Circular RNAs in Acute Kidney Injury: Roles in Pathophysiology and Implications for Clinical Management
Abstract
1. Introduction
2. CircRNAs in Health and in Kidney Disease
3. CircRNA Expression in AKI Due to Sepsis
4. CircRNA Expression in AKI Due to Ischaemia-Reperfusion Injury
5. CircRNA Expression in Drug-Induced AKI
6. CircRNA Expression in AKI Due to Urinary Tract Obstruction
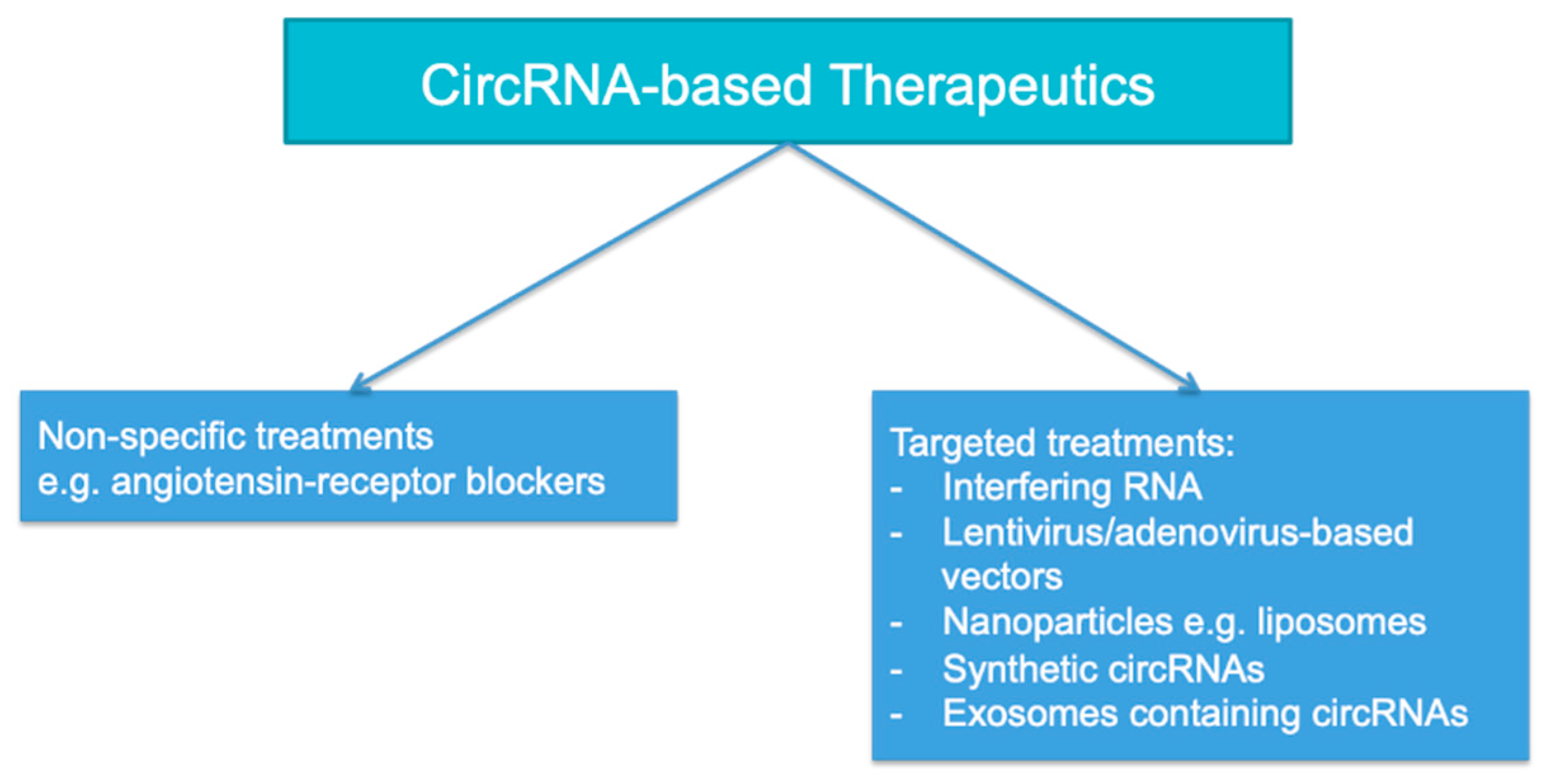
7. Potential Clinical Applications of circRNAs in AKI
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Brimhall, B.B.; Lezotte, D.C.; Glazner, J.E.; Parikh, C.R. Uncomplicated acute renal failure and hospital resource utilization: A retrospective multicenter analysis. Am. J. Kidney Dis. 2005, 46, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Clermont, G.; Kersten, A.; Venkataraman, R.; Angus, D.C.; De Bacquer, D.; Kellum, J.A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit. Care 2006, 10, R73. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Lee, B.J.; Hsu, C.Y.; Parikh, R.V.; Leong, T.K.; Tan, T.C.; Walia, S.; Liu, K.D.; Hsu, R.K.; Go, A.S. Non-recovery from dialysis-requiring acute kidney injury and short-term mortality and cardiovascular risk: A cohort study. BMC Nephrol. 2018, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin. Nephrol. 2020, 40, 206–215. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef]
- Lee-Son, K.; Jetton, J.G. AKI and Genetics: Evolving Concepts in the Genetics of Acute Kidney Injury: Implications for Pediatric AKI. J. Pediatr. Genet. 2016, 5, 61–68. [Google Scholar] [CrossRef][Green Version]
- Ledeganck, K.J.; Gielis, E.M.; Abramowicz, D.; Stenvinkel, P.; Shiels, P.G.; Van Craenenbroeck, A.H. MicroRNAs in AKI and Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. Non-coding RNAs in kidney injury and repair. Am. J. Physiol. Cell Physiol. 2019, 317, C177–C188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shan, G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021, 505, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lodde, V.; Murgia, G.; Simula, E.R.; Steri, M.; Floris, M.; Idda, M.L. Long Noncoding RNAs and Circular RNAs in Autoimmune Diseases. Biomolecules 2020, 10, 1044. [Google Scholar] [CrossRef]
- Ruffo, P.; Strafella, C.; Cascella, R.; Caputo, V.; Conforti, F.L.; Andò, S.; Giardina, E. Deregulation of ncRNA in Neurodegenerative Disease: Focus on circRNA, lncRNA and miRNA in Amyotrophic Lateral Sclerosis. Front. Genet. 2021, 12, 784996. [Google Scholar] [CrossRef] [PubMed]
- Verduci, L.; Tarcitano, E.; Strano, S.; Yarden, Y.; Blandino, G. CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 2021, 12, 468. [Google Scholar] [CrossRef]
- Wang, P.L.; Bao, Y.; Yee, M.C.; Barrett, S.P.; Hogan, G.J.; Olsen, M.N.; Dinneny, J.R.; Brown, P.O.; Salzman, J. Circular RNA is expressed across the eukaryotic tree of life. PLoS ONE 2014, 9, e90859. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Zhang, C.; Yang, W.; Yong, T.; Awan, F.M.; Yang, B.B. Identifying and Characterizing circRNA-Protein Interaction. Theranostics 2017, 7, 4183–4191. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Enuka, Y.; Lauriola, M.; Feldman, M.E.; Sas-Chen, A.; Ulitsky, I.; Yarden, Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016, 44, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Sun, H.; Wang, J.; Liang, Y.; Wang, Y.; Wong, G. The bioinformatics toolbox for circRNA discovery and analysis. Brief. Bioinform. 2021, 22, 1706–1728. [Google Scholar] [CrossRef]
- Xu, T.; Wu, J.; Han, P.; Zhao, Z.; Song, X. Circular RNA expression profiles and features in human tissues: A study using RNA-seq data. BMC Genom. 2017, 18, 680. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Gong, W.; Li, S.; Yin, B.; Zhao, C.; Liu, W.; Chen, X.; Luo, C.; Huang, Q.; Chen, T.; et al. circRNA_010383 Acts as a Sponge for miR-135a, and Its Downregulated Expression Contributes to Renal Fibrosis in Diabetic Nephropathy. Diabetes 2021, 70, 603–615. [Google Scholar] [CrossRef]
- Cui, X.; Fu, J.; Luan, J.; Qi, H.; Jiao, C.; Ran, M.; Wang, D.; Hao, X.; Zhang, Y.; Kopp, J.B.; et al. CircZNF609 is involved in the pathogenesis of focal segmental glomerulosclerosis by sponging miR-615-5p. Biochem. Biophys. Res. Commun. 2020, 531, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Kölling, M.; Seeger, H.; Haddad, G.; Kistler, A.; Nowak, A.; Faulhaber-Walter, R.; Kielstein, J.; Haller, H.; Fliser, D.; Mueller, T.; et al. The Circular RNA ciRs-126 Predicts Survival in Critically Ill Patients With Acute Kidney Injury. Kidney Int. Rep. 2018, 3, 1144–1152. [Google Scholar] [CrossRef]
- Bijkerk, R.; van Solingen, C.; de Boer, H.C.; van der Pol, P.; Khairoun, M.; de Bruin, R.G.; van Oeveren-Rietdijk, A.M.; Lievers, E.; Schlagwein, N.; van Gijlswijk, D.J.; et al. Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J. Am. Soc. Nephrol. 2014, 25, 1710–1722. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Wang, P.; Zhou, X.; Liang, H.; Li, C. Knockdown of circ-FANCA alleviates LPS-induced HK2 cell injury via targeting miR-93-5p/OXSR1 axis in septic acute kidney injury. Diabetol. Metab. Syndr. 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qing, M.; Xu, M. Circ-BNIP3L knockdown alleviates LPS-induced renal tubular epithelial cell injury during sepsis-associated acute kidney injury by miR-370-3p/MYD88 axis. J. Bioenerg. Biomembr. 2021, 53, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, W.; Zhang, J.; Guan, Y.; Huang, Y.; Li, X. Mechanism of circHIPK3-miRNA-124-3p/miRNA-148b-3p-Mediated Inflammatory Responses and Cell Senescence in Candida albicans-Induced Septic Acute Kidney Injury. Gerontology 2022, 1–21. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Y. Circular RNA HIPK3 aggravates sepsis-induced acute kidney injury via modulating the microRNA-338/forkhead box A1 axis. Bioengineered 2022, 13, 4798–4809. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, H.; Xu, P.; Nie, M.; Zhao, C. Circ_0114427 promotes LPS-induced septic acute kidney injury by modulating miR-495-3p/TRAF6 through the NF-κB pathway. Autoimmunity 2022, 55, 52–64. [Google Scholar] [CrossRef]
- He, Y.; Sun, Y.; Peng, J. Circ_0114428 Regulates Sepsis-Induced Kidney Injury by Targeting the miR-495-3p/CRBN Axis. Inflammation 2021, 44, 1464–1477. [Google Scholar] [CrossRef]
- Xu, H.P.; Ma, X.Y.; Yang, C. Circular RNA TLK1 Promotes Sepsis-Associated Acute Kidney Injury by Regulating Inflammation and Oxidative Stress Through miR-106a-5p/HMGB1 Axis. Front. Mol. Biosci. 2021, 8, 660269. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Shi, D.; Zhu, L.; Song, L. Circ_RASGEF1B Promotes LPS-Induced Apoptosis and Inflammatory Response by Targeting MicroRNA-146a-5p/Pdk1 Axis in Septic Acute Kidney Injury Cell Model. Nephron 2021, 145, 748–759. [Google Scholar] [CrossRef]
- Wei, W.; Yao, Y.; Bi, H.; Xu, W.; Gao, Y. Circular RNA circ_0068,888 protects against lipopolysaccharide-induced HK-2 cell injury via sponging microRNA-21-5p. Biochem. Biophys. Res. Commun. 2021, 540, 1–7. [Google Scholar] [CrossRef]
- Tan, M.; Bei, R. Circ_0091702 serves as a sponge of miR-545-3p to attenuate sepsis-related acute kidney injury by upregulating THBS2. J. Mol. Histol. 2021, 52, 717–728. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Tian, H.; Li, Y.; Xu, Q.; He, Z. Circ-PRKCI Alleviates Lipopolysaccharide-induced Human Kidney 2 Cell Injury by Regulating miR-106b-5p/GAB1 Axis. J. Cardiovasc. Pharmacol. 2021, 78, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhu, G.; Jiao, T.; Shao, F. Effects of circular RNA Ttc3/miR-148a/Rcan2 axis on inflammation and oxidative stress in rats with acute kidney injury induced by sepsis. Life Sci. 2021, 272, 119233. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.C.; Pan, L.Y.; Peng, Z.Y.; Li, J.G. CircMTO1 Attenuated Acute Kidney Injury Through Regulating miR-337. Inflammation 2020, 43, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, C.F.; Ge, W.H.; Du, Y.P.; Hu, N.B. Circular RNA VMA21 ameliorates sepsis-associated acute kidney injury by regulating miR-9-3p/SMG1/inflammation axis and oxidative stress. J. Cell. Mol. Med. 2020, 24, 11397–11408. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Li, H.; Zhong, L.; Lin, Y.; Xie, J.; Zheng, D. Circ_0023404 sponges miR-136 to induce HK-2 cells injury triggered by hypoxia/reoxygenation via up-regulating IL-6R. J. Cell. Mol. Med. 2021, 25, 4912–4921. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, W.; Zhong, L.; Li, H.; Bai, L.; Chen, X.; Lin, Y.; Zheng, D. circ-AKT3 aggravates renal ischaemia-reperfusion injury via regulating miR-144-5p /Wnt/β-catenin pathway and oxidative stress. J. Cell. Mol. Med. 2022, 26, 1766–1775. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, W.; Guo, C.; Huang, T. GATA1 regulates the microRNA-328-3p/PIM1 axis via circular RNA ITGB1 to promote renal ischemia/reperfusion injury in HK-2 cells. Int. J. Mol. Med. 2022, 50, 100. [Google Scholar] [CrossRef]
- Hou, J.; Li, A.L.; Xiong, W.Q.; Chen, R. Hsa Circ 001839 Promoted Inflammation in Renal Ischemia-Reperfusion Injury Through NLRP3 by miR-432-3p. Nephron 2021, 145, 540–552. [Google Scholar] [CrossRef]
- Huang, T.; Gao, Y.; Cao, Y.; Wang, Q.; Dong, Z. Downregulation of mmu_circ_0000943 ameliorates renal ischemia reperfusion-triggered inflammation and oxidative stress via regulating mmu-miR-377-3p/Egr2 axis. Int. Immunopharmacol. 2022, 106, 108614. [Google Scholar] [CrossRef]
- Meng, F.; Chen, Q.; Gu, S.; Cui, R.; Ma, Q.; Cao, R.; Zhao, M. Inhibition of Circ-Snrk ameliorates apoptosis and inflammation in acute kidney injury by regulating the MAPK pathway. Ren. Fail. 2022, 44, 672–681. [Google Scholar] [CrossRef]
- Wei, L.; Yu, Z.; Liu, L.; Zhou, Y.; Bai, X.; Wang, L.; Bai, M.; Sun, S. Integrated Analysis of the CircRNA-Based ceRNA Network in Renal Fibrosis Induced by Ischemia Reperfusion Injury. Front. Genet. 2021, 12, 793182. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Lass, N.; Gaber, A.O.; Jones, J.D.; Burnett, J.C., Jr. Radiocontrast medium-induced declines in renal function: A role for oxygen free radicals. Am. J. Physiol. 1990, 258, F115–F120. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cao, Y.; Wang, H.; Wang, Q.; Ji, J.; Sun, X.; Dong, Z. Circular RNA YAP1 acts as the sponge of microRNA-21-5p to secure HK-2 cells from ischaemia/reperfusion-induced injury. J. Cell. Mol. Med. 2020, 24, 4707–4715. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, S.; Zhou, Y.; Deng, Y.; Yin, Q.; Hu, L.; Ouyang, X.; Hou, Y.; Chen, C. Circular RNA involved in the protective effect of losartan on ischemia and reperfusion induced acute kidney injury in rat model. Am. J. Transl. Res. 2019, 11, 1129–1144. [Google Scholar] [PubMed]
- Cao, Y.; Mi, X.; Zhang, D.; Wang, Z.; Zuo, Y.; Tang, W. Transcriptome sequencing of circular RNA reveals a novel circular RNA-has_circ_0114427 in the regulation of inflammation in acute kidney injury. Clin. Sci. 2020, 134, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yin, X.; Zhao, Q.; Yang, Y. Hsa_circRNA_0045861 promotes renal injury in ureteropelvic junction obstruction via the microRNA-181d-5p/sirtuin 1 signaling axis. Ann. Transl. Med. 2021, 9, 1571. [Google Scholar] [CrossRef]
- Yi, L.; Ai, K.; Li, H.; Qiu, S.; Li, Y.; Wang, Y.; Li, X.; Zheng, P.; Chen, J.; Wu, D.; et al. CircRNA_30032 promotes renal fibrosis in UUO model mice via miRNA-96-5p/HBEGF/KRAS axis. Aging 2021, 13, 12780–12799. [Google Scholar] [CrossRef]
- Fu, H.; Gu, Y.H.; Tan, J.; Yang, Y.N.; Wang, G.H. CircACTR2 in macrophages promotes renal fibrosis by activating macrophage inflammation and epithelial-mesenchymal transition of renal tubular epithelial cells. Cell. Mol. Life Sci. 2022, 79, 253. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ai, K.; Yi, L.; Liu, W.; Li, Y.; Wang, Y.; Zhang, D. The mmu_circRNA_37492/hsa_circ_0012138 function as potential ceRNA to attenuate obstructive renal fibrosis. Cell Death Dis. 2022, 13, 207. [Google Scholar] [CrossRef]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Langenberg, C.; Wan, L.; Egi, M.; May, C.N.; Bellomo, R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006, 69, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Gobe, G.; Hood, S.; May, C.N.; Bellomo, R. Renal histopathology during experimental septic acute kidney injury and recovery. Crit. Care Med. 2014, 42, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Dellepiane, S.; Marengo, M.; Cantaluppi, V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care 2016, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Post, E.H.; Kellum, J.A.; Bellomo, R.; Vincent, J.L. Renal perfusion in sepsis: From macro- to microcirculation. Kidney Int. 2017, 91, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Takasu, O.; Gaut, J.P.; Watanabe, E.; To, K.; Fagley, R.E.; Sato, B.; Jarman, S.; Efimov, I.R.; Janks, D.L.; Srivastava, A.; et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 509–517. [Google Scholar] [CrossRef]
- Verma, S.K.; Molitoris, B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015, 35, 96–107. [Google Scholar] [CrossRef]
- Calzavacca, P.; Evans, R.G.; Bailey, M.; Bellomo, R.; May, C.N. Cortical and Medullary Tissue Perfusion and Oxygenation in Experimental Septic Acute Kidney Injury. Crit. Care Med. 2015, 43, e431–e439. [Google Scholar] [CrossRef]
- Gómez, H.; Kellum, J.A.; Ronco, C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat. Rev. Nephrol. 2017, 13, 143–151. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 2009, 119, 2868–2878. [Google Scholar] [CrossRef]
- Wichterman, K.A.; Baue, A.E.; Chaudry, I.H. Sepsis and septic shock—A review of laboratory models and a proposal. J. Surg. Res. 1980, 29, 189–201. [Google Scholar] [CrossRef]
- Remick, D.G.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 2000, 13, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.; Ho, J.; Ow, C.P.; Eppel, G.A.; Rajapakse, N.W.; Schlaich, M.P.; Evans, R.G. Renal oxygenation in acute renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2014, 306, F1026–F1038. [Google Scholar] [CrossRef]
- Regner, K.R.; Roman, R.J. Role of medullary blood flow in the pathogenesis of renal ischemia-reperfusion injury. Curr. Opin. Nephrol. Hypertens. 2012, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Gruszczyk, A.V.; Beach, T.E.; Murphy, M.P.; Saeb-Parsy, K. Mitochondrial mechanisms and therapeutics in ischaemia reperfusion injury. Pediatr. Nephrol. 2019, 34, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Winklewski, P.J. Autonomic nervous system in acute kidney injury. Clin. Exp. Pharmacol. Physiol. 2017, 44, 162–171. [Google Scholar] [CrossRef]
- Danobeitia, J.S.; Ziemelis, M.; Ma, X.; Zitur, L.J.; Zens, T.; Chlebeck, P.J.; Van Amersfoort, E.S.; Fernandez, L.A. Complement inhibition attenuates acute kidney injury after ischemia-reperfusion and limits progression to renal fibrosis in mice. PLoS ONE 2017, 12, e0183701. [Google Scholar] [CrossRef]
- Singh, A.P.; Junemann, A.; Muthuraman, A.; Jaggi, A.S.; Singh, N.; Grover, K.; Dhawan, R. Animal models of acute renal failure. Pharmacol. Rep. 2012, 64, 31–44. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Winston, J.A.; Safirstein, R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am. J. Physiol. 1985, 249, F490–F496. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Brezis, M.; Epstein, F.H.; Spokes, K.; Silva, P.; Rosen, S. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991, 40, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Sendeski, M.; Patzak, A.; Pallone, T.L.; Cao, C.; Persson, A.E.; Persson, P.B. Iodixanol, constriction of medullary descending vasa recta, and risk for contrast medium-induced nephropathy. Radiology 2009, 251, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Vlachopanos, G.; Schizas, D.; Hasemaki, N.; Georgalis, A. Pathophysiology of Contrast-Induced Acute Kidney Injury (CIAKI). Biomed. Res. Int. 2019, 25, 4642–4647. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Schmerbach, K.; Lu, Y.; Perlewitz, A.; Nikitina, T.; Cantow, K.; Seeliger, E.; Persson, P.B.; Patzak, A.; Liu, R.; et al. Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am. J. Physiol. Ren. Physiol. 2014, 306, F864–F872. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Hamar, P. Histopathological Evaluation of Contrast-Induced Acute Kidney Injury Rodent Models. Biomed. Res. Int. 2016, 2016, 3763250. [Google Scholar] [CrossRef]
- Li, C.M.; Li, M.; Ye, Z.C.; Huang, J.Y.; Li, Y.; Yao, Z.Y.; Peng, H.; Lou, T.Q. Circular RNA expression profiles in cisplatin-induced acute kidney injury in mice. Epigenomics 2019, 11, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.W.; Xiao, Y.Q.; Duan, S.B. Non-coding RNA-Associated ceRNA Networks in a New Contrast-Induced Acute Kidney Injury Rat Model. Mol. Ther. Nucleic Acids 2019, 17, 102–112. [Google Scholar] [CrossRef]
- Chávez-Iñiguez, J.S.; Navarro-Gallardo, G.J.; Medina-González, R.; Alcantar-Vallin, L.; García-García, G. Acute Kidney Injury Caused by Obstructive Nephropathy. Int. J. Nephrol. 2020, 2020, 8846622. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.E.; Vaughn, E.D., Jr.; Gillenwater, J.Y. Relationship between renal blood flow and ureteral pressure during 18 hours of total unilateral uretheral occlusion. Implications for changing sites of increased renal resistance. Investig. Urol. 1975, 13, 246–251. [Google Scholar]
- Klahr, S.; Harris, K.; Purkerson, M.L. Effects of obstruction on renal functions. Pediatr. Nephrol. 1988, 2, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, G.F.; Harris, K.P.; Purkerson, M.L.; Klahr, S. Immunological aspects of acute ureteral obstruction: Immune cell infiltrate in the kidney. Kidney Int. 1988, 34, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A.; López-Guisa, J.M.; Okamura, D.M.; Yamaguchi, I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr. Nephrol. 2012, 27, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Z.; Liu, B.; Gao, Y.; Nie, J.; Wen, S.; Lai, X.; Liang, H. Identification of circular RNA expression profiles in renal fibrosis induced by obstructive injury. Ren. Fail. 2021, 43, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, E.; Reiman, R.; Winarta, J.; Beecroft, T.; Richholt, R.; De Both, M.; Shahbander, K.; Carlson, E.; Janss, A.; Siniard, A.; et al. Extracellular circular RNA profiles in plasma and urine of healthy, male college athletes. Sci. Data 2021, 8, 276. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Cao, S.; Huang, Y.; Dai, Z.; Liao, Y.; Zhang, J.; Wang, L.; Hao, Z.; Wang, F.; Wang, D.; Liu, L. Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis. Bioengineered 2022, 13, 6323–6331. [Google Scholar] [CrossRef]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744. [Google Scholar] [CrossRef]
- Meganck, R.M.; Liu, J.; Hale, A.E.; Simon, K.E.; Fanous, M.M.; Vincent, H.A.; Wilusz, J.E.; Moorman, N.J.; Marzluff, W.F.; Asokan, A. Engineering highly efficient backsplicing and translation of synthetic circRNAs. Mol. Ther. Nucleic Acids 2021, 23, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Circular RNAs as Therapeutic Agents and Targets. Front. Physiol. 2018, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]

| AKI Syndrome | CircRNA | Dysregulation |
|---|---|---|
| Undifferentiated | hsa_circ_0003266/ciRs-126 | Upregulated [29] |
| Sepsis | hsa_circ_0040994/circ-FANCA | Upregulated [31] |
| hsa_circ_0002131/circ-BNIP3L | Upregulated [32] | |
| Circ-HIPK3 | Upregulated [33,34] | |
| hsa_circ_0114427 | Upregulated [35] | |
| hsa_circ_0114428 | Upregulated [36] | |
| Circ-TLK1 | Upregulated [37] | |
| Circ-RASGEF1B | Upregulated [38] | |
| hsa_circ_0068,888 | Downregulated [39] | |
| hsa_circ_0091702 | Downregulated [40] | |
| Circ-PRKC1 | Downregulated [41] | |
| Circ-Ttc3 | Downregulated [42] | |
| hsa_circ_0007847/circ-MTO1 | Downregulated [43] | |
| Circ-VMA21 | Downregulated [44] | |
| Ischaemia-reperfusion injury | hsa_circ_0023404 | Upregulated [45] |
| Circ-AKT3 | Upregulated [46] | |
| hsa_circ_0018148/circ-ITGB1 | Upregulated [47] | |
| hsa_circ_001839 | Upregulated [48] | |
| mmu_circ_0000943 | Upregulated [49] | |
| Circ-SNRK | Upregulated [50] | |
| mmu_circ_0000823/circ-SLC8A1 | Upregulated [51] | |
| mmu_circ_0014064/circ-APOE | Upregulated [52] | |
| Circ-YAP1 | Downregulated [53] | |
| Circ-DNMT3A | Downregulated [54] | |
| Circ-PLEKHA7 | Downregulated [20] | |
| Circ-ME1 | Downregulated [52] | |
| Cisplatin nephrotoxicity | hsa_circ_0114427 | Upregulated [55] |
| Urinary tract obstruction | hsa_circ_0045861 | Upregulated [56] |
| mmu_circ_30032 | Upregulated [57] | |
| hsa_circ_0008529/circ-ACTR2 | Upregulated [58] | |
| mmu_circ_37492 | Upregulated [59] | |
| hsa_circ_0012138 | Upregulated [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, B.Y.F.; Yap, D.Y.H.; Chan, T.M. Circular RNAs in Acute Kidney Injury: Roles in Pathophysiology and Implications for Clinical Management. Int. J. Mol. Sci. 2022, 23, 8509. https://doi.org/10.3390/ijms23158509
So BYF, Yap DYH, Chan TM. Circular RNAs in Acute Kidney Injury: Roles in Pathophysiology and Implications for Clinical Management. International Journal of Molecular Sciences. 2022; 23(15):8509. https://doi.org/10.3390/ijms23158509
Chicago/Turabian StyleSo, Benjamin Y. F., Desmond Y. H. Yap, and Tak Mao Chan. 2022. "Circular RNAs in Acute Kidney Injury: Roles in Pathophysiology and Implications for Clinical Management" International Journal of Molecular Sciences 23, no. 15: 8509. https://doi.org/10.3390/ijms23158509
APA StyleSo, B. Y. F., Yap, D. Y. H., & Chan, T. M. (2022). Circular RNAs in Acute Kidney Injury: Roles in Pathophysiology and Implications for Clinical Management. International Journal of Molecular Sciences, 23(15), 8509. https://doi.org/10.3390/ijms23158509







