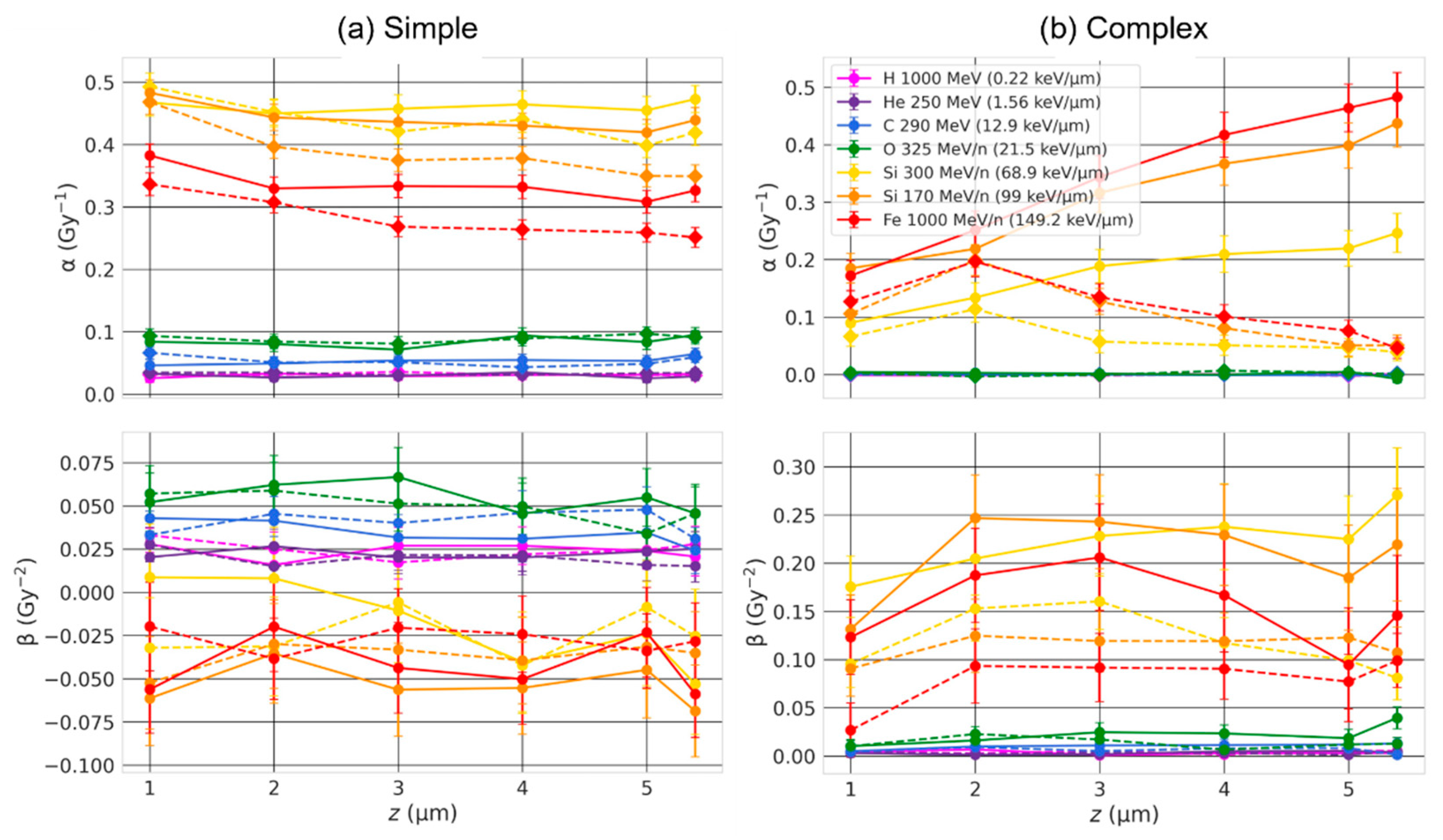
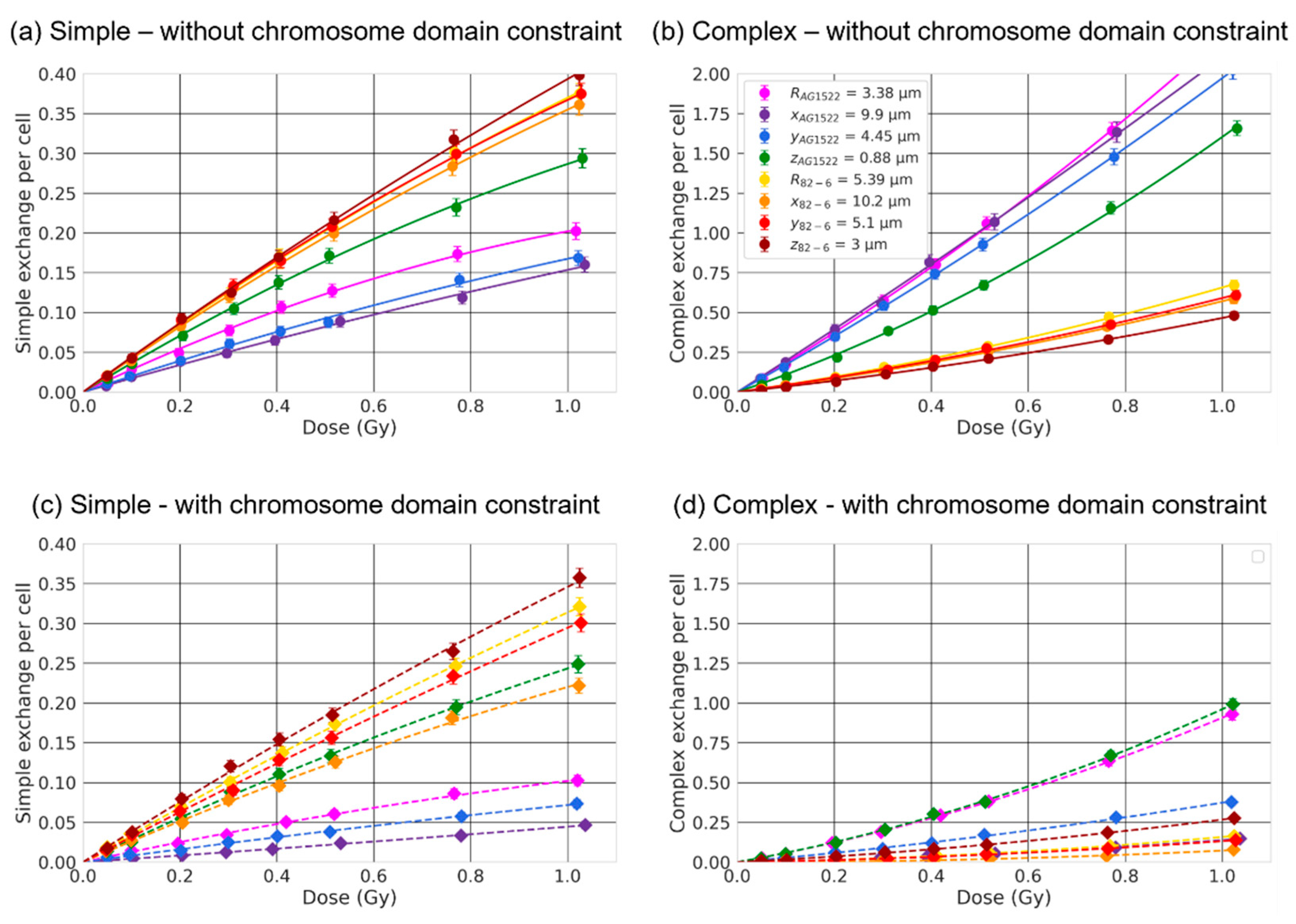
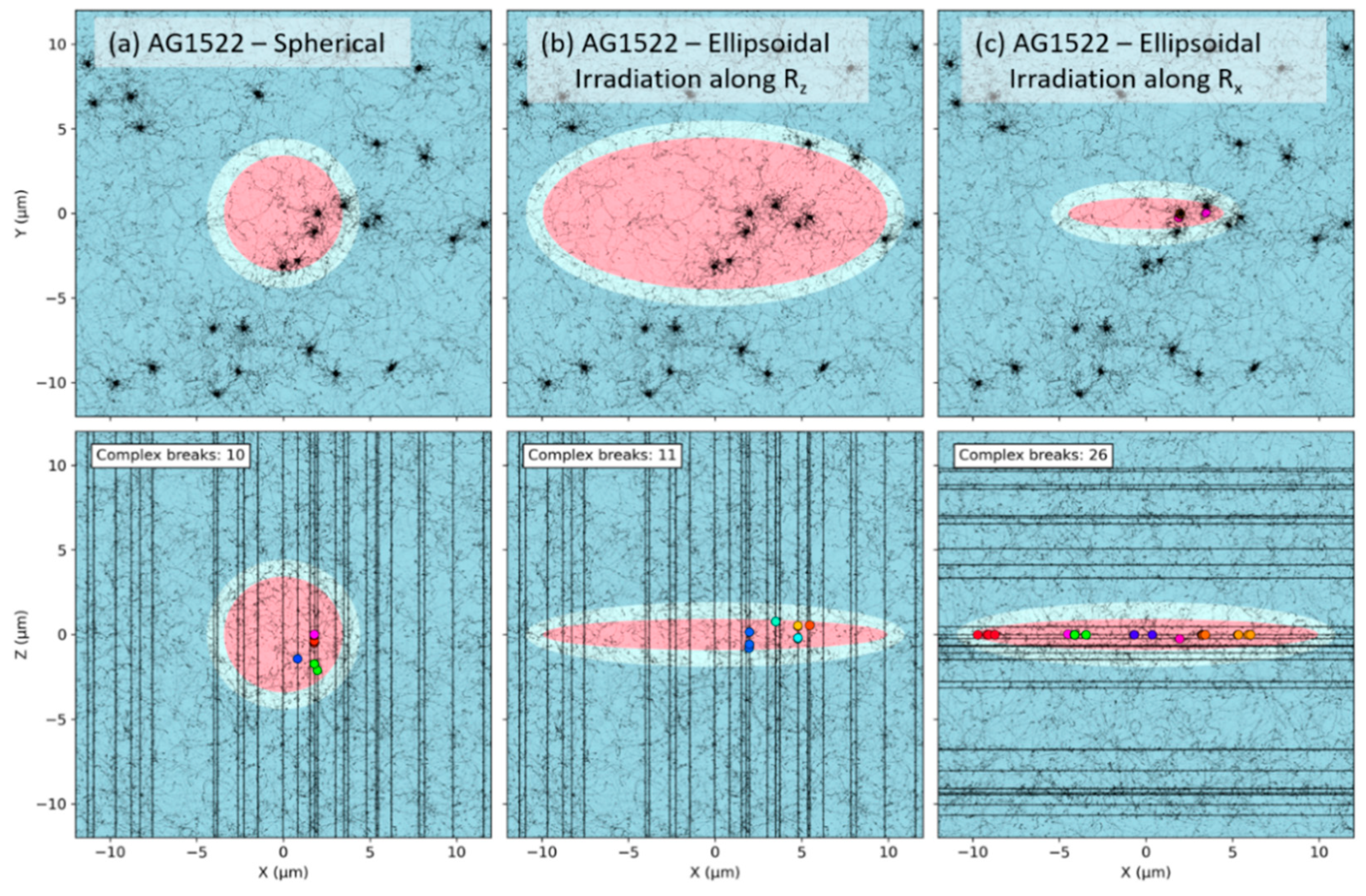
2.4. Effect of the Beam Orientation
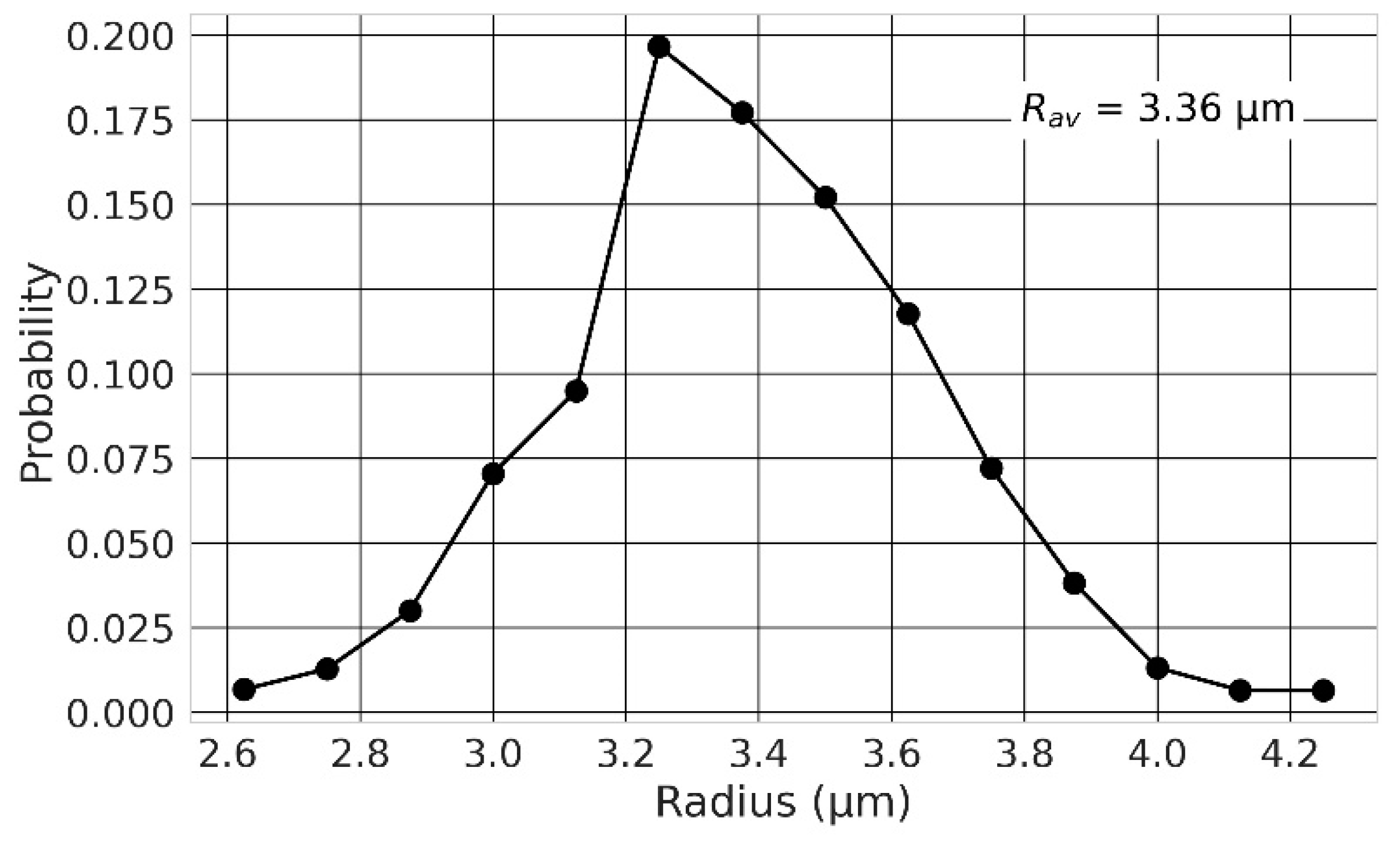
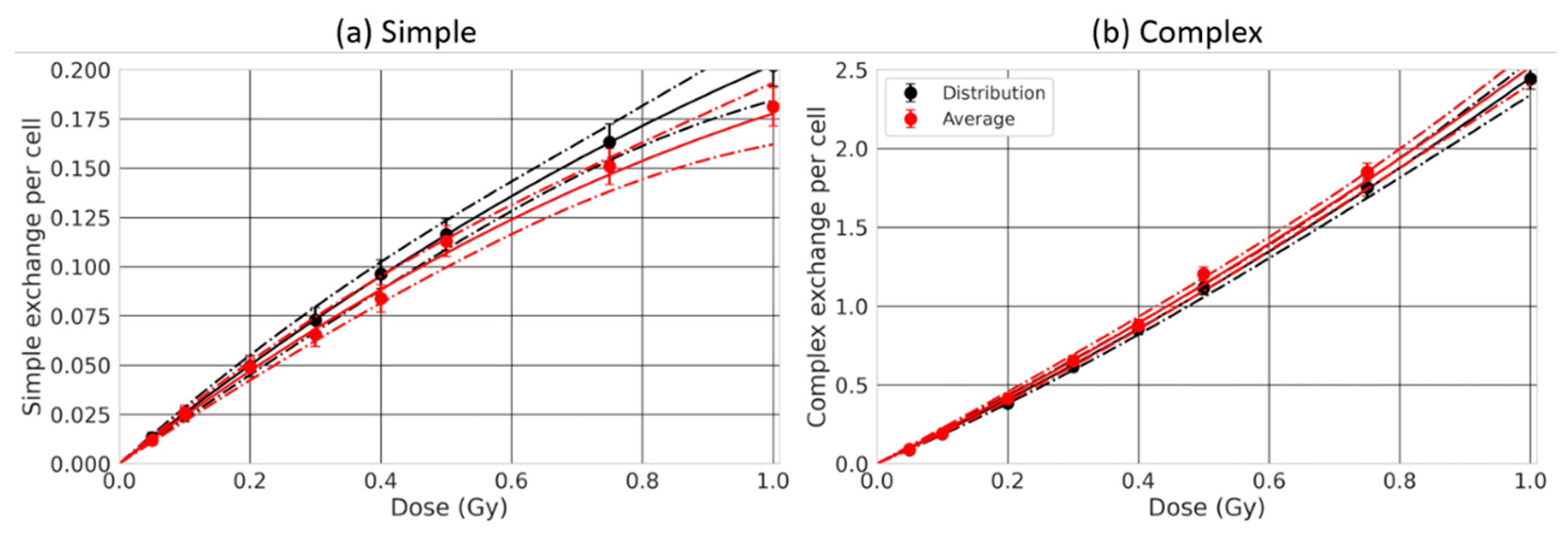
2.5. Effect of the Nuclear Size Variability
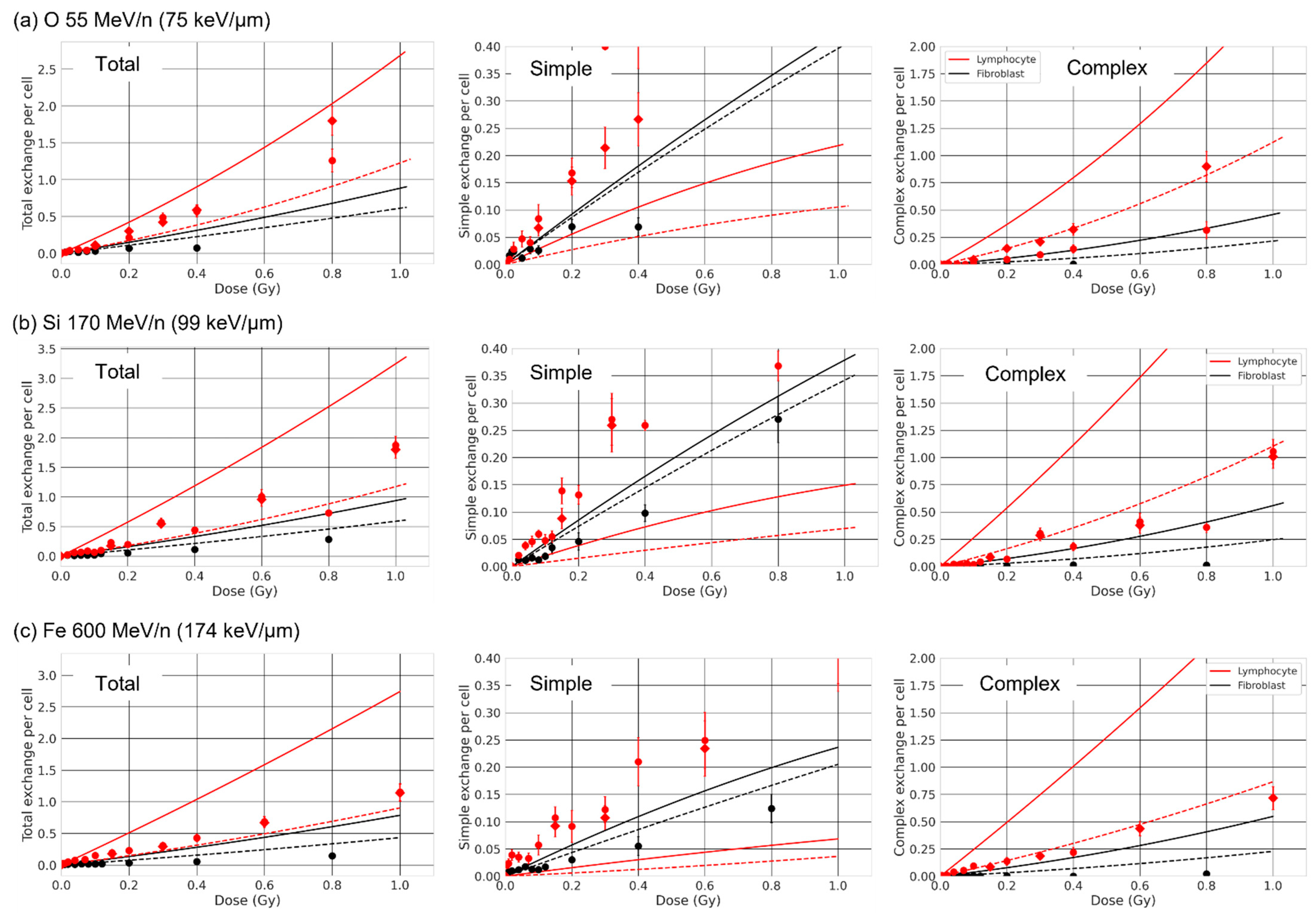
3. Discussion
3.1. Effect of the Nuclear Volume
3.2. Effect of the Nuclear Shape
3.3. Effect of the Beam Orientation
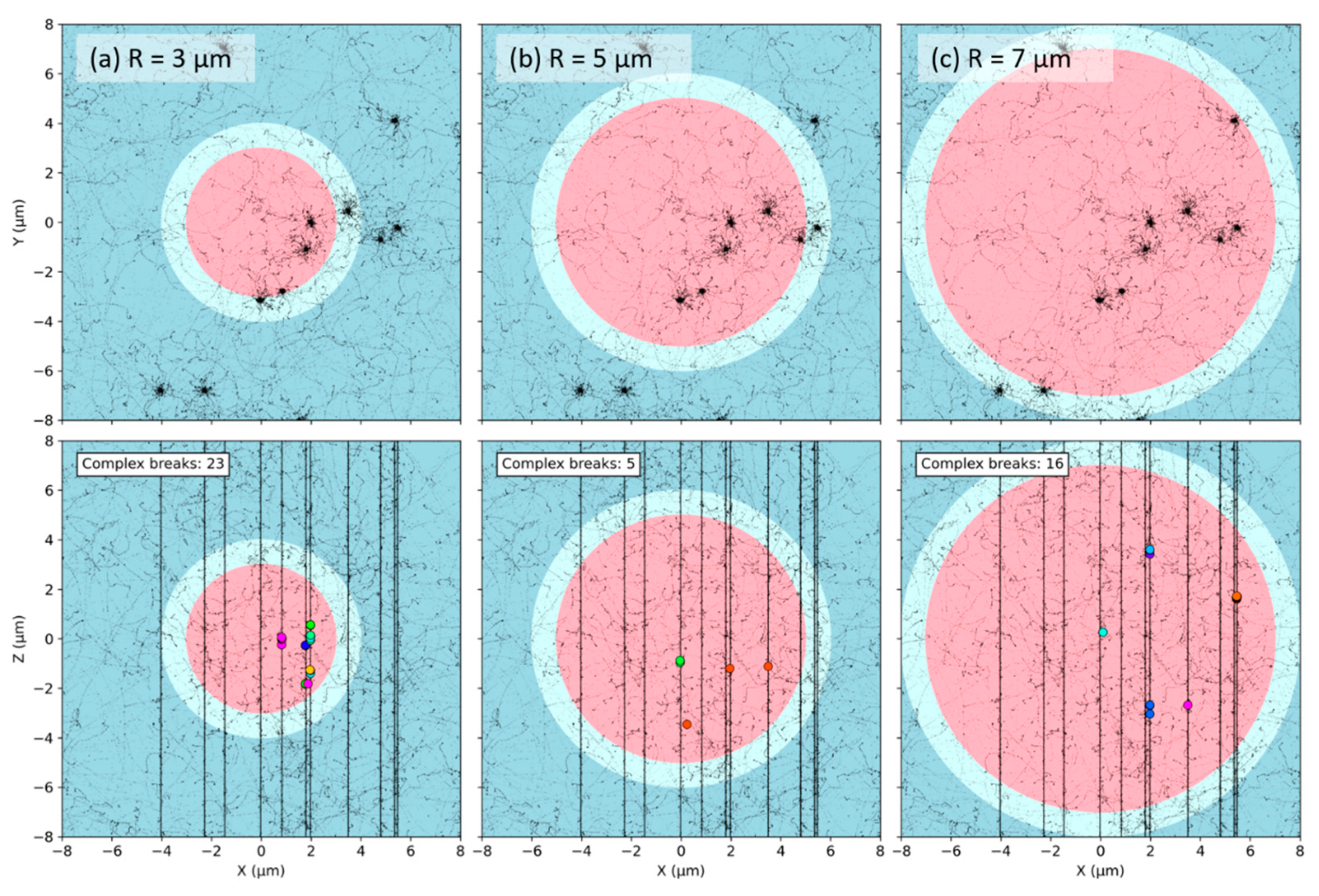
3.4. Effect of Chromosome Intermingling
4. Materials and Methods
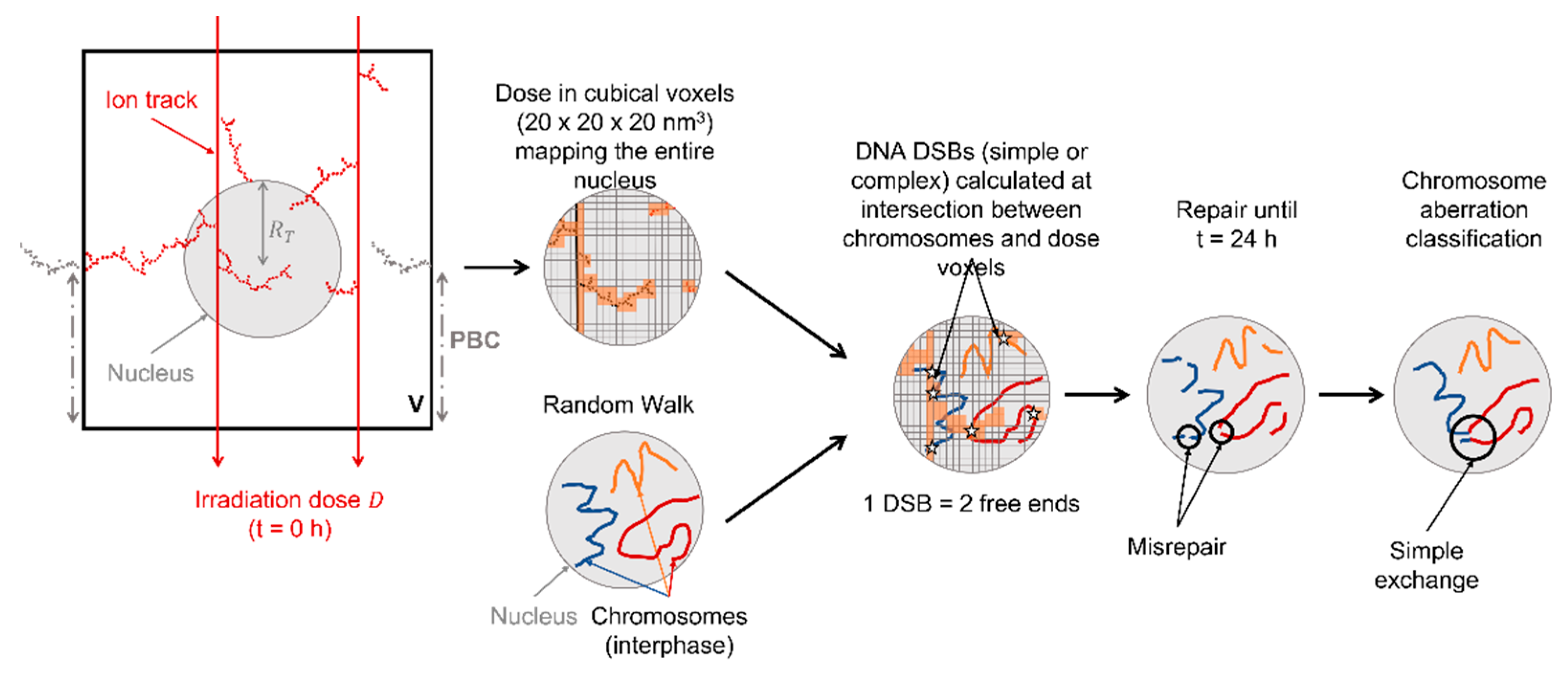
4.1. Nucleus Irradiation
4.2. Random Walk and Chromosome Geometries
4.3. DNA Damage
4.4. DSB Repair
4.5. Chromosome Aberrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. NPJ Microgravity 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, A.; Gauny, S.; Kwoh, E.; Connolly, L.; Dan, C.; Lasarev, M.; Turker, M.S. Comparative analysis of cell killing and autosomal mutation in mouse kidney epithelium exposed to 1 GeV/nucleon iron ions in vitro or in situ. Radiat. Res. 2009, 172, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, A.; Gauny, S.; Kwoh, E.; Grossi, G.; Dan, C.; Grygoryev, D.; Lasarev, M.; Turker, M.S. Comparative analysis of cell killing and autosomal mutation in mouse kidney epithelium exposed to 1 GeV protons in vitro or in vivo. Radiat. Res. 2013, 179, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Themis, M.; Garimberti, E.; Hill, M.A.; Anderson, R.M. Reduced chromosome aberration complexity in normal human bronchial epithelial cells exposed to low-LET γ-rays and high-LET α-particles. Int. J. Radiat. Biol. 2013, 89, 934–943. [Google Scholar] [CrossRef]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Niimi, A.; Isono, M.; Yamauchi, M.; Yasuhara, T.; Limsirichaikul, S.; Oike, T.; Sato, H.; Held, K.D.; Nakano, T.; et al. 3D-structured illumination microscopy reveals clustered DNA double-strand break formation in widespread γH2AX foci after high LET heavy-ion particle radiation. Oncotarget 2017, 8, 109370–109381. [Google Scholar] [CrossRef] [Green Version]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy–the heavy burden to repair. DNA Repair 2015, 28, 93–106. [Google Scholar] [CrossRef]
- Ballarini, F.; Ottolenghi, A. Chromosome aberrations as biomarkers of radiation exposure: Modelling basic mechanisms. Adv. Space Res. 2003, 31, 1557–1568. [Google Scholar] [CrossRef]
- Durante, M. Biomarkers of space radiation risk. Radiat. Res. 2005, 164, 467–473. [Google Scholar] [CrossRef]
- Huang, L.; Snyder, A.R.; Morgan, W.F. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene 2003, 22, 5848–5854. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, D.M.; Asaithamby, A.; Blattnig, S.R.; Costes, S.V.; Doetsch, P.W.; Dynan, W.S.; Hahnfeldt, P.; Hlatky, L.; Kidane, Y.; Kronenberg, A.; et al. Evaluating biomarkers to model cancer risk post cosmic ray exposure. Life Sci. Space Res. 2016, 9, 19–47. [Google Scholar] [CrossRef] [Green Version]
- Bach, D.H.; Zhang, W.; Sood, A.K. Chromosomal instability in tumor initiation and development. Cancer Res. 2019, 79, 3995–4002. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Kawata, T.; Gotoh, E.; Durante, M.; Wu, H.; George, K.; Furusawa, Y.; Cucinotta, F.A. High-LET radiation-induced aberrations in prematurely condensed G2 chromosomes of human fibroblasts. Int. J. Radiat. Biol. 2000, 76, 929–937. [Google Scholar] [CrossRef]
- Kawata, T.; Durante, M.; Furusawa, Y.; George, K.; Takai, N.; Wu, H.; Cucinotta, F.A. Dose–response of initial G2-chromatid breaks induced in normal human fibroblasts by heavy ions. Int. J. Radiat. Biol. 2001, 77, 165–174. [Google Scholar] [CrossRef]
- Tsuruoka, C.; Suzuki, M.; Hande, M.P.; Furusawa, Y.; Anzai, K.; Okayasu, R. The difference in LET and ion species dependence for induction of initially measured and non-rejoined chromatin breaks in normal human fibroblasts. Radiat. Res. 2008, 170, 163–171. [Google Scholar] [CrossRef]
- George, K.; Durante, M.; Wu, H.; Willingham, V.; Cucinotta, F.A. In vivo and in vitro measurements of complex-type chromosomal exchanges induced by heavy ions. Adv. Space Res. 2003, 31, 1525–1535. [Google Scholar] [CrossRef]
- George, K.; Durante, M.; Willingham, V.; Wu, H.; Yang, T.C.; Cucinotta, F.A. Biological effectiveness of accelerated particles for the induction of chromosome damage measured in metaphase and interphase human lymphocytes. Radiat. Res. 2003, 160, 425–435. [Google Scholar] [CrossRef]
- Durante, M.; George, K.; Gialanella, G.; Grossi, G.; La Tessa, C.; Manti, L.; Miller, J.; Pugliese, M.; Scampoli, P.; Cucinotta, F.A. Cytogenetic effects of high-energy iron ions: Dependence on shielding thickness and material. Radiat. Res. 2005, 164, 571–576. [Google Scholar] [CrossRef]
- George, K.; Cucinotta, F.A. The influence of shielding on the biological effectiveness of accelerated particles for the induction of chromosome damage. Adv. Space Res. 2007, 39, 1076–1081. [Google Scholar] [CrossRef]
- George, K.A.; Hada, M.; Chappell, L.; Cucinotta, F.A. Biological effectiveness of accelerated particles for the induction of chromosome damage: Track structure effects. Radiat. Res. 2013, 180, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Cera, F.; Cherubini, R.; Ianzini, F.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A.; Tiveron, P. Mutation induction and RBE-LET relationship of low-energy protons in V79 cells. Int. J. Radiat. Biol. 1991, 59, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Cera, F.; Cherubini, R.A.; Haque, A.M.I.; Ianzini, F.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A.; Tiveron, P. Inactivation and mutation induction in V79 cells by low energy protons: Re-evaluation of the results at the LNL facility. Int. J. Radiat. Biol. 1993, 63, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Masson, W.K. Mutation and inactivation of cultured mammalian cells exposed to beams of accelerated heavy ions: III. Human diploid fibroblasts. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1979, 36, 149–160. [Google Scholar] [CrossRef]
- Bolzer, A.; Kreth, G.; Solovei, I.; Koehler, D.; Saracoglu, K.; Fauth, C.; Müller, S.; Eils, R.; Cremer, C.; Speicher, M.R.; et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 2005, 3, e157. [Google Scholar] [CrossRef] [Green Version]
- Loiko, V.A.; Ruban, G.I.; Gritsai, O.A.; Gruzdev, A.D.; Kosmacheva, S.M.; Goncharova, N.V.; Miskevich, A.A. Morphometric model of lymphocyte as applied to scanning flow cytometry. J. Quant. Spectrosc. Radiat. Transf. 2006, 102, 73–84. [Google Scholar] [CrossRef]
- Dos Santos, M.; Villagrasa, C.; Clairand, I.; Incerti, S. Influence of the DNA density on the number of clustered damages created by protons of different energies. Nucl. Instrum. Methods Phys. Res. B 2013, 298, 47–54. [Google Scholar] [CrossRef]
- Sun, H.B.; Shen, J.; Yokota, H. Size-dependent positioning of human chromosomes in interphase nuclei. Biophys. J. 2000, 79, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Costes, S.V.; Boissière, A.; Ravani, S.; Romano, R.; Parvin, B.; Barcellos-Hoff, M.H. Imaging features that discriminate between foci induced by high-and low-LET radiation in human fibroblasts. Radiat. Res. 2006, 165, 505–515. [Google Scholar] [CrossRef]
- Seaman, L.; Meixner, W.; Snyder, J.; Rajapakse, I. Periodicity of nuclear morphology in human fibroblasts. Nucleus 2015, 6, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Cornforth, M.N.; Schillaci, M.E.; Goodhead, D.T.; Carpenter, S.G.; Wilder, M.E.; Sebring, R.J.; Raju, M.R. Radiobiology of ultrasoft x rays: III. normal human fibroblasts and the significance of terminal track structure in cell inactivation. Radiat. Res. 1989, 119, 511–522. [Google Scholar] [CrossRef]
- Durante, M.; Pignalosa, D.; Jansen, J.A.; Walboomers, X.F.; Ritter, S. Influence of nuclear geometry on the formation of genetic rearrangements in human cells. Radiat. Res. 2010, 174, 20–26. [Google Scholar] [CrossRef]
- Schmid, T.E.; Oestreicher, U.; Molls, M.; Schmid, E. Alpha particles induce different F values in monocellular layers of settled and attached human lymphocytes. Radiat. Res. 2011, 176, 226–233. [Google Scholar] [CrossRef]
- Sachs, R.K.; Chen, A.M.; Brenner, D.J. Proximity effects in the production of chromosome aberrations by ionizing radiation. Int. J. Radiat. Biol. 1997, 71, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Foster, H.A.; Estrada-Girona, G.; Themis, M.; Garimberti, E.; Hill, M.A.; Bridger, J.M.; Anderson, R.M. Relative proximity of chromosome territories influences chromosome exchange partners in radiation-induced chromosome rearrangements in primary human bronchial epithelial cells. Mutat. Res. Genet. Toxicol Environ. Mutagen. 2013, 756, 66–77. [Google Scholar] [CrossRef]
- Ingram, S.P.; Henthorn, N.T.; Warmenhoven, J.W.; Kirkby, N.F.; Mackay, R.I.; Kirkby, K.J.; Merchant, M.J. Hi-C implementation of genome structure for in silico models of radiation-induced DNA damage. PLoS Comput. Biol. 2020, 16, e1008476. [Google Scholar] [CrossRef]
- Cremer, T.; Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2001, 2, 292–301. [Google Scholar] [CrossRef]
- Branco, M.R.; Pombo, A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 2006, 4, e138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepińska, T.; Rusek, A.M.; Plewczynski, D. Intermingling of chromosome territories. Genes Chromosom. Cancer 2019, 58, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Jakob, B.; Splinter, J.; Durante, M.; Taucher-Scholz, G. Live cell microscopy analysis of radiation-induced DNA double-strand break motion. Proc. Natl. Acad. Sci. USA 2009, 106, 3172–3177. [Google Scholar] [CrossRef] [Green Version]
- Friedland, W.; Kundrát, P.; Jacob, P. Stochastic modelling of DSB repair after photon and ion irradiation. Int. J. Radiat. Biol. 2012, 88, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, T.; Swenson, J.; Pham, C.; Polyzos, A.; Lo, A.T.; Yang, P.; Dyball, J.; Asaithamby, A.; Chen, D.J.; Bissell, M.J.; et al. Evidence for formation of DNA repair centers and dose-response nonlinearity in human cells. Proc. Natl. Acad. Sci. USA 2012, 109, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García Fernández, F.; Fabre, E. The Dynamic Behavior of Chromatin in Response to DNA Double-Strand Breaks. Genes 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- McCord, R.P.; Balajee, A. 3D genome organization influences the chromosome translocation pattern. In Chromosome Translocation; Springer: Singapore, 2018; pp. 113–133. [Google Scholar] [CrossRef]
- Roukos, V.; Voss, T.C.; Schmidt, C.K.; Lee, S.; Wangsa, D.; Misteli, T. Spatial dynamics of chromosome translocations in living cells. Science 2013, 341, 660–664. [Google Scholar] [CrossRef]
- Edwards, A.A.; Moiseenko, V.V.; Nikjoo, H. Modelling of DNA breaks and the formation of chromosome aberrations. Int. J. Radiat. Biol. 1994, 66, 633–637. [Google Scholar] [CrossRef]
- Ponomarev, A.L.; George, K.; Cucinotta, F.A. Computational model of chromosome aberration yield induced by high-and low-LET radiation exposures. Radiat. Res. 2012, 177, 727–737. [Google Scholar] [CrossRef]
- Cajiao, J.J.T.; Carante, M.P.; Rodriguez, M.A.B.; Ballarini, F. Proximity effects in chromosome aberration induction by low-LET ionizing radiation. DNA Repair 2017, 58, 38–46. [Google Scholar] [CrossRef]
- Cajiao, J.J.T.; Carante, M.P.; Rodriguez, M.A.B.; Ballarini, F. Proximity effects in chromosome aberration induction: Dependence on radiation quality, cell type and dose. DNA Repair 2018, 64, 45–52. [Google Scholar] [CrossRef]
- Plante, I.; Ponomarev, A.; Patel, Z.; Slaba, T.; Hada, M. RITCARD: Radiation-Induced Tracks, Chromosome Aberrations, Repair and Damage. Radiat. Res. 2019, 192, 282–298. [Google Scholar] [CrossRef]
- Plante, I.; Cucinotta, F.A. Monte-Carlo Simulation of Ionizing Radiation Tracks. In Applications of Monte Carlo Methods in Biology, Medicine and Other Fields of Science; Mode, C.B., Ed.; InTechOpen: London, UK, 2011; pp. 315–356. [Google Scholar] [CrossRef] [Green Version]
- Plante, I.; Slaba, T.C.; Shavers, Z.; Hada, M. A bi-exponential repair algorithm for radiation-induced double-strand breaks: Application to simulation of chromosome aberrations. Genes 2019, 10, 936. [Google Scholar] [CrossRef] [Green Version]
- Slaba, T.C.; Plante, I.; Ponomarev, A.; Patel, Z.S.; Hada, M. Determination of chromosome aberrations in human fibroblasts irradiated by mixed fields generated with shielding. Radiat. Res. 2020, 194, 246–258. [Google Scholar] [CrossRef]
- Saha, B.; Zitnik, G.; Johnson, S.C.; Nguyen, Q.; Risques, R.A.; Martin, G.M.; Oshima, J. DNA damage accumulation and TRF2 degradation in atypical Werner syndrome fibroblasts with LMNA mutations. Front. Genet. 2013, 4, 129. [Google Scholar] [CrossRef] [Green Version]
- Loucas, B.D.; Durante, M.; Bailey, S.M.; Cornforth, M.N. Chromosome damage in human cells by γ rays, α particles and heavy ions: Track interactions in basic dose-response relationships. Radiat. Res. 2013, 179, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, F.; Campa, A.; Esposito, G.; Giardullo, P.; Belli, M.; Dini, V.; Meschini, S.; Simone, G.; Sorrentino, E.; Gerardi, S.; et al. Induction and repair of DNA DSB as revealed by H2AX phosphorylation foci in human fibroblasts exposed to low-and high-LET radiation: Relationship with early and delayed reproductive cell death. Radiat. Res. 2015, 183, 417–431. [Google Scholar] [CrossRef]
- Raju, M.R.; Eisen, Y.; Carpenter, S.; Inkret, W.C. Radiobiology of α particles: III. Cell inactivation by α-particle traversals of the cell nucleus. Radiat. Res. 1991, 128, 204–209. [Google Scholar] [CrossRef]
- Bettega, D.; Calzolari, P.; Doneda, L.; Durante, M.; Tallone, L. Early and delayed reproductive death in human cells exposed to high energy iron ion beams. Adv. Space Res. 2005, 35, 280–285. [Google Scholar] [CrossRef]
- Ponomarev, A.L.; Huff, J.; Cucinotta, F.A. The analysis of the densely populated patterns of radiation-induced foci by a stochastic, Monte Carlo model of DNA double-strand breaks induction by heavy ions. Int. J. Radiat. Biol. 2010, 86, 507–515. [Google Scholar] [CrossRef]
- Townsend, K.M.S.; Marsden, S.J. Nuclear area measurement on viable cells, using confocal microscopy. Int. J. Radiat. Biol. 1992, 61, 549–551. [Google Scholar] [CrossRef]
- Elbakrawy, E.M.; Mayah, A.; Hill, M.A.; Kadhim, M. Induction of genomic instability in a primary human fibroblast cell line following low-dose alpha-particle exposure and the potential role of exosomes. Biology 2020, 10, 11. [Google Scholar] [CrossRef]
- Belli, D.; Bettega, P.; Calzolari, F.; Cera, R.; Cherubini, M.; Dalla Vecchia, M.; Durante, S.; Favaretto, G.; Gialanella, G.; Grossi, M. Inactivation of human normal and tumour cells irradiated with low energy protons. Int. J. Radiat. Biol. 2000, 76, 831–839. [Google Scholar] [CrossRef]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 241–250. [Google Scholar] [CrossRef]
- Griffin, C.S.; Marsden, S.J.; Stevens, D.L.; Simpson, P.; Savage, J.R.K. Frequencies of complex chromosome exchange aberrations induced by 238Pu α-particles and detected by fluorescence in situ hybridization using single chromosome-specific probes. Int. J. Radiat. Biol. 1995, 67, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tracy, B.L.; Stevens, D.L.; Goodhead, D.T.; Hill, M.A. Variation in RBE for survival of V79-4 cells as a function of alpha-particle (helium ion) energy. Radiat. Res. 2015, 184, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Rohrig, N.; Colvett, R.D.; Geard, C.R.; Marino, S.A. Inactivation of synchronized Chinese hamster V79 cells with charged-particle track segments. Radiat. Res. 1980, 82, 277–289. [Google Scholar] [CrossRef]
- Hill, M.A.; Herdman, M.T.; Stevens, D.L.; Jones, N.J.; Thacker, J.; Goodhead, D.T. Relative sensitivities of repair-deficient mammalian cells for clonogenic survival after α-particle irradiation. Radiat. Res. 2004, 162, 667–676. [Google Scholar] [CrossRef]
- Hada, M.; Chappell, L.J.; Wang, M.; George, K.A.; Cucinotta, F.A. Induction of chromosomal aberrations at fluences of less than one HZE particle per cell nucleus. Radiat. Res. 2014, 182, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.A. Fishing for radiation quality: Chromosome aberrations and the role of radiation track structure. Radiat. Prot. Dosimetry 2015, 166, 295–301. [Google Scholar] [CrossRef]
- Ponomarev, A.L.; George, K.; Cucinotta, F.A. Generalized time-dependent model of radiation-induced chromosomal aberrations in normal and repair-deficient human cells. Radiat. Res. 2014, 181, 284–292. [Google Scholar] [CrossRef]
- Ponomarev, A.L.; Cucinotta, F.A. Chromatin loops are responsible for higher counts of small DNA fragments induced by high-LET radiation, while chromosomal domains do not affect the fragment sizes. Int. J. Radiat. Biol. 2006, 82, 293–305. [Google Scholar] [CrossRef]
- Turner, J. Atoms, Radiation and Radiation Protection, 3rd ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Poignant, F.; Plante, I.; Crespo, L.; Slaba, T. Impact of Radiation Quality on Microdosimetry and Chromosome Aberrations for High-Energy (>250 MeV/n) Ions. Life 2022, 12, 358. [Google Scholar] [CrossRef]
- Plante, I.; Poignant, F.; Slaba, T. Track structure components: Characterizing energy deposited in spherical cells from direct and peripheral HZE ion hits. Life 2021, 11, 1112. [Google Scholar] [CrossRef]
- Morton, N.E. Parameters of the human genome. Proc. Natl. Acad. Sci. USA 1991, 88, 7474–7476. [Google Scholar] [CrossRef] [Green Version]
- Fritz, A.J.; Barutcu, A.R.; Martin-Buley, L.; Van Wijnen, A.J.; Zaidi, S.K.; Imbalzano, A.N.; Lian, J.B.; Stein, J.L.; Stein, G.S. Chromosomes at work: Organization of chromosome territories in the interphase nucleus. J. Cell. Biochem. 2016, 117, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Pariset, E.; Plante, I.; Ponomarev, A.; Viger, L.; Evain, T.; Blattnig, S.R.; Costes, S.V. DNA break clustering inside repair domains predicts cell death and mutation frequency in human fibroblasts and in Chinese hamster cells for a 10^ 3× range of linear energy transfers. bioRxiv 2020. [Google Scholar] [CrossRef]
- Asaithamby, A.; Uematsu, N.; Chatterjee, A.; Story, M.D.; Burma, S.; Chen, D.J. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat. Res. 2008, 169, 437–446. [Google Scholar] [CrossRef]
- Savage, J.R. Classification and relationships of induced chromosomal structural changes. J. Med. Genet. 1976, 13, 103–122. [Google Scholar] [CrossRef] [Green Version]
- Cornforth, M.N.; Bedford, J.S. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat. Res. 1987, 111, 385–405. [Google Scholar] [CrossRef]
| Average Nuclear Ion Traversal/1 Gy | |||||||
|---|---|---|---|---|---|---|---|
| (µm) | (µm) | (µm) | (µm2) | (µm3) | H 1000 MeV/n | Si 170 MeV/n | Fe 450 MeV/n |
| Volume study | |||||||
| 2 | 2 | 2 | 12.6 | 33.5 | 356 | 0.8 | 0.4 |
| 3 | 3 | 3 | 28.3 | 113.1 | 802 | 1.8 | 0.9 |
| 4 | 4 | 4 | 50.3 | 268.1 | 1426 | 3.2 | 1.6 |
| 5 | 5 | 5 | 78.5 | 523.6 | 2228 | 5.0 | 2.5 |
| 6 | 6 | 6 | 113.1 | 904.8 | 3208 | 7.1 | 3.6 |
| 7 | 7 | 7 | 153.9 | 1436.8 | 4366 | 9.7 | 4.9 |
| 8 | 8 | 8 | 201.1 | 2144.7 | 5703 | 12.7 | 6.4 |
| Shape study | |||||||
| Fibroblast 82-6 | |||||||
| 12.51 | 12.51 | 1.00 | 492.0 | 656 | 13,954 | 31.0 | 15.7 |
| 8.85 | 8.85 | 2.00 | 246.0 | 656 | 6978 | 15.5 | 7.9 |
| 7.23 | 7.23 | 3.00 | 164.0 | 656 | 4651 | 10.3 | 5.2 |
| 6.26 | 6.26 | 4.00 | 123.0 | 656 | 3489 | 7.8 | 3.9 |
| 5.60 | 5.60 | 5.00 | 98.4 | 656 | 2791 | 6.2 | 3.1 |
| 5.39 | 5.39 | 5.39 | 91.3 | 656 | 2589 | 5.8 | 2.9 |
| Fibroblast AG1522 | |||||||
| 8.81 | 8.81 | 0.50 | 243.6 | 162 | 6909 | 15.4 | 7.8 |
| 6.23 | 6.23 | 1.00 | 121.8 | 162 | 3454 | 7.7 | 3.9 |
| 4.40 | 4.40 | 2.00 | 60.9 | 162 | 1727 | 3.8 | 1.9 |
| 3.59 | 3.59 | 3.00 | 40.6 | 162 | 1151 | 2.6 | 1.3 |
| 3.38 | 3.38 | 3.38 | 36.0 | 162 | 1021 | 2.3 | 1.2 |
| Beam orientation study | |||||||
| Fibroblast 82-6 | |||||||
| 10.22 | 5.11 | 3.00 | 164.0 | 656 | 4652 | 10.3 | 5.2 |
| 3.00 | 10.22 | 5.11 | 96.3 | 656 | 2731 | 6.1 | 3.1 |
| 5.11 | 3.00 | 10.22 | 48.2 | 656 | 1366 | 5.8 | 1.5 |
| 5.39 | 5.39 | 5.39 | 91.3 | 656 | 2589 | 3.0 | 2.9 |
| Fibroblast AG1522 | |||||||
| 9.90 | 4.45 | 0.88 | 138.4 | 162 | 3926 | 8.7 | 4.4 |
| 0.88 | 9.90 | 4.45 | 27.4 | 162 | 776 | 2.3 | 0.9 |
| 4.45 | 0.88 | 9.90 | 12.3 | 162 | 349 | 1.7 | 0.4 |
| 3.38 | 3.38 | 3.38 | 36.0 | 162 | 1021 | 0.8 | 1.2 |
| Simple | Complex | |||||||
|---|---|---|---|---|---|---|---|---|
| AG1255 | ||||||||
| = 3.38 μm | 0.29 | 0.13 | −0.09 | −0.03 | 1.78 | 0.54 | 0.46 | 0.36 |
| = 9.9 μm | 0.17 | 0.04 | −0.02 | 0.00 | 1.94 | 0.05 | 0.16 | 0.08 |
| = 4.45 μm | 0.20 | 0.08 | −0.04 | −0.01 | 1.70 | 0.27 | 0.28 | 0.11 |
| = 0.88 μm | 0.37 | 0.29 | −0.08 | −0.04 | 1.04 | 0.54 | 0.56 | 0.42 |
| 82-6 | ||||||||
| R82-6 = 5.39 μm | 0.44 | 0.35 | −0.07 | −0.04 | 0.44 | 0.05 | 0.22 | 0.11 |
| x82-6 = 10.22 μm | 0.42 | 0.27 | −0.07 | −0.05 | 0.39 | 0.00 | 0.18 | 0.07 |
| y82-6 = 5.11 μm | 0.45 | 0.32 | −0.08 | −0.03 | 0.42 | 0.05 | 0.17 | 0.08 |
| z82-6 = 3.00 μm | 0.44 | 0.39 | −0.05 | −0.04 | 0.33 | 0.16 | 0.14 | 0.11 |
| Ref. | Cell Line | X Semi-Axis (µm) | Y Semi-Axis (µm) | Z Semi-Axis (µm) | Irradiation Area (µm3) | Volume (µm3) |
|---|---|---|---|---|---|---|
| Normal human lymphocytes | ||||||
| [33] a | Blood (1 donor) | 3.5–5 | 3.5–5 | 1.5 | 38–79 | 77–157 |
| 2.75 | 2.75 | 2.75 | 24 | 87 | ||
| [26] b | Blood (5 donors) | 2.05–3.55 | 13.2–39.6 | 11.5–59.7 | ||
| Normal human fibroblast | ||||||
| [54] c | 82-6 | 8.0 | 5.0 | 126 | ||
| [53] | 82-6 | 7.23 | 7.23 | 164 (10) | ||
| [31] | AG1522 (male foreskin) | 4.7 (<0.5) | 0.55 (<0.05) | |||
| [55] | AG1521 | 6.43 | 6.43 | 0.6 | 130 | 104 |
| [32] | AG1522 | 9.9 (0.9) | 4.45 (35) | 0.88 (0.1) | 138 | 162 |
| [56] | AG01522 | 8.18 | 8.18 | 1.5 | 210 (20) | |
| [57] | AG1522 | 6.77 | 6.77 | 0.6 | 144 (45) | 115 |
| [58] | AG1522 | 7.36 | 7.36 | 170 | ||
| [27] | Not Specified | 9.85 | 7.1 | 2.5 | 220 | 732 |
| [59] | HF19 (female fetal lung) | 6 | 6 | 6 | 113 | 904 |
| [60] | HF19 | 8.41 | 8.41 | 222 (56) | ||
| [61] | HF19 | 7.76 | 7.76 | 189 | ||
| [62] | HF19 | <1.75 | ||||
| [63] | HFL-III (male fetal lung) | 6.74 | 6.74 | 142.9 (1.9) | ||
| [29] | HCA2 (male foreskin) | 7.6 | 7.6 | 1.25 | 180 | 302 |
| [64] | HF12 (male fetal lung) | 8.46 | 8.46 | 1.93 (0.28) | 225 (106–444) | 579 |
| [28] | HLF-CLL (male lung) | 16.1 (2.35) | 9.0 (0.95) | 455 | ||
| [63] | NB1RGB (male skin) | 7.41 | 7.41 | 172.3 (2.8) | ||
| [25] | Skin biopsy (male) | 10 | 5 | 2.5 | 157 | 524 |
| [30] | Male foreskin (6 donors) | 8.36–9.15 | 6.42–7.83 | 2.02–3.17 | 492–1010 | |
| Normal human epithelial/endothelial cells | ||||||
| [27] | Endothelial | 9.55 | 5.5 | 1 | 165 | 220 |
| [4] | Bronchial Epithelial | 5.8 | 5.8 | 1.7 | 105 | 240 |
| Hamster fibroblast | ||||||
| [65] | V79 (male) | 6.00 | 6.00 | 2 | 113 (34) | 377 |
| [66] | V79 (late S) | 8.41 | 8.41 | 222 | ||
| [66] | V79 (asynchronous) | 6.68 | 6.68 | 140 | ||
| [66] | V79 (G1/S) | 7.25 | 7.25 | 165 | ||
| [57] | V79 | 4.95 | 4.95 | 1.9 | 77 (25) | 195 |
| [67] | V79 | 6.98 | 6.98 | 153 | ||
| [60] | V79 | 6.53 | 6.53 | 134 (36) | ||
| Ion | 1H1+ | 4He2+ | 12C6+ | 16O8+ | 28Si14+ | 48Ti22+ | 56Fe26+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy | 1000 | 250 | 1000 | 250 | 150 | 290 | 325 | 250 | 128 | 600 | 300 | 170 | 600 | 1000 | 600 | 450 |
| LET | 0.22 | 0.39 | 0.89 | 1.56 | 2.2 | 12.9 | 21.5 | 25 | 39 | 50 | 68.9 | 99 | 125 | 149.2 | 174 | 195 |
| R | 322 | 37.5 | 323 | 37.6 | 15.6 | 16.4 | 14.6 | 9.4 | 3.0 | 22 | 7.3 | 2.8 | 15.6 | 27.4 | 13.1 | 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poignant, F.; Plante, I.; Patel, Z.S.; Huff, J.L.; Slaba, T.C. Geometrical Properties of the Nucleus and Chromosome Intermingling Are Possible Major Parameters of Chromosome Aberration Formation. Int. J. Mol. Sci. 2022, 23, 8638. https://doi.org/10.3390/ijms23158638
Poignant F, Plante I, Patel ZS, Huff JL, Slaba TC. Geometrical Properties of the Nucleus and Chromosome Intermingling Are Possible Major Parameters of Chromosome Aberration Formation. International Journal of Molecular Sciences. 2022; 23(15):8638. https://doi.org/10.3390/ijms23158638
Chicago/Turabian StylePoignant, Floriane, Ianik Plante, Zarana S. Patel, Janice L. Huff, and Tony C. Slaba. 2022. "Geometrical Properties of the Nucleus and Chromosome Intermingling Are Possible Major Parameters of Chromosome Aberration Formation" International Journal of Molecular Sciences 23, no. 15: 8638. https://doi.org/10.3390/ijms23158638
APA StylePoignant, F., Plante, I., Patel, Z. S., Huff, J. L., & Slaba, T. C. (2022). Geometrical Properties of the Nucleus and Chromosome Intermingling Are Possible Major Parameters of Chromosome Aberration Formation. International Journal of Molecular Sciences, 23(15), 8638. https://doi.org/10.3390/ijms23158638







