Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden
Abstract
1. Introduction
2. Results
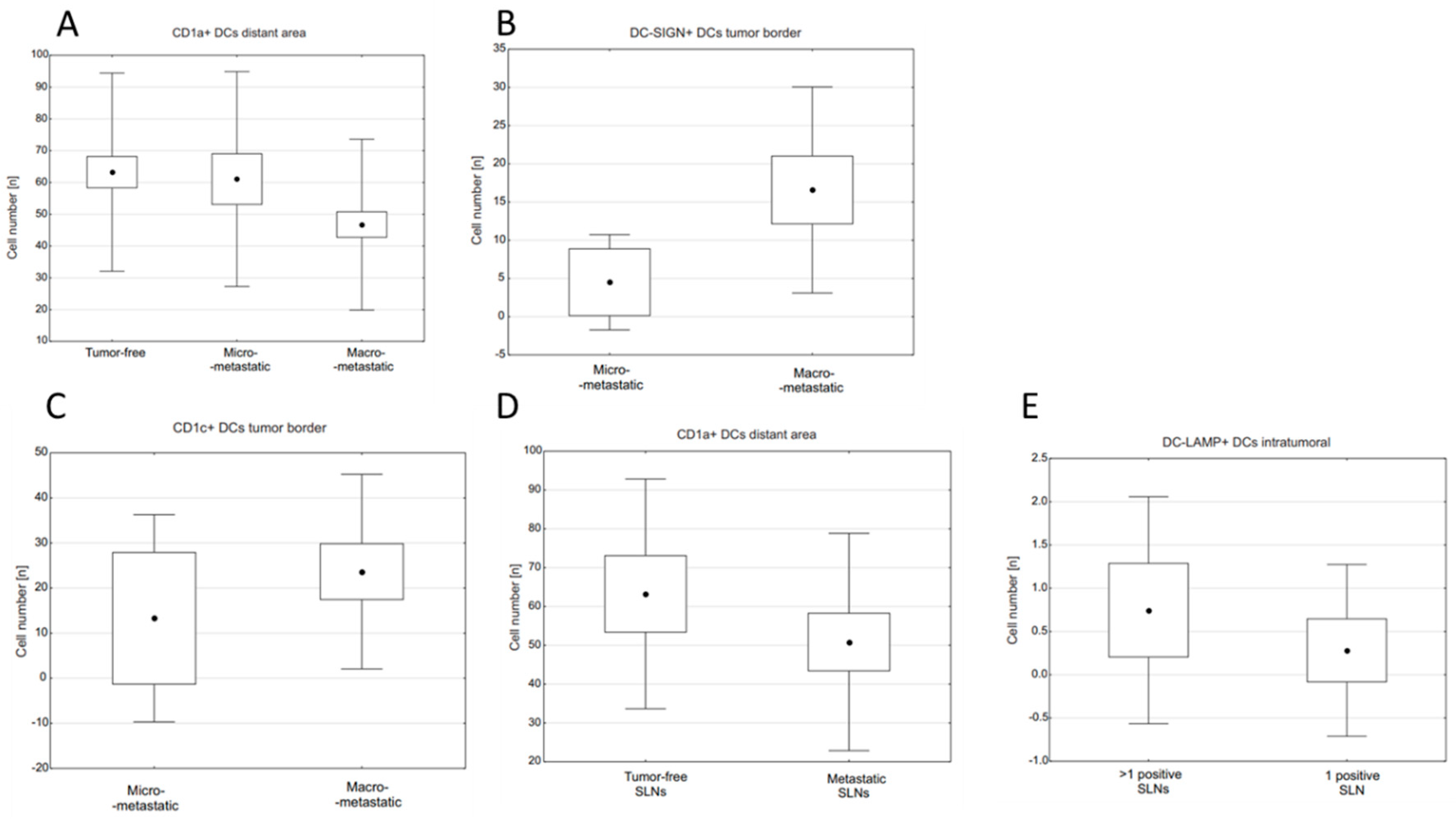
2.1. Relationships between Densities of DC Populations and Metastatic Burden of SLNs
2.2. Differences in Densities of DC Populations in SLNs and Other Prognostic Indicators in BC
2.3. Correlations between Densities of DC Populations
3. Discussion
4. Material and Methods
4.1. Selection of Cases
4.2. Detection of SLNs and Identification of Nodal Metastases
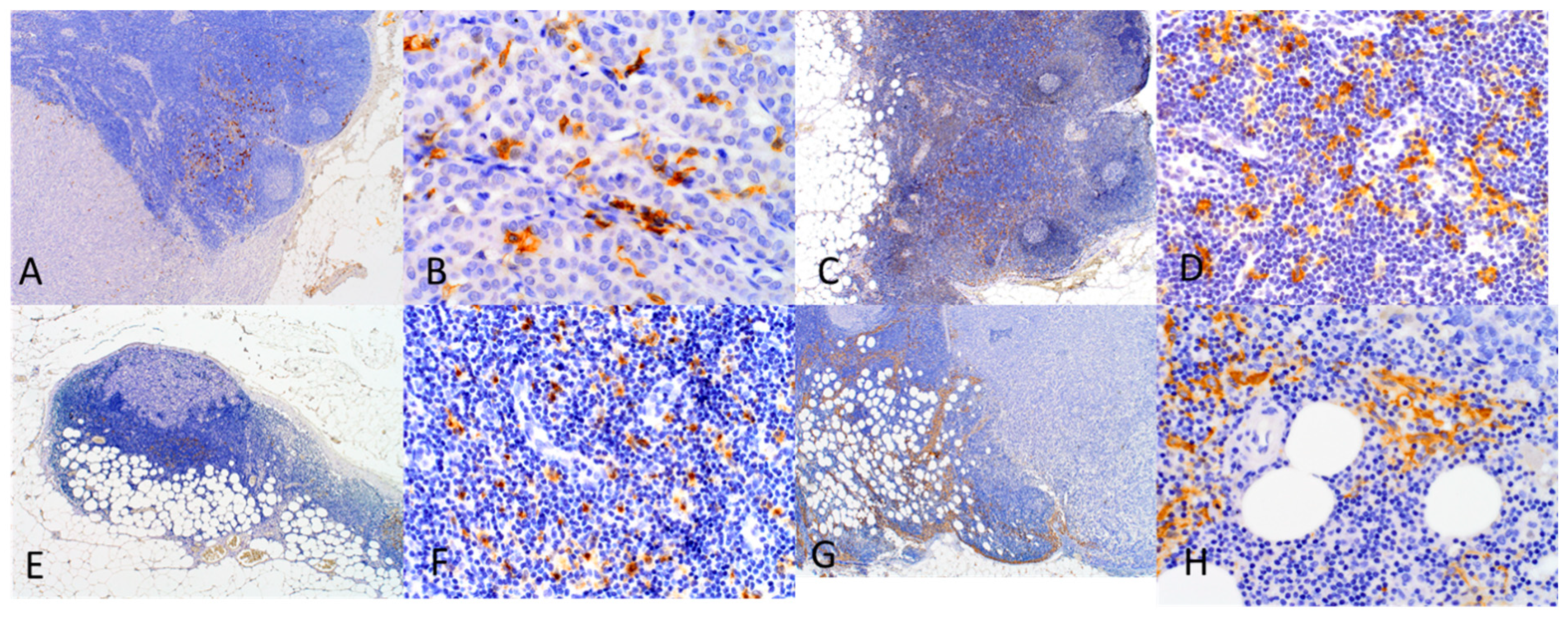
4.3. Immunohistochemistry
4.4. Evaluation of DCs Densities in SLNs
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charalampoudis, P.; Markopoulos, C.; Kovacs, T. Controversies and recommendations regarding sentinel lymph node biopsy in primary breast cancer: A comprehensive review of current data. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Huang, R.-R.; Lee, J.; Itakura, E.; Leong, S.P.L.; Essner, R. Tumour–induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 2006, 6, 659–670. [Google Scholar] [CrossRef]
- Naidoo, K.; Pinder, S.E. Micro- and macro-metastasis in the axillary lymph node: A review. Surgeon 2017, 15, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, C.A.; Rebaza, P.; Castillo, M.; Gomez, H.L.; De La Cruz, M.; Calderon, G.; Dunstan, J.; Cotrina, J.M.; Abugattas, J.; Vidaurre, T. Critical review of axillary recurrence in early breast cancer. Crit. Rev. Oncol. 2018, 129, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, N.J.; Sahin, A.; Hunt, K.K.; Grimm, E. Analysis of dendritic cells in tumor-free and tumor-containing sentinel lymph nodes from patients with breast cancer. Breast Cancer Res. 2004, 6, R408–R415. [Google Scholar] [CrossRef] [PubMed]
- Kara, P.P.; Ayhan, A.; Caner, B.; Gultekin, M.; Ugur, O.; Bozkurt, M.F.; Usubutun, A.; Uner, A. Analysis of Dendritic Cells in Sentinel Lymph Nodes of Patients with Endometrial and Patients with Cervical Cancers. Int. J. Gynecol. Cancer 2009, 19, 1239–1243. [Google Scholar] [CrossRef]
- Hossain, K.; Wall, K.A. Use of Dendritic Cell Receptors as Targets for Enhancing Anti-Cancer Immune Responses. Cancers 2019, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Zhang, S.; Li, Y. Inhibition of vascular endothelial growth factor by small interfering RNA upregulates differentiation, maturation and function of dendritic cells. Exp. Ther. Med. 2014, 9, 120–124. [Google Scholar] [CrossRef]
- Romoli, M.R.; Di Gennaro, P.; Gerlini, G.; Sestini, S.; Brandani, P.; Ferrone, S.; Borgognoni, L. High Antigen Processing Machinery component expression in Langerhans cells from melanoma patients’ sentinel lymph nodes. Cell. Immunol. 2017, 320, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Laumbacher, B.; Gu, S.; Wank, R. Activated Monocytes Prime Naïve T Cells Against Autologous Cancer: Vigorous Cancer Destruction In Vitro and In Vivo. Scand. J. Immunol. 2012, 75, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Tabarkiewicz, J.; Rybojad, P.; Jablonka, A.; Rolinski, J.M. CD1c+ and CD303+ dendritic cells in peripheral blood, lymph nodes and tumor tissue of patients with non-small cell lung cancer. Oncol. Rep. 2008, 19, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Blenman, K.R.M.; He, T.-F.; Frankel, P.H.; Ruel, N.H.; Schwartz, E.J.; Krag, D.N.; Tan, L.K.; Yim, J.H.; Mortimer, J.E.; Yuan, Y.; et al. Sentinel lymph node B cells can predict disease-free survival in breast cancer patients. NPJ Breast Cancer 2018, 4, 28. [Google Scholar] [CrossRef]
- Pedersen, A.E.; Thorn, M.; Gad, M.; Walter, M.R.; Johnsen, H.E.; Gaarsdal, E.; Nikolajsen, K.; Buus, S.; Claesson, M.H.; Svane, I.M. Phenotypic and Functional Characterization of Clinical Grade Dendritic Cells Generated from Patients with Advanced Breast Cancer for Therapeutic Vaccination. Scand. J. Immunol. 2005, 61, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Yamaguchi, Y.; Ueno, H.; Osaki, A.; Arihiro, K.; Toge, T. Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 2006, 106, 1227–1236. [Google Scholar] [CrossRef]
- Vasir, B.; Wu, Z.; Crawford, K.; Rosenblatt, J.; Zarwan, C.; Bissonnette, A.; Kufe, D.; Avigan, D. Fusions of Dendritic Cells with Breast Carcinoma Stimulate the Expansion of Regulatory T Cells while Concomitant Exposure to IL-12, CpG Oligodeoxynucleotides, and Anti-CD3/CD28 Promotes the Expansion of Activated Tumor Reactive Cells. J. Immunol. 2008, 181, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Szpor, J.; Streb, J.; Glajcar, A.; Frączek, P.; Winiarska, A.; Tyrak, K.; Basta, P.; Okoń, K.; Jach, R.; Hodorowicz-Zaniewska, D. Dendritic Cells Are Associated with Prognosis and Survival in Breast Cancer. Diagnostics 2021, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.S.; Costa, N.L.; Arantes, D.A.C.; Alencar, R.D.C.G.; Silva, T.A.; Batista, A.C. Immune response in cervical lymph nodes from patients with primary oral squamous cell carcinoma. J. Oral Pathol. Med. 2013, 42, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Van De Ven, R.; Hout, M.F.C.M.V.D.; Lindenberg, J.J.; Sluijter, B.J.R.; Van Leeuwen, P.A.M.; Lougheed, S.M.; Meijer, S.; Tol, M.P.V.D.; Scheper, R.J.; De Gruijl, T.D. Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to T-cell activation. Blood 2011, 118, 2502–2510. [Google Scholar] [CrossRef]
- Ven, R.; Lindenberg, J.J.; Reurs, A.W.; Scheper, R.J.; Scheffer, G.L.; Gruijl, T.D. Preferential Langerhans cell differentiation from CD34+precursors upon introduction of ABCG2 (BCRP). Immunol. Cell Biol. 2012, 90, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Vermi, W.; Bonecchi, R.; Facchetti, F.; Bianchi, D.; Sozzani, S.; Festa, S.; Berenzi, A.; Cella, M.; Colonna, M. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J. Pathol. 2003, 200, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Thomachot, M.C.; Bendriss-Vermare, N.; Massacrier, C.; Biota, C.; Treilleux, I.; Goddard, S.; Caux, C.; Bachelot, T.; Blay, J.Y.; Menetrier-Caux, C. Breast carcinoma cells promote the differentiation of CD34+ progenitors towards 2 different subpopulations of dendritic cells with CD1ahighCD86?Langerin- and CD1a+CD86+Langerin+ phenotypes. Int. J. Cancer 2004, 110, 710–720. [Google Scholar] [CrossRef]
- La Rocca, G.; Anzalone, R.; Cappello, F.; Corrao, S.; Magno, F.; Rappa, F.; Marasà, S.; Czarnecka, A.M.; Marasà, L.; Sergi, C.; et al. CD1a down-regulation in primary invasive ductal breast carcinoma may predict regional lymph node invasion and patient outcome. Histopathology 2008, 52, 203–212. [Google Scholar] [CrossRef]
- Giorello, M.B.; Matas, A.; Marenco, P.; Davies, K.M.; Borzone, F.R.; Calcagno, M.D.L.; García-Rivello, H.; Wernicke, A.; Martinez, L.M.; Labovsky, V.; et al. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer 2021, 28, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, H.; Nouri, N.; Nowels, K.; Johnson, D.; Holmes, S.; Lee, P.P. Profile of Immune Cells in Axillary Lymph Nodes Predicts Disease-Free Survival in Breast Cancer. PLoS Med. 2005, 2, e284. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Heikkila, P.; Von Smitten, K.; Vakkila, J.; Leidenius, M. Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch. 2011, 459, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J. Diverse antigen presentation by the Group 1 CD1 molecule, CD1c. Mol. Immunol. 2013, 55, 182–185. [Google Scholar] [CrossRef][Green Version]
- Bourdely, P.; Anselmi, G.; Vaivode, K.; Ramos, R.N.; Missolo-Koussou, Y.; Hidalgo, S.; Tosselo, J.; Nuñez, N.; Richer, W.; Vincent-Salomon, A.; et al. Transcriptional and Functional Analysis of CD1c+ Human Dendritic Cells Identifies a CD163+ Subset Priming CD8+CD103+ T Cells. Immunity 2020, 53, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Tang-Huau, T.-L.; Gueguen, P.; Goudot, C.; Durand, M.; Bohec, M.; Baulande, S.; Pasquier, B.; Amigorena, S.; Segura, E. Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway. Nat. Commun. 2018, 9, 2570. [Google Scholar] [CrossRef]
- Kassianos, A.J.; Hardy, M.Y.; Ju, X.; Vijayan, D.; Ding, Y.; Vulink, A.J.E.; McDonald, K.J.; Jongbloed, S.L.; Wadley, R.B.; Wells, C.; et al. Human CD1c (BDCA-1)+ myeloid dendritic cells secrete IL-10 and display an immuno-regulatory phenotype and function in response to Escherichia coli. Eur. J. Immunol. 2012, 42, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef]
- Zekri, A.-R.N.; El Deeb, S.; Bahnassy, A.; Badr, A.M.; Lateif, M.A.; Esmat, G.; Salama, H.; Mohanad, M.; El-Dien, A.E.; Rabah, S.; et al. Role of relevant immune-modulators and cytokines in hepatocellular carcinoma and premalignant hepatic lesions. World J. Gastroenterol. 2018, 24, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Dyduch, G.; Tyrak, K.E.; Glajcar, A.; Szpor, J.; Ulatowska-Białas, M.; Okoń, K. Melanomas and Dysplastic Nevi Differ in Epidermal CD1c+ Dendritic Cell Count. BioMed Res. Int. 2017, 2017, 6803756. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.K.; Mick, R.; Feldman, M.; Hino, S.; Wang, Y.; Brose, M.S.; Muschel, R.J. Distribution of dendritic cell subtypes in primary oral squamous cell carcinoma is inconsistent with a functional response. Cancer Lett. 2007, 255, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, M.; Spatz, A.; Davoust, J.; Lebecque, S.; Romero, P.; Pittet, M.; Rimoldi, D.; Liénard, D.; Gugerli, O.; Ferradini, L.; et al. Selective Accumulation of Mature DC-Lamp+ Dendritic Cells in Tumor Sites Is Associated with Efficient T-Cell-Mediated Antitumor Response and Control of Metastatic Dissemination in Melanoma. Cancer Res. 2004, 64, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.; Scolyer, R.A.; Suciu, S.; Lebecque, S.; Rimoldi, D.; Gugerli, O.; Musat, E.; Sharma, R.N.; Lienard, D.; Keilholz, U.; et al. Long-Term Protective Effect of Mature DC-LAMP+ Dendritic Cell Accumulation in Sentinel Lymph Nodes Containing Micrometastatic Melanoma. Clin. Cancer Res. 2007, 13, 3825–3830. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, Y.; Hao, L.; Zhang, Y. DC-SIGN and immunoregulation. Cell. Mol. Immunol. 2006, 3, 279–283. [Google Scholar]
- Deluce-Kakwata-Nkor, N.; Lamendour, L.; Chabot, V.; Héraud, A.; Ivanovic, Z.; Halary, F.; Dehaut, F.; Velge-Roussel, F. Differentiation of human dendritic cell subsets for immune tolerance induction. Transfus. Clin. Biol. 2018, 25, 90–95. [Google Scholar] [CrossRef]
- Domínguez-Soto, A.; Sierra-Filardi, E.; Puig-Kröger, A.; Pérez-Maceda, B.; Gómez-Aguado, F.; Corcuera, M.T.; Sánchez-Mateos, P.; Corbí, A.L. Dendritic Cell-Specific ICAM-3–Grabbing Nonintegrin Expression on M2-Polarized and Tumor-Associated Macrophages Is Macrophage-CSF Dependent and Enhanced by Tumor-Derived IL-6 and IL-10. J. Immunol. 2011, 186, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Merlotti, A.; Malizia, A.L.; Michea, P.; Bonté, P.-E.; Goudot, C.; Carregal, M.S.; Nuñez, N.; Sedlik, C.; Ceballos, A.; Soumelis, V.; et al. Aberrant fucosylation enables breast cancer clusterin to interact with dendritic cell-specific ICAM-grabbing non-integrin (DC-SIGN). OncoImmunology 2019, 8, e1629257. [Google Scholar] [CrossRef] [PubMed]
- Spary, L.K.; Salimu, J.; Webber, J.P.; Clayton, A.; Mason, M.D.; Tabi, Z. Tumor stroma-derived factors skew monocyte to dendritic cell differentiation toward a suppressive CD14+PD-L1+phenotype in prostate cancer. OncoImmunology 2014, 3, e955331. [Google Scholar] [CrossRef]
- Jubb, A.M.; Soilleux, E.J.; Turley, H.; Steers, G.; Parker, A.; Low, I.; Blades, J.; Li, J.-L.; Allen, P.; Leek, R.; et al. Expression of Vascular Notch Ligand Delta-Like 4 and Inflammatory Markers in Breast Cancer. Am. J. Pathol. 2010, 176, 2019–2028. [Google Scholar] [CrossRef]
- Ammar, A.; Mohammed, R.A.A.; Salmi, M.; Pepper, M.; Paish, E.C.; Ellis, I.O.; Martin, S.G. Lymphatic expression of CLEVER-1 in breast cancer and its relationship with lymph node metastasis. Anal. Cell. Pathol. 2011, 34, 67–78. [Google Scholar] [CrossRef]
- Cernadas, M.; Cavallari, M.; Watts, G.; Mori, L.; De Libero, G.; Brenner, M.B. Early Recycling Compartment Trafficking of CD1a Is Essential for Its Intersection and Presentation of Lipid Antigens. J. Immunol. 2010, 184, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, A.; Li, J.; Loddenkemper, C.; Kemmner, W.; Stein, H.; Wernecke, K.; Schlag, P. Presence of mature DC-Lamp+ dendritic cells in sentinel and non-sentinel lymph nodes of breast cancer patients. Eur. J. Surg. Oncol. (EJSO) 2008, 34, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.; Veronesi, U. The isotope technique. Sentinel Lymph Node Biopsy; Hiram, S.C., III, Eds.; Martin Dunitz Ltd.: London, UK, 2002; pp. 105–110. [Google Scholar]
- Schauer, A.; Becker, W.; Reiser, M.F. Sentinel Lymph Node Concept; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number of Cases (Total = 123) | % |
|---|---|---|
| Age (years): | ||
| Range | 29–87 | |
| Mean | 55 | |
| Nodal burden: | ||
| Tumor-free | 43 | 35 |
| Micrometastases | 22 | 17.9 |
| Macrometastases | 58 | 47.1 |
| Patients with positive SLNs: | 83 | 100 |
| 1 involved LN | 52 | 62.7 |
| >1 involved LNs | 31 | 37.3 |
| Tumor size: | ||
| pT1 | 82 | 66.7 |
| pT2 | 35 | 28.5 |
| pT3 | 3 | 2.4 |
| pT4 | 2 | 1.6 |
| Lymph nodes status: | ||
| pN0 | 41 | 33.3 |
| pN1 | 70 | 56.9 |
| pN2 | 8 | 6.5 |
| pN3 | 4 | 3.2 |
| Nottingham Histologic Grade: | ||
| G1 | 21 | 17.1 |
| G2 | 50 | 40.7 |
| G3 | 51 | 41.5 |
| Histologic type: | ||
| NOS * | 105 | 85.4 |
| ILC ** | 15 | 12.2 |
| Other | 3 | 2.4 |
| Hormone receptor status: | ||
| Negative | 16 | 13 |
| Positive | 102 | 83 |
| HER2 status: | ||
| Normal | 92 | 74.8 |
| Overexpression | 26 | 21.1 |
| Tumor-Free | Micro-Metastatic | Macro-Metastatic | p-Value | ||
|---|---|---|---|---|---|
| CD1a | intratumoral | - | - | 1.55 ± 2.09 | - |
| tumor margin | - | 10.58 ± 10.38 | 18.74 ± 20.19 | NS ** | |
| distant area | 63.25 ± 31.16 | 61.08 ± 33.79 | 46.76 ± 26.87 | 0.055 * | |
| CD1c | intratumoral | - | - | 0.45 ± 0.82 | - |
| tumor margin | - | 13.28 ± 24.21 | 23.66 ± 22.73 | 0.031 ** | |
| distant area | 42.22 ± 34.64 | 49.13 ± 29.90 | 51.83 ± 38.52 | NS * | |
| DC-LAMP | intratumoral | - | 0.70 ± 1.56 | 0.47 ± 1.19 | - |
| tumor margin | - | 36.34 ± 36.46 | 68.69 ± 55.19 | 0.073 ** | |
| distant area | 143.09 ± 57.65 | 147.22 ± 57.71 | 136.11 ± 53.30 | NS * | |
| DC-SIGN | intratumoral | - | - | 0.19 ± 0.42 | - |
| tumor margin | - | 4.51 ± 6.55 | 16.58 ± 14.19 | 0.002 ** | |
| distant area | 18.71 ± 9.88 | 18.59 ± 9.73 | 22.45 ± 16.26 | NS * |
| DC Marker | First Author, Date | Material | Conclusions | Reference |
|---|---|---|---|---|
| CD1a+ | Studies investigating function of the DC subset | |||
| Gonçalves A.S., 2013 | Cervical LNs from primary OSCC | Marker of immature DCs; Their accumulation in LNs associated with immunosuppressive microenvironment | [17] | |
| Cochran A.J., 2018 | review | Expression of CD1a is not restricted to immature DCs exclusively | [2] | |
| Van de Ven R., 2011 | SLNs from melanoma patients | CD1a+ DCs are poor activators of T cells; Contribute to immune tolerance even in their mature state | [18] | |
| Van de Ven R., 2012 | DCs generated from their precursors | Marker of Langerhans cells | [19] | |
| Vermi W., 2003 | Primary cutaneous melanoma patients (skin tumor & SLNs) | DCs exhibit capacity to coexpress molecules attributed to mature phenotype of LCs | [20] | |
| Thomachot M.C., 2004 | Primary breast carcinoma | DCs with decreased ability to stimulate T cel proliferation; Immature DCs recruited to tumor site, where their maturation is impaired | [21] | |
| Studies investigating prognostic significance of the DC subset and its relationships with cancer progression | ||||
| Gonçalves A.S., 2013 | Cervical LNs from primary OSCC | Accumulation in LNs associated with occurrence of metastases | [17] | |
| La Rocca G., 2008 | Primary invasive ductal breast carcinoma tumors and LNs | Accumulation of DCs associated with absence of nodal metastases | [22] | |
| Giorello M.B., 2021 | Early invasive ductal breast carcinoma | Higher numbers of DCs associated with lower risk of metastatic disease | [23] | |
| Szpor J., 2021 | Primary invasive breast cancer | Higher numbers of DCs associated with longer progression-free survival | [16] | |
| Kohrt H.E., 2005 | LNs from breast cancer patients | Lower numbers of DCs associated with nodal metastases and recurrence | [24] | |
| Poindexter N.J., 2004 Blenman K.R.M., 2018 Mansfield A.S., 2011 | SLNs from breast cancer patients | No relationship between DCs density and metastases in SLNs | [5,12,25] | |
| CD1c+ | Studies investigating function of the DC subset | |||
| Adams E.J., 2013 | review | Molecule expressed on LCs | [26] | |
| Bourdely P., 2020 | DCs progenitors from human blood | Inflammatory CD1c+ DC subset is distributed in numerous tissues and solid tumors; DCs capable of stimulate cytotoxic and helper T cells | [27] | |
| Tang-Huau T.L., 2018 | Peritoneal ascites from cancer patients | DCs with capacity of stimulating effector cytotoxic T cells | [28] | |
| Kassianos A.J., 2012 | Human blood DCs | CD1c+ DCs produce immunosuppressive and regulatory factors as well as exhibit a tolerogenic phenotype in response to bacterial stimulation | [29] | |
| Lavin Y., 2017 | NSCLC tumor tissues and blood samples | Represent monocyte-derived DCs population generated at the tumor site | [30] | |
| Zekri A.R.N., 2018 | Blood samples from chronic liver disease patients | Marker expressed on myeloid DCs producing IL-12 | [31] | |
| Tabarkiewicz J., 2008 | Tumor tissue, draining LNs and blood samples from NSCLC patients | CD1c+ DCs are trapped and accumulate at the tumor site | [11] | |
| Studies investigating prognostic significance of the DC subset and its relationships with cancer progression | ||||
| Lavin Y., 2017 | NSCLC tumor tissues and blood samples | More abundant in tumor tissue than in normal | [30] | |
| Zekri A.R.N., 2018 | Blood samples from chronic liver disease patients | Less abundant in HCC patients than in normal | [31] | |
| Dyduch G., 2017 | Cutaneous samples | Less abundant epidermal DCs in pre- and invasive melanoma than in bening nevi | [32] | |
| Tabarkiewicz J., 2008 | Tumor tissue, draining LNs and blood samples from NSCLC patients | Higher DC numbers in tumors associated with worse survival | [11] | |
| DC-LAMP | Studies investigating function of the DC subset | |||
| Cochran A.J., 2018 | review | Marker of DC maturity | [2] | |
| Vermi W., 2003 | Primary cutaneous melanoma patients (skin tumor & SLNs) | Marker of mature dermal DCs; Mutually exclusive expression with DC-SIGN; Mature DCs at tumor site show impaired ability to stimulate T cells | [20] | |
| O’Donell R.K., 2007 | LNs from primary OSCC patients | Represent mature DCs; | [33] | |
| Movassagh M., 2004 | Melanoma-positive SLNs | Mature DCs are pivotal contributors to melanoma immunosurveillance at the initial site of tumor spread | [34] | |
| Mansfield A.S., 2011 | SLNs from breast cancer patients | In BC maturation and antigen presentation of DCs are arrested in SLNs | [25] | |
| Studies investigating prognostic significance of the DC subset and its relationships with cancer progression | ||||
| Elliott B., 2007 | Melanoma containing SLNs | Higher numbers of mature DCs in SLNs associated with longer survival and with antimetastatic immune response | [35] | |
| Movassagh M., 2004 | Melanoma-positive SLNs | Higher numbers associated with occurence of tumor-free non-SLNs | [34] | |
| O’Donell R.K., 2007 | LNs from primary OSCC patients | Represent mature DCs; Higher numbers in LNs associated with absence of metasases | [33] | |
| Mansfield A.S., 2011 | SLNs from breast cancer patients | More dense infiltration related to absence of nodal metastases | [25] | |
| DC-SIGN | Studies investigating function of the DC subset | |||
| Vermi W., 2003 | Primary cutaneous melanoma patients (skin tumor and SLNs) | Marker expressed on immature dermal LCs; Mutually exclusive expression with DC-LAMP | [20] | |
| O’Donell R.K., 2007 | LNs from primary OSCC patients | DC-SIGN+ DCs represent immature DCs with impaired antigen capture | [33] | |
| Zhou T., 2006 | review | Marker expressed both on mature and immature DCs in dermis and mucosa; Expression of DC-SIGN on DCs contribute to tumor immune escape | [36] | |
| Van de Ven R., 2012 | DCs generated from their precursors | Expression on interstitial DCs | [19] | |
| Deluce-Kakwata-nkor N., 2018 | Monocyte-derived DCs from human blood samples | Expression on monocyte-derived DCs; After stimulation with LPS support pro-tolerant microenvironment | [37] | |
| Hossain M.K., 2019 | review | Expression attributed primarily to dermal DCs; Represent immature and mature subsets in peripheral tissues and lymphoid organs, respectively | [7] | |
| Van de Ven R., 2011 | SLNs from melanoma patients | Lower expression in SLN DCs attributed to maturation and migration of DCs | [18] | |
| Domínguez-Soto A., 2011 | Monocytes from human blood samples | Expression observed on tumor-associated pro-tolerant macrophages; DC-SIGN+ cells present in stroma of several carcinoma tissues | [38] | |
| Merlotti A., 2019 | Breast tumor and juxtatumoral samples | Expression observed on tumor-associated macrophages | [39] | |
| Spary L.K., 2014 | Primary prostate cancer, prostate cancer cel lines and human blood samples | DCs represent immunosuppressive subset induced by stromal factors and cancer cells; | [40] | |
| Jubb A.M., 2010 | Primary breast adenocarcinoma tissues | Expression on immature myeloid DCs | [41] | |
| Ammar A., 2011 | Primary invasive breast cancer tissues | Marker of immature DCs | [42] | |
| Studies investigating prognostic significance of the DC subset and its relationships with cancer progression | ||||
| O’Donell R.K., 2007 | LNs from primary OSCC patients | Presence in primary tumor associated with poor survival | [33] | |
| Domínguez-Soto A., 2011 | Monocytes from human blood samples | Interplay of DC-SIGN+ and cancer cells contribute to cancer progression | [38] | |
| Merlotti A., 2019 | Breast tumor and juxtatumoral samples | Interaction between DC-SIGN+ macrophages and cancer cells contribute to cancer progression | [39] | |
| Jubb A.M., 2010 | Primary breast adenocarcinoma tissues | Immature DCs related to worse survival | [41] | |
| Ammar A., 2011 | Primary invasive breast cancer tissues | Immature DCs related to early recurrence | [42] | |
| Clone | Dilution | Antigen Retrieval | Incubation Time | Manufacturer | |
|---|---|---|---|---|---|
| CD1a | MTB1 | 1:10 | Citrate (40 min) | overnight | Novocastra (Leica Biosystems, Deer Park, IL, USA) |
| CD1c | 5B8 | 1:200 | EDTA (30 min) | 30 min | Abcam (Cambridge, UK) |
| DC-LAMP | Rabbit polyclonal | 1:50 | EDTA (30 min) | 30 min | Novus Bilogicals (Centennial, CO, USA) |
| DC-SIGN | 5D7 | 1:50 | EDTA (30 min) | 30 min | Abcam (Cambridge, UK) |
| ER | 6F11 | 1:100 | Citrate (40 min) | 30 min | Novocastra (Leica Biosystems, Deer Park, IL, USA) |
| PR | PgR636 | 1:100 | Citrate (40 min) | 60 min | Dako (Agilent Technologies, Santa Clara, CA, USA) |
| Ki67 | MIB-1 | 1:100 | Citrate (40 min) | 30 min | Dako (Agilent Technologies, Santa Clara, CA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpor, J.; Streb, J.; Glajcar, A.; Sadowski, P.; Streb-Smoleń, A.; Jach, R.; Hodorowicz-Zaniewska, D. Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden. Int. J. Mol. Sci. 2022, 23, 8461. https://doi.org/10.3390/ijms23158461
Szpor J, Streb J, Glajcar A, Sadowski P, Streb-Smoleń A, Jach R, Hodorowicz-Zaniewska D. Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden. International Journal of Molecular Sciences. 2022; 23(15):8461. https://doi.org/10.3390/ijms23158461
Chicago/Turabian StyleSzpor, Joanna, Joanna Streb, Anna Glajcar, Piotr Sadowski, Anna Streb-Smoleń, Robert Jach, and Diana Hodorowicz-Zaniewska. 2022. "Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden" International Journal of Molecular Sciences 23, no. 15: 8461. https://doi.org/10.3390/ijms23158461
APA StyleSzpor, J., Streb, J., Glajcar, A., Sadowski, P., Streb-Smoleń, A., Jach, R., & Hodorowicz-Zaniewska, D. (2022). Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden. International Journal of Molecular Sciences, 23(15), 8461. https://doi.org/10.3390/ijms23158461







