Vitamin D–VDR Novel Anti-Inflammatory Molecules—New Insights into Their Effects on Liver Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection of Studies
3. Hepatocytes and Liver Damage
4. Physiology and Metabolism of Vitamin D
5. Vitamin D Receptor (VDR)
6. Vitamin D, VDR, and Liver Diseases
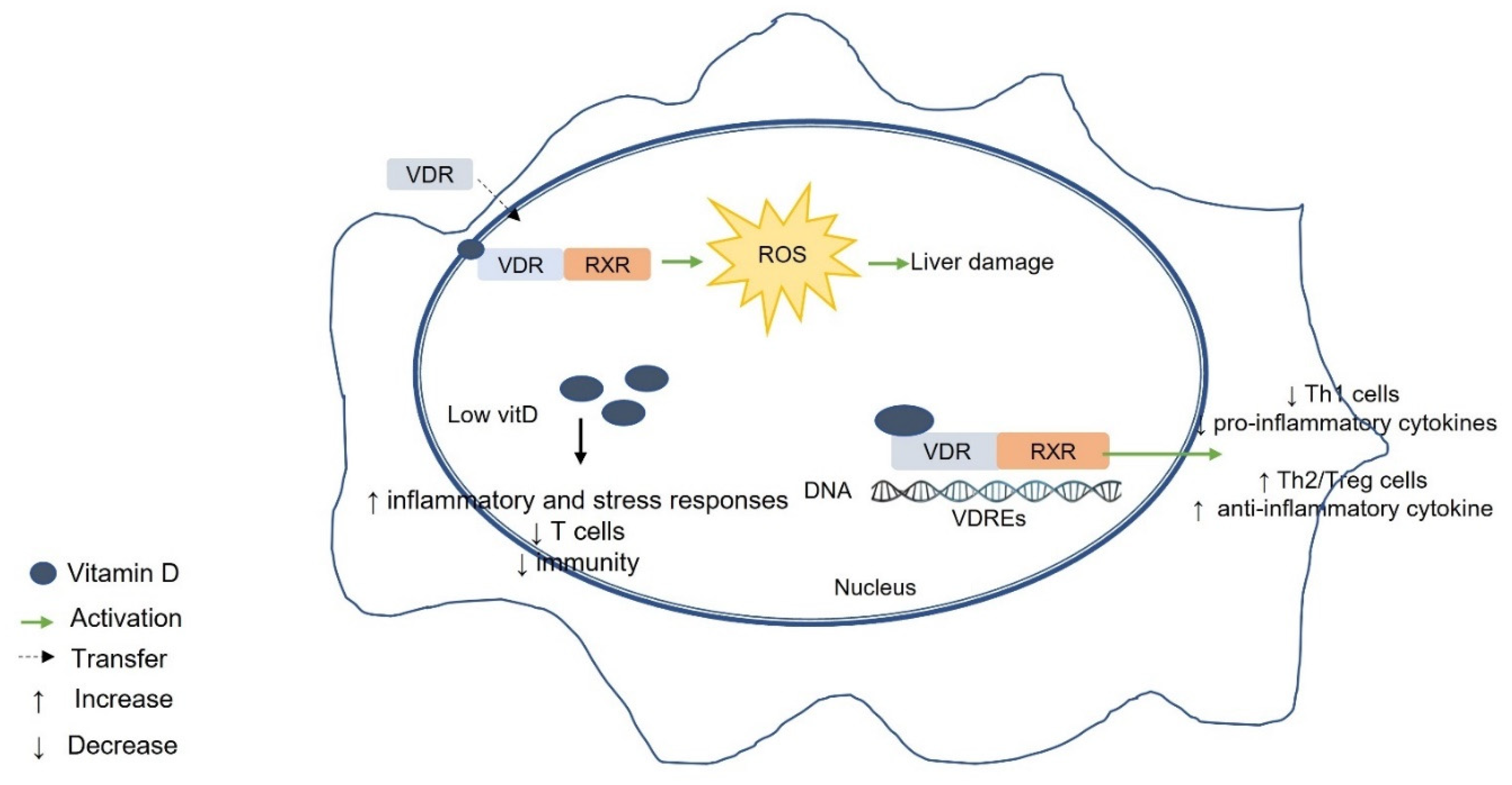
7. Possible Mechanisms of the Association between Vitamin D–VDR and Liver Disease
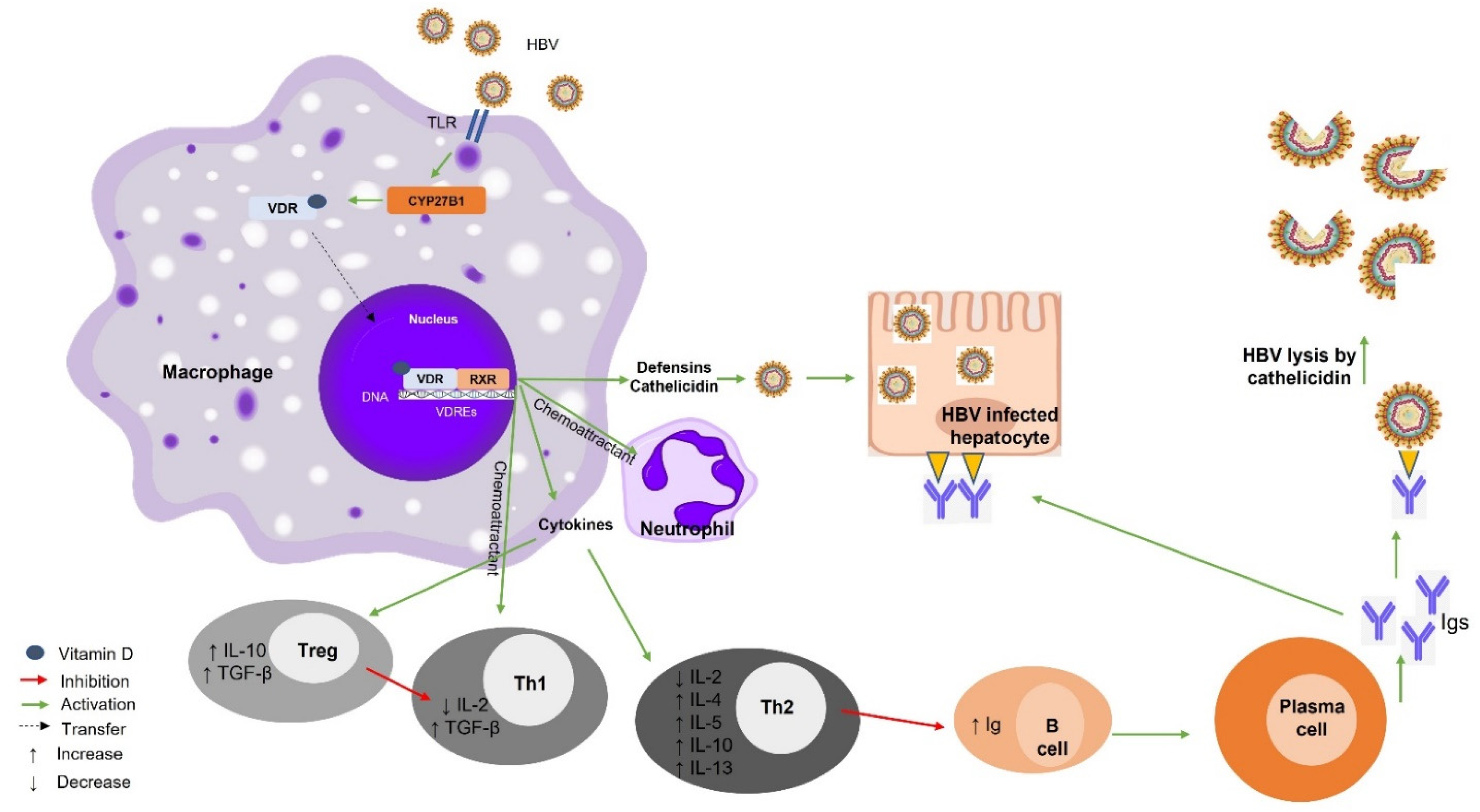
7.1. Vitamin D–VDR and Hepatitis B Virus (HBV)
Vitamin D Supplementation and HBV
7.2. Vitamin D–VDR and Hepatitis C Virus (HCV)
Vitamin D Supplementation and HCV
7.3. Vitamin D–VDR and Autoimmune Hepatitis
Vitamin D Supplementation and AIH
7.4. Vitamin D–VDR and Primary Biliary Cholangitis (PBC)
Vitamin D Supplementation and PBC
7.5. Vitamin D–VDR and NAFLD
Vitamin D Supplementation and NAFLD
8. Vitamin D–VDR-Related Genetic Polymorphisms in Liver Disease
9. Vitamin D Signaling and Gut Microbiota in Liver Disease
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Tarantino, G.; Scalera, A.; Finelli, C. Liver-spleen axis: Intersection between immunity, infections and metabolism. World J. Gastroenterol. 2013, 19, 3534–3542. [Google Scholar] [CrossRef]
- Barrea, L.; Di Somma, C. Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutr. 2018, 58, 3141–3158. [Google Scholar] [CrossRef]
- van der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Rowe, I.A. Lessons from Epidemiology: The Burden of Liver Disease. Dig. Dis. 2017, 35, 304–309. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Recent advances in understanding liver fibrosis: Bridging basic science and individualized treatment concepts. F1000Research 2018, 7, 27. [Google Scholar] [CrossRef]
- Campana, L.; Iredale, J.P. Regression of Liver Fibrosis. Semin. Liver Dis. 2017, 37, 1–10. [Google Scholar]
- Ramachandran, P.; Pellicoro, A.; Vernon, M.A.; Boulter, L.; Aucott, R.L.; Ali, A.; Hartland, S.N.; Snowdon, V.K.; Cappon, A.; Gordon-Walker, T.T.; et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 24. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007, 56, 284–292. [Google Scholar] [CrossRef]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review article: Vitamin D and inflammatory bowel diseases. Aliment. Pharm. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A. Vitamin D–liver disease association: Biological basis and mechanisms of action. Hepatology 2021, 74, 1065–1073. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef]
- Ronti, T.; Lupattelli, G.; Mannarino, E. The endocrine function of adipose tissue: An update. Clin. Endocrinol. 2006, 64, 355–365. [Google Scholar] [CrossRef]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef]
- Kongsbak, M.; von Essen, M.R.; Boding, L.; Levring, T.B.; Schjerling, P.; Lauritsen, J.P.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS ONE 2014, 9, e96695. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate–Induced Colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Abramovitch, S.; Dahan-Bachar, L.; Sharvit, E.; Weisman, Y.; Ben Tov, A.; Brazowski, E.; Reif, S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut 2011, 60, 1728–1737. [Google Scholar] [CrossRef]
- Petta, S.; Camma, C.; Scazzone, C.; Tripodo, C.; Di Marco, V.; Bono, A.; Cabibi, D.; Licata, G.; Porcasi, R.; Marchesini, G.; et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010, 51, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Putz-Bankuti, C.; Pilz, S.; Stojakovic, T.; Scharnagl, H.; Pieber, T.R.; Trauner, M.; Obermayer-Pietsch, B.; Stauber, R.E. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012, 32, 845–851. [Google Scholar] [CrossRef]
- Stokes, C.S.; Krawczyk, M.; Reichel, C.; Lammert, F.; Grunhage, F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur. J. Clin. Investig. 2014, 44, 176–183. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Wu, X.X.; Ling, Z.X.; Cheng, Y.W.; Yuan, L.; Xiang, C. Association between serum vitamin D and severity of liver fibrosis in chronic hepatitis C patients: A systematic meta-analysis. J. Zhejiang Univ. Sci. B 2014, 15, 900–906. [Google Scholar] [CrossRef]
- Trepo, E.; Ouziel, R.; Pradat, P.; Momozawa, Y.; Quertinmont, E.; Gervy, C.; Gustot, T.; Degre, D.; Vercruysse, V.; Deltenre, P.; et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J. Hepatol. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Guo, G.Y.; Shi, Y.Q.; Wang, L.; Ren, X.; Han, Z.Y.; Guo, C.C.; Cui, L.N.; Wang, J.B.; Zhu, J.; Wang, N.; et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment. Pharm. 2015, 42, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Fisher, A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Malham, M.; Jorgensen, S.P.; Ott, P.; Agnholt, J.; Vilstrup, H.; Borre, M.; Dahlerup, J.F. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J. Gastroenterol. 2011, 17, 922–925. [Google Scholar] [CrossRef]
- Triantos, C.; Kalafateli, M.; Aggeletopoulou, I.; Diamantopoulou, G.; Spantidea, P.I.; Michalaki, M.; Vourli, G.; Konstantakis, C.; Assimakopoulos, S.F.; Manolakopoulos, S.; et al. Vitamin D-related immunomodulation in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, M.; Pineda-Tenor, D.; Jimenez-Sousa, M.A.; Fernandez-Rodriguez, A.; Guzman-Fulgencio, M.; Resino, S. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: A meta-analysis. Hepatology 2014, 60, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Carrat, F.; Geri, G.; Pol, S.; Piroth, L.; Halfon, P.; Poynard, T.; Souberbielle, J.C.; Cacoub, P. Low 25-OH vitamin D serum levels correlate with severe fibrosis in HIV-HCV co-infected patients with chronic hepatitis. J. Hepatol. 2011, 55, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.C.; Slater, S.; Jurutka, P.W. Vitamin D receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008, 66, 1753–4887. [Google Scholar] [CrossRef]
- Carlberg, C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D genomics: From in vitro to in vivo. Front. Endocrinol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Campbell, M.J. Vitamin D and the RNA transcriptome: More than mRNA regulation. Front. Physiol. 2014, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Jittikoon, J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed. Pharm. 2019, 109, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Cave, M.C.; Clair, H.B.; Hardesty, J.E.; Falkner, K.C.; Feng, W.; Clark, B.J.; Sidey, J.; Shi, H.; Aqel, B.A.; McClain, C.J.; et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, H.; Chahal, S.; Gunton, J.E. Vitamin D in liver disease: Current evidence and potential directions. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 4, 907–916. [Google Scholar] [CrossRef]
- Norman, A.W. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Gascon-Barre, M.; Demers, C.; Mirshahi, A.; Neron, S.; Zalzal, S.; Nanci, A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 2003, 37, 1034–1042. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L.; Tangpricha, V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef]
- Griffin, M.D.; Lutz, W.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6800–6805. [Google Scholar] [CrossRef]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; Jeong, Y.; Downes, M.; Yu, R.T.; Evans, R.M.; Mangelsdorf, D.J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006, 126, 789–799. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Konstantakis, C.; Tselekouni, P.; Kalafateli, M.; Triantos, C. Vitamin D deficiency in patients with liver cirrhosis. Ann. Gastroenterol. 2016, 29, 297–306. [Google Scholar] [CrossRef]
- Chan, H.L.; Elkhashab, M.; Trinh, H.; Tak, W.Y.; Ma, X.; Chuang, W.L.; Kim, Y.J.; Martins, E.B.; Lin, L.; Dinh, P.; et al. Association of baseline vitamin D levels with clinical parameters and treatment outcomes in chronic hepatitis B. J. Hepatol. 2015, 63, 1086–1092. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Chan, H.Y.; Tse, C.H.; Chim, A.M.; Lo, A.O.; Wong, V.W. Adverse effects of vitamin D deficiency on outcomes of patients with chronic hepatitis B. Clin. Gastroenterol. Hepatol. 2015, 13, 783–790. [Google Scholar] [CrossRef]
- Chen, E.Q.; Bai, L.; Zhou, T.Y.; Fe, M.; Zhang, D.M.; Tang, H. Sustained suppression of viral replication in improving vitamin D serum concentrations in patients with chronic hepatitis B. Sci. Rep. 2015, 5, 15441. [Google Scholar] [CrossRef]
- Farnik, H.; Bojunga, J.; Berger, A.; Allwinn, R.; Waidmann, O.; Kronenberger, B.; Keppler, O.T.; Zeuzem, S.; Sarrazin, C.; Lange, C.M. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology 2013, 58, 1270–1276. [Google Scholar] [CrossRef]
- Hoan, N.X.; Khuyen, N.; Binh, M.T.; Giang, D.P.; Van Tong, H.; Hoan, P.Q.; Trung, N.T.; Anh, D.T.; Toan, N.L.; Meyer, C.G.; et al. Association of vitamin D deficiency with hepatitis B virus—Related liver diseases. BMC Infect. Dis. 2016, 16, 016–1836. [Google Scholar] [CrossRef]
- Gotlieb, N.; Tachlytski, I.; Lapidot, Y.; Sultan, M.; Safran, M.; Ben-Ari, Z. Hepatitis B virus downregulates vitamin D receptor levels in hepatoma cell lines, thereby preventing vitamin D-dependent inhibition of viral transcription and production. Mol. Med. 2018, 24, 018–0055. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Mushtaq, S.; Ahmed, S.Z.; Shahid, M.A. The Role of Vitamin D in HBV infection. Eur. J. Biotechnol. Biosci. 2015, 3, 35–41. [Google Scholar]
- Cantorna, M.T.; Waddell, A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014, 1317, 70–75. [Google Scholar] [CrossRef]
- Khoo, A.L.; Joosten, I.; Michels, M.; Woestenenk, R.; Preijers, F.; He, X.H.; Netea, M.G.; van der Ven, A.J.; Koenen, H.J. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology 2011, 134, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Ojaimi, S.; Skinner, N.A.; Strauss, B.J.G.; Sundararajan, V.; Woolley, I.; Visvanathan, K. Vitamin d deficiency impacts on expression of toll-like receptor-2 and cytokine profile: A pilot study. J. Transl. Med. 2013, 11, 176. [Google Scholar] [CrossRef]
- He, Q.; Huang, Y.; Zhang, L.; Yan, Y.; Liu, J.; Song, X.; Chen, W. Association between vitamin D receptor polymorphisms and hepatitis B virus infection susceptibility: A meta-analysis study. Gene 2018, 645, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Youssry, S.; Shalaby, T.; Maher, A.S.; Ghoneim, H. Association of hepatitis B vaccine response to vitamin D supplementation and ultraviolet B (UVB) exposure during different time intervals in experimental animals. Immunol. Res. 2022, 70, 537–545. [Google Scholar] [CrossRef]
- Kashi, D.S.; Oliver, S.J. Vitamin D and the hepatitis B vaccine response: A prospective cohort study and a randomized, placebo-controlled oral vitamin D(3) and simulated sunlight supplementation trial in healthy adults. Eur. J. Nutr. 2021, 60, 475–491. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Sun, H.-Y.; Chiu, W.-T.; Su, H.-C.; Chien, Y.-C.; Chong, L.-W.; Chang, H.-C.; Bai, C.-H.; Young, K.-C.; Tsao, C.-W. Calcitriol inhibits HCV infection via blockade of activation of PPAR and interference with endoplasmic reticulum-associated degradation. Viruses 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef]
- Murayama, A.; Saitoh, H.; Takeuchi, A.; Yamada, N.; Matsumura, T.; Shiina, M.; Muramatsu, M.; Wakita, T.; Imawari, M.; Kato, T. Vitamin D derivatives inhibit hepatitis C virus production through the suppression of apolipoprotein. Antivir. Res. 2018, 160, 55–63. [Google Scholar] [CrossRef]
- Ravid, A.; Rapaport, N.; Issachar, A.; Erman, A.; Bachmetov, L.; Tur-Kaspa, R.; Zemel, R. 25-Hydroxyvitamin D Inhibits Hepatitis C Virus Production in Hepatocellular Carcinoma Cell Line by a Vitamin D Receptor-Independent Mechanism. Int. J. Mol. Sci. 2019, 20, 2367. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Kato, T.; Sugiyama, N.; Tasaka-Fujita, M.; Murayama, A.; Masaki, T.; Wakita, T.; Imawari, M. 25-hydroxyvitamin D3 suppresses hepatitis C virus production. Hepatology 2012, 56, 1231–1239. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Jones, K.A.; Flores, R.; Singhania, A.; Woelk, C.H.; Schooley, R.T.; Wyles, D.L. Vitamin D metabolites inhibit hepatitis C virus and modulate cellular gene expression. J. Virol. Antivir. Res. 2014, 3. [Google Scholar] [CrossRef]
- Saleh, M.; Welsch, C.; Cai, C.; Döring, C.; Gouttenoire, J.; Friedrich, J.; Haselow, K.; Sarrazin, C.; Badenhoop, K.; Moradpour, D.; et al. Differential modulation of hepatitis C virus replication and innate immune pathways by synthetic calcitriol-analogs. J. Steroid Biochem. Mol. Biol. 2018, 183, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.M.; Gouttenoire, J.; Duong, F.H.; Morikawa, K.; Heim, M.H.; Moradpour, D. Vitamin D receptor and Jak–STAT signaling crosstalk results in calcitriol-mediated increase of hepatocellular response to IFN-α. J. Immunol. 2014, 192, 6037–6044. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Kitson, M.T.; Sarrazin, C.; Toniutto, P.; Eslick, G.D.; Roberts, S.K. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: A systematic review and meta-analysis. J. Hepatol. 2014, 61, 1247–1252. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J.; Sukkho, S.; Pojarassangkul, N.; Sangroongruangsri, S.; Chaikledkaew, U. Decreased circulating vitamin D reflects adverse outcomes of hepatitis C virus infection: A systematic review and meta-analysis. J. Infect. 2020, 15, 30402–30407. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, L.A.; Matte, U.; da Silveira, T.R.; Álvares-da-Silva, M.R. Genetic variants underlying vitamin D metabolism and VDR-TGFβ-1-SMAD3 interaction may impact on HCV progression: A study based on dbGaP data from the HALT-C study. J. Hum. Genet. 2017, 62, 969–977. [Google Scholar] [CrossRef]
- Murayama, A.; Kato, T. Chapter Nine—Inhibition of hepatitis C virus by vitamin D. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 117, pp. 227–238. [Google Scholar]
- Sriphoosanaphan, S.; Thanapirom, K.; Kerr, S.J.; Suksawatamnuay, S.; Thaimai, P.; Sittisomwong, S.; Sonsiri, K.; Srisoonthorn, N.; Teeratorn, N.; Tanpowpong, N.; et al. Effect of vitamin D supplementation in patients with chronic hepatitis C after direct-acting antiviral treatment: A randomized, double-blind, placebo-controlled trial. PeerJ 2021, 9, e10709. [Google Scholar] [CrossRef]
- Abu-Mouch, S.; Fireman, Z.; Jarchovsky, J.; Zeina, A.R.; Assy, N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J. Gastroenterol. 2011, 17, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Bitetto, D.; Fabris, C.; Fornasiere, E.; Pipan, C.; Fumolo, E.; Cussigh, A.; Bignulin, S.; Cmet, S.; Fontanini, E.; Falleti, E.; et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl. Int. 2011, 24, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Nimer, A.; Mouch, A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J. Gastroenterol. 2012, 18, 800–805. [Google Scholar] [CrossRef]
- Vosoghinia, H.; Esmaeilzadeh, A.; Ganji, A.; Hosseini, S.M.-R.; Jamehdar, S.A.; Salehi, M.; Bahari, A.; Ghanaei, O.; Sahebari, M.; Rajabzadeh, F. Vitamin D in standard HCV regimen (PEG-interferon plus ribavirin), its effect on the early virologic response rate: A clinical trial. Razavi Int. J. Med. 2016, 4. [Google Scholar] [CrossRef]
- Villar, L.M.; Del Campo, J.A.; Ranchal, I.; Lampe, E.; Romero-Gomez, M. Association between vitamin D and hepatitis C virus infection: A meta-analysis. World J. Gastroenterol. 2013, 19, 5917–5924. [Google Scholar] [CrossRef]
- Kim, H.B.; Myung, S.K.; Lee, Y.J.; Park, B.J.; Group, K.M.A.S. Efficacy of vitamin D supplementation in combination with conventional antiviral therapy in patients with chronic hepatitis C infection: A meta—Analysis of randomised controlled trials. J. Hum. Nutr. Diet. 2018, 31, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.K.; Shukla, S.K.; Dixit, V.K.; Nath, P.; Abhilash, V.B.; Asati, P.K.; Jain, A.K. Effect of vitamin D supplementation on sustained virological response in genotype 1/4 chronic hepatitis C treatment-naïve patients from India. Indian J. Med. Res. 2018, 148, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ladero, J.M.; Torrejón, M.J.; Sánchez-Pobre, P.; Suárez, A.; Cuenca, F.; de la Orden, V.; Devesa, M.J.; Rodrigo, M.; Estrada, V.; López-Alonso, G.; et al. Vitamin D deficiency and vitamin D therapy in chronic hepatitis C. Ann. Hepatol. 2013, 12, 199–204. [Google Scholar] [CrossRef]
- Esmat, G.; El Raziky, M.; Elsharkawy, A.; Sabry, D.; Hassany, M.; Ahmed, A.; Assem, N.; El Kassas, M.; Doss, W. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. J. Interferon Cytokine Res. 2015, 35, 49–54. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Jun, D.W.; Park, S.J.; Sohn, J.H.; Kim, S.G.; Lee, S.W.; Jeong, S.W.; Kim, M.Y.; Kim, W.; Shim, J.J.; et al. Effects of vitamin D supplements in patients with chronic hepatitis C: A randomized, multi-center, open label study. Korean J. Intern. Med. 2020, 35, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, M.; Nikolova, D.; Bjelakovic, G.; Gluud, C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst. Rev. 2021, 8, Cd011564. [Google Scholar] [CrossRef]
- Kondo, Y.; Kato, T.; Kimura, O.; Iwata, T.; Ninomiya, M.; Kakazu, E.; Miura, M.; Akahane, T.; Miyazaki, Y.; Kobayashi, T. 1 (OH) vitamin D3 supplementation improves the sensitivity of the immune-response during Peg-IFN/RBV therapy in chronic hepatitis C patients-case controlled trial. PLoS ONE 2013, 8, e63672. [Google Scholar] [CrossRef] [PubMed]
- Omori-Mizuno, Y.; Nakayama, N.; Inao, M.; Funyu, J.; Asabe, S.; Tomita, K.; Nishikawa, K.; Hosoda, Y.; Tanaka, M.; Hashimoto, Y.; et al. Randomized study comparing vitamin D3 and 1α-Hydroxyvitamin D3 in combination with pegylated interferon/ribavirin therapy for chronic hepatitis C. J. Gastroenterol. Hepatol. 2015, 30, 1384–1390. [Google Scholar] [CrossRef]
- Chen, H.W.; Chiu, Y.L. Relationships Between Vitamin D Status and Cytokine: Results from Interferon-Based Therapy in Non-Cirrhotic, Treatment-Naïve Patients with Chronic Hepatitis C Infection. J. Inflamm. Res. 2020, 13, 1207–1218. [Google Scholar] [CrossRef]
- Sergi, C.M. Vitamin D supplementation for autoimmune hepatitis: A need for further investigation. World J. Hepatol. 2022, 14, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhang, H.; Zhao, Q.; Bu, H.; Wang, H.; Guo, H. Correlation of vitamin D with inflammatory factors, oxidative stress and T cell subsets in patients with autoimmune hepatitis. Exp. Med. 2020, 19, 3419–3424. [Google Scholar] [CrossRef]
- Umeda, N.; Endo-Umeda, K.; Nakashima, H.; Kato, S.; Seki, S.; Makishima, M. Frontline Science: Concanavalin A-induced acute hepatitis is attenuated in vitamin D receptor knockout mice with decreased immune cell function. J. Leukoc. Biol. 2019, 106, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Kav, T.; Aydin, C.; Cengiz, M.; Imga, N.N.; Purnak, T.; Smyk, D.S.; Torgutalp, M.; Turhan, T.; Ozenirler, S.; et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig. Dis. Sci. 2014, 59, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J.; Montano-Loza, A.J. Evolving Role of Vitamin D in Immune-Mediated Disease and Its Implications in Autoimmune Hepatitis. Dig. Dis. Sci. 2019, 64, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Luong, K.V.Q.; Nguyễn, L.T.H. The role of vitamin d in primary biliary cirrhosis: Possible genetic and cell signaling mechanisms. Gastroenterol. Res. Pract. 2013, 2013, 602321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Kempinska-Podhorodecka, A.; Milkiewicz, M.; Wasik, U.; Ligocka, J.; Zawadzki, M.; Krawczyk, M.; Milkiewicz, P. Decreased Expression of Vitamin D Receptor Affects an Immune Response in Primary Biliary Cholangitis via the VDR-miRNA155-SOCS1 Pathway. Int. J. Mol. Sci. 2017, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, C.; Wang, P.; Sui, J.; Wang, Y.; Sun, G.; Liu, M. Serum vitamin D level is related to disease progression in primary biliary cholangitis. Scand. J. Gastroenterol. 2020, 55, 1333–1340. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 2012, 287, 42324–42332. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Elfers, C.T.; Figlewicz, D.P.; Melhorn, S.J.; Morton, G.J.; Hoofnagle, A.; Yeh, M.M.; Nelson, J.E.; Kowdley, K.V. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology 2012, 55, 1103–1111. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Chai, Y.; Liu, Y.; Li, F.; Wang, B.; Zhu, C.; Cui, J.; Qu, H.; Zhu, M. 1,25(OH)2D3 downregulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats. J. Endocrinol. Investig. 2015, 38, 1083–1091. [Google Scholar] [CrossRef]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Savastano, S.; Capone, D.; Colao, A. Spleen: A new role for an old player? World J. Gastroenterol. 2011, 17, 3776–3784. [Google Scholar] [CrossRef]
- Mohamed, H.K. Effect of vitamin D on the spleen of adult male rats fed on diet with high fat: A histological and immunohistochemical study. Egypt. J. Histol. 2019, 42, 1001–1017. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, M.; Pan, L.; Chen, Y.; Guo, S.; Luo, D.; Zhu, L.; Liu, Y.; Xu, S.; Zhang, R.; et al. Vitamin D signaling maintains intestinal innate immunity and gut microbiota: Potential intervention for metabolic syndrome and NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G542–G553. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E. Vitamin D: A new player in non-alcoholic fatty liver disease? World J. Gastroenterol. 2015, 21, 1718–1727. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Z.; Lin, Y.; Zhang, J.; Zhang, Y.; Liu, P.; Zeng, H.; Yu, M.; Chen, X.; Ning, L.; et al. Vitamin D receptor targets hepatocyte nuclear factor 4α and mediates protective effects of vitamin D in nonalcoholic fatty liver disease. J. Biol. Chem. 2020, 295, 3891–3905. [Google Scholar] [CrossRef]
- Shin, D.-J.; Osborne, T.F. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J. Biol. Chem. 2009, 284, 11110–11120. [Google Scholar] [CrossRef] [PubMed]
- Bozic, M.; Guzmán, C.; Benet, M.; Sánchez-Campos, S.; García-Monzón, C.; Gari, E.; Gatius, S.; Valdivielso, J.M.; Jover, R. Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis. J. Hepatol. 2016, 65, 748–757. [Google Scholar] [CrossRef] [PubMed]
- García-Monzón, C.; Petrov, P.D.; Rey, E.; Marañón, P.; Del Pozo-Maroto, E.; Guzmán, C.; Rodríguez de Cía, J.; Casado-Collado, A.J.; Vargas-Castrillón, J.; Saez, A.; et al. Angiopoietin-Like Protein 8 Is a Novel Vitamin D Receptor Target Gene Involved in Nonalcoholic Fatty Liver Pathogenesis. Am. J. Pathol. 2018, 188, 2800–2810. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Koeda, M.; Yoshida, Y.; Okubo, T.; Nakagawa, A.; Itokawa, N.; Kondo, C.; Nakatsuka, K.; et al. Association of vitamin D levels and vitamin D-related gene polymorphisms with liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dig. Liver Dis. 2019, 51, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D Supplementation and Non-Alcoholic Fatty Liver Disease: Present and Future. Nutrients 2017, 9, 1015. [Google Scholar] [CrossRef]
- Kitson, M.T.; Roberts, S.K. D-livering the message: The importance of vitamin D status in chronic liver disease. J. Hepatol. 2012, 57, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Del Ben, M.; Angelico, F.; Di Martino, M.; Fraioli, A.; La Torre, G.; Saulle, R.; Perri, L.; Morini, S.; Tiberti, C.; et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. BMC Med. 2016, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Amani, R.; Hajiani, E.; Cheraghian, B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine 2014, 47, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, S.; Meng, Y.; Yu, Q.; Wang, Q.; Xu, H.; Yuan, H.; Li, X.; Chen, L. Effects of Vitamin D Supplementation in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. Metab. 2020, 18, e97205. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.I.; Elmedames, M.R.; Abdelhai, A.R.; Marei, A.M.; Abdel-Ghani, H.A. Efficacy of vitamin D supplementation on adult patients with non-alcoholic fatty liver disease: A single-center experience. Gastroenterol. Hepatol. Bed Bench 2021, 14, 44–52. [Google Scholar] [PubMed]
- Rezaei, S.; Tabrizi, R.; Nowrouzi-Sohrabi, P.; Jalali, M.; Shabani-Borujeni, M.; Modaresi, S.; Gholamalizadeh, M.; Doaei, S. The Effects of Vitamin D Supplementation on Anthropometric and Biochemical Indices in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Pharmacol. 2021, 12, 732496. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, L.; Asadi, S.; Al-Mousavi, Z.; Niknam, R. A randomized controlled clinical trial comparing calcitriol versus cholecalciferol supplementation to reduce insulin resistance in patients with non-alcoholic fatty liver disease. Clin. Nutr. 2021, 40, 2999–3005. [Google Scholar] [CrossRef]
- Sindhughosa, D.A.; Wibawa, I.D.N.; Mariadi, I.K.; Somayana, G. Additional treatment of vitamin D for improvement of insulin resistance in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 7716. [Google Scholar] [CrossRef]
- Hamzehzadeh Alamdari, A.; Ahrabi, S.; Khoshbaten, M.; Roustaei, S.; Araqchin Ahrabi, S.; Asghari Jafarabadi, M. Effect of Oral and parenteral routes of vitamin D supplementation on serum 25(OH) vitamin D levels in patients with non-alcoholic fatty liver disease. Casp. J. Intern. Med. 2022, 13, 23–28. [Google Scholar] [CrossRef]
- Guo, X.F.; Wang, C.; Yang, T.; Ma, W.J.; Zhai, J.; Zhao, T.; Xu, T.C.; Li, J.; Liu, H.; Sinclair, A.J.; et al. The effects of fish oil plus vitamin D(3) intervention on non-alcoholic fatty liver disease: A randomized controlled trial. Eur. J. Nutr. 2022, 61, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.S.; Quaglia, A.; Dhawan, A.; Wu, H.; Lanham-New, S.; Hart, K.H.; Fitzpatrick, E.; Moore, J.B. Vitamin D status and associated genetic polymorphisms in a cohort of UK children with non-alcoholic fatty liver disease. Pediatr. Obes. 2018, 13, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; White, S.W.; Marsh, J.A.; Lye, S.J.; Connor, K.L.; Maganga, R.; Ayonrinde, O.T.; Olynyk, J.K.; Mori, T.A.; Beilin, L.J.; et al. Association between liver-specific gene polymorphisms and their expression levels with nonalcoholic fatty liver disease. Hepatology 2013, 57, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2018, 233, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Strassburg, C.P.; Manns, M.P. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology 2002, 35, 126–131. [Google Scholar] [CrossRef]
- Kempinska-Podhorecka, A.; Wunsch, E.; Jarowicz, T.; Raszeja-Wyszomirska, J.; Loniewska, B.; Kaczmarczyk, M.; Milkiewicz, M.; Milkiewicz, P. Vitamin d receptor polymorphisms predispose to primary biliary cirrhosis and severity of the disease in polish population. Gastroenterol. Res. Pract. 2012, 408723, 29. [Google Scholar] [CrossRef]
- Halmos, B.; Szalay, F.; Cserniczky, T.; Nemesanszky, E.; Lakatos, P.; Barlage, S.; Schmitz, G.; Romics, L.; Csaszar, A. Association of primary biliary cirrhosis with vitamin D receptor BsmI genotype polymorphism in a Hungarian population. Dig. Dis. Sci. 2000, 45, 1091–1095. [Google Scholar] [CrossRef]
- Lakatos, L.P.; Bajnok, E.; Hegedus, D.; Toth, T.; Lakatos, P.; Szalay, F. Vitamin D receptor, oestrogen receptor-alpha gene and interleukin-1 receptor antagonist gene polymorphisms in Hungarian patients with primary biliary cirrhosis. Eur. J. Gastroenterol. Hepatol. 2002, 14, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nezu, S.; Uegaki, S.; Kikuchi, K.; Shibuya, A.; Miyakawa, H.; Takahashi, S.; Bianchi, I.; Zermiani, P.; Podda, M.; et al. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J. Hepatol. 2009, 50, 1202–1209. [Google Scholar] [CrossRef]
- Falleti, E.; Bitetto, D.; Fabris, C.; Cussigh, A.; Fontanini, E.; Fornasiere, E.; Fumolo, E.; Bignulin, S.; Cmet, S.; Minisini, R.; et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J. Gastroenterol. 2010, 16, 3016–3024. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zeng, H.; Zhang, G.; Zhou, W.; Yan, Q.; Dai, L.; Wang, X. The associated ion between the VDR gene polymorphisms and susceptibility to hepatocellular carcinoma and the clinicopathological features in subjects infected with HBV. Biomed. Res. Int. 2013, 953974, 23. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, P.V.; Sarin, S.K.; Goyal, A.; Kumar, G.T.; Shukla, D.K.; Hissar, S. Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J. Hepatol. 2006, 44, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Tu, X.; Zhu, Y.; Zhou, L.; Pfeiffer, T.; Feltens, R.; Stoecker, W.; Zhong, R. Genetic association of vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the Chinese. J. Gastroenterol. Hepatol. 2005, 20, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tsounis, E.P.; Tourkochristou, E.; Sapsani, A.; Aggeletopoulou, I.; Lourida, T.; Ζisimopoulos, Κ.; Tzikopoulos, T.; Diamantopoulou, G.; Tsintoni, A.; Thomopoulos, K.; et al. The role of vitamin D receptor polymorphisms in the course of chronic hepatitis C infection. Ann. Gastroenterol. 2022, 35, 203–212. [Google Scholar] [CrossRef]
- Baur, K.; Mertens, J.C.; Schmitt, J.; Iwata, R.; Stieger, B.; Eloranta, J.J.; Frei, P.; Stickel, F.; Dill, M.T.; Seifert, B.; et al. Combined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patients. Liver Int. 2012, 32, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Triantos, C.; Aggeletopoulou, I.; Kalafateli, M.; Spantidea, P.I.; Vourli, G.; Diamantopoulou, G.; Tapratzi, D.; Michalaki, M.; Manolakopoulos, S.; Gogos, C.; et al. Prognostic significance of vitamin D receptor (VDR) gene polymorphisms in liver cirrhosis. Sci. Rep. 2018, 8, 14065. [Google Scholar] [CrossRef]
- Beilfuss, A.; Sowa, J.P.; Sydor, S.; Beste, M.; Bechmann, L.P.; Schlattjan, M.; Syn, W.K.; Wedemeyer, I.; Mathe, Z.; Jochum, C.; et al. Vitamin D counteracts fibrogenic TGF-beta signalling in human hepatic stellate cells both receptor-dependently and independently. Gut 2015, 64, 791–799. [Google Scholar] [CrossRef]
- Petta, S.; Grimaudo, S.; Marco, V.D.; Scazzone, C.; Macaluso, F.S.; Camma, C.; Cabibi, D.; Pipitone, R.; Craxi, A. Association of vitamin D serum levels and its common genetic determinants, with severity of liver fibrosis in genotype 1 chronic hepatitis C patients. J. Viral Hepat. 2013, 20, 486–493. [Google Scholar] [CrossRef]
- Grunhage, F.; Hochrath, K.; Krawczyk, M.; Hoblinger, A.; Obermayer-Pietsch, B.; Geisel, J.; Trauner, M.; Sauerbruch, T.; Lammert, F. Common genetic variation in vitamin D metabolism is associated with liver stiffness. Hepatology 2012, 56, 1883–1891. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Bouillon, R. Extra-Skeletal Effects of Vitamin D. Front. Horm. Res. 2018, 50, 72–88. [Google Scholar] [PubMed]
- Damoiseaux, J.; Smolders, J. The Engagement Between Vitamin D and the Immune System: Is Consolidation by a Marriage to Be Expected? EBioMedicine 2018, 31, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef] [PubMed]
- Barbáchano, A.; Fernández-Barral, A.; Ferrer-Mayorga, G.; Costales-Carrera, A.; Larriba, M.J.; Muñoz, A. The endocrine vitamin D system in the gut. Mol. Cell. Endocrinol. 2017, 453, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Tonohouan, M.; Ferrari-Panaia, P.; Piche, T.; Pariente, A.; Anstee, Q.M.; Gual, P.; Tran, A. Low Levels of 25-Hydroxy Vitamin D are Independently Associated with the Risk of Bacterial Infection in Cirrhotic Patients. Clin. Transl. Gastroenterol. 2014, 5, e56. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Hsieh, Y.C.; Huo, T.I.; Yang, U.C.; Lin, C.H.; Li, C.P.; Huang, Y.H.; Hou, M.C.; Lin, H.C.; Lee, K.C. Active Vitamin D(3) Treatment Attenuated Bacterial Translocation via Improving Intestinal Barriers in Cirrhotic Rats. Mol. Nutr. Food Res. 2021, 65, e2000937. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, R.; Luo, M.; Zhang, T.; Pan, L.; Xu, S.; Pan, L.; Ren, F.; Ji, C.; Hu, R. Impaired 25-hydroxylation of vitamin D in liver injury suppresses intestinal Paneth cell defensins, leading to gut dysbiosis and liver fibrogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G685–G695. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shang, X.; Jin, S.; Ma, Z.; Wang, H.; Ao, N.; Yang, J.; Du, J. Vitamin D ameliorates high-fat-diet-induced hepatic injury via inhibiting pyroptosis and alters gut microbiota in rats. Arch. Biochem. Biophys. 2021, 705, 108894. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A.; Triantos, C. Vitamin D–VDR Novel Anti-Inflammatory Molecules—New Insights into Their Effects on Liver Diseases. Int. J. Mol. Sci. 2022, 23, 8465. https://doi.org/10.3390/ijms23158465
Aggeletopoulou I, Thomopoulos K, Mouzaki A, Triantos C. Vitamin D–VDR Novel Anti-Inflammatory Molecules—New Insights into Their Effects on Liver Diseases. International Journal of Molecular Sciences. 2022; 23(15):8465. https://doi.org/10.3390/ijms23158465
Chicago/Turabian StyleAggeletopoulou, Ioanna, Konstantinos Thomopoulos, Athanasia Mouzaki, and Christos Triantos. 2022. "Vitamin D–VDR Novel Anti-Inflammatory Molecules—New Insights into Their Effects on Liver Diseases" International Journal of Molecular Sciences 23, no. 15: 8465. https://doi.org/10.3390/ijms23158465
APA StyleAggeletopoulou, I., Thomopoulos, K., Mouzaki, A., & Triantos, C. (2022). Vitamin D–VDR Novel Anti-Inflammatory Molecules—New Insights into Their Effects on Liver Diseases. International Journal of Molecular Sciences, 23(15), 8465. https://doi.org/10.3390/ijms23158465








