Vascularization in Bioartificial Parenchymal Tissue: Bioink and Bioprinting Strategies
Abstract
:1. Introduction
1.1. Highlights
1.2. Outlook
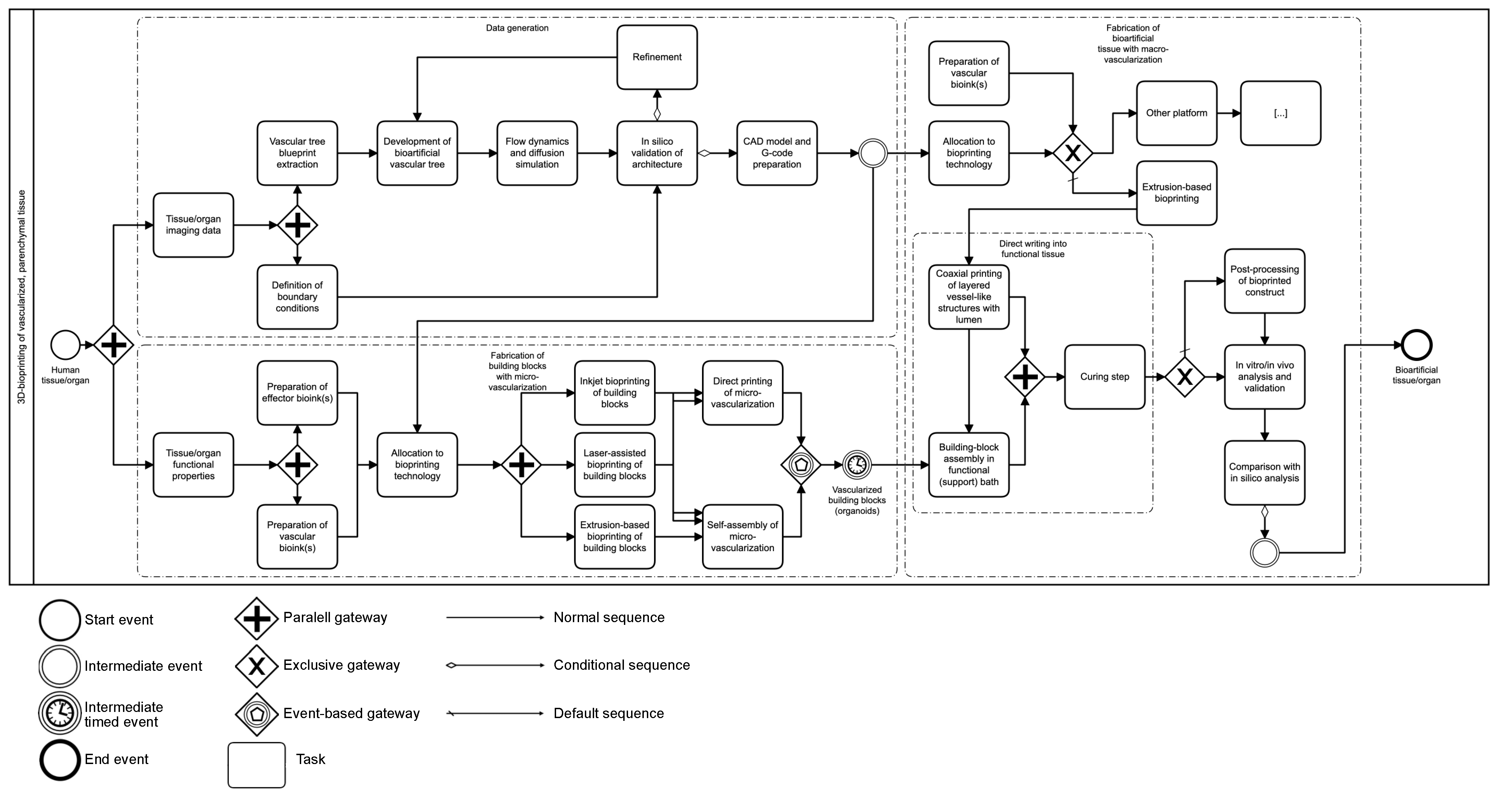
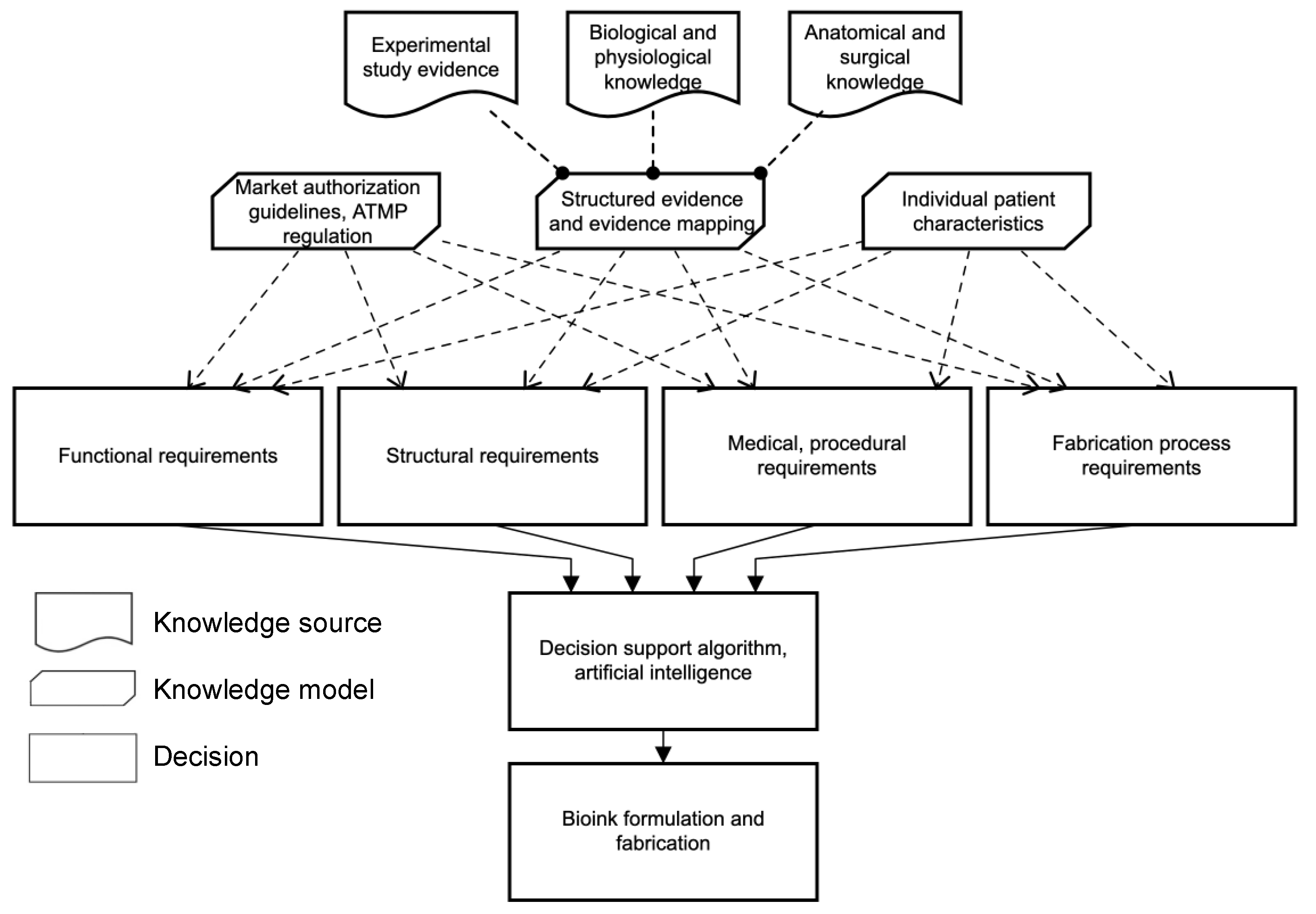
2. Methodology
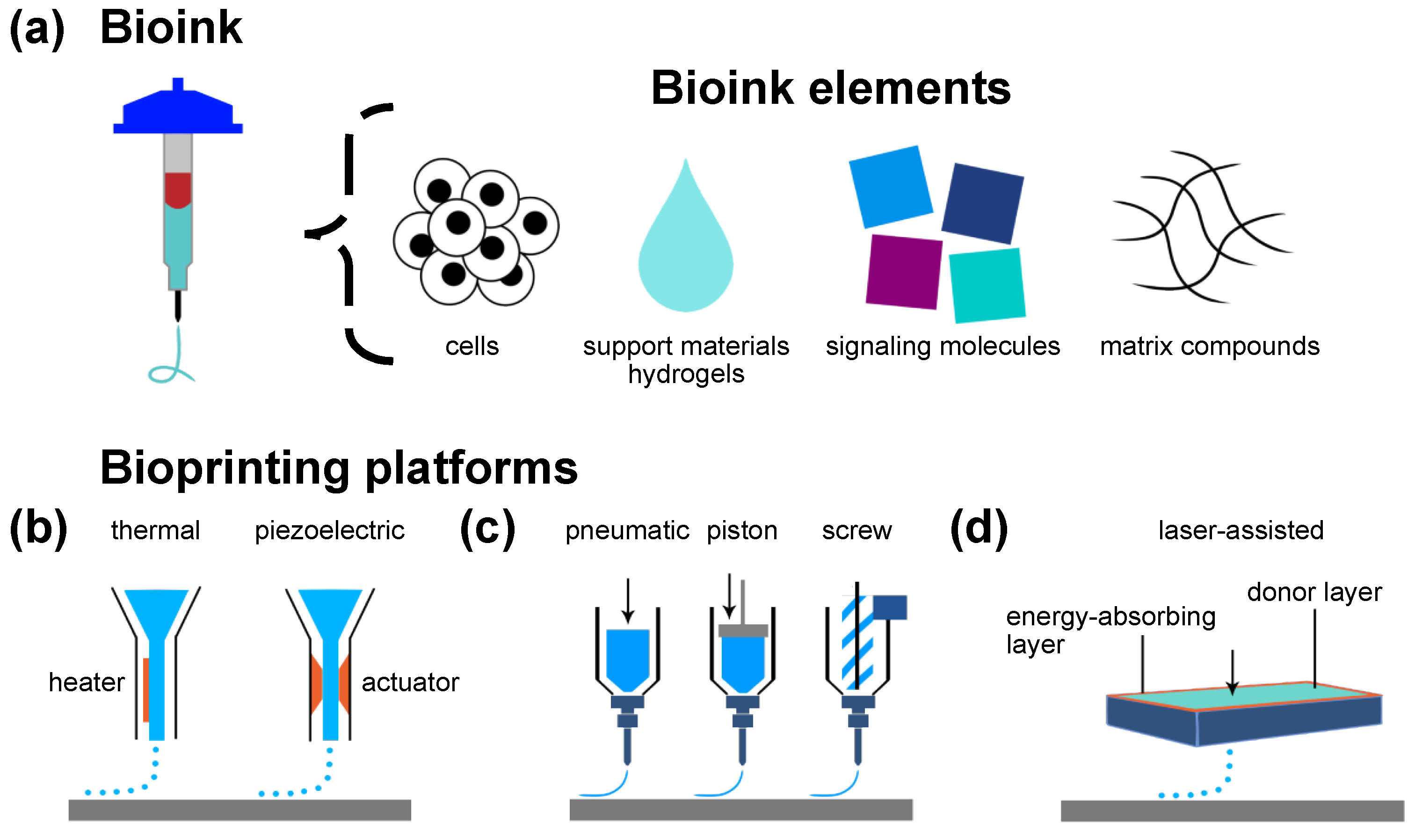
3. Bioprinting Technology: A Brief Overview
3.1. Jet-Based Drop-on-Demand Bioprinting Inspired by Inkjet Printing
3.2. Microextrusion-Based Bioprinting Inspired by Fused-Deposition Modeling
3.3. Laser-Assisted Bioprinting
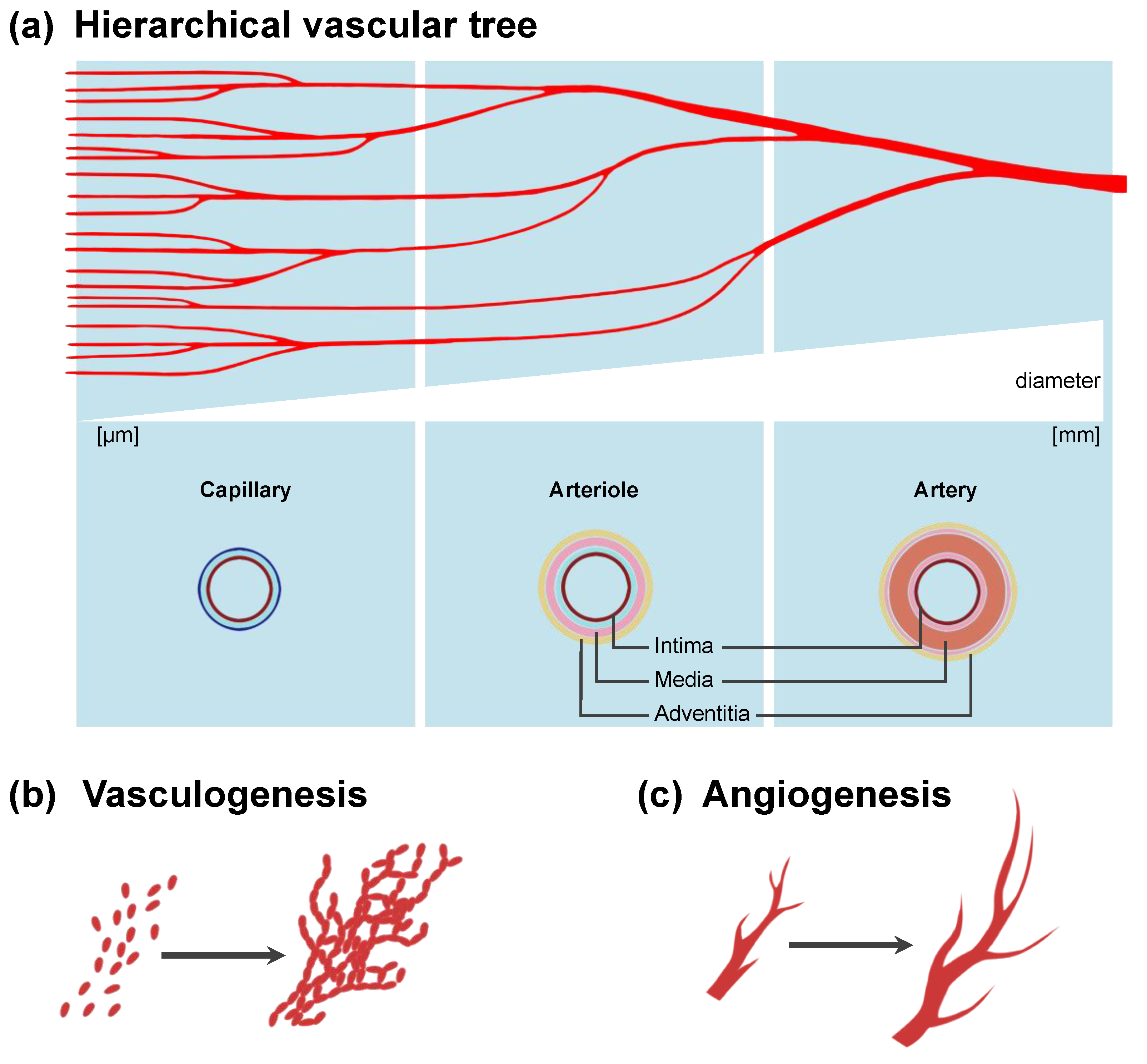
4. Vascularization in Bioprinted Tissue: Different Approaches to Macro- and Microvascularization
5. Strategies before 3D Bioprinting: Material Properties and Bioink Formulation Can Alter Vascularization Processes
5.1. Physical Properties of Bioink and Geometry of Construct Can Positively Influence Vascularization
5.2. Biochemical Properties of Bioink Can Positively Influence Vascularization
5.3. Cellular Composition in Bioink Can Positively Influence Vascularization
| Physical properties and geometry | Porosity | |
| High porosity and pore interconnectivity increases mass transfer | [4,23,47] | |
| Pore size | ||
| Larger pore size leads to increased neovascularization and accelerated vascularization process | [48] | |
| Architecture (in silico modeling) | ||
| Adaption of model to boundary conditions such as maximum diffusion distance of nutrient flow might enable uniform perfusion | [50,51,52] | |
| Biochemical properties | Structural binding motifs | |
| RGD motifs promote endothelial cell adherence | [42,47] | |
| Motifs can be offered naturally or added to bioink formulations | [42,55,58,61,62,63] | |
| Hydrogel concentration | ||
| Lower collagen, Matrigel® and gelatin concentrations enable enhanced proliferation | [64] | |
| Proangiogenic signaling molecules | ||
| VEGF addition causes dose-dependent increase in vessel formation | [23,69] | |
| Oxygen-producing bioinks | ||
| Critical oxygen-supply before self-assembly of microvasculature OxySite® can address locally insufficient oxygenation | [52,73,74] | |
| Cellular composition | Endothelial cell coating | [18] |
| Diminished thrombogenicity | ||
| Organ-specific endothelial cell sources | [77,88,89] | |
| More natural microenvironment | [21,42,90] | |
| Human induced pluripotent stem cells (hiPSCs) | [46,91,96,97,98] | |
| Autologous cell source for patient-specific tissue engineering | [46,98] | |
| Pericytes, smooth muscle cells and fibroblasts | ||
| Endothelial-stabilizing cells for co-culture | [33,42,60,63,89,99] | |
| Growth factor secretion and cell–cell interactions promote vascularization | ||
| Mesenchymal stem cells (MSCs) | ||
| VEGF secretion promotes functional vascularization | [17,39,42,58,59,101] | |
| Differentiation into smooth muscle cells to resemble a natural cellular environment | [42,84,100] |
6. Strategies during 3D Bioprinting: Modifications in Material Deposition Enable Fabrication of Vessel-like Networks
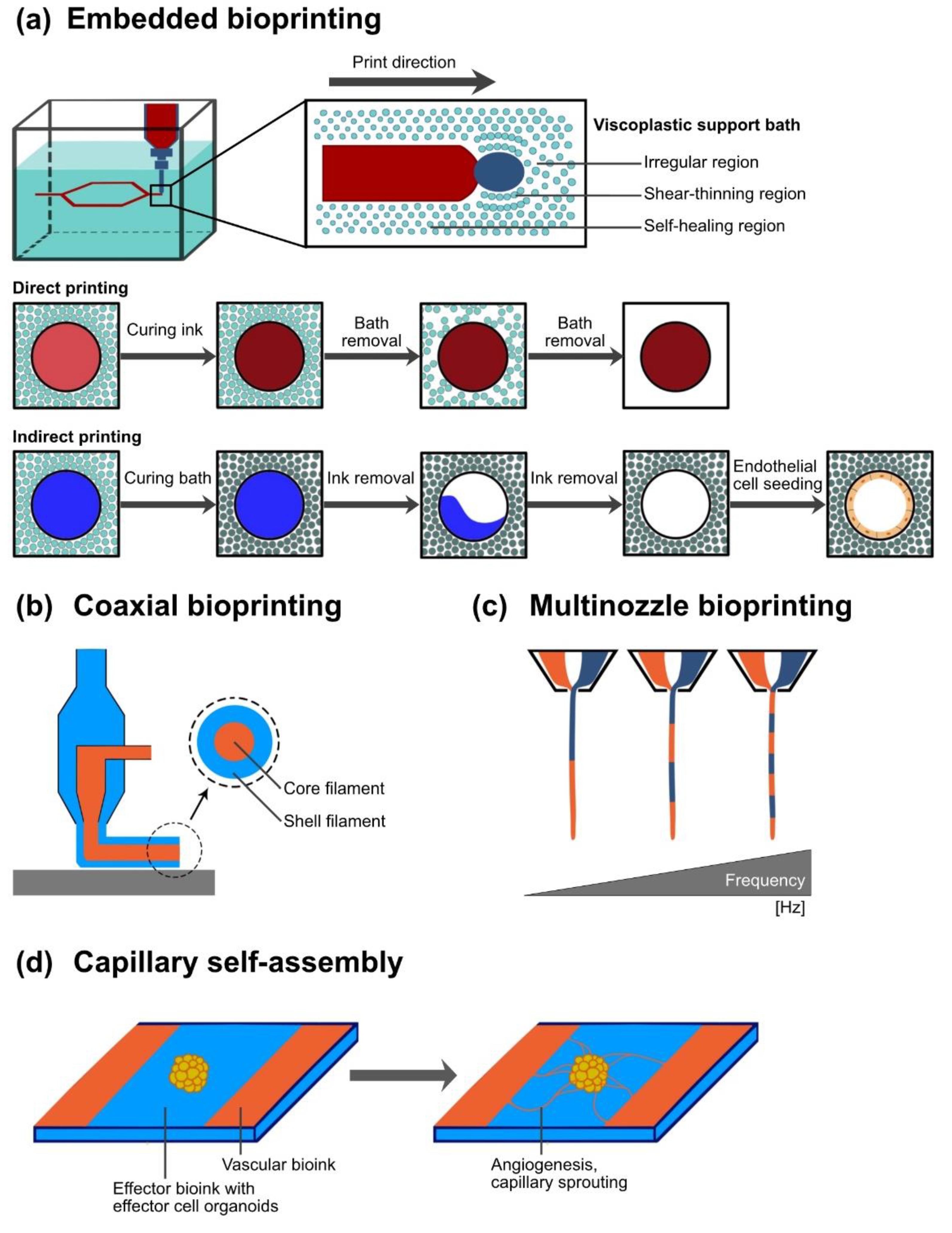
6.1. Application of Sacrificial Material and Sacrificial Writing into Functional Tissue for Vascular Network Integration
6.2. Coaxial Printing Technology for Vessel-Like Structure Fabrication
| Explanation | Advantages | Disadvantages | Example Materials | ||
|---|---|---|---|---|---|
| Sacrificial bioprinting | Deposition of a material, that can be removed in a subsequent stage | Easy removal of sacrificial material High degree of geometrical freedom | Printed structure prone to drying out Limited to sacrificial materials removable under cytocompatible conditions | Natural and synthetic hydrogels: gelatin, | [14,22,27,39,47,63,83] |
| agarose, | [108] | ||||
| and alginate | [87,102,103] | ||||
| Pluronic® 127 | [68,85,86,104,105,106,107] | ||||
| Thermoplastics: PVA | [109] | ||||
| Carbohydrate mixtures | [43,88] | ||||
| Sacrificial Writing | Extrusion of sacrificial material into functional tissue or merged organoids, respectively | High cell density, exceeding capability of microextrusion bioprinting Native ECM secreted by organoids | Limited resolution of vessel diameter (400 µm) | Sacrificial gelatin | [83] |
| Submerged bioprinting | Bioinks containing cells are 3D-printed into a support bath (e.g., high-density liquids and hydrogel slurries) | Placement into support bath prevents printed structure from drying Mechanical support for shape fidelity and geometrical freedom | Large volume of surrounding matrix necessary Postprocessing (washing) can damage fine structures | Perfluorocarbon Hydrogel slurry: | [116] |
| gelatin → FRESH | [71] | ||||
| agarose → CLASS | [124] | ||||
| Coaxial bioprinting | Simultaneous printing of at least two materials by same cantilever axis | Direct printing of vessel-like structures with core and shell Printing of layered vessel wall | Challenges in printing branched structures | Sacrificial materials Ionic crosslinking agents | [68] [16,32,84,120] |
7. Adjuvant Strategies for Vascularization
7.1. Prevascularization of Transplantation Site to Accelerate Graft Function
7.2. Generation of a Bioartificial Vascular Tree
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, G.; Berni, M.; Cassiolas, G.; Vivarelli, L.; Lopomo, N.F.; Fini, M.; Dallari, D.; Govoni, M. Extra-Corporeal Membrane Oxygenation Cadaver Donors: What about Tissues Used as Allografts? Membranes 2021, 11, 545. [Google Scholar] [CrossRef]
- Ortega-Deballon, I.; Hornby, L.; Shemie, S.D. Protocols for uncontrolled donation after circulatory death: A systematic review of international guidelines, practices and transplant outcomes. Crit. Care 2015, 19, 268. [Google Scholar] [CrossRef] [Green Version]
- Salg, G.A.; Giese, N.A.; Schenk, M.; Huttner, F.J.; Felix, K.; Probst, P.; Diener, M.K.; Hackert, T.; Kenngott, H.G. The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J. Tissue Eng. 2019, 10, 2041731419884708. [Google Scholar] [CrossRef]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two Decades of Global Progress in Authorized Advanced Therapy Medicinal Products: An Emerging Revolution in Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef] [PubMed]
- Oberweis, C.V.; Marchal, J.A.; Lopez-Ruiz, E.; Galvez-Martin, P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B Rev. 2020, 26, 181–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, M.P.; Budharaju, H.; Zennifer, A.; Sethuraman, S.; Vermeulen, N.; Sundaramurthi, D.; Kalaskar, D.M. Current standards and ethical landscape of engineered tissues-3D bioprinting perspective. J. Tissue Eng. 2021, 12, 20417314211027677. [Google Scholar] [CrossRef] [PubMed]
- Armoiry, X.; Cummins, E.; Connock, M.; Metcalfe, A.; Royle, P.; Johnston, R.; Rodrigues, J.; Waugh, N.; Mistry, H. Autologous Chondrocyte Implantation with Chondrosphere for Treating Articular Cartilage Defects in the Knee: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics 2019, 37, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, J.L.; Remmers, A.E.; Flanigan, D.C. Use of MACI (Autologous Cultured Chondrocytes on Porcine Collagen Membrane) in the United States: Preliminary Experience. Orthop. J. Sports Med. 2020, 8, 2325967120941816. [Google Scholar] [CrossRef]
- Tilkin, C.; Duchesne, B.; Camby, S. Holoclar(R), an autologous stem cells graft for sight recovery after ocular burns. Rev. Med. De Liege 2021, 76, 776–782. [Google Scholar]
- Pellegrini, G.; Ardigo, D.; Milazzo, G.; Iotti, G.; Guatelli, P.; Pelosi, D.; De Luca, M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl. Med. 2018, 7, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagli, G.; Akbarov, I.; Heidenreich, A.; Zugor, V.; Olianas, R.; Aragona, M.; Romano, G.; Balsmeyer, U.; Fahlenkamp, D.; Rebmann, U.; et al. Anterior Urethroplasty Using a New Tissue Engineered Oral Mucosa Graft: Surgical Techniques and Outcomes. J. Urol. 2018, 200, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Schoneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.C.; Kiessling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef] [Green Version]
- Sekine, H.; Okano, T. Capillary Networks for Bio-Artificial Three-Dimensional Tissues Fabricated Using Cell Sheet Based Tissue Engineering. Int. J. Mol. Sci. 2020, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef] [PubMed]
- De Moor, L.; Smet, J.; Plovyt, M.; Bekaert, B.; Vercruysse, C.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Engineering microvasculature by 3D bioprinting of prevascularized spheroids in photo-crosslinkable gelatin. Biofabrication 2021, 13, 045021. [Google Scholar] [CrossRef]
- McGuigan, A.P.; Sefton, M.V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl. Acad. Sci. USA 2006, 103, 11461–11466. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Barrs, R.W.; Jia, J.; Silver, S.E.; Yost, M.; Mei, Y. Biomaterials for Bioprinting Microvasculature. Chem. Rev. 2020, 120, 10887–10949. [Google Scholar] [CrossRef] [PubMed]
- Brumm, P.; Fritschen, A.; Doss, L.; Dorsam, E.; Blaeser, A. Fabrication of biomimetic networks using viscous fingering in flexographic printing. Biomed. Mater. 2022, 17, 045012. [Google Scholar] [CrossRef] [PubMed]
- Accolla, R.P.; Simmons, A.M.; Stabler, C.L. Integrating Additive Manufacturing (AM) Techniques to Improve Cell-Based Implants for the Treatment of Type 1 Diabetes. Adv. Healthc. Mater. 2022, 11, e2200243. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Kincaid, H.; Atala, A.; Yoo, J.J. High-Throughput Production of Single-Cell Microparticles Using an Inkjet Printing Technology. J. Manuf. Sci. Eng. 2008, 130, 021017. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.S.; Vincent, P.A.; Dai, G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [CrossRef] [PubMed]
- Lovecchio, J.; Cortesi, M.; Zani, M.; Govoni, M.; Dallari, D.; Giordano, E. Fiber Thickness and Porosity Control in a Biopolymer Scaffold 3D Printed through a Converted Commercial FDM Device. Materials 2022, 15, 2394. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, K.; Danalatos, R.I.; Champeris Tsaniras, S.; Kaplani, K.; Lokka, G.; Kanellou, A.; Papachristou, D.J.; Bokias, G.; Lygerou, Z.; Taraviras, S. A Custom Ultra-Low-Cost 3D Bioprinter Supports Cell Growth and Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 580889. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, e1902026. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2017, 29, 1604983. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Chichkov, B. Capillary-like Formations of Endothelial Cells in Defined Patterns Generated by Laser Bioprinting. Micromachines 2021, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Niklason, L.E.; Lawson, J.H. Bioengineered human blood vessels. Science 2020, 370, eaaw8682. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Ramos, R.; Alexis Chando, P.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef]
- Lawson, J.H.; Glickman, M.H.; Ilzecki, M.; Jakimowicz, T.; Jaroszynski, A.; Peden, E.K.; Pilgrim, A.J.; Prichard, H.L.; Guziewicz, M.; Przywara, S.; et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: Two phase 2 single-arm trials. Lancet 2016, 387, 2026–2034. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, X.; Zhang, L.; Sun, L.; Wang, H.; Zhao, H.; Zhang, Z.; Liu, W.; Huang, Y.; Ji, S.; et al. 3D Liver Tissue Model with Branched Vascular Networks by Multimaterial Bioprinting. Adv. Healthc. Mater. 2021, 10, e2101405. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Blaeser, A.; Duarte Campos, D.F.; Fischer, H. 3D bioprinting of cell-laden hydrogels for advanced tissue engineering. Curr. Opin. Biomed. Eng. 2017, 2, 58–66. [Google Scholar] [CrossRef]
- Fritschen, A.; Blaeser, A. Biosynthetic, biomimetic, and self-assembled vascularized Organ-on-a-Chip systems. Biomaterials 2021, 268, 120556. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Dogan, L.; Scheuring, R.; Wagner, N.; Ueda, Y.; Schmidt, S.; Worsdorfer, P.; Groll, J.; Ergun, S. Human iPSC-derived mesodermal progenitor cells preserve their vasculogenesis potential after extrusion and form hierarchically organized blood vessels. Biofabrication 2021, 13, 045028. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Nico, B.; Roncali, L.; Dammacco, F. Postnatal vasculogenesis. Mech. Dev. 2001, 100, 157–163. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.H.; Highley, C.B.; Rouff, A.; Burdick, J.A. Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 2018, 28, 1801331. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Cheng, M.H.; Engel, H.; Kao, S.W.; Larson, J.C.; Gupta, S.; Brey, E.M. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: A quantitative study. Tissue Eng. Part A 2010, 16, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Salg, G.A.; Poisel, E.; Neulinger-Munoz, M.; Gerhardus, J.; Cebulla, D.; Bludszuweit-Philipp, C.; Vieira, V.; Nickel, F.; Herr, I.; Blaeser, A.; et al. Toward 3D-bioprinting of an endocrine pancreas: A building-block concept for bioartificial insulin-secreting tissue. J. Tissue Eng. 2022, 13, 20417314221091033. [Google Scholar] [CrossRef]
- Han, E.X.; Wang, J.; Kural, M.; Jiang, B.; Leiby, K.L.; Chowdhury, N.; Tellides, G.; Kibbey, R.G.; Lawson, J.H.; Niklason, L.E. Development of a Bioartificial Vascular Pancreas. J. Tissue Eng. 2021, 12, 20417314211027714. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, E.; Coronel, M.M.; Fraker, C.A.; Ricordi, C.; Stabler, C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA 2012, 109, 4245–4250. [Google Scholar] [CrossRef] [Green Version]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the retention of tissue niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, H.W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Soliman, B.G.; Major, G.S.; Atienza-Roca, P.; Murphy, C.A.; Longoni, A.; Alcala-Orozco, C.R.; Rnjak-Kovacina, J.; Gawlitta, D.; Woodfield, T.B.F.; Lim, K.S. Development and Characterization of Gelatin-Norbornene Bioink to Understand the Interplay between Physical Architecture and Micro-Capillary Formation in Biofabricated Vascularized Constructs. Adv. Healthc. Mater. 2022, 11, e2101873. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Y.; Chen, T.H.; Kao, F.S.; Hsu, Y.J.; Chen, Y.C. Strategy for improving cell-mediated vascularized soft tissue formation in a hydrogen peroxide-triggered chemically-crosslinked hydrogel. J. Tissue Eng. 2022, 13, 20417314221084096. [Google Scholar] [CrossRef] [PubMed]
- Stratesteffen, H.; Kopf, M.; Kreimendahl, F.; Blaeser, A.; Jockenhoevel, S.; Fischer, H. GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication 2017, 9, 045002. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.H.; Sriram, G.; Cao, T. 3D bioprinting and microscale organization of vascularized tissue constructs using collagen-based bioink. Biotechnol. Bioeng. 2021, 118, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Saik, J.E.; Poche, R.A.; Leslie-Barbick, J.E.; Lee, S.H.; Smith, A.A.; Dickinson, M.E.; West, J.L. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 2010, 31, 3840–3847. [Google Scholar] [CrossRef] [Green Version]
- Son, J.; Hong, S.J.; Lim, J.W.; Jeong, W.; Jeong, J.H.; Kang, H.W. Engineering Tissue-Specific, Multiscale Microvasculature with a Capillary Network for Prevascularized Tissue. Small Methods 2021, 5, e2100632. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Gutzweiler, L.; Trondle, K.; Riba, J.; Zengerle, R.; Koltay, P.; Zimmermann, S.; Stark, G.B.; Finkenzeller, G. Assessment of hydrogels for bioprinting of endothelial cells. J. Biomed. Mater. Res. A 2018, 106, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, N.; Sudo, R.; Ikeda, M.; Tanishita, K. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng. 2007, 13, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial Cell Printing of Freestanding, Perfusable, and Functional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater. 2018, 7, e1801102. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Ballerini, A.; Fraga, D.W.; Nicolov, E.; Hogan, M.; Demarchi, D.; Scaglione, F.; Sabek, O.M.; Horner, P.; Thekkedath, U.; et al. 3D Printed Vascularized Device for Subcutaneous Transplantation of Human Islets. Biotechnol. J. 2017, 12, 1700169. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Headen, D.M.; Hunckler, M.D.; Coronel, M.M.; Stabler, C.L.; Garcia, A.J. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018, 172, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- van Duinen, V.; Zhu, D.; Ramakers, C.; van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronel, M.M.; Geusz, R.; Stabler, C.L. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 2017, 129, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Coronel, M.M.; Liang, J.P.; Li, Y.; Stabler, C.L. Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials 2019, 210, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.P.; Accolla, R.P.; Soundirarajan, M.; Emerson, A.; Coronel, M.M.; Stabler, C.L. Engineering a macroporous oxygen-generating scaffold for enhancing islet cell transplantation within an extrahepatic site. Acta Biomater. 2021, 130, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to cell-hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Zhang, S.; Kreimendahl, F.; Kopf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef]
- Zhang, T.; Day, J.H.; Su, X.; Guadarrama, A.G.; Sandbo, N.K.; Esnault, S.; Denlinger, L.C.; Berthier, E.; Theberge, A.B. Investigating Fibroblast-Induced Collagen Gel Contraction Using a Dynamic Microscale Platform. Front. Bioeng. Biotechnol. 2019, 7, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenz, A.; Tjoeng, I.; Schneider, I.; Kluger, P.J.; Borchers, K. Improved vasculogenesis and bone matrix formation through coculture of endothelial cells and stem cells in tissue-specific methacryloyl gelatin-based hydrogels. Biotechnol. Bioeng. 2018, 115, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.; Welch, Z.; Kovala, A.T.; Garcia, J.G.; English, D. Comparative analysis of in vitro angiogenic activities of endothelial cells of heterogeneous origin. Microvasc. Res. 2002, 63, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Uzel, S.G.M.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch-Rue, E.; Diez-Tercero, L.; Delgado, L.M.; Perez, R.A. Biofabrication of Collagen Tissue-Engineered Blood Vessels with Direct Co-Axial Extrusion. Int. J. Mol. Sci. 2022, 23, 5618. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Rue, E.; Delgado, L.M.; Gil, F.J.; Perez, R.A. Direct extrusion of individually encapsulated endothelial and smooth muscle cells mimicking blood vessel structures and vascular native cell alignment. Biofabrication 2020, 13, 015003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Shen, Y.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X.; Tang, B. Direct writing alginate bioink inside pre-polymers of hydrogels to create patterned vascular networks. J. Mater. Sci. 2019, 54, 7883–7892. [Google Scholar] [CrossRef]
- Mirabella, T.; MacArthur, J.W.; Cheng, D.; Ozaki, C.K.; Woo, Y.J.; Yang, M.; Chen, C.S. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng. 2017, 1, 0083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.K.; Ringeisen, B.R. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP). Biofabrication 2010, 2, 014111. [Google Scholar] [CrossRef]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef] [PubMed]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Lin, N.Y.C.; Homan, K.A.; Robinson, S.S.; Kolesky, D.B.; Duarte, N.; Moisan, A.; Lewis, J.A. Renal reabsorption in 3D vascularized proximal tubule models. Proc. Natl. Acad. Sci. USA 2019, 116, 5399–5404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.H.; Lin, R.Z.; Melero-Martin, J.M.; Chen, Y.C. Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue. Artif. Cells Nanomed. Biotechnol. 2018, 46, S434–S447. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.B.; Christoforou, N.; Leong, K.W.; Truskey, G.A.; West, J.L. Poly(ethylene glycol) Hydrogel Scaffolds Containing Cell-Adhesive and Protease-Sensitive Peptides Support Microvessel Formation by Endothelial Progenitor Cells. Cell. Mol. Bioeng. 2016, 9, 38–54. [Google Scholar] [CrossRef] [Green Version]
- Szepes, M.; Melchert, A.; Dahlmann, J.; Hegermann, J.; Werlein, C.; Jonigk, D.; Haverich, A.; Martin, U.; Olmer, R.; Gruh, I. Dual Function of iPSC-Derived Pericyte-Like Cells in Vascularization and Fibrosis-Related Cardiac Tissue Remodeling In Vitro. Int. J. Mol. Sci. 2020, 21, 8947. [Google Scholar] [CrossRef] [PubMed]
- Sances, S.; Ho, R.; Vatine, G.; West, D.; Laperle, A.; Meyer, A.; Godoy, M.; Kay, P.S.; Mandefro, B.; Hatata, S.; et al. Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Stem Cell Rep. 2018, 10, 1222–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosyakova, N.; Kao, D.D.; Figetakis, M.; Lopez-Giraldez, F.; Spindler, S.; Graham, M.; James, K.J.; Won Shin, J.; Liu, X.; Tietjen, G.T.; et al. Differential functional roles of fibroblasts and pericytes in the formation of tissue-engineered microvascular networks in vitro. NPJ Regen. Med. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Mayer, H.; Bertram, H.; Lindenmaier, W.; Korff, T.; Weber, H.; Weich, H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: Autocrine and paracrine role on osteoblastic and endothelial differentiation. J. Cell Biochem. 2005, 95, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Dew, L.; Kelly, A.G.; Chong, C.K.; MacNeil, S.; Claeyssens, F. Fabrication of biodegradable synthetic perfusable vascular networks via a combination of electrospinning and robocasting. Biomater. Sci. 2015, 3, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Contessi Negrini, N.; Bonnetier, M.; Giatsidis, G.; Orgill, D.P.; Fare, S.; Marelli, B. Tissue-mimicking gelatin scaffolds by alginate sacrificial templates for adipose tissue engineering. Acta Biomater. 2019, 87, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [Green Version]
- Forget, A.; Derme, T.; Mitterberger, D.; Heiny, M.; Sweeney, C.; Mudili, L.; Dargaville, T.R.; Shastri, V.P. Architecture-inspired paradigm for 3D bioprinting of vessel-like structures using extrudable carboxylated agarose hydrogels. Emergent Mater. 2019, 2, 233–243. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, Y.Y.; Liu, L.J.; Hu, Q.X. A Versatile Method for Fabricating Tissue Engineering Scaffolds with a Three-Dimensional Channel for Prevasculature Networks. ACS Appl. Mater. Interfaces 2016, 8, 25096–25103. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Zhianmanesh, M.; Abbasgholizadeh, R.; Haghniaz, R.; Baidya, A.; Pourmohammadali, H.; Annabi, N.; Weiss, P.S.; Toyserkani, E.; et al. Template-Enabled Biofabrication of Thick 3D Tissues with Patterned Perfusable Macrochannels. Adv. Healthc. Mater. 2022, 11, e2102123. [Google Scholar] [CrossRef] [PubMed]
- Millik, S.C.; Dostie, A.M.; Karis, D.G.; Smith, P.T.; McKenna, M.; Chan, N.; Curtis, C.D.; Nance, E.; Theberge, A.B.; Nelson, A. 3D printed coaxial nozzles for the extrusion of hydrogel tubes toward modeling vascular endothelium. Biofabrication 2019, 11, 045009. [Google Scholar] [CrossRef]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From arteries to capillaries: Approaches to engineering human vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [Green Version]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Weber, M.; Neuss, S.; Theek, B.; Fischer, H.; Jahnen-Dechent, W. Biofabrication under fluorocarbon: A novel freeform fabrication technique to generate high aspect ratio tissue-engineered constructs. BioResearch Open Access 2013, 2, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Paszkowiak, J.J.; Dardik, A. Arterial wall shear stress: Observations from the bench to the bedside. Vasc. Endovasc. Surg. 2003, 37, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Carter, S.D.; Renes, M.J.; Kim, J.; Rojas-Canales, D.M.; Penko, D.; Angus, C.; Beirne, S.; Drogemuller, C.J.; Yue, Z.; et al. Development of a Coaxial 3D Printing Platform for Biofabrication of Implantable Islet-Containing Constructs. Adv. Healthc. Mater. 2019, 8, e1801181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 025004. [Google Scholar] [CrossRef] [PubMed]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B.; van Genderen, A.M.; Robledo-Padilla, F.; Parra-Saldivar, R.; Hu, N.; Jia, W.; et al. Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues. Adv. Mater. 2018, 30, e1706913. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Mueller, J.; Visser, C.W.; Lewis, J.A. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 2019, 575, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.C.; Trujillo-de Santiago, G.; et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv. Mater. 2017, 29, 1604630. [Google Scholar] [CrossRef]
- Mirdamadi, E.; Muselimyan, N.; Koti, P.; Asfour, H.; Sarvazyan, N. Agarose Slurry as a Support Medium for Bioprinting and Culturing Freestanding Cell-Laden Hydrogel Constructs. 3d Print. Addit. Manuf. 2019, 6, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Chua, C.Y.X.; Ballerini, A.; Thekkedath, U.; Alexander, J.F.; Rhudy, J.R.; Torchio, G.; Fraga, D.; Pathak, R.R.; Villanueva, M.; et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials 2018, 177, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Smink, A.M.; Li, S.; Hertsig, D.T.; de Haan, B.J.; Schwab, L.; van Apeldoorn, A.A.; de Koning, E.; Faas, M.M.; Lakey, J.R.; de Vos, P. The Efficacy of a Prevascularized, Retrievable Poly(D,L,-lactide-co-epsilon-caprolactone) Subcutaneous Scaffold as Transplantation Site for Pancreatic Islets. Transplantation 2017, 101, e112. [Google Scholar] [CrossRef]
- Laschke, M.W.; Rucker, M.; Jensen, G.; Carvalho, C.; Mulhaupt, R.; Gellrich, N.C.; Menger, M.D. Improvement of vascularization of PLGA scaffolds by inosculation of in situ-preformed functional blood vessels with the host microvasculature. Ann. Surg. 2008, 248, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.M.; Memarnejadian, A.; Boyce, G.K.; Nguyen, A.; Walker, B.A.; Holmes, D.T.; Welch, I.D.; Mazzuca, D.M.; Toleikis, P.M. Subcutaneous transplantation of human thyroid tissue into a pre-vascularized Cell Pouch device in a Mus musculus model: Evidence of viability and function for thyroid transplantation. PLoS ONE 2022, 17, e0262345. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Pawlick, R.; Gala-Lopez, B.; MacGillivary, A.; Mazzuca, D.M.; White, D.J.; Toleikis, P.M.; Shapiro, A.M. Diabetes Is Reversed in a Murine Model by Marginal Mass Syngeneic Islet Transplantation Using a Subcutaneous Cell Pouch Device. Transplantation 2015, 99, 2294–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepper, A.R.; Pawlick, R.; Bruni, A.; Wink, J.; Rafiei, Y.; O’Gorman, D.; Yan-Do, R.; Gala-Lopez, B.; Kin, T.; MacDonald, P.E.; et al. Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes Post Transplantation in a Prevascularized Subcutaneous Site. Stem Cell Rep. 2017, 8, 1689–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, E.; Steinbach, M.C.; Debbaut, C.; Schillinger, D. Rigorous mathematical optimization of synthetic hepatic vascular trees. J. R Soc. Interface 2022, 19, 20220087. [Google Scholar] [CrossRef] [PubMed]
- Brassard, J.A.; Nikolaev, M.; Hubscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 16997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govoni, M.; Muscari, C.; Guarnieri, C.; Giordano, E. Mechanostimulation protocols for cardiac tissue engineering. Biomed. Res. Int. 2013, 2013, 918640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahi, S.; Davis, A.; Miller, J.H.; Feinberg, A.W. Expert-guided optimization for 3D printing of soft and liquid materials. PLoS ONE 2018, 13, e0194890. [Google Scholar] [CrossRef]
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Li, Z.; Hu, J.; Park, S.S.; Kim, K. Optimized 3D Bioprinting Technology Based on Machine Learning: A Review of Recent Trends and Advances. Micromachines 2022, 13, 363. [Google Scholar] [CrossRef]
- Fu, Z.; Angeline, V.; Sun, W. Evaluation of Printing Parameters on 3D Extrusion Printing of Pluronic Hydrogels and Machine Learning Guided Parameter Recommendation. Int. J. Bioprinting 2021, 7, 434. [Google Scholar] [CrossRef]
- Tian, S.; Stevens, R.; McInnes, B.T.; Lewinski, N.A. Machine Assisted Experimentation of Extrusion-Based Bioprinting Systems. Micromachines 2021, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chua, C.K.; Mironov, V. Application of Machine Learning in 3D Bioprinting: Focus on Development of Big Data and Digital Twin. Int. J. Bioprinting 2021, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Bone, J.M.; Childs, C.M.; Menon, A.; Poczos, B.; Feinberg, A.W.; LeDuc, P.R.; Washburn, N.R. Hierarchical Machine Learning for High-Fidelity 3D Printed Biopolymers. ACS Biomater. Sci. Eng. 2020, 6, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McKee, J.A.; Fontenot, J.J.; Jung, J.P. Engineering Tissue Fabrication With Machine Intelligence: Generating a Blueprint for Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 443. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salg, G.A.; Blaeser, A.; Gerhardus, J.S.; Hackert, T.; Kenngott, H.G. Vascularization in Bioartificial Parenchymal Tissue: Bioink and Bioprinting Strategies. Int. J. Mol. Sci. 2022, 23, 8589. https://doi.org/10.3390/ijms23158589
Salg GA, Blaeser A, Gerhardus JS, Hackert T, Kenngott HG. Vascularization in Bioartificial Parenchymal Tissue: Bioink and Bioprinting Strategies. International Journal of Molecular Sciences. 2022; 23(15):8589. https://doi.org/10.3390/ijms23158589
Chicago/Turabian StyleSalg, Gabriel Alexander, Andreas Blaeser, Jamina Sofie Gerhardus, Thilo Hackert, and Hannes Goetz Kenngott. 2022. "Vascularization in Bioartificial Parenchymal Tissue: Bioink and Bioprinting Strategies" International Journal of Molecular Sciences 23, no. 15: 8589. https://doi.org/10.3390/ijms23158589
APA StyleSalg, G. A., Blaeser, A., Gerhardus, J. S., Hackert, T., & Kenngott, H. G. (2022). Vascularization in Bioartificial Parenchymal Tissue: Bioink and Bioprinting Strategies. International Journal of Molecular Sciences, 23(15), 8589. https://doi.org/10.3390/ijms23158589








