BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders
Abstract
1. Introduction
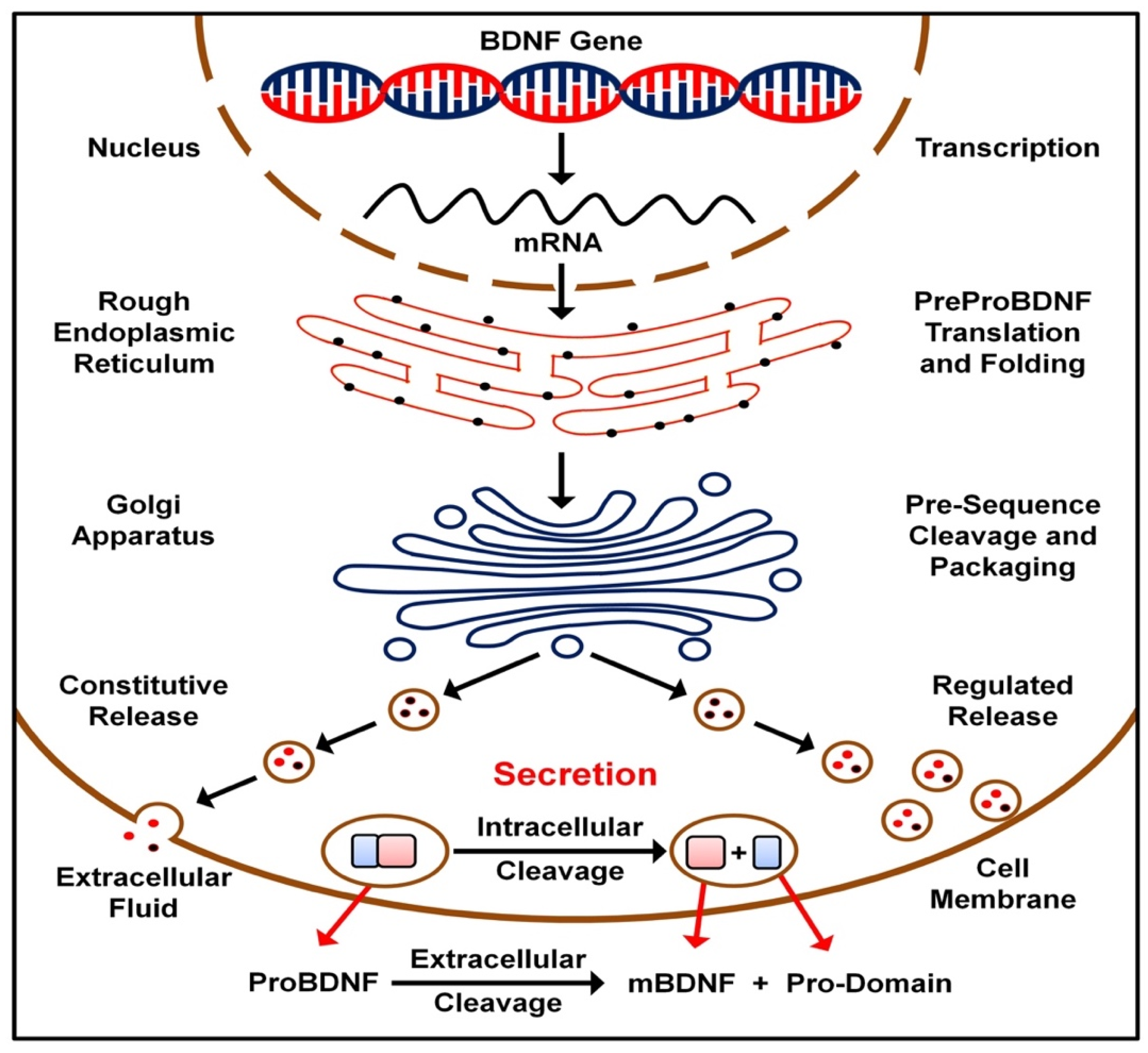
2. BDNF Transmission
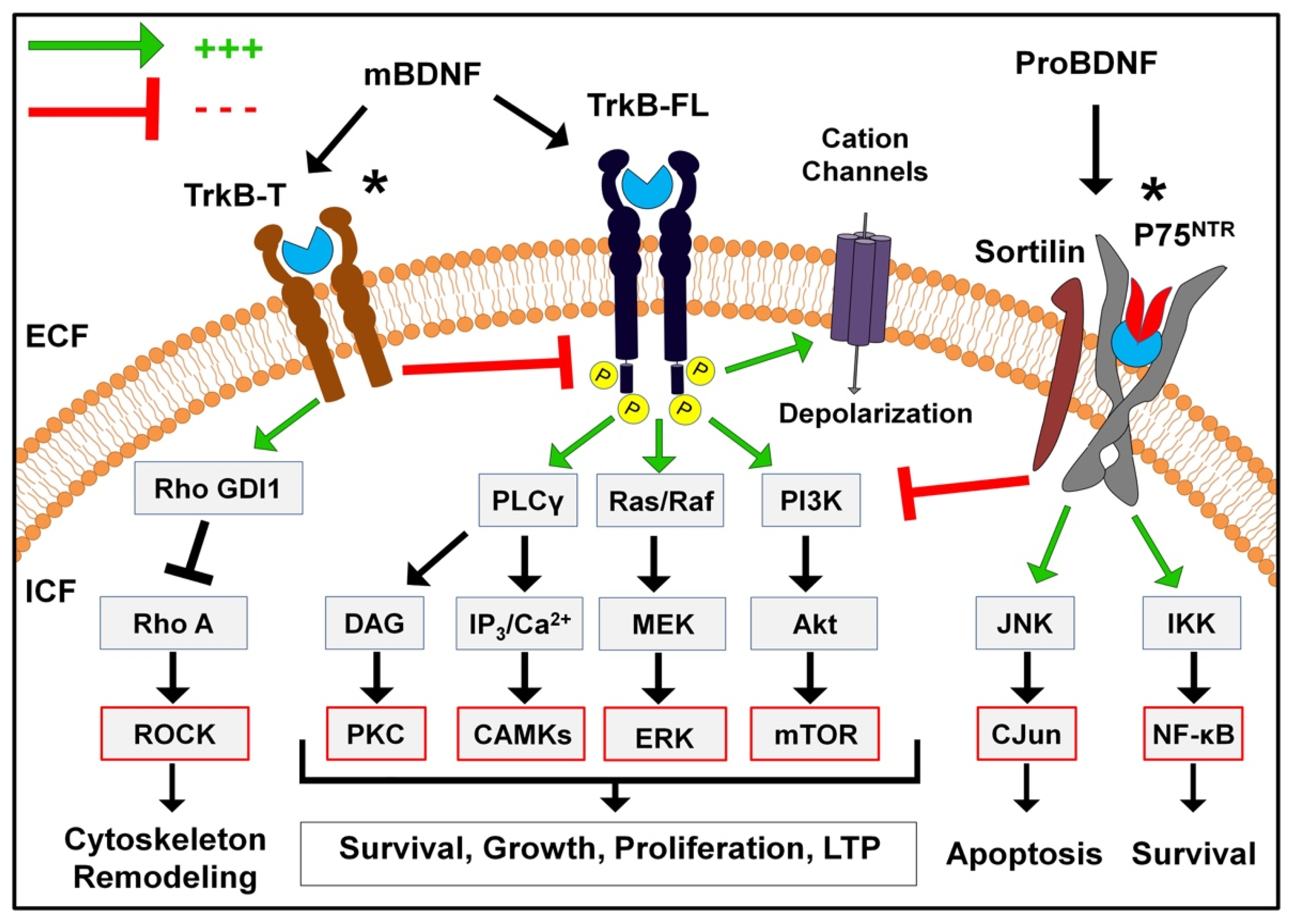
3. TrkB Receptor Signaling and Cellular Functions
3.1. TrkB Receptor Isoforms
3.2. Truncated TrkB Receptor
3.3. Full-Length TrkB Receptor
3.3.1. The PI3K/Akt Pathway
3.3.2. The MAPK Pathway
3.3.3. The PLC Pathway
3.3.4. Rapid Modulation of Ion Channels
4. Therapeutic BDNF Mechanisms
4.1. Neuronal Protection and Survival
4.2. Synaptic Maintenence
4.3. Immunomodulation
4.4. Plasticity Facilitation
4.5. Secondary Neuromodulation
4.6. Preservation of Neurovascular Unit Integrity
5. Neuropsychiatric Therapies Converge on BDNF: A Common Mediator
6. Current Status and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sahay, A.; Kale, A.; Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020, 83, 102075. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, R.H.; Marini, A.M. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann. N. Y. Acad. Sci. 2007, 1122, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Brigadski, T.; Leßmann, V. The physiology of regulated BDNF release. Cell Tissue Res. 2020, 382, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Boyle, P.A.; Schneider, J.A.; De Jager, P.L.; Bennett, D.A. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016, 86, 735–741. [Google Scholar] [CrossRef]
- Šerý, O.; Šťastný, F.; Zvolský, P.; Hlinomazová, Z.; Balcar, V.J. Association between Val66Met polymorphism of Brain-Derived Neurotrophic Factor (BDNF) gene and a deficiency of colour vision in alcohol-dependent male patients. Neurosci. Lett. 2011, 499, 154–157. [Google Scholar] [CrossRef]
- Singer, W.; Panford-Walsh, R.; Knipper, M. The function of BDNF in the adult auditory system. Neuropharmacology 2014, 76, 719–728. [Google Scholar] [CrossRef]
- Deveci, S.Ş.; Matur, Z.; Kesim, Y.Y.; Senturk, G.G.; Sargın-Kurt, G.G.; Ugur, S.A.; Öge, A.E. Effect of the brain-derived neurotrophic factor gene Val66Met polymorphism on sensory-motor integration during a complex motor learning exercise. Brain Res. 2020, 1732, 146652. [Google Scholar] [CrossRef]
- Kambeitz, J.P.; Bhattacharyya, S.; Kambeitz-Ilankovic, L.M.; Valli, I.; Collier, D.A.; McGuire, P. Effect of BDNF val66met polymorphism on declarative memory and its neural substrate: A meta-analysis. Neurosci. Biobehav. Rev. 2012, 36, 2165–2177. [Google Scholar] [CrossRef]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003, 23, 6690–6694. [Google Scholar] [CrossRef]
- Lipovich, L.; Dachet, F.; Cai, J.; Bagla, S.; Balan, K.; Jia, H.; Loeb, J.A. Activity-dependent human brain coding/noncoding gene regulatory networks. Genetics 2012, 192, 1133–1148. [Google Scholar] [CrossRef]
- Nanda, S.A.; Mack, K.J. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Mol. Brain Res. 2000, 78, 1–14. [Google Scholar] [CrossRef]
- Valles, A.; Boender, A.J.; Gijsbers, S.; Haast, R.A.; Martens, G.J.; de Weerd, P. Genomewide analysis of rat barrel cortex reveals time-and layer-specific mRNA expression changes related to experience-dependent plasticity. J. Neurosci. 2011, 31, 6140–6158. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Ying, Z.; Agoncillo, T.; Frostig, R. The influence of naturalistic experience on plasticity markers in somatosensory cortex and hippocampus: Effects of whisker use. Brain Res. 2011, 1388, 39–47. [Google Scholar] [CrossRef]
- Karpova, N.N.; Rantamäki, T.; Di Lieto, A.; Lindemann, L.; Hoener, M.C.; Castrén, E. Darkness reduces BDNF expression in the visual cortex and induces repressive chromatin remodeling at the BDNF gene in both hippocampus and visual cortex. Cell. Mol. Neurobiol. 2010, 30, 1117–1123. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef]
- Devlin, P.; Cao, X.; Stanfill, A.G. Genotype-expression interactions for BDNF across human brain regions. BMC Genom. 2021, 22, 207. [Google Scholar] [CrossRef]
- Shen, T.; You, Y.; Joseph, C.; Mirzaei, M.; Klistorner, A.; Graham, S.L.; Gupta, V. BDNF polymorphism: A review of its diagnostic and clinical relevance in neurodegenerative disorders. Aging Dis. 2018, 9, 523–536. [Google Scholar] [CrossRef]
- Ventriglia, M.; Zanardini, R.; Bonomini, C.; Zanetti, O.; Volpe, D.; Pasqualetti, P.; Gennarelli, M.; Bocchio-Chiavetto, L. Serum brain-derived neurotrophic factor levels in different neurological diseases. BioMed Res. Int. 2013, 2013, 901082. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Berk, M.; Turck, C.W.; Steiner, J.; Goncalves, C.A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: A comparative meta-analysis. Mol. Psychiatry 2014, 19, 750–751. [Google Scholar] [CrossRef]
- Hernández-Vara, J.; Sáez-Francàs, N.; Lorenzo-Bosquet, C.; Corominas-Roso, M.; Cuberas-Borròs, G.; Lucas-Del Pozo, S.; Carter, S.; Armengol-Bellapart, M.; Castell-Conesa, J. BDNF levels and nigrostriatal degeneration in “drug naïve” Parkinson’s disease patients. An “in vivo” study using I-123-FP-CIT SPECT. Parkinsonism Relat. Disord. 2020, 78, 31–35. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Mowla, S.J.; Pareek, S.; Farhadi, H.F.; Petrecca, K.; Fawcett, J.P.; Seidah, N.G.; Morris, S.J.; Sossin, W.S.; Murphy, R.A. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J. Neurosci. 1999, 19, 2069–2080. [Google Scholar] [CrossRef]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef]
- Magby, J.P.; Bi, C.; Chen, Z.Y.; Lee, F.S.; Plummer, M.R. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J. Neurosci. 2006, 26, 13531–13536. [Google Scholar] [CrossRef]
- Choo, M.; Miyazaki, T.; Yamazaki, M.; Kawamura, M.; Nakazawa, T.; Zhang, J.; Tanimura, A.; Uesaka, N.; Watanabe, M.; Sakimura, K.; et al. Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat. Commun. 2017, 8, 195. [Google Scholar] [CrossRef]
- Yang, J.; Siao, C.J.; Nagappan, G.; Marinic, T.; Jing, D.; McGrath, K.; Chen, Z.Y.; Mark, W.; Tessarollo, L.; Lee, F.S.; et al. Neuronal release of proBDNF. Nat. Neurosci. 2009, 12, 113–115. [Google Scholar] [CrossRef]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef]
- Woo, N.H.; Teng, H.K.; Siao, C.J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Ruan, C.S.; Yang, C.R.; Li, J.Y.; Kang, Z.L.; Zhou, L.; Liu, D.; Zeng, Y.Q.; Wang, T.H.; Tian, C.F.; et al. ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology 2016, 41, 2882–2892. [Google Scholar] [CrossRef]
- Zhong, F.; Liu, L.; Wei, J.L.; Hu, Z.L.; Li, L.; Wang, S.; Xu, J.M.; Zhou, X.F.; Li, C.Q.; Yang, Z.Y.; et al. Brain-derived neurotrophic factor precursor in the hippocampus regulates both depressive and anxiety-like behaviors in rats. Front. Psychiatry 2019, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Bartrup, J.T.; Moorman, J.M.; Newberry, N.R. BDNF enhances neuronal growth and synaptic activity in hippocampal cell cultures. Neuroreport 1997, 8, 3791–3794. [Google Scholar] [CrossRef] [PubMed]
- De Vincenti, A.P.; Ríos, A.S.; Paratcha, G.; Ledda, F. Mechanisms that modulate and diversify BDNF functions: Implications for hippocampal synaptic plasticity. Front. Cell. Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lu, Y.; Yang, F.; Shen, W.; Tang, T.T.; Feng, L.; Duan, S.; Lu, B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010, 13, 302–309. [Google Scholar] [CrossRef]
- Guo, W.; Nagappan, G.; Lu, B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev. Neurobiol. 2018, 78, 647–659. [Google Scholar] [CrossRef]
- Nagappan, G.; Lu, B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005, 28, 464–471. [Google Scholar] [CrossRef]
- Jang, M.; Gould, E.; Xu, J.; Kim, E.J.; Kim, J.H. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. eLife 2019, 8, e42156. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef]
- Bergami, M.; Santi, S.; Formaggio, E.; Cagnoli, C.; Verderio, C.; Blum, R.; Berninger, B.; Matteoli, M.; Canossa, M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J. Cell Biol. 2008, 183, 213–221. [Google Scholar] [CrossRef]
- Lalo, U.; Bogdanov, A.; Moss, G.W.; Pankratov, Y. Astroglia-derived BDNF and MSK-1 mediate experience-and diet-dependent synaptic plasticity. Brain Sci. 2020, 10, 462. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Stoilov, P.; Castren, E.; Stamm, S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 2002, 290, 1054–1065. [Google Scholar] [CrossRef]
- Baxter, G.T.; Radeke, M.J.; Kuo, R.C.; Makrides, V.; Hinkle, B.; Hoang, R.; Medina-Selby, A.; Coit, D.; Valenzuela, P.; Feinstein, S.C. Signal transduction mediated by the truncated trkB receptor isoforms, trkB. T1 and trkB. T2. J. Neurosci. 1997, 17, 2683–2690. [Google Scholar] [CrossRef]
- Cheng, A.; Coksaygan, T.; Tang, H.; Khatri, R.; Balice-Gordon, R.J.; Rao, M.S.; Mattson, M.P. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J. Neurochem. 2007, 100, 1515–1530. [Google Scholar] [CrossRef]
- Yacoubian, T.A.; Lo, D.C. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat. Neurosci. 2000, 3, 342–349. [Google Scholar] [CrossRef]
- Fenner, B.M. Truncated TrkB: Beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012, 23, 15–24. [Google Scholar] [CrossRef]
- Rose, C.R.; Blum, R.; Pichler, B.; Lepier, A.; Kafitz, K.W.; Konnerth, A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature 2003, 426, 74–78. [Google Scholar] [CrossRef]
- Holt, L.M.; Hernandez, R.D.; Pacheco, N.L.; Ceja, B.T.; Hossain, M.; Olsen, M.L. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB. T1. eLife 2019, 8, e44667. [Google Scholar] [CrossRef]
- Ameroso, D.; Meng, A.; Chen, S.; Felsted, J.; Dulla, C.G.; Rios, M. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat. Metab. 2022, 4, 627–643. [Google Scholar] [CrossRef]
- Ohira, K.; Homma, K.J.; Hirai, H.; Nakamura, S.; Hayashi, M. TrkB-T1 regulates the RhoA signaling and actin cytoskeleton in glioma cells. Biochem. Biophys. Res. Commun. 2006, 342, 867–874. [Google Scholar] [CrossRef]
- Aroeira, R.I.; Sebastião, A.M.; Valente, C.A. BDNF, via truncated TrkB receptor, modulates GlyT1 and GlyT2 in astrocytes. Glia 2015, 63, 2181–2197. [Google Scholar] [CrossRef] [PubMed]
- Carim-Todd, L.; Bath, K.G.; Fulgenzi, G.; Yanpallewar, S.; Jing, D.; Barrick, C.A.; Becker, J.; Buckley, H.; Dorsey, S.G.; Lee, F.S.; et al. Endogenous truncated TrkB. T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J. Neurosci. 2009, 29, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Eide, F.F.; Vining, E.R.; Eide, B.L.; Zang, K.; Wang, X.Y.; Reichardt, L.F. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J. Neurosci. 1996, 16, 3123–3129. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Matyas, J.J.; Renn, C.L.; Faden, A.I.; Dorsey, S.G.; Wu, J. Function and mechanisms of truncated BDNF receptor TrkB. T1 in neuropathic pain. Cells 2020, 9, 1194. [Google Scholar] [CrossRef]
- Ninkina, N.; Adu, J.; Fischer, A.; Pinon, L.G.; Buchman, V.L.; Davies, A.M. Expression and function of TrkB variants in developing sensory neurons. EMBO J. 1996, 15, 6385–6393. [Google Scholar] [CrossRef]
- He, J.; Gong, H.; Luo, Q. BDNF acutely modulates synaptic transmission and calcium signalling in developing cortical neurons. Cell. Physiol. Biochem. 2005, 16, 69–76. [Google Scholar] [CrossRef]
- Elmariah, S.B.; Crumling, M.A.; Parsons, T.D.; Balice-Gordon, R.J. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J. Neurosci. 2004, 24, 2380–2393. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Gupta, V.K.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. TrkB receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. Pi3k-pkb/akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- Yao, R.Q.; Qi, D.S.; Yu, H.L.; Liu, J.; Yang, L.H.; Wu, X.X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF–TrkB–PI3K/Akt signaling pathway. Neurochem. Res. 2012, 37, 2777–2786. [Google Scholar] [CrossRef]
- Qi, D.; Ouyang, C.; Wang, Y.; Zhang, S.; Ma, X.; Song, Y.; Yu, H.; Tang, J.; Fu, W.; Sheng, L.; et al. HO-1 attenuates hippocampal neurons injury via the activation of BDNF–TrkB–PI3K/Akt signaling pathway in stroke. Brain Res. 2014, 1577, 69–76. [Google Scholar] [CrossRef]
- Xiang, J.; Pan, J.; Chen, F.; Zheng, L.; Chen, Y.; Zhang, S.; Feng, W. L-3-n-butylphthalide improves cognitive impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int. J. Clin. Exp. Med. 2014, 7, 1706–1713. [Google Scholar]
- Jin, T.; Zhang, Y.; Benson, O.A.; Zhang, J.; Fan, R.; Zhang, Y.; Liu, X. Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food. Chem. Toxicol. 2022, 164, 113091. [Google Scholar] [CrossRef]
- Wu, H.; Lu, D.; Jiang, H.; Xiong, Y.; Qu, C.; Li, B.; Mahmood, A.; Zhou, D.; Chopp, M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma 2008, 25, 130–139. [Google Scholar] [CrossRef]
- Mao, X.Y.; Zhou, H.H.; Li, X.; Liu, Z.Q. Huperzine A alleviates oxidative glutamate toxicity in hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway. Cell. Mol. Neurobiol. 2016, 36, 915–925. [Google Scholar] [CrossRef]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 2007, 10, 702–711. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, Y.; He, T.; Li, W.; Li, Q.; Miao, Y. Brain-derived neurotrophic factor inhibits hyperglycemia-induced apoptosis and downregulation of synaptic plasticity-related proteins in hippocampal neurons via the PI3K/Akt pathway. Int. J. Mol. Med. 2019, 43, 294–304. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Chenodeoxycholic acid ameliorates AlCl3-induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules 2019, 24, 1992. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Chenodeoxycholic acid reduces neuroinflammation and oxidative stress in aluminium chloride-induced rat model of Alzheimer’s disease. Br. J. Pharmacol. 2021, 178, 458. [Google Scholar]
- Kumar, V.; Zhang, M.X.; Swank, M.W.; Kunz, J.; Wu, G.Y. Regulation of dendritic morphogenesis by Ras–PI3K–Akt–mTOR and Ras–MAPK signaling pathways. J. Neurosci. 2005, 25, 11288–11299. [Google Scholar] [CrossRef]
- Aksamitiene, E.; Kiyatkin, A.; Kholodenko, B.N. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: A fine balance. Biochem. Soc. Trans. 2012, 40, 139–146. [Google Scholar] [CrossRef]
- Wilkinson, M.G.; Millar, J.B. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J. 2000, 14, 2147–2157. [Google Scholar] [CrossRef]
- Bonni, A.; Brunet, A.; West, A.E.; Datta, S.R.; Takasu, M.A.; Greenberg, M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science 1999, 286, 1358–1362. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Cohen, M.S.; Orth, C.B.; Kim, H.J.; Jeon, N.L.; Jaffrey, S.R. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc. Natl. Acad. Sci. USA 2011, 20108, 11246–11251. [Google Scholar] [CrossRef]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef]
- Marte, A.; Messa, M.; Benfenati, F.; Onofri, F. Synapsins are downstream players of the BDNF-mediated axonal growth. Mol. Neurobiol. 2017, 54, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Song, S.H.; Augustine, G.J. Calcium-dependent and synapsin-dependent pathways for the presynaptic actions of BDNF. Front. Cell. Neurosci. 2017, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Keifer, J. BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol. Learn. Mem. 2009, 91, 243–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reimers, J.M.; Loweth, J.A.; Wolf, M.E. BDNF contributes to both rapid and homeostatic alterations in AMPA receptor surface expression in nucleus accumbens medium spiny neurons. Eur. J. Neurosci. 2014, 39, 1159–1169. [Google Scholar] [CrossRef]
- Quintero, G.C. Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatr. Dis. Treat. 2013, 9, 1499–1512. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Li, H.S.; Xu, X.Z.; Montell, C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 1999, 24, 261–273. [Google Scholar] [CrossRef]
- Crozier, R.A.; Black, I.B.; Plummer, M.R. Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn. Mem. 1999, 6, 257–266. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Monji, A.; Nabekura, J. Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons. Eur. J. Neurosci. 2002, 16, 1417–1424. [Google Scholar] [CrossRef]
- Nieto-Gonzalez, J.L.; Jensen, K. BDNF depresses excitability of parvalbumin-positive interneurons through an M-like current in rat dentate gyrus. PLoS ONE 2013, 8, e67318. [Google Scholar] [CrossRef]
- Matsumoto, T.; Numakawa, T.; Adachi, N.; Yokomaku, D.; Yamagishi, S.; Takei, N.; Hatanaka, H. Brain-derived neurotrophic factor enhances depolarization-evoked glutamate release in cultured cortical neurons. J. Neurochem. 2001, 79, 522–530. [Google Scholar] [CrossRef]
- Takei, N.; Numakawa, T.; Kozaki, S.; Sakai, N.; Endo, Y.; Takahashi, M.; Hatanaka, H. Brain-derived neurotrophic factor induces rapid and transient release of glutamate through the non-exocytotic pathway from cortical neurons. J. Biol. Chem. 1998, 273, 27620–27624. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Matsumoto, T.; Adachi, N.; Yokomaku, D.; Kojima, M.; Takei, N.; Hatanaka, H. Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca2+ and Na+ in cultured cerebellar neurons. J. Neurosci. Res. 2001, 66, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Kanhema, T.; Ying, S.W.; Nairn, A.C.; Bramham, C.R. BDNF-induced long-term potentiation in the dentate gyrus in vivo is associated with phosphorylation of elongation factor-2. Soc. Neurosci. Abstr. 2001, 27, 920.17. [Google Scholar]
- Caldeira, M.V.; Melo, C.V.; Pereira, D.B.; Carvalho, R.; Correia, S.S.; Backos, D.S.; Carvalho, A.L.; Esteban, J.A.; Duarte, C.B. Brain-derived neurotrophic factor regulates the Expression and synaptic delivery ofα-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Biol. Chem. 2007, 282, 12619–12628. [Google Scholar] [CrossRef]
- Blanquet, P.R.; Mariani, J.; Derer, P. A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience 2003, 18, 477–490. [Google Scholar] [CrossRef]
- Hellmann, J.; Rommelspacher, H.; Wernicke, C. Long-term ethanol exposure impairs neuronal differentiation of human neuroblastoma cells involving neurotrophin-mediated intracellular signaling and in particular protein kinase C. Alcohol. Clin. Exp. Res. 2009, 33, 538–550. [Google Scholar] [CrossRef]
- Zirrgiebel, U.; Ohga, Y.; Carter, B.; Berninger, B.; Inagaki, N.; Thoenen, H.; Lindholm, D. Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: Involvement of protein kinase C in neuronal survival. J. Neurochem. 1995, 65, 2241–2250. [Google Scholar] [CrossRef]
- Sotogaku, N.; Tully, S.E.; Gama, C.I.; Higashi, H.; Tanaka, M.; Hsieh-Wilson, L.C.; Nishi, A. Activation of phospholipase C pathways by a synthetic chondroitin sulfate-E tetrasaccharide promotes neurite outgrowth of dopaminergic neurons. J. Neurochem. 2007, 103, 749–760. [Google Scholar] [CrossRef]
- Liu, M.; Kay, J.C.; Shen, S.; Qiao, L.Y. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J. Neuroinflamm. 2015, 12, 151. [Google Scholar] [CrossRef]
- Tao, W.; Chen, Q.; Zhou, W.; Wang, Y.; Wang, L.; Zhang, Z. Persistent inflammation-induced up-regulation of brain-derived neurotrophic factor (BDNF) promotes synaptic delivery of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor GluA1 subunits in descending pain modulatory circuits. J. Biol. Chem. 2014, 289, 22196–22204. [Google Scholar] [CrossRef]
- Roberts, D.S.; Hu, Y.; Lund, I.V.; Brooks-Kayal, A.R.; Russek, S.J. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type a GABA Receptorα4 subunits in hippocampal neurons. J. Biol. Chem. 2006, 281, 29431–29435. [Google Scholar] [CrossRef]
- Mou, L.; Heldt, S.A.; Ressler, K. Rapid BDNF-dependent sequestration of amygdala and hippocampal GABAA receptors via different TrkB-mediated phosphorylation pathways. Neuroscience 2011, 176, 72–85. [Google Scholar] [CrossRef]
- Agosto-Marlin, I.M.; Mitchell, G.S. Spinal BDNF-induced phrenic motor facilitation requires PKCθ activity. J. Neurophysiol. 2017, 118, 2755–2762. [Google Scholar] [CrossRef]
- Colgan, L.A.; Hu, M.; Misler, J.A.; Parra-Bueno, P.; Moran, C.M.; Leitges, M.; Yasuda, R. PKCα integrates spatiotemporally distinct Ca2+ and autocrine BDNF signaling to facilitate synaptic plasticity. Nat. Neurosci. 2018, 21, 1027–1037. [Google Scholar] [CrossRef]
- Kafitz, K.W.; Rose, C.R.; Thoenen, H.; Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 1999, 401, 918–921. [Google Scholar] [CrossRef]
- Kovalchuk, Y.; Holthoff, K.; Konnerth, A. Neurotrophin action on a rapid timescale. Curr. Opin. Neurobiol. 2004, 14, 558–563. [Google Scholar] [CrossRef]
- Blum, R.; Kafitz, K.W.; Konnerth, A. Neurotrophin-evoked depolarization requires the sodium channel NaV1. 9. Nature 2002, 419, 687–693. [Google Scholar] [CrossRef]
- Kovalchuk, Y.; Hanse, E.; Kafitz, K.W.; Konnerth, A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 2002, 295, 1729–1734. [Google Scholar] [CrossRef]
- Ahn, M.; Beacham, D.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Regulation of NaV1. 2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J. Neurosci. 2007, 27, 11533–11542. [Google Scholar] [CrossRef][Green Version]
- Linnarsson, S.; Willson, C.A.; Ernfors, P. Cell death in regenerating populations of neurons in BDNF mutant mice. Mol. Brain Res. 2000, 75, 61–69. [Google Scholar] [CrossRef]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Nonomura, T.; Enokido, Y.; Hatanaka, H. Brain-derived neurotrophic factor (BDNF) can prevent apoptosis of rat cerebellar granule neurons in culture. Dev. Brain Res. 1995, 85, 249–258. [Google Scholar] [CrossRef]
- Han, B.H.; D’Costa, A.; Back, S.A.; Parsadanian, M.; Patel, S.; Shah, A.R.; Gidday, J.M.; Srinivasan, A.; Deshmukh, M.; Holtzman, D.M. BDNF blocks caspase-3 activation in neonatal hypoxia–ischemia. Neurobiol. Dis. 2000, 7, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, F.; Naska, S.; Bozzi, Y. BDNF down-regulates the caspase 3 pathway in injured geniculo-cortical neurones. Neuroreport 2004, 15, 2045–2049. [Google Scholar] [CrossRef]
- Hua, Z.; Zhan, Y.; Zhang, S.; Dong, Y.; Jiang, M.; Tan, F.; Liu, Z.; Thiele, C.J.; Li, Z. P53/PUMA are potential targets that mediate the protection of brain-derived neurotrophic factor (BDNF)/TrkB from etoposide-induced cell death in neuroblastoma (NB). Apoptosis 2018, 23, 408–419. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Graos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death. Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Glazner, G.W.; Mattson, M.P. Differential effects of BDNF, ADNF9, and TNFα on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp. Neurol. 2000, 161, 442–452. [Google Scholar] [CrossRef]
- Bifrare, Y.D.; Kummer, J.; Joss, P.; Täuber, M.G.; Leib, S.L. Brain-derived neurotrophic factor protects against multiple forms of brain injury in bacterial meningitis. J. Infect. Dis. 2005, 191, 40–45. [Google Scholar] [CrossRef][Green Version]
- Hisatomi, T.; Sakamoto, T.; Murata, T.; Yamanaka, I.; Oshima, Y.; Hata, Y.; Ishibashi, T.; Inomata, H.; Susin, S.A.; Kroemer, G. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am. J. Pathol. 2001, 158, 1271–1278. [Google Scholar] [CrossRef]
- Tong, L.; Perez-Polo, R. Brain-derived neurotrophic factor (BDNF) protects cultured rat cerebellar granule neurons against glucose deprivation-induced apoptosis. J. Neural Transm. 1998, 105, 905–914. [Google Scholar] [CrossRef]
- Spina, M.B.; Squinto, S.P.; Miller, J.; Lindsay, R.M.; Hyman, C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: Involvement of the glutathione system. J. Neurochem. 1992, 59, 99–106. [Google Scholar] [CrossRef]
- Mattson, M.P.; Lovell, M.A.; Furukawa, K.; Markesbery, W.R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995, 65, 1740–1751. [Google Scholar] [CrossRef]
- Ikeda, O.; Murakami, M.; Ino, H.; Yamazaki, M.; Koda, M.; Nakayama, C.; Moriya, H. Effects of brain-derived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of superoxide dismutase expression and promotes up-regulation of myelin basic protein expression. J. Neuropathol. Exp. Neurol. 2002, 61, 142–153. [Google Scholar] [CrossRef]
- Wu, C.L.; Chen, C.H.; Hwang, C.S.; Chen, S.D.; Hwang, W.C.; Yang, D.I. Roles of p62 in BDNF-dependent autophagy suppression and neuroprotection against mitochondrial dysfunction in rat cortical neurons. J. Neurochem. 2017, 140, 845–861. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More insight into BDNF against neurodegeneration: Anti-apoptosis, anti-oxidation, and suppression of autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Subramanian, J.; Tremblay, M.È. Synaptic Loss and Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 681029. [Google Scholar] [CrossRef]
- Bolton, M.M.; Pittman, A.J.; Lo, D.C. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J. Neurosci. 2000, 20, 3221–3232. [Google Scholar] [CrossRef]
- Jin, X.; Hu, H.; Mathers, P.H.; Agmon, A. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J. Neurosci. 2003, 23, 5662–5673. [Google Scholar] [CrossRef]
- Lazo, O.M.; Gonzalez, A.; Ascano, M.; Kuruvilla, R.; Couve, A.; Bronfman, F.C. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J. Neurosci. 2013, 33, 6112–6122. [Google Scholar] [CrossRef]
- Bamji, S.X.; Rico, B.; Kimes, N.; Reichardt, L.F. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin–β-catenin interactions. J. Cell Biol. 2006, 174, 289–299. [Google Scholar] [CrossRef]
- Horch, H.W. Local effects of BDNF on dendritic growth. Rev. Neurosci. 2004, 15, 117–130. [Google Scholar] [CrossRef]
- Horvath, P.M.; Chanaday, N.L.; Alten, B.; Kavalali, E.T.; Monteggia, L.M. A subthreshold synaptic mechanism regulating BDNF expression and resting synaptic strength. Cell Rep. 2021, 36, 109467. [Google Scholar] [CrossRef]
- Dean, C.; Liu, H.; Mark Dunning, F.; Chang, P.Y.; Jackson, M.B.; Chapman, E.R. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat. Neurosci. 2009, 12, 767–776. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Sun, M.K.; Alkon, D.L. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J. Neurosci. 2011, 31, 630–643. [Google Scholar] [CrossRef]
- de Pins, B.; Cifuentes-Díaz, C.; Farah, A.T.; López-Molina, L.; Montalban, E.; Sancho-Balsells, A.; López, A.; Ginés, S.; Delgado-García, J.M.; Alberch, J.; et al. Conditional BDNF delivery from astrocytes rescues memory deficits, spine density, and synaptic properties in the 5xFAD mouse model of Alzheimer disease. J. Neurosci. 2019, 39, 2441–2458. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7, 8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Alterations in immune cells and mediators in the brain: It’s not always neuroinflammation! Brain Pathol. 2014, 24, 623–630. [Google Scholar] [CrossRef]
- Hong, H.; Kim, B.S.; Im, H.I. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int. Neurourol. J. 2016, 20, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, L.; Yu, T.; Xu, Y.; Pu, S.; Du, D.; Jiang, W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell. Physiol. Biochem. 2014, 34, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, J.; Su, M.; Lin, Z.; Zhan, H.; Yang, F.; Li, W.; Xie, J.; Huang, Y.; Liu, X.; et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflamm. 2020, 17, 19. [Google Scholar] [CrossRef]
- Onodera, J.; Nagata, H.; Nakashima, A.; Ikegaya, Y.; Koyama, R. Neuronal brain-derived neurotrophic factor manipulates microglial dynamics. Glia 2021, 69, 890–904. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.; Bromberg, E.; de Vries, E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Han, R.; Liu, Z.; Sun, N.; Liu, S.; Li, L.; Shen, Y.; Xiu, J.; Xu, Q. BDNF alleviates neuroinflammation in the hippocampus of type 1 diabetic mice via blocking the aberrant HMGB1/RAGE/NF-κB pathway. Aging Dis. 2019, 10, 611. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef]
- Kim, O.Y.; Song, J. The importance of BDNF and RAGE in diabetes-induced dementia. Pharmacol. Res. 2020, 160, 105083. [Google Scholar] [CrossRef]
- Wu, S.Y.; Pan, B.S.; Tsai, S.F.; Chiang, Y.T.; Huang, B.M.; Mo, F.E.; Kuo, Y.M. BDNF reverses aging-related microglial activation. J. Neuroinflamm. 2020, 17, 210. [Google Scholar] [CrossRef]
- Bovolenta, R.; Zucchini, S.; Paradiso, B.; Rodi, D.; Merigo, F.; Mora, G.N.; Osculati, F.; Berto, E.; Marconi, P.; Marzola, A.; et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J. Neuroinflamm. 2010, 7, 81. [Google Scholar] [CrossRef]
- Hsu, C.C.; Kuo, T.W.; Liu, W.P.; Chang, C.P.; Lin, H.J. Calycosin preserves BDNF/TrkB signaling and reduces post-stroke neurological injury after cerebral ischemia by reducing accumulation of hypertrophic and TNF-α-containing microglia in rats. J. Neuroimmune Pharmacol. 2020, 15, 326–339. [Google Scholar] [CrossRef]
- Li, W.; Ali, T.; Zheng, C.; He, K.; Liu, Z.; Shah, F.A.; Li, N.; Yu, Z.J.; Li, S. Anti-depressive-like behaviors of APN KO mice involve Trkb/BDNF signaling related neuroinflammatory changes. Mol. Psychiatry 2022, 27, 1047–1058. [Google Scholar] [CrossRef]
- Makar, T.K.; Nimmagadda, V.K.; Singh, I.S.; Lam, K.; Mubariz, F.; Judge, S.I.; Trisler, D.; Bever, C.T., Jr. TrkB agonist, 7,8-dihydroxyflavone, reduces the clinical and pathological severity of a murine model of multiple sclerosis. J. Neuroimmunol. 2016, 292, 9–20. [Google Scholar] [CrossRef]
- Yin, R.; Zhao, S.; Qiu, C. Brain-derived neurotrophic factor fused with a collagen-binding domain inhibits neuroinflammation and promotes neurological recovery of traumatic brain injury mice via TrkB signalling. J. Pharm. Pharmacol. 2020, 72, 539–550. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic plasticity forms and functions. Ann. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Bazzari, A.H.; Parri, H.R. Neuromodulators and long-term synaptic plasticity in learning and memory: A steered-glutamatergic perspective. Brain Sci. 2019, 9, 300. [Google Scholar] [CrossRef]
- Nakata, H.; Nakamura, S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007, 581, 2047–2054. [Google Scholar] [CrossRef]
- Gangarossa, G.; Perez, S.; Dembitskaya, Y.; Prokin, I.; Berry, H.; Venance, L. BDNF controls bidirectional endocannabinoid plasticity at corticostriatal synapses. Cereb. Cortex 2020, 30, 197–214. [Google Scholar] [CrossRef]
- Gottschalk, W.; Pozzo-Miller, L.D.; Figurov, A.; Lu, B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J. Neurosci. 1998, 18, 6830–6839. [Google Scholar] [CrossRef] [PubMed]
- Kramár, E.A.; Lin, B.; Lin, C.Y.; Arai, A.C.; Gall, C.M.; Lynch, G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J. Neurosci. 2004, 24, 5151–5161. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cheng, P.L.; Lim, B.K.; Khoshnevisrad, N.; Poo, M.M. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 2010, 67, 821–833. [Google Scholar] [CrossRef]
- Fujisawa, S.; Yamada, M.K.; Nishiyama, N.; Matsuki, N.; Ikegaya, Y. BDNF boosts spike fidelity in chaotic neural oscillations. Biophys. J. 2004, 86, 1820–1828. [Google Scholar] [CrossRef]
- Niculescu, D.; Michaelsen-Preusse, K.; Güner, Ü.; van Dorland, R.; Wierenga, C.J.; Lohmann, C. A BDNF-mediated push-pull plasticity mechanism for synaptic clustering. Cell Rep. 2018, 24, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Sidiropoulou, K.; Kallergi, E.; Dalezios, Y.; Tavernarakis, N. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab. 2017, 26, 230–242. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Nagappan, G.; Ke, Y.; Sacktor, T.C.; Lu, B. BDNF facilitates L-LTP maintenance in the absence of protein synthesis through PKMζ. PLoS ONE 2011, 6, e21568. [Google Scholar] [CrossRef]
- Panja, D.; Kenney, J.W.; D’Andrea, L.; Zalfa, F.; Vedeler, A.; Wibrand, K.; Fukunaga, R.; Bagni, C.; Proud, C.G.; Bramham, C.R. Two-stage translational control of dentate gyrus LTP consolidation is mediated by sustained BDNF-TrkB signaling to MNK. Cell Rep. 2014, 9, 1430–1445. [Google Scholar] [CrossRef]
- Dincheva, I.; Glatt, C.E.; Lee, F.S. Impact of the BDNF Val66Met polymorphism on cognition: Implications for behavioral genetics. Neuroscientist 2012, 18, 439–451. [Google Scholar] [CrossRef]
- Ninan, I.; Bath, K.G.; Dagar, K.; Perez-Castro, R.; Plummer, M.R.; Lee, F.S.; Chao, M.V. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 2010, 30, 8866–8870. [Google Scholar] [CrossRef]
- Pattwell, S.S.; Bath, K.G.; Perez-Castro, R.; Lee, F.S.; Chao, M.V.; Ninan, I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J. Neurosci. 2012, 32, 2410–2421. [Google Scholar] [CrossRef]
- Galvin, C.; Lee, F.S.; Ninan, I. Alteration of the centromedial amygdala glutamatergic synapses by the BDNF Val66Met polymorphism. Neuropsychopharmacology 2015, 40, 2269–2277. [Google Scholar] [CrossRef]
- Jing, D.; Lee, F.S.; Ninan, I. The BDNF Val66Met polymorphism enhances glutamatergic transmission but diminishes activity-dependent synaptic plasticity in the dorsolateral striatum. Neuropharmacology 2017, 112, 84–93. [Google Scholar] [CrossRef]
- Lu, B.D.; Nagappan, G.; Lu, Y.B. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar]
- Criscuolo, C.; Fabiani, C.; Bonadonna, C.; Origlia, N.; Domenici, L. BDNF prevents amyloid-dependent impairment of LTP in the entorhinal cortex by attenuating p38 MAPK phosphorylation. Neurobiol. Aging 2015, 36, 1303–1309. [Google Scholar] [CrossRef]
- Simmons, D.A.; Rex, C.S.; Palmer, L.; Pandyarajan, V.; Fedulov, V.; Gall, C.M.; Lynch, G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4906–4911. [Google Scholar] [CrossRef]
- Lauterborn, J.C.; Rex, C.S.; Kramár, E.; Chen, L.Y.; Pandyarajan, V.; Lynch, G.; Gall, C.M. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J. Neurosci. 2007, 27, 10685–10694. [Google Scholar] [CrossRef]
- Xie, H.; Leung, K.L.; Chen, L.; Chan, Y.S.; Ng, P.C.; Fok, T.F.; Wing, Y.K.; Ke, Y.; Li, A.M.; Yung, W.H. Brain-derived neurotrophic factor rescues and prevents chronic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol. Dis. 2010, 40, 155–162. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, Y.K.; Wang, W.; Wan, J.G.; Yu, B.; Wang, M.Z.; Hu, B. Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol. Biochem. Behav. 2014, 122, 30–36. [Google Scholar] [CrossRef]
- Li, M.; Dai, F.R.; Du, X.P.; Yang, Q.D.; Zhang, X.; Chen, Y. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behav. Brain Res. 2012, 231, 146–153. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D. Neuronal plasticity: Neuronal organization is associated with neurological disorders. J. Mol. Neurosci. 2020, 70, 1684–1701. [Google Scholar] [CrossRef]
- Pittenger, C. Disorders of memory and plasticity in psychiatric disease. Dialogues Clin. Neurosci. 2013, 15, 455–463. [Google Scholar] [CrossRef]
- Vose, L.R.; Stanton, P.K. Synaptic plasticity, metaplasticity and depression. Curr. Neuropharmacol. 2017, 15, 71–86. [Google Scholar] [CrossRef]
- Zhou, L.J.; Zhong, Y.; Ren, W.J.; Li, Y.Y.; Zhang, T.; Liu, X.G. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp. Neurol. 2008, 212, 507–514. [Google Scholar] [CrossRef]
- Groth, R.; Aanonsen, L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain 2002, 100, 171–181. [Google Scholar] [CrossRef]
- Bazzari, A.H.; Bazzari, F.H. Advances in targeting central sensitization and brain plasticity in chronic pain. Egypt. J. Neurol. Psychiatr. Neurosurg. 2022, 58, 38. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.; Pu, Y.; Jiang, D.; Jiang, X.; Zhang, Y.; Tao, J. Brain-derived neurotrophic factor stimulation of T-type Ca2+ channels in sensory neurons contributes to increased peripheral pain sensitivity. Sci. Signal. 2019, 12, eaaw2300. [Google Scholar] [CrossRef]
- Luo, D.; Luo, L.; Lin, R.; Lin, L.; Lin, Q. Brain-derived neurotrophic factor and Glial cell line-derived neurotrophic factor expressions in the trigeminal root entry zone and trigeminal ganglion neurons of a trigeminal neuralgia rat model. Anat. Rec. 2020, 303, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahashi, M.; Kitagawa, J.; Kanazawa, T.; Nasu, M.; Matsumoto, S. Brain-derived neurotrophic factor enhances the excitability of small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone following masseter muscle inflammation. Mol. Pain 2013, 9, 49. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Bazzari, A.H. Orofacial neuropathic pain: A pharmacological approach. S. Afr. Pharm. J. 2019, 86, 23–28. [Google Scholar]
- Siuciak, J.A.; Boylan, C.; Fritsche, M.; Altar, C.A.; Lindsay, R.M. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996, 710, 11–20. [Google Scholar] [CrossRef]
- Altar, C.A.; Boylan, C.B.; Fritsche, M.; Jackson, C.; Hyman, C.; Lindsay, R.M. The neurotrophins NT-4/5 and BDNF augment serotonin, dopamine, and GABAergic systems during behaviorally effective infusions to the substantia nigra. Exp. Neurol. 1994, 130, 31–40. [Google Scholar] [CrossRef]
- Goggi, J.; Pullar, I.A.; Carney, S.L.; Bradford, H.F. Signalling pathways involved in the short-term potentiation of dopamine release by BDNF. Brain Res. 2003, 968, 156–161. [Google Scholar] [CrossRef]
- Paredes, D.; Granholm, A.C.; Bickford, P.C. Effects of NGF and BDNF on baseline glutamate and dopamine release in the hippocampal formation of the adult rat. Brain. Res. 2007, 1141, 56–64. [Google Scholar] [CrossRef]
- Guillin, O.; Griffon, N.; Bezard, E.; Leriche, L.; Diaz, J.; Gross, C.; Sokoloff, P. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: Therapeutic implications in Parkinson’s disease. Eur. J. Pharmacol. 2003, 480, 89–95. [Google Scholar] [CrossRef]
- Razgado-Hernandez, L.F.; Espadas-Alvarez, A.J.; Reyna-Velazquez, P.; Sierra-Sanchez, A.; Anaya-Martinez, V.; Jimenez-Estrada, I.; Bannon, M.J.; Martinez-Fong, D.; Aceves-Ruiz, J. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS ONE 2015, 10, e0117391. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Benmansour, S.; Deltheil, T.; Piotrowski, J.; Nicolas, L.; Reperant, C.; Gardier, A.M.; Frazer, A.; David, D.J. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur. J. Pharmacol. 2008, 587, 90–98. [Google Scholar] [CrossRef]
- Deltheil, T.; Guiard, B.P.; Guilloux, J.P.; Nicolas, L.; Deloménie, C.; Repérant, C.; Le Maitre, E.; Leroux-Nicollet, I.; Benmansour, S.; Coudoré, F.; et al. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: Studies in adult mice hippocampus. Pharmacol. Biochem. Behav. 2008, 90, 174–183. [Google Scholar] [CrossRef]
- Celada, P.; Siuciak, J.A.; Tran, T.M.; Altar, C.A.; Tepper, J.M. Local infusion of brain-derived neurotrophic factor modifies the firing pattern of dorsal raphe serotonergic neurons. Brain Res. 1996, 712, 293–298. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Nestor, P.G.; O’Donovan, K.; Lapp, H.E.; Hasler, V.C.; Boodai, S.B.; Hunter, R. Risk and protective effects of serotonin and BDNF genes on stress-related adult psychiatric symptoms. Neurobiol. Stress 2019, 11, 100186. [Google Scholar] [CrossRef]
- Witte, A.V.; Kürten, J.; Jansen, S.; Schirmacher, A.; Brand, E.; Sommer, J.; Flöel, A. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J. Neurosci. 2012, 32, 4553–4561. [Google Scholar] [CrossRef]
- Han, D.H.; Park, D.B.; Choi, T.Y.; Joo, S.Y.; Lee, M.K.; Park, B.R.; Nishimura, R.; Chu, C.C.; Renshaw, P.F. Effects of brain-derived neurotrophic factor–catecholamine-O-methyltransferase gene interaction on schizophrenic symptoms. Neuroreport 2008, 19, 1155–1158. [Google Scholar] [CrossRef]
- Sakata, K.; Duke, S.M. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience 2014, 260, 265–275. [Google Scholar] [CrossRef]
- Guillin, O.; Diaz, J.; Carroll, P.; Griffon, N.; Schwartz, J.C.; Sokoloff, P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 2001, 411, 86–89. [Google Scholar] [CrossRef]
- Vogel, M.; Pfeifer, S.; Schaub, R.T.; Grabe, H.J.; Barnow, S.; Freyberger, H.J.; Cascorbi, I. Decreased levels of dopamine D3 receptor mRNA in schizophrenic and bipolar patients. Neuropsychobiology 2004, 50, 305–310. [Google Scholar] [CrossRef]
- Glenthoj, B.Y.; Mackeprang, T.; Svarer, C.; Rasmussen, H.; Pinborg, L.H.; Friberg, L.; Baaré, W.; Hemmingsen, R.; Videbaek, C. Frontal dopamine D2/3 receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol. Psychiatry 2006, 60, 621–629. [Google Scholar] [CrossRef]
- Bosse, K.E.; Maina, F.K.; Birbeck, J.A.; France, M.M.; Roberts, J.J.; Colombo, M.L.; Mathews, T.A. Aberrant striatal dopamine transmitter dynamics in brain-derived neurotrophic factor-deficient mice. J. Neurochem. 2012, 120, 385–395. [Google Scholar] [CrossRef]
- Di Carlo, P.; Punzi, G.; Ursini, G. BDNF and schizophrenia. Psychiatr. Genet. 2019, 29, 200–210. [Google Scholar] [CrossRef]
- Guillin, O.; Demily, C.; Thibaut, F. Brain-derived neurotrophic factor in schizophrenia and its relation with dopamine. Int. Rev. Neurobiol. 2007, 78, 377–395. [Google Scholar]
- Homberg, J.R.; Molteni, R.; Calabrese, F.; Riva, M.A. The serotonin–BDNF duo: Developmental implications for the vulnerability to psychopathology. Neurosci. Biobehav. Rev. 2014, 43, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Lu, B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Maurel, O.M.; Torrisi, S.A.; Barbagallo, C.; Purrello, M.; Salomone, S.; Drago, F.; Ragusa, M.; Leggio, G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. Int. J. Mol. Sci. 2021, 22, 5157. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress 2021, 14, 100286. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Rosenhauer, A.M.; Beach, L.Q.; Jeffress, E.C.; Thompson, B.M.; McCann, K.E.; Partrick, K.A.; Diaz, B.; Norvelle, A.; Choi, D.C.; Huhman, K.L. Brain-derived neurotrophic factor signaling mitigates the impact of acute social stress. Neuropharmacology 2019, 148, 40–49. [Google Scholar] [CrossRef]
- Wang, J.; Gao, F.; Cui, S.; Yang, S.; Gao, F.; Wang, X.; Zhu, G. Utility of 7, 8-dihydroxyflavone in preventing astrocytic and synaptic deficits in the hippocampus elicited by PTSD. Pharmacol. Res. 2022, 176, 106079. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Bazzari, A.H. Drug-Induced Delirium: A Mini Review. BMH. Med. J. 2018, 5, 51–56. [Google Scholar]
- Soysal, P.; Isik, A.T. Pathogenesis of Delirium. In Delirium in Elderly Patients; Isik, A., Grossberg, G., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Williams, J.; Finn, K.; Melvin, V.; Meagher, D.; McCarthy, G.; Adamis, D. The association of serum levels of brain-derived neurotrophic factor with the occurrence of and recovery from delirium in older medical inpatients. BioMed Res. Int. 2017, 2017, 5271395. [Google Scholar] [CrossRef]
- Wyrobek, J.; LaFlam, A.; Max, L.; Tian, J.; Neufeld, K.J.; Kebaish, K.M.; Walston, J.D.; Hogue, C.W.; Riley, L.H.; Everett, A.D.; et al. Association of intraoperative changes in brain-derived neurotrophic factor and postoperative delirium in older adults. Br. J. Anaesth. 2017, 119, 324–332. [Google Scholar] [CrossRef]
- Cursano, S.; Battaglia, C.R.; Urrutia-Ruiz, C.; Grabrucker, S.; Schön, M.; Bockmann, J.; Braumüller, S.; Radermacher, P.; Roselli, F.; Huber-Lang, M.; et al. A CRHR1 antagonist prevents synaptic loss and memory deficits in a trauma-induced delirium-like syndrome. Mol. Psychiatry 2021, 26, 3778–3794. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol. Res. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism. 2016, 7, 49. [Google Scholar] [CrossRef]
- Van Vliet, E.A.; Aronica, E.; Gorter, J.A. Blood–brain barrier dysfunction, seizures and epilepsy. Semin. Cell Dev. Biol. 2015, 38, 26–34. [Google Scholar] [CrossRef]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.; Najjar, A.; Chong, D. Neurovascular unit dysfunction and blood–brain barrier hyperpermeability contribute to schizophrenia neurobiology: A theoretical integration of clinical and experimental evidence. Front. Psychiatry 2017, 8, 83. [Google Scholar] [CrossRef]
- Qin, L.; Kim, E.; Ratan, R.; Lee, F.S.; Cho, S. Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J. Neurosci. 2011, 31, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhu, Y.; Li, Y.; Ding, X.; Ma, W.; Han, X.; Wang, B. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis 2019, 24, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Xie, Q.; Yu, X.; Li, S.; Wu, Q.; Chen, X.; Yue, J.; Zhou, K.; Tu, W.; Jiang, S. Water treadmill training protects the integrity of the blood-spinal cord barrier following SCI via the BDNF/TrkB-CREB signalling pathway. Neurochem. Int. 2021, 143, 104945. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Yan, J.; Cheng, F.; Ma, X.; Chen, C.; Wang, W.; Wang, Q. Cholic Acid Protects In Vitro Neurovascular Units against Oxygen and Glucose Deprivation-Induced Injury through the BDNF-TrkB Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1201624. [Google Scholar] [CrossRef] [PubMed]
- Grade, S.; Weng, Y.C.; Snapyan, M.; Kriz, J.; Malva, J.O.; Saghatelyan, A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS ONE 2013, 8, e55039. [Google Scholar] [CrossRef]
- Becerra-Calixto, A.; Posada-Duque, R.; Cardona-Gómez, G.P. Recovery of neurovascular unit integrity by CDK5-KD astrocyte transplantation in a global cerebral ischemia model. Mol. Neurobiol. 2018, 55, 8563–8585. [Google Scholar] [CrossRef]
- Kim, H.; Li, Q.; Hempstead, B.L.; Madri, J.A. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J. Biol. Chem. 2004, 279, 33538–33546. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Abdallah, D.M.; El-Abhar, H.S. Pharmacological interventions to attenuate Alzheimer’s disease progression: The story so far. Curr. Alzheimer Res. 2019, 16, 261–277. [Google Scholar] [CrossRef]
- Bazzari, A.H.; Bazzari, F.H. Medicinal plants for Alzheimer’s disease: An updated review. J. Med. Plant. Stud. 2018, 6, 81–85. [Google Scholar]
- Marvanová, M.; Lakso, M.; Pirhonen, J.; Nawa, H.; Wong, G.; Castrén, E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol. Cell. Neurosci. 2001, 18, 247–258. [Google Scholar] [CrossRef]
- López-Valdés, H.E.; Clarkson, A.N.; Ao, Y.; Charles, A.C.; Carmichael, S.T.; Sofroniew, M.V.; Brennan, K.C. Memantine enhances recovery from stroke. Stroke 2014, 45, 2093–2100. [Google Scholar] [CrossRef]
- Zhu, G.; Li, J.; He, L.; Wang, X.; Hong, X. MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. Br. J. Pharmacol. 2015, 172, 2354–2368. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hidau, M.K.; Rai, S. Memantine treatment exerts an antidepressant-like effect by preventing hippocampal mitochondrial dysfunction and memory impairment via upregulation of CREB/BDNF signaling in the rat model of chronic unpredictable stress-induced depression. Neurochem. Int. 2021, 142, 104932. [Google Scholar] [CrossRef]
- Mohseni, I.; Peeri, M.; Azarbayjani, M.A. Improvement of Learning and Memory Deficits with Aerobic Training and Donepezil Co-therapy in Amyloid-β beta Injected Male Rats Through the CREB and BDNF Signaling Pathway. Pharm. Biomed. Res. 2020, 6, 181–190. [Google Scholar] [CrossRef]
- Zheng, H.; Niu, S.; Zhao, H.; Li, S.; Jiao, J. Donepezil improves the cognitive impairment in a tree shrew model of Alzheimer’s disease induced by amyloid-β1–40 via activating the BDNF/TrkB signal pathway. Metab. Brain Dis. 2018, 33, 1961–1974. [Google Scholar] [CrossRef]
- Jian, W.X.; Zhang, Z.; Zhan, J.H.; Chu, S.F.; Peng, Y.; Zhao, M.; Wang, Q.; Chen, N.H. Donepezil attenuates vascular dementia in rats through increasing BDNF induced by reducing HDAC6 nuclear translocation. Acta Pharmacol. Sin. 2020, 41, 588–598. [Google Scholar] [CrossRef]
- Muratori, B.G.; Zamberlam, C.R.; Mendes, T.B.; Nozima, B.H.; Cerutti, J.M.; Cerutti, S.M. BDNF as a Putative Target for Standardized Extract of Ginkgo biloba-Induced Persistence of Object Recognition Memory. Molecules 2021, 26, 3326. [Google Scholar] [CrossRef]
- Hamdan, M.; AS, N.; Rahayu, M.; Machfoed, M.H. Influence of ginkgo biloba (Egb) extracts in apoptosis index and number of neurons at Rattus novergicus with lead (pb) exposure. Res. J. Pharm. Technol. 2019, 12, 5883–5887. [Google Scholar] [CrossRef]
- Ben-Azu, B.; Adebayo, O.G.; Wopara, I.; Aduema, W.; Onyeleonu, I.; Umoren, E.B.; Kolawole, T.A.; Ebo, O.T.; Akpotu, A.E.; Ajibo, D.N.; et al. Lead acetate induces hippocampal pyramidal neuron degeneration in mice via up-regulation of executioner caspase-3, oxido-inflammatory stress expression and decreased BDNF and cholinergic activity: Reversal effects of Gingko biloba supplement. J. Trace Elem. Med. Biol. 2022, 71, 126919. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, D.; Zhu, H.; Wang, X. Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res. Bull. 2012, 87, 193–198. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Brivio, P.; Dell’Agli, M.; Calabrese, F. Botanicals as modulators of neuroplasticity: Focus on BDNF. Neural Plast. 2017, 2017, 5965371. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Bai, O.; Chlan-Fourney, J.; Bowen, R.; Keegan, D.; Li, X.M. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J. Neurosci. Res. 2003, 71, 127–131. [Google Scholar] [CrossRef]
- Adachi, N.; Yoshimura, A.; Chiba, S.; Ogawa, S.; Kunugi, H. Rotigotine, a dopamine receptor agonist, increased BDNF protein levels in the rat cortex and hippocampus. Neurosci. Lett. 2018, 62, 44–50. [Google Scholar] [CrossRef]
- Yang, L.; Xu, J.M.; Jiang, X.; Ruan, W.; Cui, Y.; He, L. Effect of dexmedetomidine on plasma brain-derived neurotrophic factor: A double-blind, randomized and placebo-controlled study. Upsala J. Med. Sci. 2013, 118, 235–239. [Google Scholar] [CrossRef]
- Li, Z.C.; Jia, Y.P.; Wang, Y.; Qi, J.L.; Han, X.P. Effects of dexmedetomidine post-treatment on BDNF and VEGF expression following cerebral ischemia/reperfusion injury in rats. Mol. Med. Rep. 2018, 17, 6033–6037. [Google Scholar] [CrossRef]
- Sheng, S.; Huang, J.; Ren, Y.; Zhi, F.; Tian, X.; Wen, G.; Ding, G.; Xia, T.C.; Hua, F.; Xia, Y. Neuroprotection against hypoxic/ischemic injury: δ-opioid receptors and BDNF-TrkB pathway. Cell. Physiol. Biochem. 2018, 47, 302–315. [Google Scholar] [CrossRef]
- Bazzari, A.H.; Bazzari, F.H. Promising Therapeutic Agents: Delta Opioid Receptor Agonists. Int. Ann. Med. 2017, 1, 1–5. [Google Scholar] [CrossRef][Green Version]
- Fukui, H.; Wong, H.T.; Beyer, L.A.; Case, B.G.; Swiderski, D.L.; Di Polo, A.; Ryan, A.F.; Raphael, Y. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci. Rep. 2012, 2, 838. [Google Scholar] [CrossRef]
- Dąbkowska, M.; Łuczkowska, K.; Rogińska, D.; Sobuś, A.; Wasilewska, M.; Ulańczyk, Z.; Machaliński, B. Novel design of (PEG-ylated) PAMAM-based nanoparticles for sustained delivery of BDNF to neurotoxin-injured differentiated neuroblastoma cells. J. Nanobiotechnol. 2020, 18, 120. [Google Scholar] [CrossRef]
- Jiang, Y.; Fay, J.M.; Poon, C.D.; Vinod, N.; Zhao, Y.; Bullock, K.; Qin, S.; Manickam, D.S.; Yi, X.; Banks, W.A.; et al. Nanoformulation of Brain-Derived Neurotrophic Factor with Target Receptor-Triggered-Release in the Central Nervous System. Adv. Funct. Mater. 2018, 28, 1703982. [Google Scholar] [CrossRef]
- Wang, S.; Yao, H.; Xu, Y.; Hao, R.; Zhang, W.; Liu, H.; Huang, Y.; Guo, W.; Lu, B. Therapeutic potential of a TrkB agonistic antibody for Alzheimer’s disease. Theranostics 2020, 10, 6854–6874. [Google Scholar] [CrossRef]
- Bawari, S.; Tewari, D.; Argüelles, S.; Sah, A.N.; Nabavi, S.F.; Xu, S.; Vacca, R.A.; Nabavi, S.M.; Shirooie, S. Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacol. Res. 2019, 148, 104458. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Povarnina, P.; Tarasiuk, A.V.; Seredenin, S.B. The low molecular weight brain-derived neurotrophic factor mimetics with antidepressant-like activity. Curr. Pharm. Des. 2019, 25, 729–737. [Google Scholar] [CrossRef]
- Miranda-Lourenço, C.; Ribeiro-Rodrigues, L.; Fonseca-Gomes, J.; Tanqueiro, S.R.; Belo, R.F.; Ferreira, C.B.; Rei, N.; Ferreira-Manso, M.; de Almeida-Borlido, C.; Costa-Coelho, T.; et al. Challenges of BDNF-based therapies: From common to rare diseases. Pharmacol. Res. 2020, 162, 105281. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzari, A.H.; Bazzari, F.H. BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 8417. https://doi.org/10.3390/ijms23158417
Bazzari AH, Bazzari FH. BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2022; 23(15):8417. https://doi.org/10.3390/ijms23158417
Chicago/Turabian StyleBazzari, Amjad H., and Firas H. Bazzari. 2022. "BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders" International Journal of Molecular Sciences 23, no. 15: 8417. https://doi.org/10.3390/ijms23158417
APA StyleBazzari, A. H., & Bazzari, F. H. (2022). BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders. International Journal of Molecular Sciences, 23(15), 8417. https://doi.org/10.3390/ijms23158417




