Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light
Abstract
:1. Introduction
2. Results and Discussion
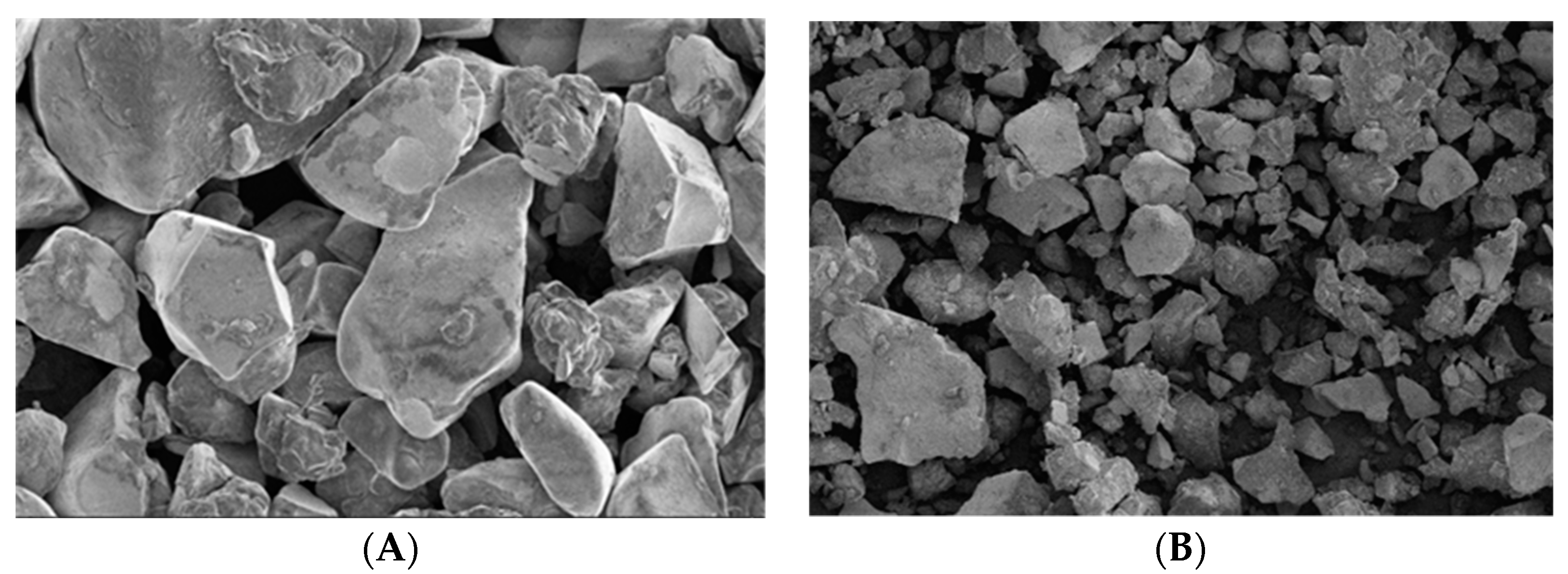
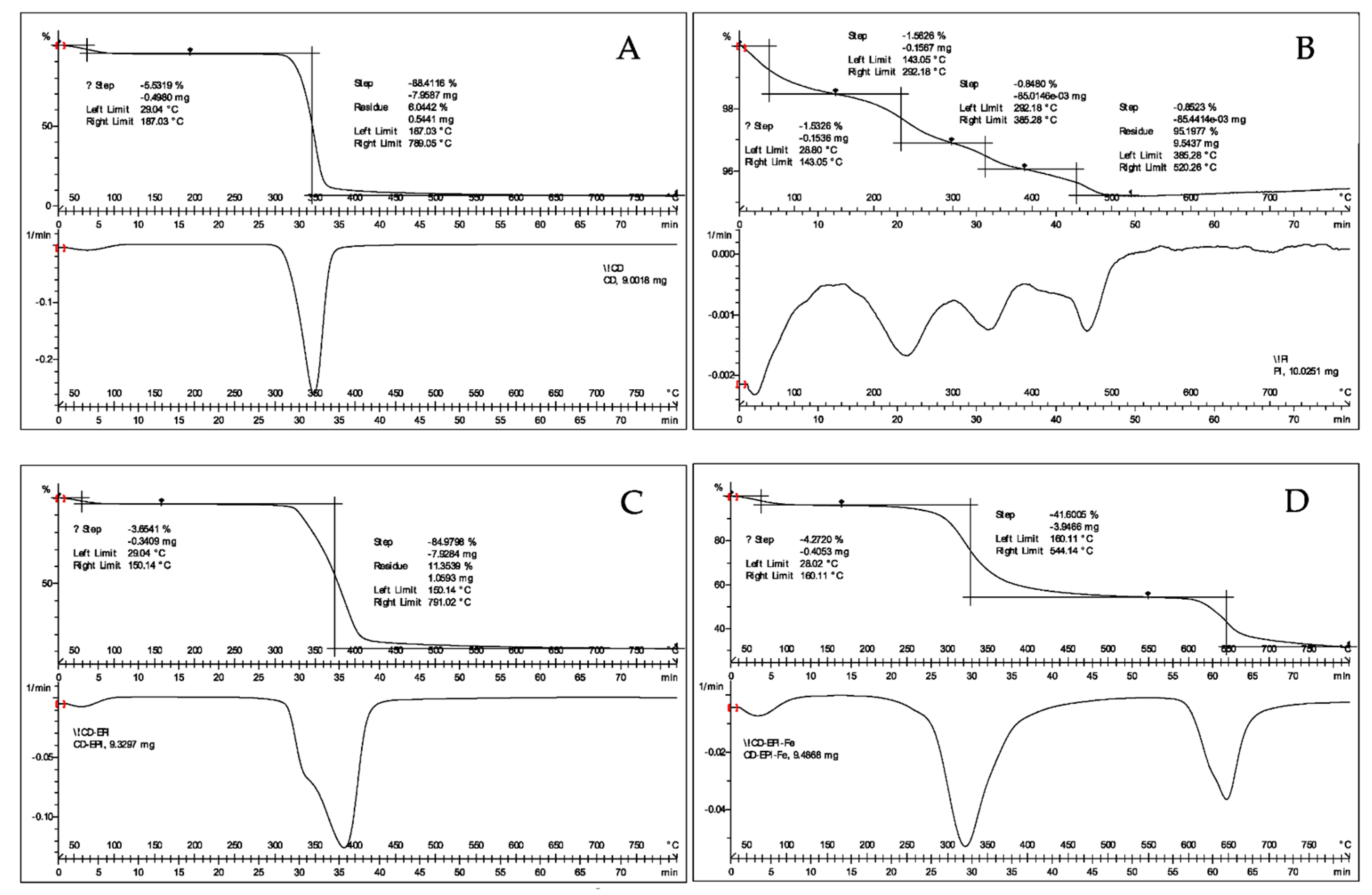
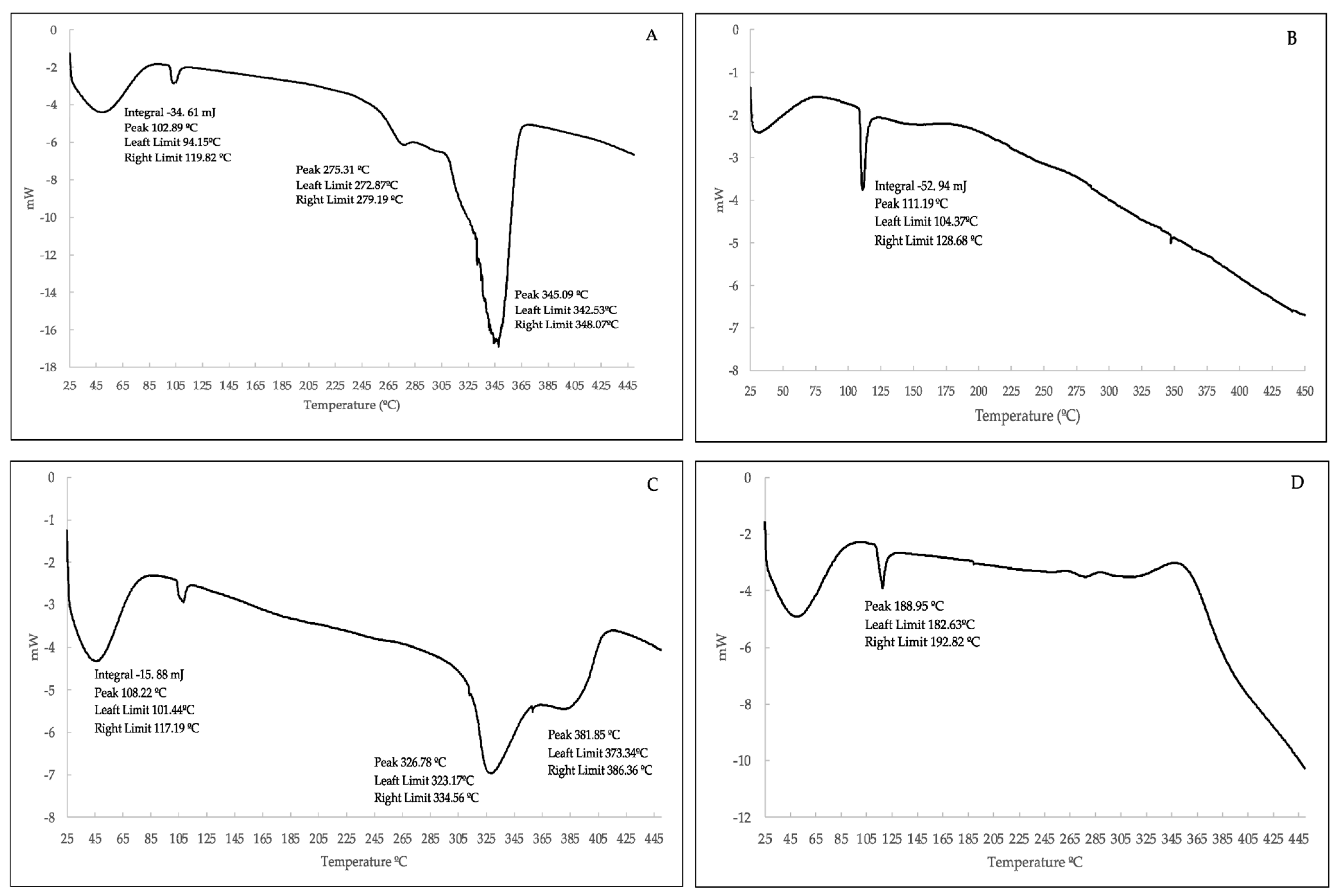
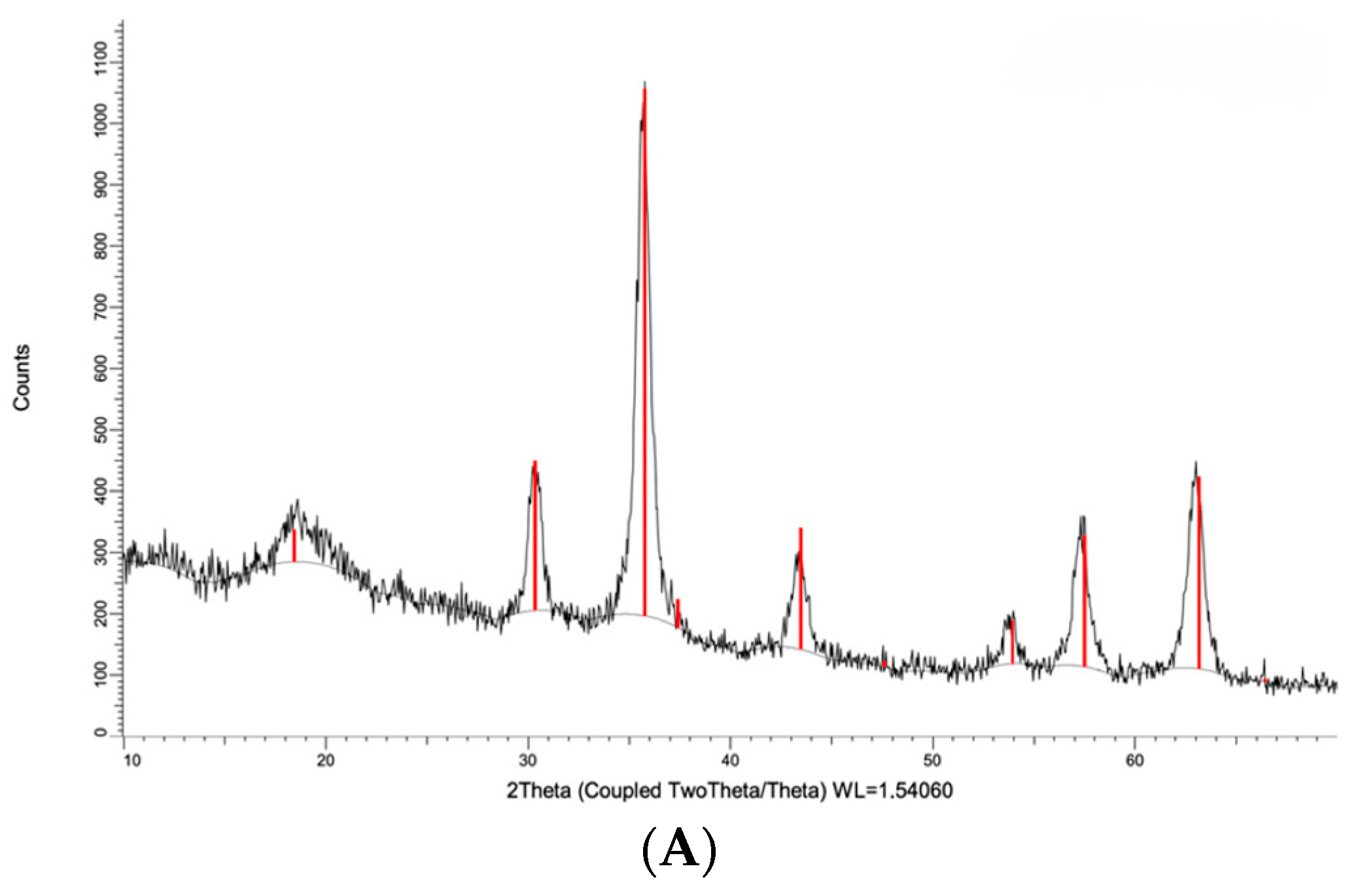
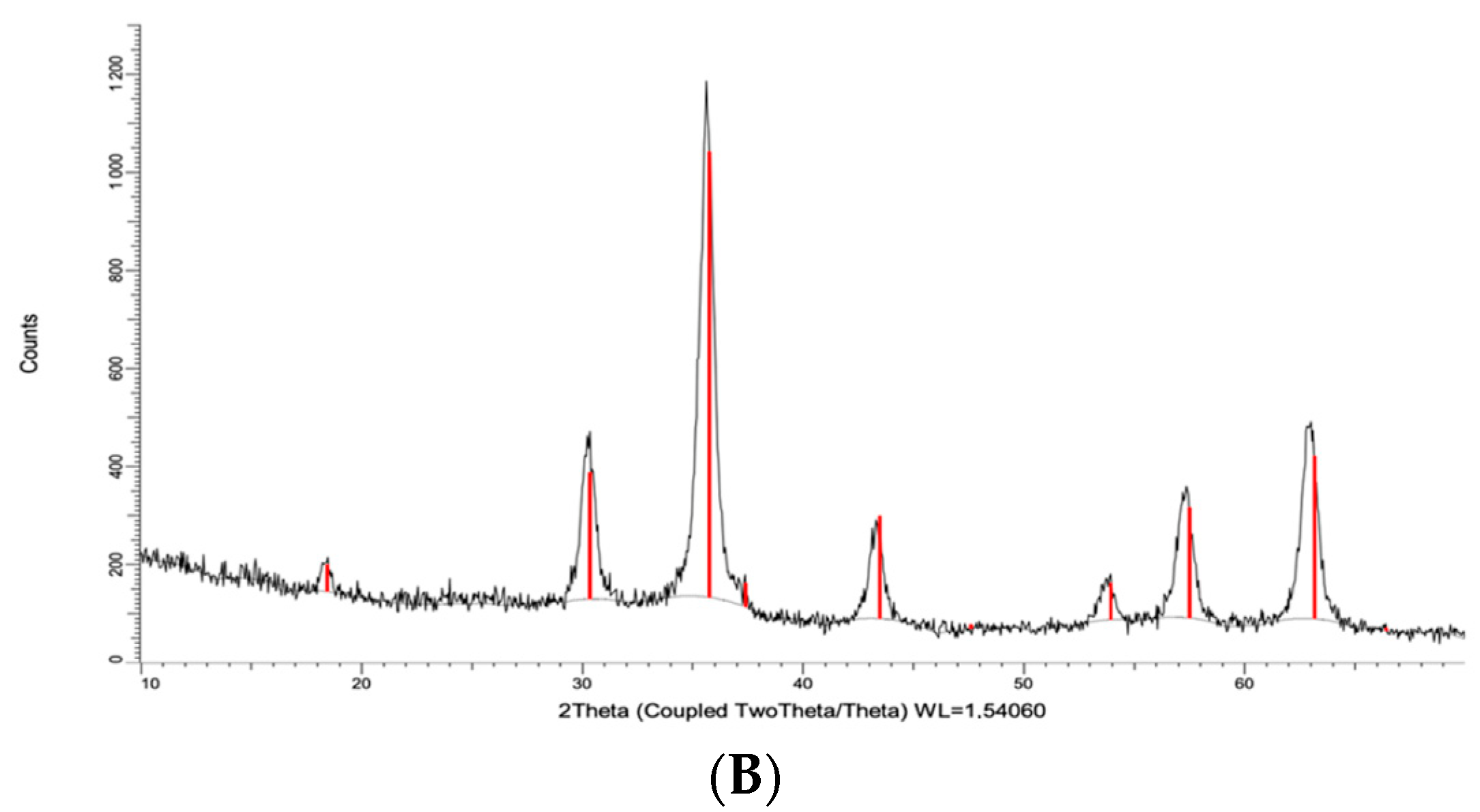
2.1. Characteristics of the Polymer Material
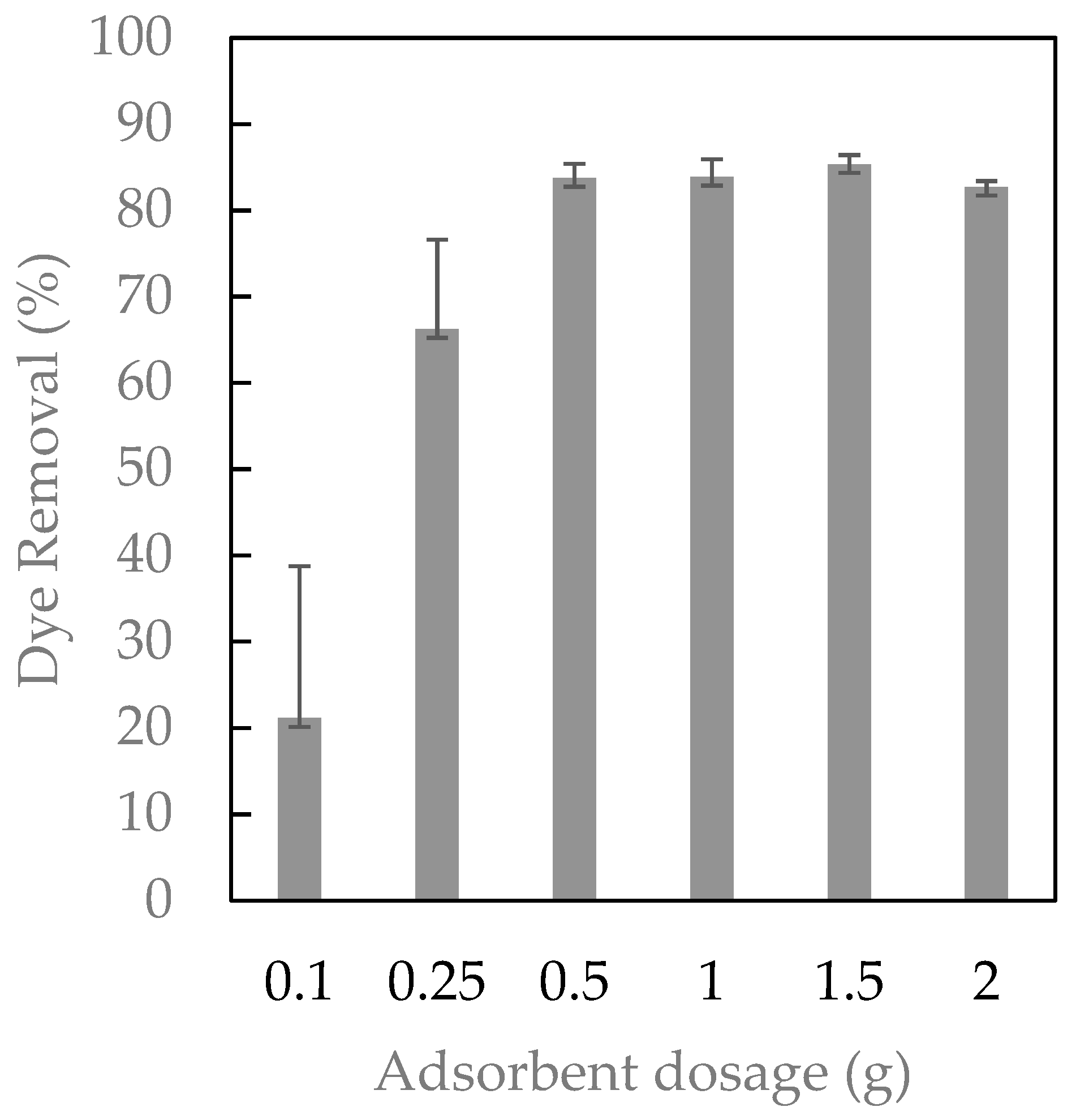
2.2. Adsorbent Dosage
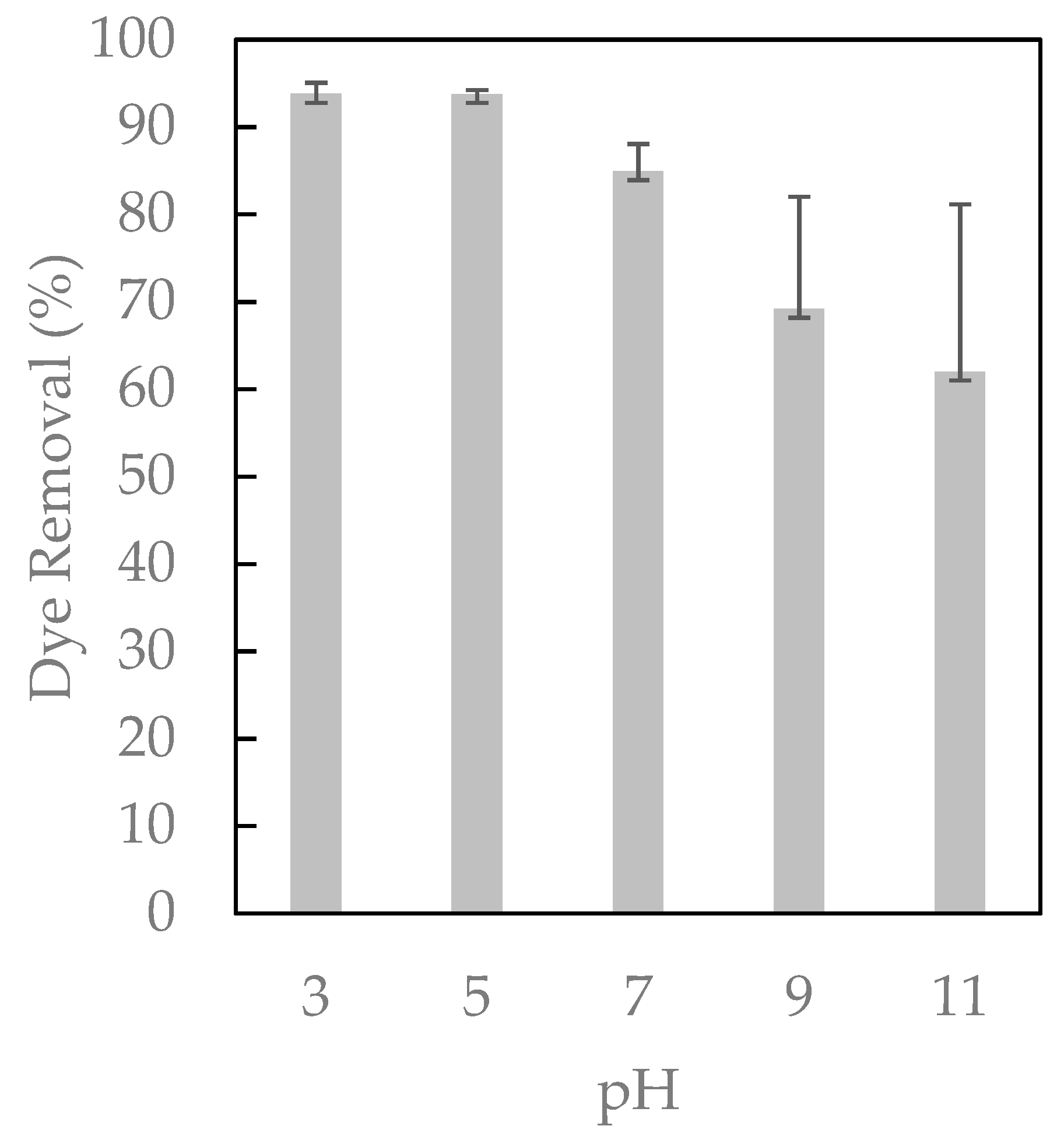
2.3. pH Effect
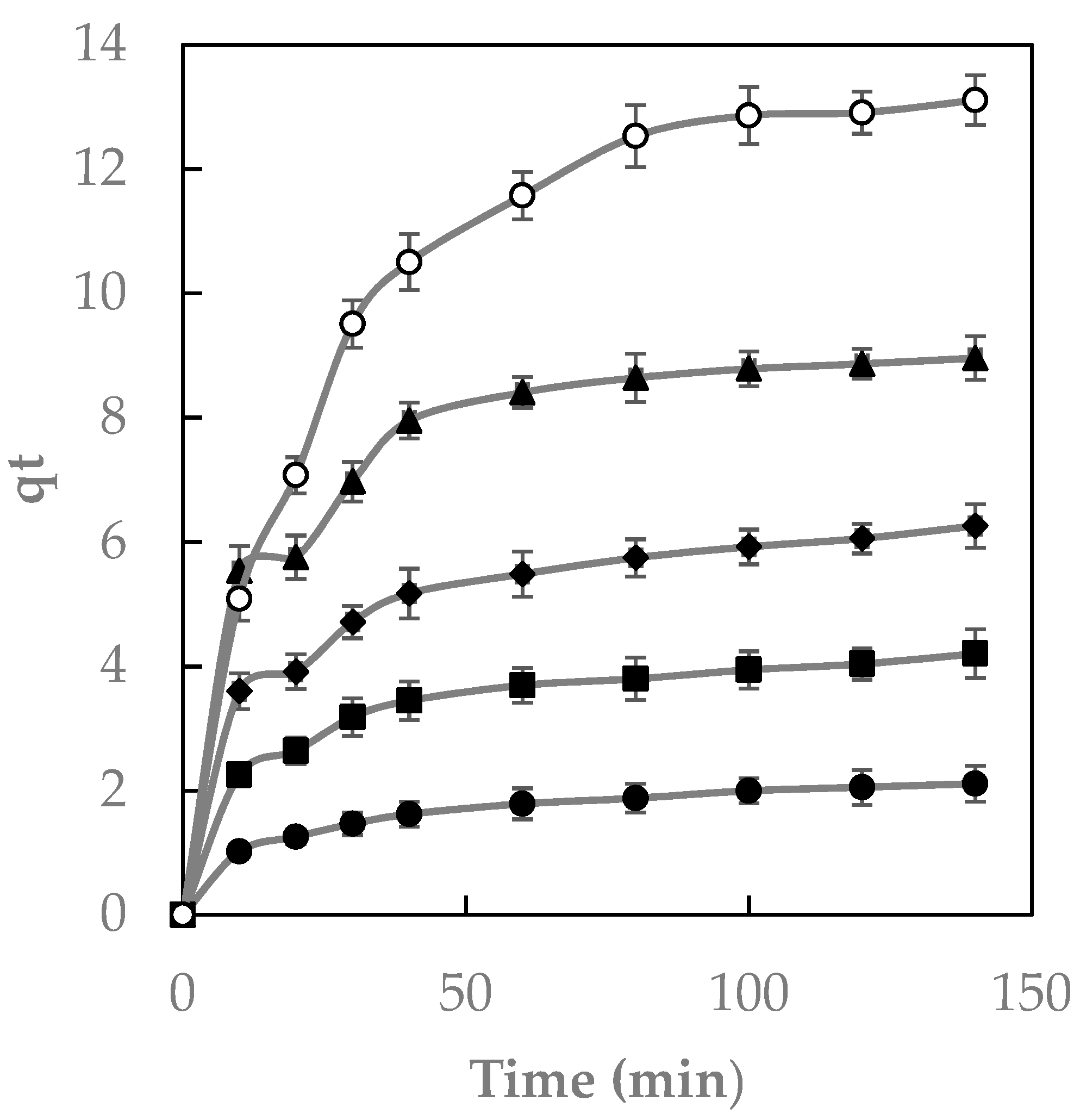
2.4. Effect of Contact Time
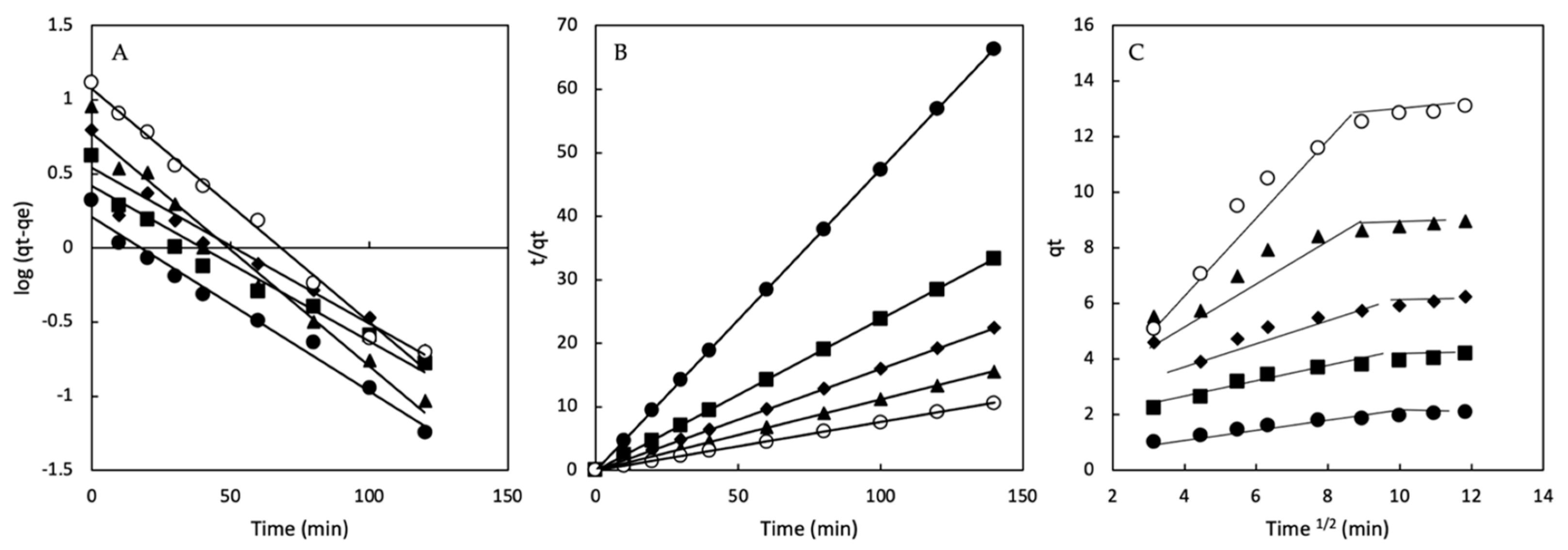
2.5. Kinetic Analysis
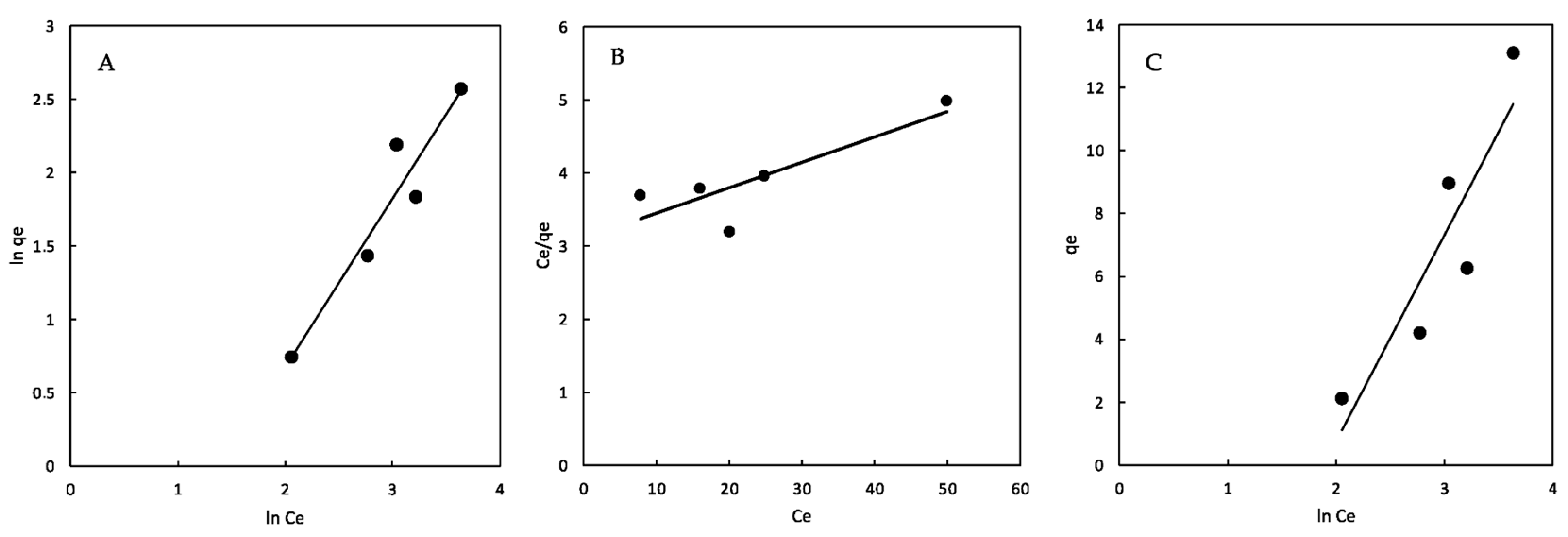
2.6. Isotherm Analysis
2.7. Thermodynamic Parameters
2.8. Advanced Oxidation Process
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Iron Nanoparticles Preparation
3.3. Epichlorohydrin-Iron-β-Cyclodextrin Polymer Preparation
3.4. Characterization of the Polymer Material
3.5. Dye Solution Preparation
3.6. Adsorption Experiments
3.7. Kinetics and Isotherm Analysis
3.8. Polymer Reusability
3.9. Dye Degradation by Pulsed Light/H2O2 Process
3.10. Molecular Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and Adsorption Application of Succinyl-Grafted Chitosan for the Simultaneous Removal of Zinc and Cationic Dye from Binary Hazardous Mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Crini, G. Non-Conventional Adsorbents for Dye Removal. In Green Chemistry for Dyes Removal from Wastewater; Sharma, S.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 359–407. [Google Scholar] [CrossRef]
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green Synthesis of a Magnetic β-Cyclodextrin Polymer for Rapid Removal of Organic Micro-Pollutants and Heavy Metals from Dyeing Wastewater. Environ. Res. 2020, 180, 108796. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of Chitosan and Its Derivatives as Adsorbents for Dye Removal from Water and Wastewater: A Review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Paździor, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Rong, J.; Mao, K.; Yang, D.; Zhang, T.; Qiu, F.; Pan, J.; Pu, Z. Boronate Affinity-Modified Magnetic β-Cyclodextrin Polymer for Selective Separation and Adsorption of Shikimic Acid. J. Mater. Sci 2021, 56, 13043–13055. [Google Scholar] [CrossRef]
- Nasiri, S.; Alizadeh, N. Hydroxypropyl-β-Cyclodextrin-Polyurethane/Graphene Oxide Magnetic Nanoconjugates as Effective Adsorbent for Chromium and Lead Ions. Carbohydr. Polym. 2021, 259, 117731. [Google Scholar] [CrossRef]
- Wang, L.K.; Wang, M.-H.S.; Shammas, N.K.; Hahn, H.H. Physicochemical Treatment Consisting of Chemical Coagulation, Precipitation, Sedimentation, and Flotation. In Integrated Natural Resources Research; Handbook of Environmental Engineering; Wang, L.K., Wang, M.-H.S., Hung, Y.-T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 22, pp. 265–397. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Zamora-Ledezma, C.; Chuya-Sumba, C.; De Sousa, F.B.; Whitehead, D.; Alexis, F.; Guerrero, V.H. Persistent Organic Pollutants: The Trade-off between Potential Risks and Sustainable Remediation Methods. J. Environ. Manag. 2021, 300, 113737. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Electrospun Crosslinked Poly-Cyclodextrin Nanofibers: Highly Efficient Molecular Filtration Thru Host-Guest Inclusion Complexation. Sci. Rep. 2017, 7, 7369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Env. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Fourmentin, S.; Crini, G.; Lichtfouse, E. (Eds.) Cyclodextrin Fundamentals, Reactivity and Analysis; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2018; Volume 16. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Aguado, R.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin Polymers and Cyclodextrin-Containing Polysaccharides for Water Remediation. Polysaccharides 2021, 2, 16–38. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Cyclodextrin-Based Adsorbents for the Removal of Pollutants from Wastewater: A Review. Environ. Sci. Pollut. Res. 2021, 28, 1317–1340. [Google Scholar] [CrossRef] [PubMed]
- Sargın, İ.; Kaya, M.; Arslan, G.; Baran, T.; Ceter, T. Preparation and Characterisation of Biodegradable Pollen–Chitosan Microcapsules and Its Application in Heavy Metal Removal. Bioresour. Technol. 2015, 177, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, L.; Jiang, X.; Yu, J.; Chen, X. Adsorption and Removal of Malachite Green from Aqueous Solution Using Magnetic β-Cyclodextrin-Graphene Oxide Nanocomposites as Adsorbents. Colloids. Surf. A Physicochem. Eng. Asp. 2015, 466, 166–173. [Google Scholar] [CrossRef]
- Moradi Shahrebabak, S.; Saber-Tehrani, M.; Faraji, M.; Shabanian, M.; Aberoomand-Azar, P. Magnetic Solid Phase Extraction Based on Poly(β-Cyclodextrin-Ester) Functionalized Silica-Coated Magnetic Nanoparticles (NPs) for Simultaneous Extraction of the Malachite Green and Crystal Violet from Aqueous Samples. Environ. Monit. Assess. 2020, 192, 262. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Y.; Li, Z.; Tan, B.H.; Tang, K.Y.; Tam, K.C. β-Cyclodextrin Functionalized Magnetic Nanoparticles for the Removal of Pharmaceutical Residues in Drinking Water. J. Ind. Eng. Chem. 2022, 109, 461–474. [Google Scholar] [CrossRef]
- Juengchareonpoon, K.; Wanichpongpan, P.; Boonamnuayvitaya, V. Functionalization of Magnetite Nanoparticles with Carboxymethyl-Beta-Cyclodextrin for Oxytetracycline Removal. Appl. Phys. A 2021, 127, 197. [Google Scholar] [CrossRef]
- Hassan, M.; Naidu, R.; Du, J.; Qi, F.; Ahsan, M.A.; Liu, Y. Magnetic Responsive Mesoporous Alginate/β-Cyclodextrin Polymer Beads Enhance Selectivity and Adsorption of Heavy Metal Ions. Int. J. Biol. Macromol. 2022, 207, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, Y.; Wang, S.; Chen, X.; Cao, X.; Wang, Z.; Li, Y. Efficient Removal of Various Coexisting Organic Pollutants in Water Based on β-Cyclodextrin Polymer Modified Flower-like Fe3O4 Particles. J. Colloid Interface Sci. 2021, 589, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ange, K.U.; Ali, N.; Yang, Y.; Khan, A.; Ali, F.; Sajid, M.; Tian, C.T.; Bilal, M. Analytical Perspective and Environmental Remediation Potentials of Magnetic Composite Nanosorbents. Chemosphere 2022, 304, 135312. [Google Scholar] [CrossRef] [PubMed]
- Bayatloo, M.R.; Nojavan, S. Rapid and Simple Magnetic Solid-Phase Extraction of Bisphenol A from Bottled Water, Baby Bottle, and Urine Samples Using Green Magnetic Hydroxyapatite/β-Cyclodextrin Polymer Nanocomposite. Microchem. J. 2022, 175, 107180. [Google Scholar] [CrossRef]
- Moradi, O.; Sharma, G. Emerging Novel Polymeric Adsorbents for Removing Dyes from Wastewater: A Comprehensive Review and Comparison with Other Adsorbents. Environ. Res. 2021, 201, 111534. [Google Scholar] [CrossRef] [PubMed]
- Nkinahamira, F.; Alsbaiee, A.; Zeng, Q.; Li, Y.; Zhang, Y.; Feng, M.; Yu, C.-P.; Sun, Q. Selective and Fast Recovery of Rare Earth Elements from Industrial Wastewater by Porous β-Cyclodextrin and Magnetic β-Cyclodextrin Polymers. Water Res. 2020, 181, 115857. [Google Scholar] [CrossRef] [PubMed]
- Salazar, S.; Yutronic, N.; Jara, P. Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. IJMS 2020, 21, 4079. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A.C. Adsorption of Cationic Dyes, Drugs and Metal from Aqueous Solutions Using a Polymer Composite of Magnetic/β-Cyclodextrin/Activated Charcoal/Na Alginate: Isotherm, Kinetics and Regeneration Studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, S.; Lucas-Abellán, C.; Serrano-Martínez, A.; Mercader-Ros, M.T.; Cuartero, N.; Navarro, P.; Pérez, S.; Gabaldón, J.A.; Gómez-López, V.M. Pulsed Light for a Cleaner Dyeing Industry: Azo Dye Degradation by an Advanced Oxidation Process Driven by Pulsed Light. J. Clean. Prod. 2019, 217, 757–766. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.; Haron, M.; Namvar, F.; Nadi, B.; Rahman, M.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeraro, P.; Fini, P.; D’Addabbo, M.; Rizzi, V.; Cosma, P. Removal from Wastewater and Recycling of Azo Textile Dyes by Alginate-Chitosan Beads. IJEAB 2017, 2, 1835–1850. [Google Scholar] [CrossRef] [Green Version]
- Murcia-Salvador, A.; Pellicer, J.A.; Fortea, M.I.; Gómez-López, V.M.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A. Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents. Polymers 2019, 11, 1003. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Chen, S.; Zhao, H.; Liu, Y.; Long, F.; Pan, X. A Versatile β-Cyclodextrin and Polyethyleneimine Bi-Functionalized Magnetic Nanoadsorbent for Simultaneous Capture of Methyl Orange and Pb (II) from Complex Wastewater. Chemosphere 2019, 216, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Pan, S.; Xu, J. Fabrication of Fe3O4@ Cyclodextrin Magnetic Composite for the High-Efficient Removal of Eu (III). J. Mol. Liq. 2015, 206, 272–277. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Z.; Li, M.; Sun, P.; Yu, T.; Zhou, L. Novel Porous Magnetic Nanospheres Functionalized by β-Cyclodextrin Polymer and Its Application in Organic Pollutants from Aqueous Solution. Environ. Pollut. 2019, 250, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Gimbert, F.; Robert, C.; Martel, B.; Adam, O.; Morin-Crini, N.; De Giorgi, F.; Badot, P.-M. The Removal of Basic Blue 3 from Aqueous Solutions by Chitosan-Based Adsorbent: Batch Studies. J. Hazard. Mater. 2008, 153, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Gimbert, F.; Morin-Crini, N.; Renault, F.; Badot, P.-M.; Crini, G. Adsorption Isotherm Models for Dye Removal by Cationized Starch-Based Material in a Single Component System: Error Analysis. J. Hazard. Mater. 2008, 157, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-T.; Shi, L.-Q.; Shen, H.; Chen, X.; Xie, K.-P. Equilibrium, Isotherm, Kinetic and Thermodynamic Studies for Removal of Tetracycline Antibiotics by Adsorption onto Hazelnut Shell Derived Activated Carbons from Aqueous Media. RSC Adv. 2016, 6, 109983–109991. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Gómez-López, V.M.; Auñón, D.; Núñez-Delicado, E.; Gabaldón, J.A. Synthesis of New Cyclodextrin-Based Adsorbents to Remove Direct Red 83:1. Polymers 2020, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-Insoluble β-Cyclodextrin–Epichlorohydrin Polymers for Removal of Pollutants from Aqueous Solutions by Sorption Processes Using Batch Studies: A Review of Inclusion Mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Gabaldón Hernández, J.A.; Lucas-Abellán, C.; Mercader-Ros, M.T.; Serrano-Martínez, A.; Núñez-Delicado, E.; Cosma, P.; Fini, P.; et al. Removing of Direct Red 83:1 Using α- and HP-α-CDs Polymerized with Epichlorohydrin: Kinetic and Equilibrium Studies. Dye. Pigment. 2018, 149, 736–746. [Google Scholar] [CrossRef]
- Pellicer, J.; Rodríguez-López, M.; Fortea, M.; Lucas-Abellán, C.; Mercader-Ros, M.; López-Miranda, S.; Gómez-López, V.; Semeraro, P.; Cosma, P.; Fini, P.; et al. Adsorption Properties of β- and Hydroxypropyl-β-Cyclodextrins Cross-Linked with Epichlorohydrin in Aqueous Solution. A Sustainable Recycling Strategy in Textile Dyeing Process. Polymers 2019, 11, 252. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhou, X. The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Murcia-Salvador, A.; Pellicer, J.A.; Rodríguez-López, M.I.; Gómez-López, V.M.; Núñez-Delicado, E.; Gabaldón, J.A. Egg By-Products as a Tool to Remove Direct Blue 78 Dye from Wastewater: Kinetic, Equilibrium Modeling, Thermodynamics and Desorption Properties. Materials 2020, 13, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, P.; Chowdhury, S. Insight into Adsorption Thermodynamics. Thermodynamics 2011, 16, 349–364. [Google Scholar]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of CI Basic Green 4 (Malachite Green) from Aqueous Solutions by Adsorption Using Cyclodextrin-Based Adsorbent: Kinetic and Equilibrium Studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.-S. Review of Second-Order Models for Adsorption Systems. ChemInform 2006, 37. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Temkin, M.I. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Cudemos, E.; Izquier, A.; Medina-Martínez, M.S.; Gómez-López, V.M. Effects of Shading and Growth Phase on the Microbial Inactivation by Pulsed Light. Czech J. Food Sci. 2013, 31, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A Software Program for PK a Prediction and Protonation State Generation for Drug-like Molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the Comprehensive, Rapid, and Accurate Prediction of the Favorable Tautomeric States of Drug-like Molecules in Aqueous Solution. J. Comput. Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Carrasco, J.P.; Jacquemin, D. Using Theory To Extend the Scope of Azobenzene Drugs in Chemotherapy: Novel Combinations for a Specific Delivery. ChemMedChem 2021, 16, 1765–1775. [Google Scholar] [CrossRef]
- Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Contreras-García, J.; Pérez-Sánchez, H. Exploiting the Cyclodextrins Ability for Antioxidants Encapsulation: A Computational Approach to Carnosol and Carnosic Acid Embedding. Comput. Theor. Chem. 2016, 1077, 65–73. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of the Crystal Environment in Determining Protein Side-Chain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]



| PFOM | β-CDs-EPI Magnetic Polymer | |||
| Co (mg/L) | qeexp | qecal | K1(min−1) | R2 |
| 50 | 2.10 | 1.61 | 0.027 | 0.982 |
| 100 | 4.20 | 2.60 | 0.024 | 0.950 |
| 150 | 6.26 | 3.48 | 0.024 | 0.924 |
| 200 | 8.95 | 5.93 | 0.036 | 0.978 |
| 300 | 13.10 | 11.78 | 0.036 | 0.989 |
| PSOM | β-CDs-EPI Magnetic Polymer | |||
| Co (mg/L) | qeexp | qecal | K2(g/mg min) | R2 |
| 50 | 2.10 | 2.10 | 0.225 | 0.999 |
| 100 | 4.20 | 4.20 | 0.168 | 0.999 |
| 150 | 6.26 | 6.26 | 0.105 | 0.999 |
| 200 | 8.95 | 8.62 | 0.035 | 0.999 |
| 300 | 13.10 | 13.10 | 0.013 | 0.999 |
| IDM | β-CDs-EPI Magnetic Polymer | |||
| Co (mg/L) | qeexp | qecal | Ki(mg/g min 1/2) | R2 |
| 50 | 2.10 | 0.74 | 0.120 | 0.959 |
| 100 | 4.20 | 1.85 | 0.21 | 0.920 |
| 150 | 6.26 | 3.46 | 0.24 | 0.872 |
| 200 | 8.95 | 4.58 | 0.41 | 0.861 |
| 300 | 13.10 | 3.75 | 0.89 | 0.878 |
| Isotherm | Parameter | CDs |
|---|---|---|
| Freundlich | KF | 0.190 |
| nF | 1.040 | |
| R2 | 0.911 | |
| Langmuir | qmax | 32 |
| KL | 0.320 | |
| aL | 0.010 | |
| R2 | 0.747 | |
| RL | 0.667–0.250 | |
| Tempkin | aT | 0.150 |
| bT | 0.370 | |
| R2 | 0.807 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-López, M.I.; Pellicer, J.A.; Gómez-Morte, T.; Auñón, D.; Gómez-López, V.M.; Yáñez-Gascón, M.J.; Gil-Izquierdo, Á.; Cerón-Carrasco, J.P.; Crini, G.; Núñez-Delicado, E.; et al. Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light. Int. J. Mol. Sci. 2022, 23, 8406. https://doi.org/10.3390/ijms23158406
Rodríguez-López MI, Pellicer JA, Gómez-Morte T, Auñón D, Gómez-López VM, Yáñez-Gascón MJ, Gil-Izquierdo Á, Cerón-Carrasco JP, Crini G, Núñez-Delicado E, et al. Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light. International Journal of Molecular Sciences. 2022; 23(15):8406. https://doi.org/10.3390/ijms23158406
Chicago/Turabian StyleRodríguez-López, María Isabel, José Antonio Pellicer, Teresa Gómez-Morte, David Auñón, Vicente M. Gómez-López, María José Yáñez-Gascón, Ángel Gil-Izquierdo, José Pedro Cerón-Carrasco, Grégorio Crini, Estrella Núñez-Delicado, and et al. 2022. "Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light" International Journal of Molecular Sciences 23, no. 15: 8406. https://doi.org/10.3390/ijms23158406
APA StyleRodríguez-López, M. I., Pellicer, J. A., Gómez-Morte, T., Auñón, D., Gómez-López, V. M., Yáñez-Gascón, M. J., Gil-Izquierdo, Á., Cerón-Carrasco, J. P., Crini, G., Núñez-Delicado, E., & Gabaldón, J. A. (2022). Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light. International Journal of Molecular Sciences, 23(15), 8406. https://doi.org/10.3390/ijms23158406











