Zileuton Alleviates Radiation-Induced Cutaneous Ulcers via Inhibition of Senescence-Associated Secretory Phenotype in Rodents
Abstract
:1. Introduction
2. Results
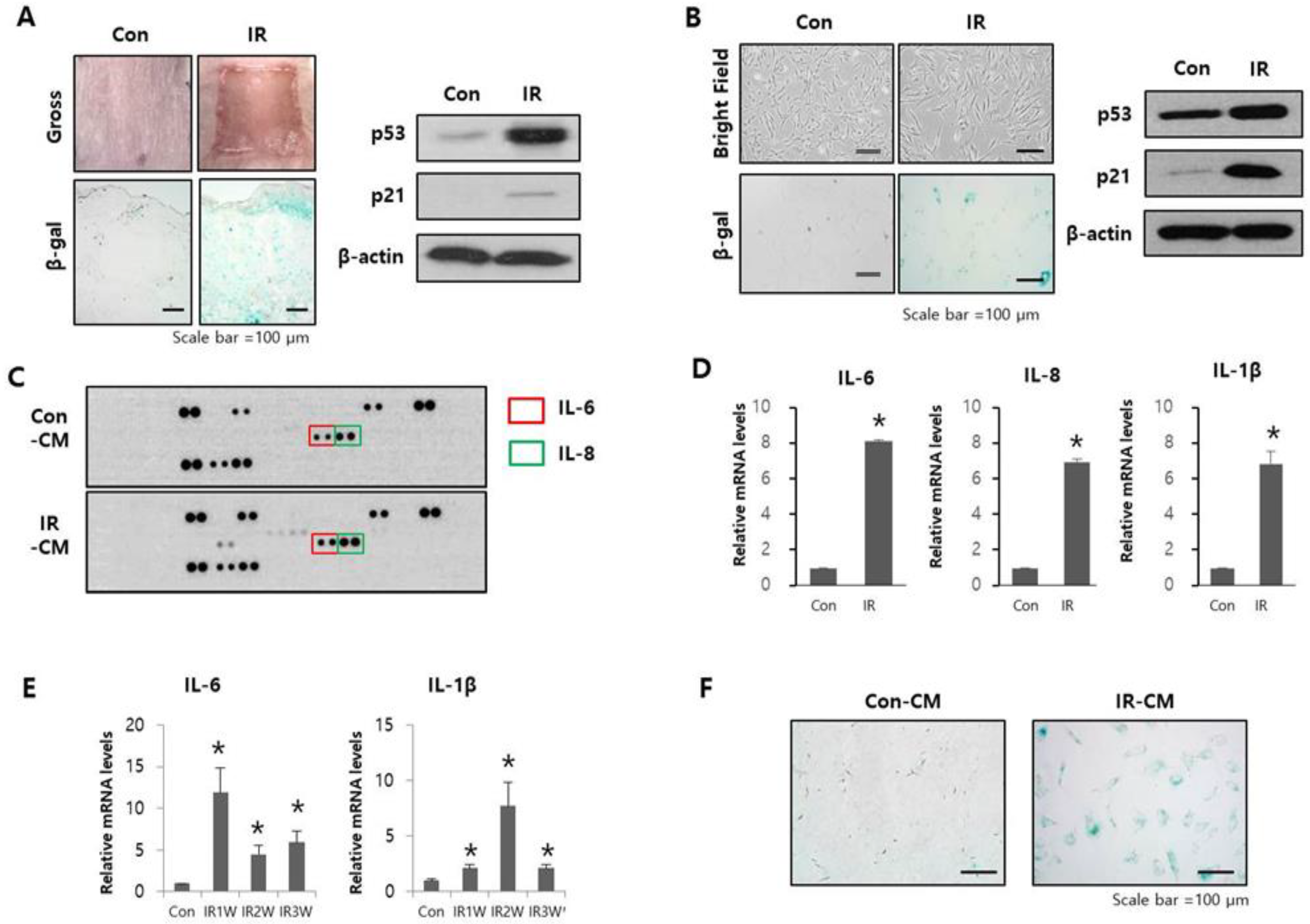
2.1. Radiation-Induced SASP Triggered Senescence in Adjacent Normal Cells
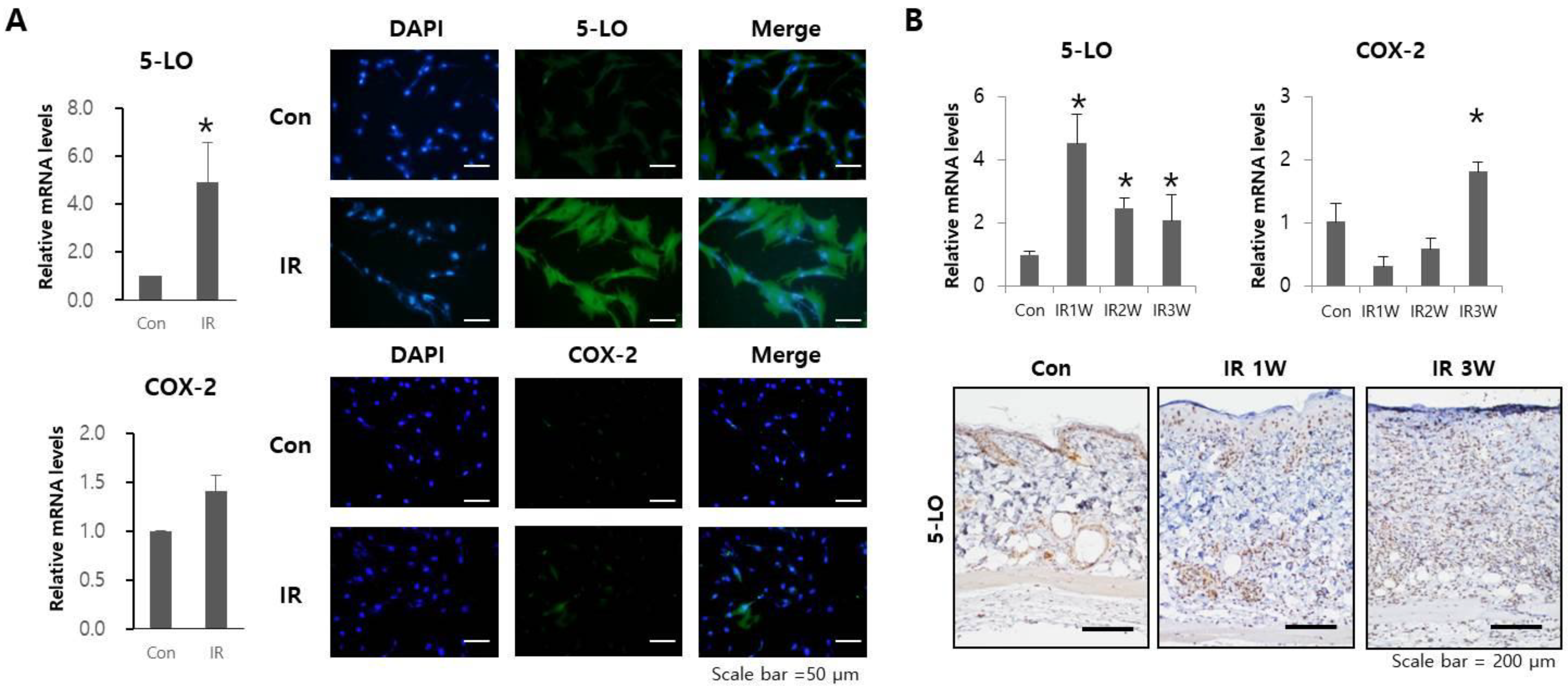
2.2. Radiation Increases 5-LO Expression
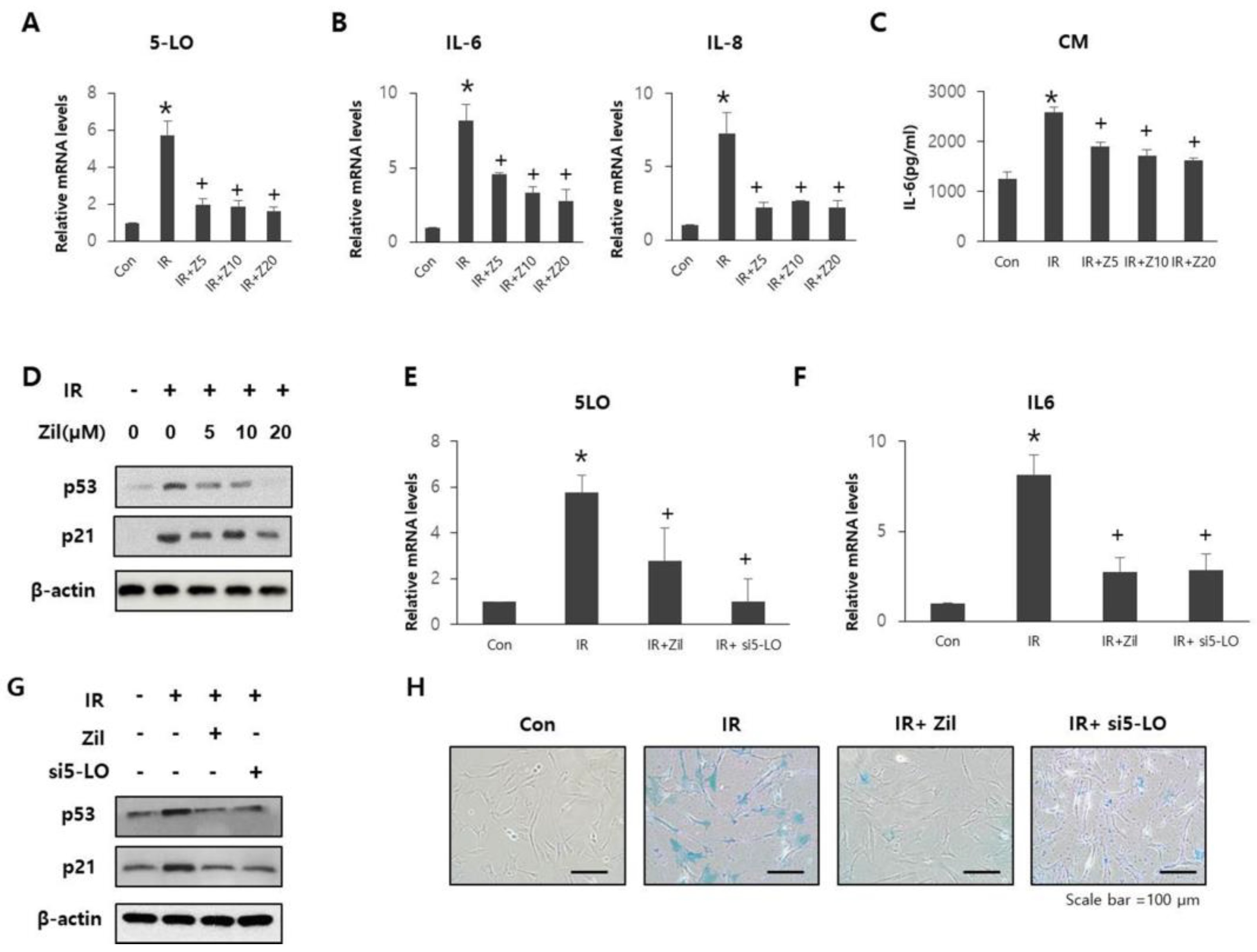
2.3. Radiation-Induced 5-LO Promotes Cell Senescence and SASP
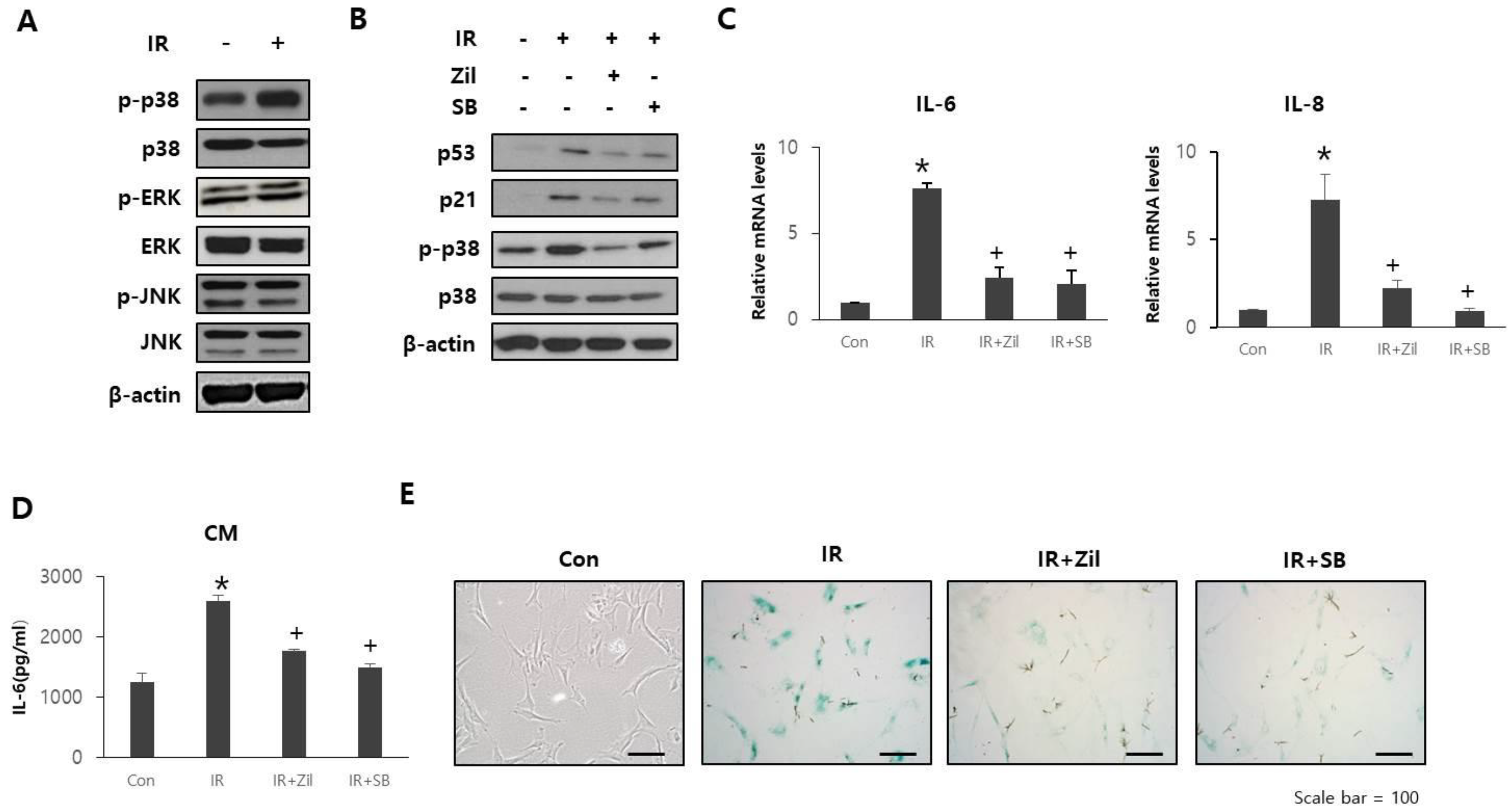
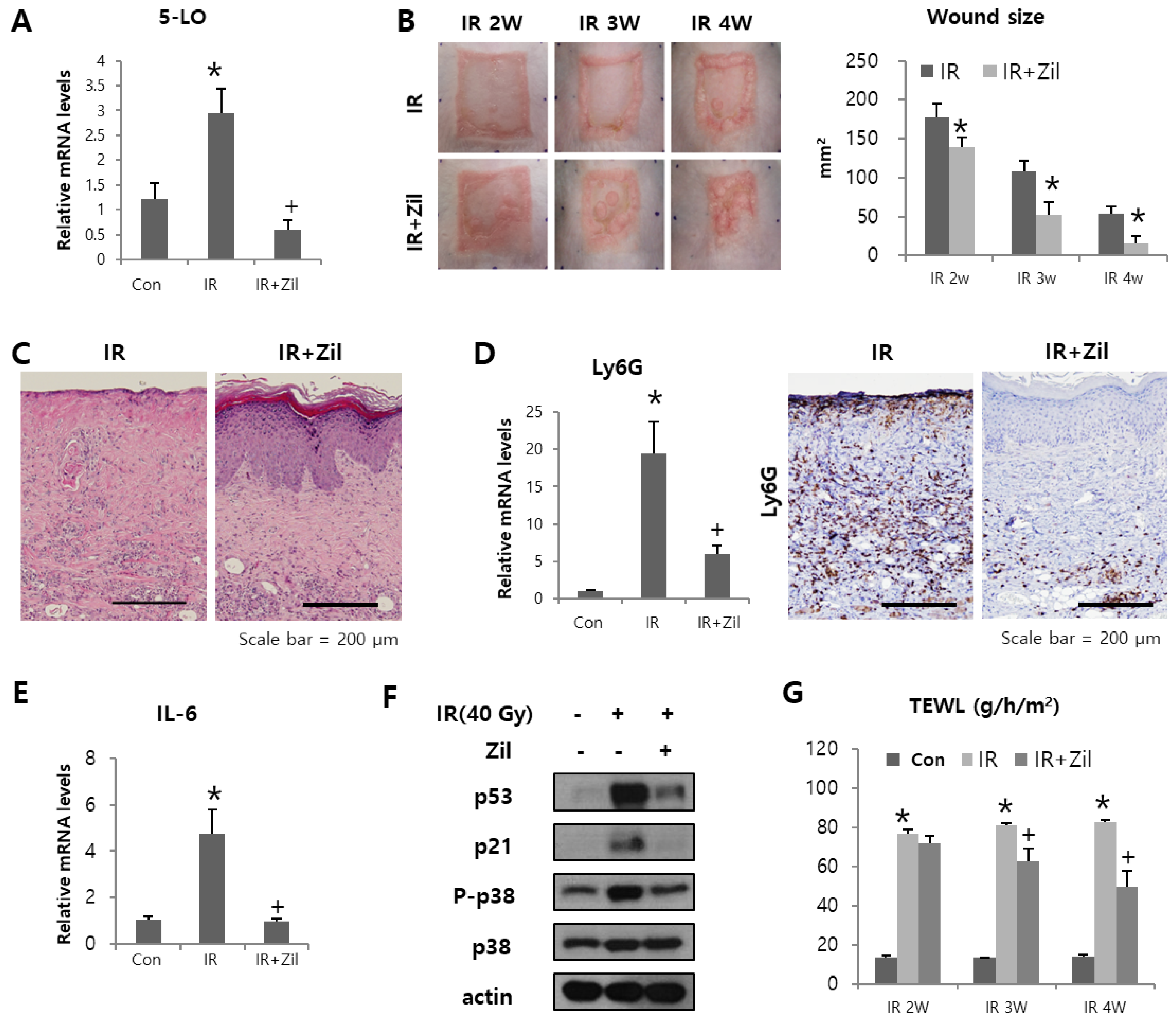
2.4. Zileuton Prevents Cell Senescence and SASP through p38 Phosphorylation
2.5. Zileuton Attenuates Radiation-Induced Cutaneous Ulcers
3. Discussion
4. Materials and Methods
4.1. Cell Culture & Irradiation
4.2. Conditioned Medium (CM)
4.3. Cytokine Array and Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Small Interfering RNA Transfection
4.5. Animal Irradiation
4.6. Wound Analysis
4.7. Transepidermal Water Loss Analysis
4.8. Histological Examination
4.9. Immunostaining
4.10. RNA Isolation and Quantitative Real-Time PCR
4.11. SA-β-Gal Staining
4.12. Western Blot
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, E.D.; Song, F.; Smith, J.D.; Ayan, A.S.; Mo, X.; Weldon, M.; Lu, L.; Campbell, P.G.; Bhatt, A.D.; Chakravarti, A.; et al. Plasma-based biomaterials for the treatment of cutaneous radiation injury. Wound Repair Regen. 2019, 27, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCarlo, A.L.; Bandremer, A.C.; Hollingsworth, B.A.; Kasim, S.; Laniyonu, A.; Todd, N.F.; Wang, S.J.; Wertheimer, E.R.; Rios, C.I. Cutaneous radiation injuries: Models, assessment and treatments. Radiat. Res. 2020, 194, 315–344. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Benson, R.; Rath, G.K. Radiation induced oral mucositis: A review of current literature on prevention and management. Eur. Arch. Otorhinolaryngol. 2016, 273, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Le, O.N.; Rodier, F.; Fontaine, F.; Coppe, J.P.; Campisi, J.; DeGregori, J.; Laverdière, C.; Kokta, V.; Haddad, E.; Beauséjour, C.M. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 2010, 9, 398–409. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Schulte, B.A.; LaRue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; You, L.; Xue, J.; Lu, Y. Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: A mini review. Front. Pharmacol. 2018, 9, 522. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Vernot, J.P. Senescence-associated pro-inflammatory cytokines and tumor cell plasticity. Front. Mol. Biosci. 2020, 7, 63. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Krtolica, A.; Beauséjour, C.M.; Parrinello, S.; Hodgson, J.G.; Chin, K.; Desprez, P.Y.; Campisi, J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS ONE 2010, 5, e9188. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic ras and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. Mtor regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting il1a translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Zdanov, S.; Bernard, D.; Debacq-Chainiaux, F.; Martien, S.; Gosselin, K.; Vercamer, C.; Chelli, F.; Toussaint, O.; Abbadie, C. Normal or stress-induced fibroblast senescence involves COX-2 activity. Exp. Cell Res. 2007, 313, 3046–3056. [Google Scholar] [CrossRef]
- Catalano, A.; Rodilossi, S.; Caprari, P.; Coppola, V.; Procopio, A. 5-Lipoxygenase regulates senescence-like growth arrest by promoting ros-dependent p53 activation. EMBO J. 2005, 24, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Saul, M.J.; Emmerich, A.C.; Steinhilber, D.; Suess, B. Regulation of eicosanoid pathways by microRNAs. Front. Pharmacol. 2019, 10, 824. [Google Scholar] [CrossRef] [Green Version]
- Khanapure, S.P.; Garvey, D.S.; Janero, D.R.; Letts, L.G. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 2007, 7, 311–340. [Google Scholar] [CrossRef]
- Jozefowski, S.; Biedroń, R.; Bobek, M.; Marcinkiewicz, J. Leukotrienes modulate cytokine release from dendritic cells. Immunology 2005, 116, 418–428. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Nakamura, H.; Ohara, N.; Naito, Y. Eicosanoids derived from arachidonic acid and their family prostaglandins and cyclooxygenase in psychiatric disorders. Curr. Neuropharmacol. 2015, 13, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Wiley, C.D.; Sharma, R.; Davis, S.S.; Lopez-Dominguez, J.A.; Mitchell, K.P.; Wiley, S.; Alimirah, F.; Kim, D.E.; Payne, T.; Rosko, A.; et al. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metab. 2021, 33, 1124–1136.e5. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Brumwell, A.N.; Davis, S.S.; Jackson, J.R.; Valdovinos, A.; Calhoun, C.; Alimirah, F.; Castellanos, C.A.; Ruan, R.; Wei, Y.; et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight 2019, 4, e130056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Roh, M.S.; Park, C.H.; Park, K.C.; Cho, K.H.; Kim, K.H.; Eun, H.C.; Chung, J.H. Selective COX-2 inhibitor, NS-398, inhibits the replicative senescence of cultured dermal fibroblasts. Mech. Ageing Dev. 2004, 125, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Martien, S.; Pluquet, O.; Vercamer, C.; Malaquin, N.; Martin, N.; Gosselin, K.; Pourtier, A.; Abbadie, C. Cellular senescence involves an intracrine prostaglandin E2 pathway in human fibroblasts. Biochim. Biophys. Acta 2013, 1831, 1217–1227. [Google Scholar] [CrossRef]
- Jiao, W.; Kiang, J.G.; Cary, L.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. COX-2 inhibitors are contraindicated for treatment of combined injury. Radiat. Res. 2009, 172, 686–697. [Google Scholar] [CrossRef]
- Budamagunta, V.; Manohar-Sindhu, S.; Yang, Y.; He, Y.; Traktuev, D.O.; Foster, T.C.; Zhou, D. Senescence-associated hyper-activation to inflammatory stimuli in vitro. Aging 2021, 13, 19088–19107. [Google Scholar] [CrossRef]
- Freund, A.; Patil, C.K.; Campisi, J. P38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef] [Green Version]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. Mapk pathways in radiation responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Han, J. The p38 signal transduction pathway: Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, B.; Lin, A. Novel strategies for inhibition of the p38 mapk pathway. Trends Pharmacol. Sci. 2007, 28, 286–295. [Google Scholar] [CrossRef]
- Bolderston, A.; Lloyd, N.S.; Wong, R.K.; Holden, L.; Robb-Blenderman, L.; Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based Care. The prevention and management of acute skin reactions related to radiation therapy: A systematic review and practice guideline. Support. Care Cancer 2006, 14, 802–817. [Google Scholar] [CrossRef]
- Hoang, T.T.; Vu, V.Q.; Trinh, D.T. Management of radiation-induced ulcers by single stage reconstructive surgery: A prospective study. Ann. Burns Fire Disasters 2019, 32, 294–300. [Google Scholar]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.Y.; Chang, K.W.; Liu, C.J.; Tseng, Y.H.; Lu, H.H.; Lee, S.Y.; Lin, S.C. Ripe areca nut extract induces g1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis 2006, 27, 1273–1284. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D.; Lebedeva, I.V.; Emdad, L.; Kang, D.C.; Baldwin, A.S., Jr.; Fisher, P.B. Human polynucleotide phosphorylase (hpnpaseold-35): A potential link between aging and inflammation. Cancer Res. 2004, 64, 7473–7478. [Google Scholar] [CrossRef] [Green Version]
- Rodier, F.; Coppé, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Muñoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Fornace, A.J., Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef]
- Woodmansee, D.P.; Simon, R.A. A pilot study examining the role of zileuton in atopic dermatitis. Ann. Allergy Asthma Immunol. 1999, 83, 548–552. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Lajeunesse, D.; Reboul, P.; Pelletier, J.P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann. Rheum. Dis. 2003, 62, 501–509. [Google Scholar] [CrossRef]
- Suryakumari, C.; Narender, M.; Umasankar, K.; Panda, S.P.; Koteswara Rao, S.N.; Panda, S. Formulation and evaluation of tacrolimus transdermal gel. J. Drug Deliv. Ther. 2019, 9, 110–118. [Google Scholar] [CrossRef]
| Species | Gene | Forward Primer | Reverse Primer |
|---|---|---|---|
| Mouse | IL-1β | CAGCTCATATGGGTCCGACA | CTGTGTCTTTCCCGTGGACC |
| IL-6 | AGCCAGAGTCCTTCAGAGAG | GATGGTCTTGGTCCTTAGCC | |
| COX-2 | CCAGCACTTCACCCATCAGTT | ACCCAGGTCCTCGCTTATGA | |
| 5-LO | GCCGGACTGATGTACCTGTT | CGCTTCCGAAGAAGAAGATG | |
| Ly6G | GAGAGGAAGTTTTATCTGTGCAGC | TCTCAGGTGGGACCCCAATA | |
| Β-actin | CTTTTCACGGTTGGCCTTAG | CCCTGAAGTACCCCATTGAAC | |
| Human | IL-1β | ATGATGGC TTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
| IL-6 | ATGGGAAACAATGTCACGAAC | TGTATTCCGTCTCCTTGGTTC | |
| IL-8 | CTCTTGGCAGCCTTC CTGATT | ACTCTCAATCACTCTCAGTTCT | |
| 5-LO | ACAAGCCCTTCTACAACGACT | AACTGGG CGAGATCCAGCT | |
| COX-2 | GTTCCACCCGCAGTACAGAA | AGGGCTTCAGCATAAAGCGT | |
| GAPDH | GGACTCATGACCACAGTCCATGCC | TCAGGGATGACCTTGCCCACAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Na, J.; Kwak, S.Y.; Park, S.; Kim, H.; Lee, S.-J.; Jang, W.-S.; Lee, S.B.; Jang, W.I.; Jang, H.; et al. Zileuton Alleviates Radiation-Induced Cutaneous Ulcers via Inhibition of Senescence-Associated Secretory Phenotype in Rodents. Int. J. Mol. Sci. 2022, 23, 8390. https://doi.org/10.3390/ijms23158390
Park M, Na J, Kwak SY, Park S, Kim H, Lee S-J, Jang W-S, Lee SB, Jang WI, Jang H, et al. Zileuton Alleviates Radiation-Induced Cutaneous Ulcers via Inhibition of Senescence-Associated Secretory Phenotype in Rodents. International Journal of Molecular Sciences. 2022; 23(15):8390. https://doi.org/10.3390/ijms23158390
Chicago/Turabian StylePark, Mineon, Jiyoung Na, Seo Young Kwak, Sunhoo Park, Hyewon Kim, Sun-Joo Lee, Won-Suk Jang, Seung Bum Lee, Won Il Jang, Hyosun Jang, and et al. 2022. "Zileuton Alleviates Radiation-Induced Cutaneous Ulcers via Inhibition of Senescence-Associated Secretory Phenotype in Rodents" International Journal of Molecular Sciences 23, no. 15: 8390. https://doi.org/10.3390/ijms23158390
APA StylePark, M., Na, J., Kwak, S. Y., Park, S., Kim, H., Lee, S.-J., Jang, W.-S., Lee, S. B., Jang, W. I., Jang, H., & Shim, S. (2022). Zileuton Alleviates Radiation-Induced Cutaneous Ulcers via Inhibition of Senescence-Associated Secretory Phenotype in Rodents. International Journal of Molecular Sciences, 23(15), 8390. https://doi.org/10.3390/ijms23158390






