COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR
Abstract
:1. Introduction
2. Results
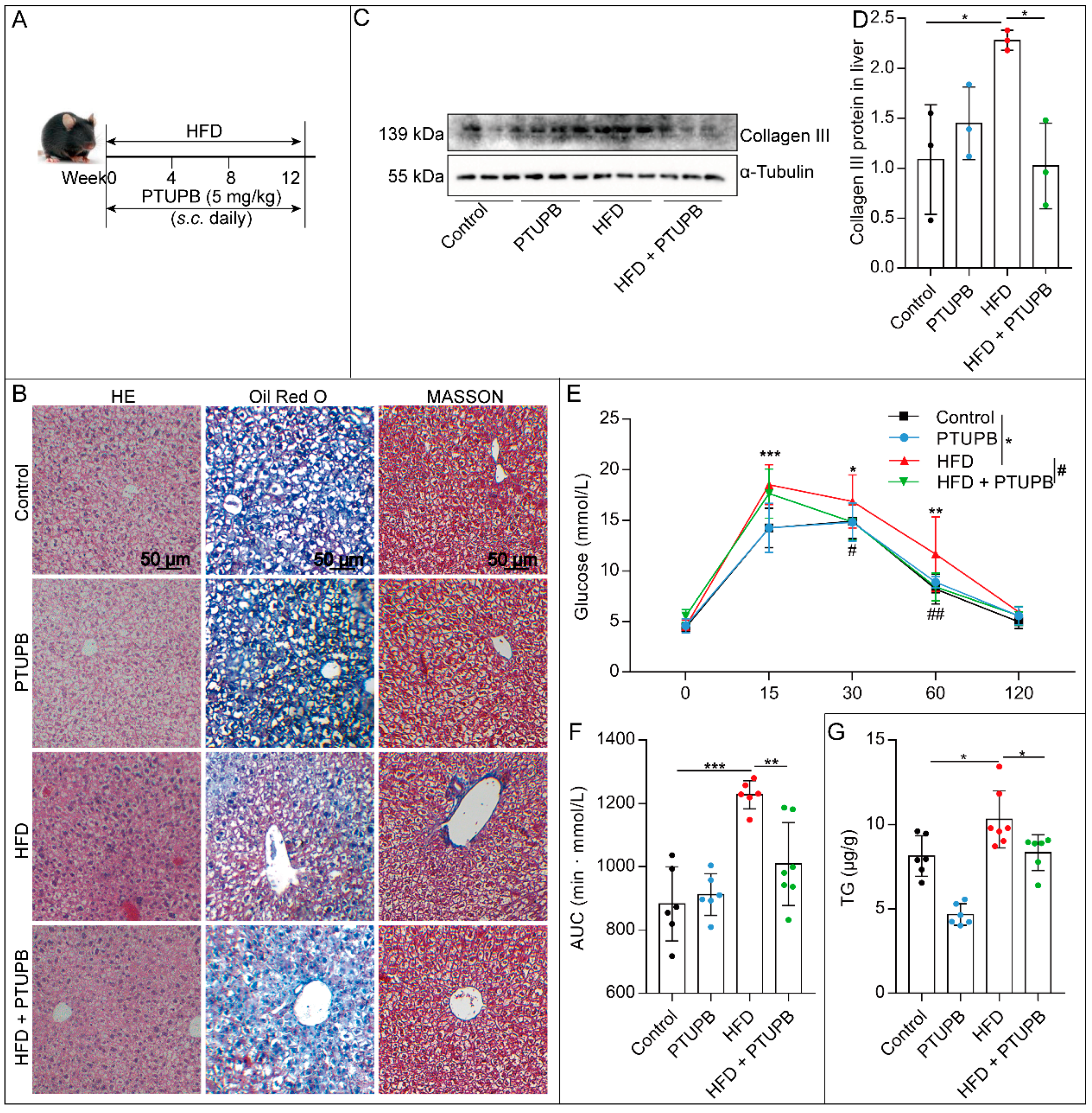
2.1. PTUPB Attenuates HFD-Induced NAFLD in Mice
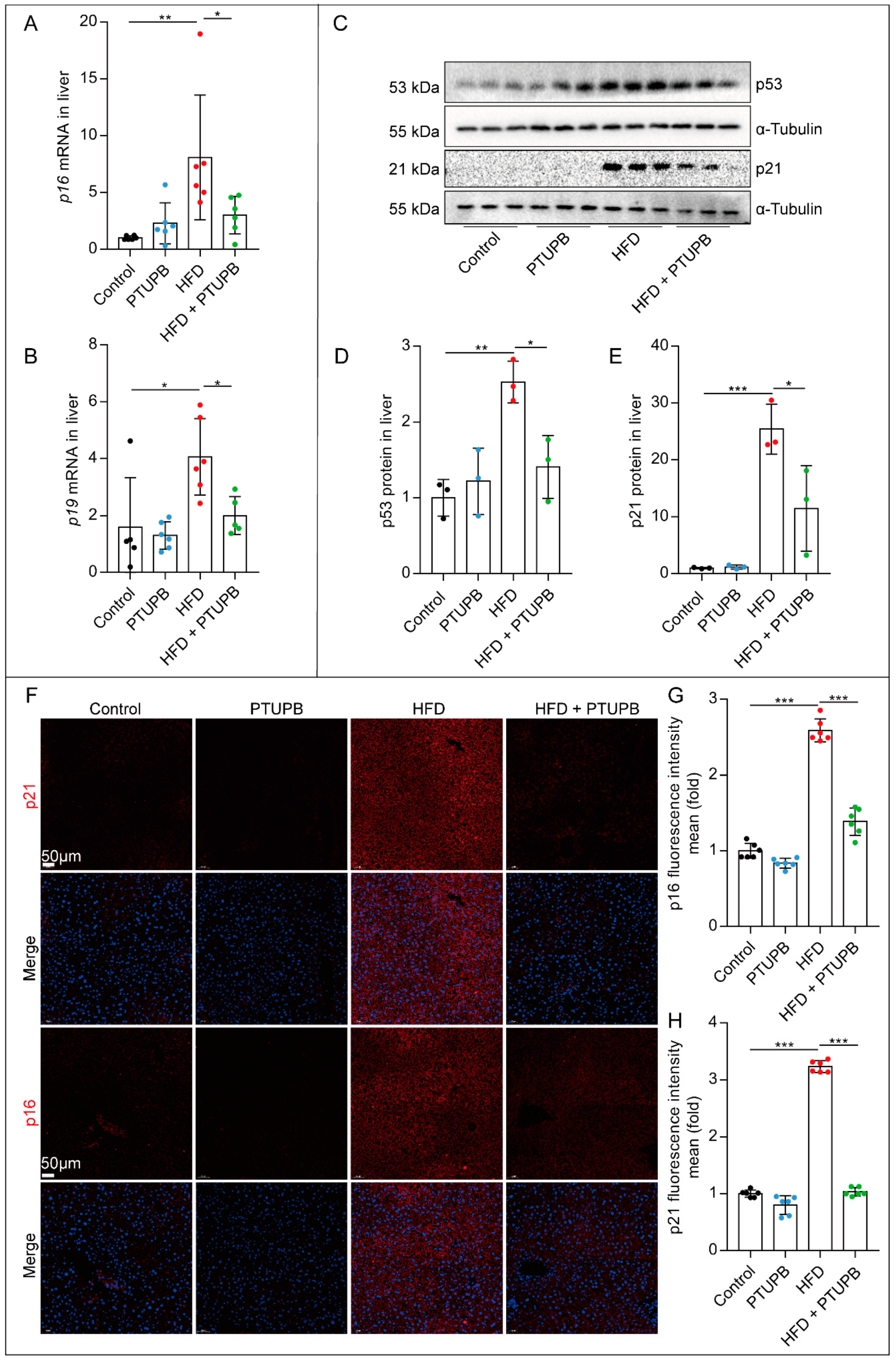
2.2. PTUPB Inhibits Hepatocyte Senescence Induced by HFD in Mice
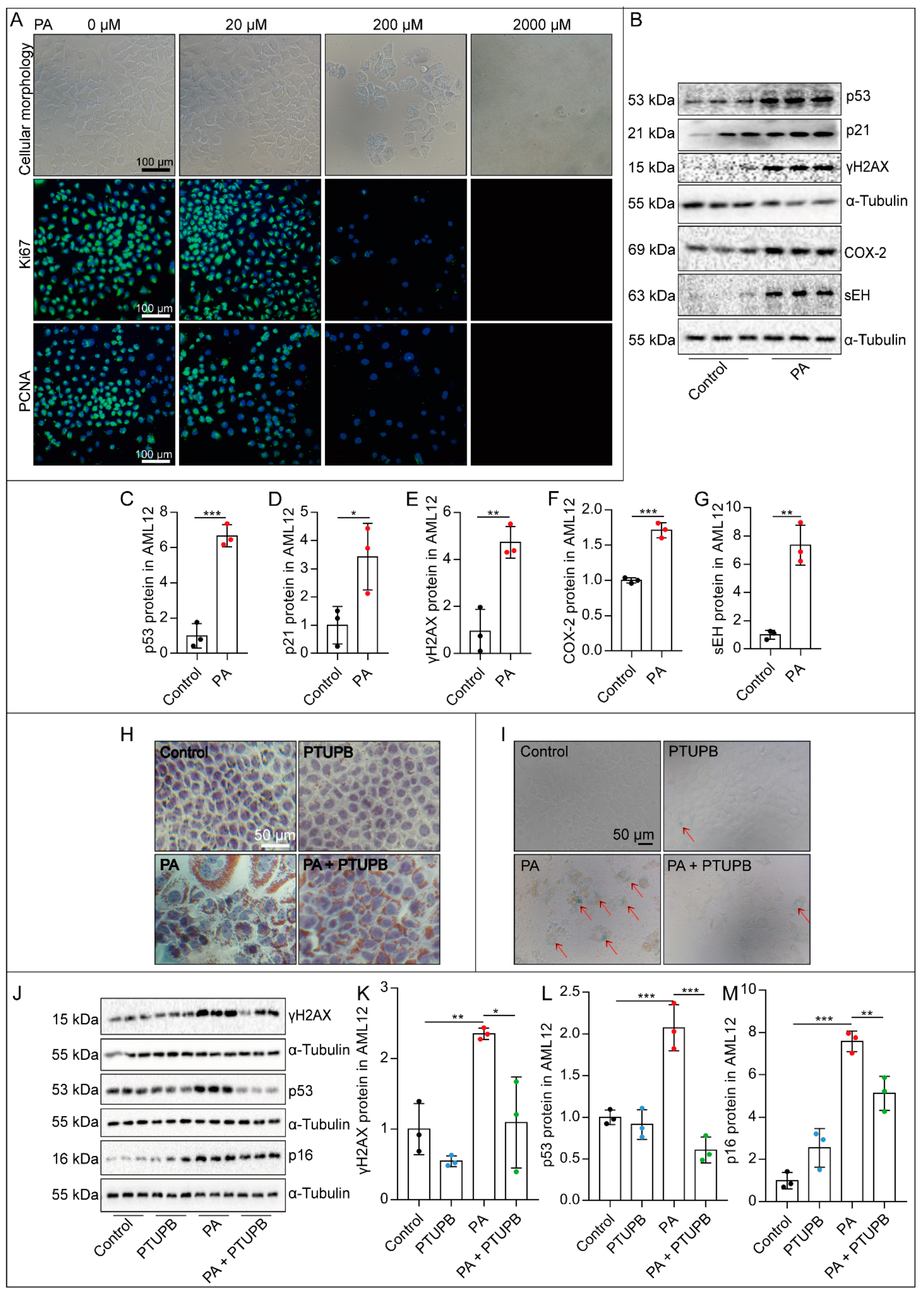
2.3. PTUPB Attenuates PA-Induced Hepatocyte Senescence In Vitro
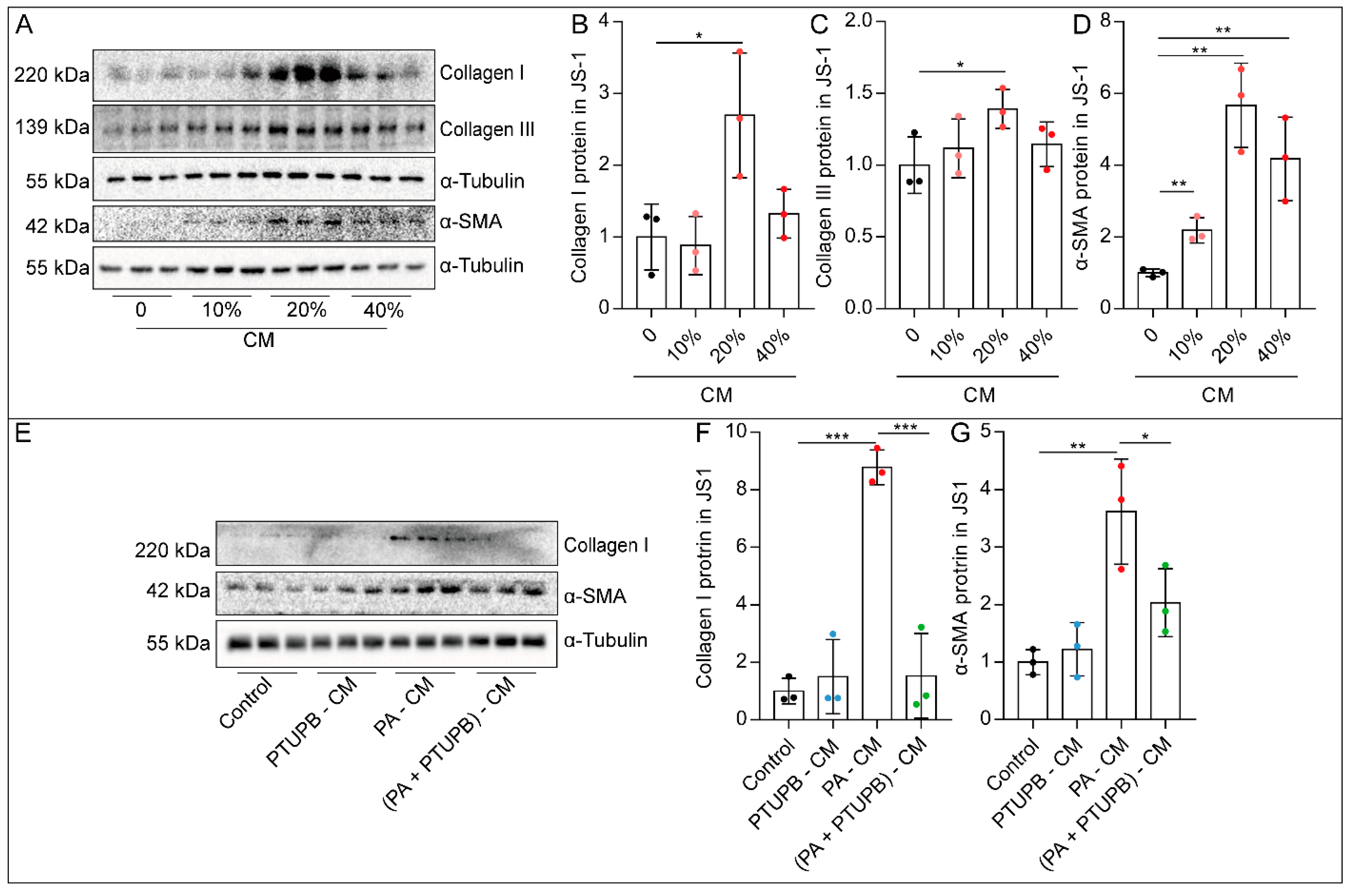
2.4. PTUPB Reduces the Hepatic Astrocytes Activation by Inhibiting Hepatocyte Senescence
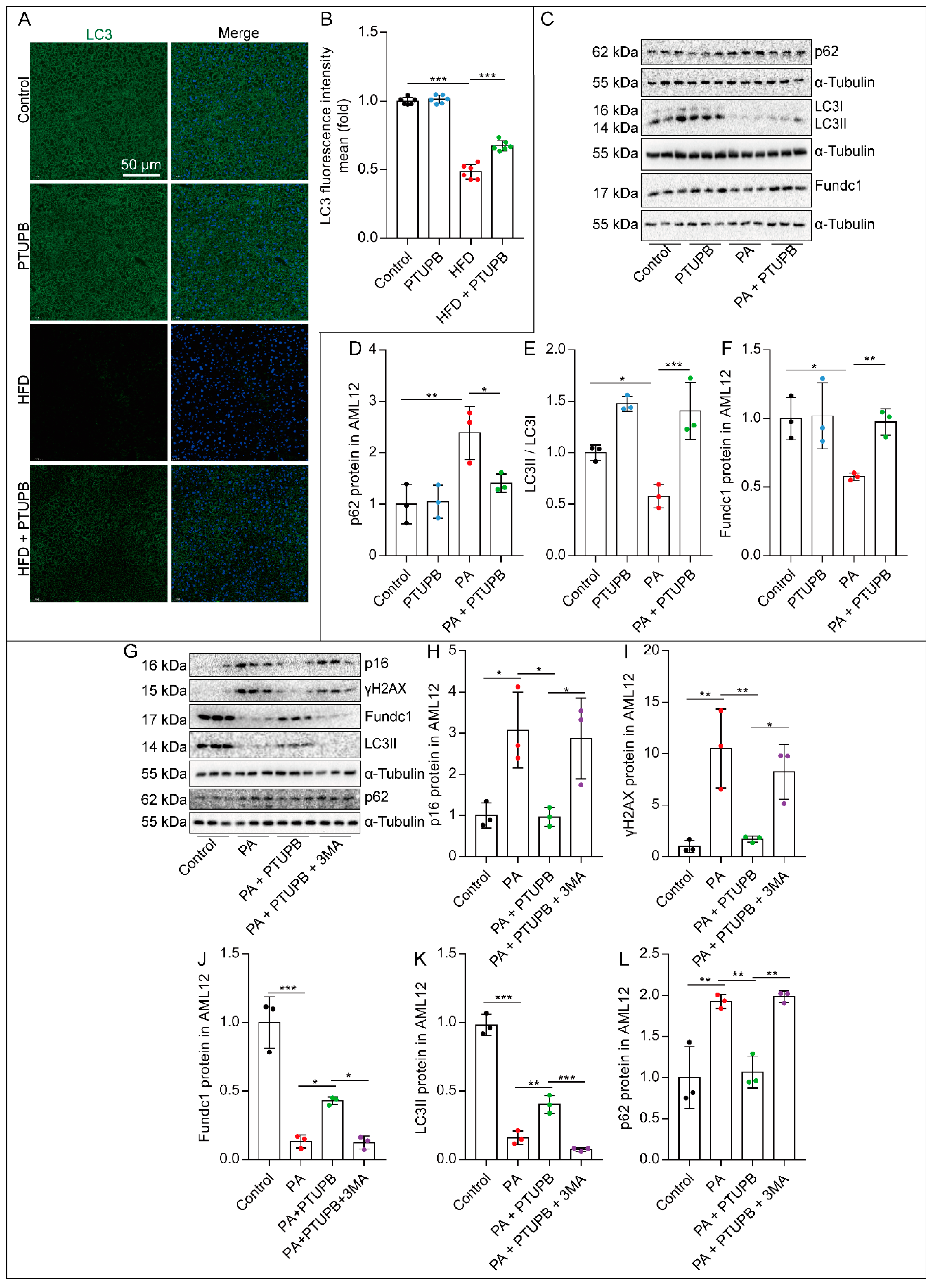
2.5. PTUPB Attenuates Hepatocyte Senescence by Enhancing Autophagy
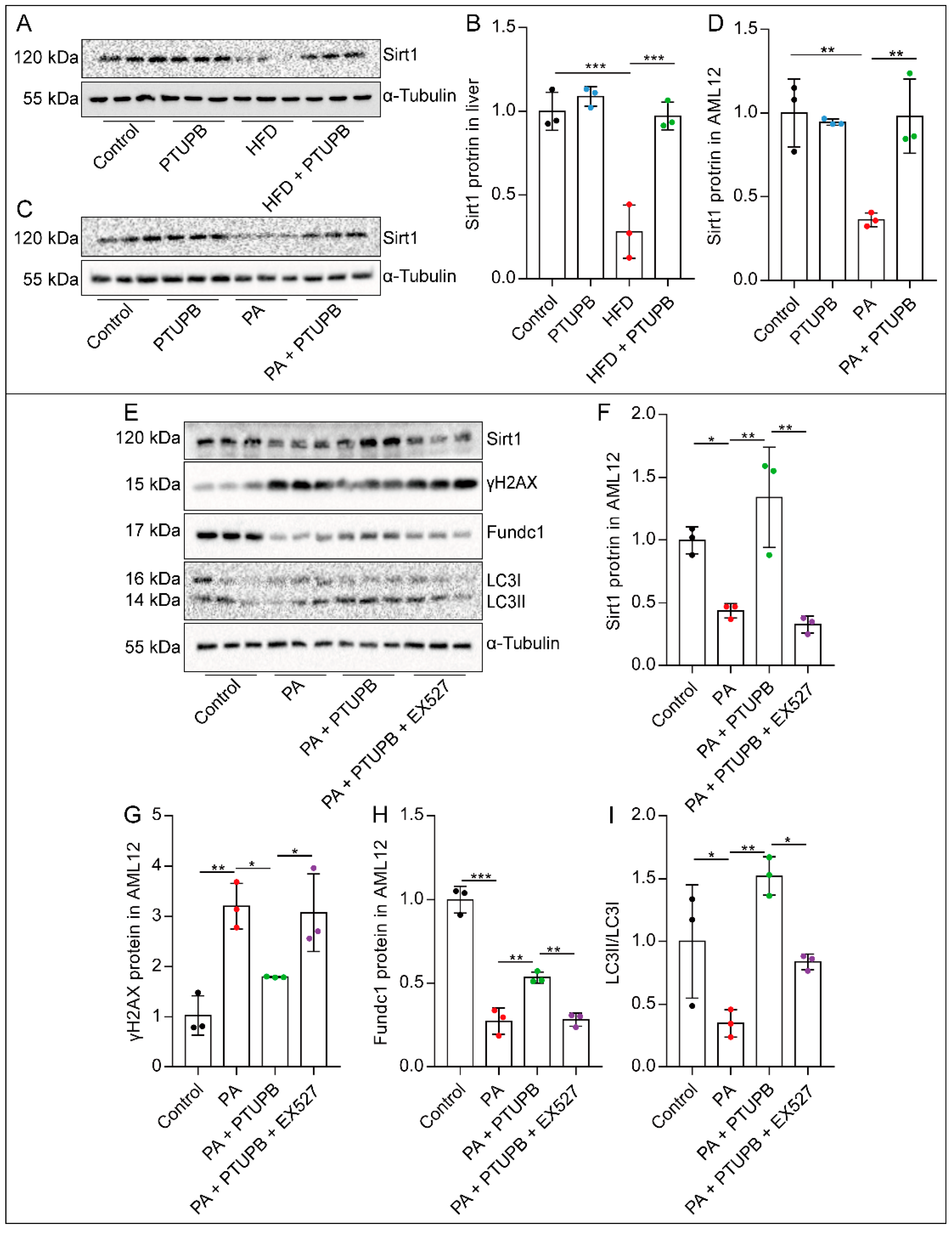
2.6. PTUPB Enhances Hepatocyte Autophagy by Promoting Sirt1 Expression
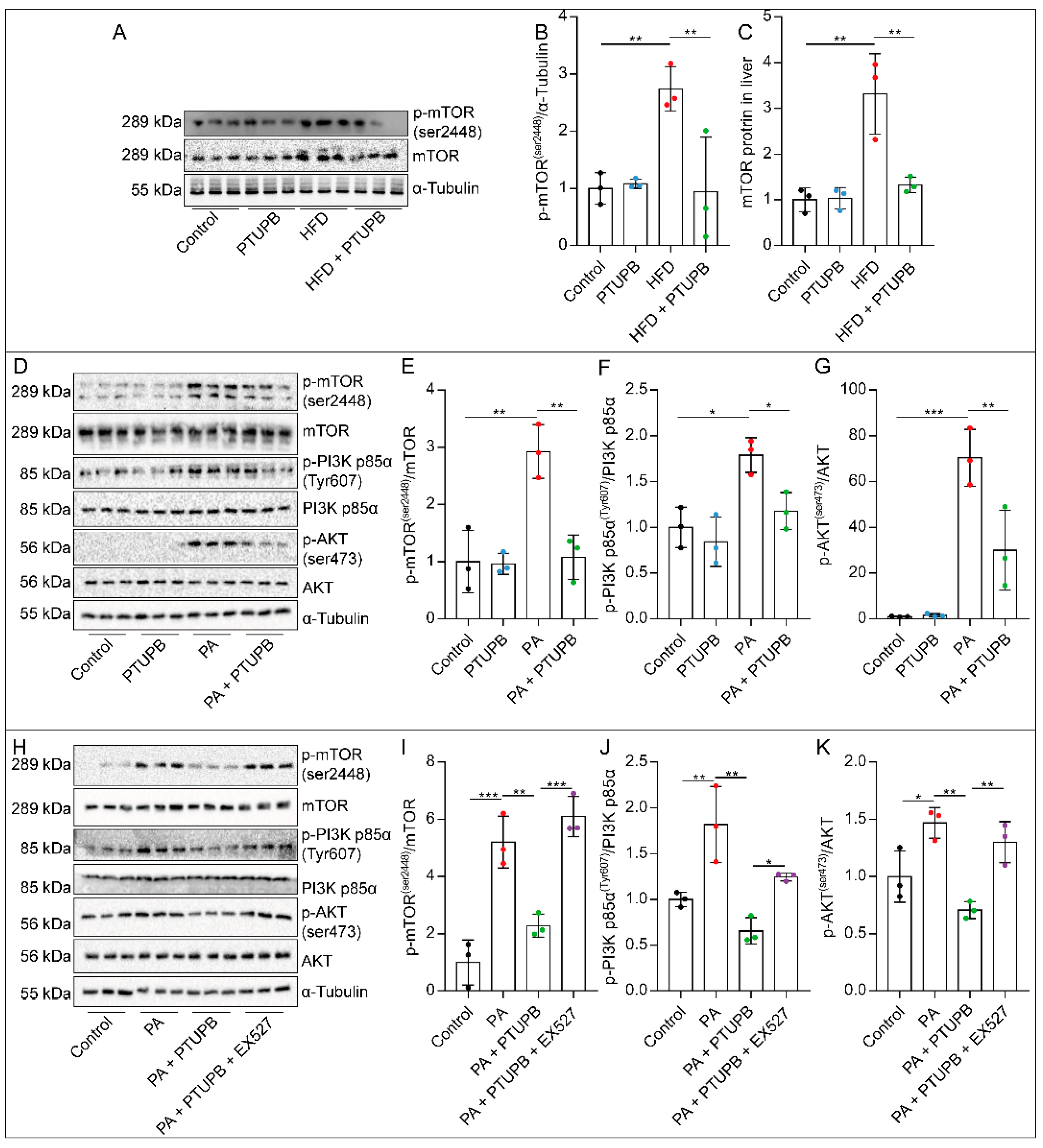
2.7. PTUPB Inhibits the PI3K/AKT/mTOR Signaling Pathway by Promoting Sirt1 Expression
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. HFD Mouse Model
4.3. Liver Histopathology Analysis
4.4. Triglycerides Measurement
4.5. Cell Culture and Treatment
4.6. Oil Red O Stain
4.7. Senescence-Associated β-Galactosidase Staining
4.8. Glucose Tolerance Measurement
4.9. Western Blot
4.10. Real-Time PCR
4.11. Immunofluorescent Staining
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARA | arachidonic acid |
| α-SMA | α-smooth muscle actin |
| AUC | area under the curve |
| CM | conditioned medium |
| COX-2 | cyclooxygenase-2 |
| CYP | cytochrome P450 oxidase |
| EET | epoxyeicosatrienoic acid |
| HFD | high-fat diet |
| NAFLD | non-alcoholic fatty liver disease |
| PA | palmitic acid |
| PTUPB | 4-(5-phenyl-3-{3-[3-(4-trifluoromethylphenyl)-ureido]-propyl} pyrazol 1-yl)-benzenesulfonamide |
| SASP | senescence-related secretory phenotype |
| sEH | soluble epoxide hydrolase |
| TG | triglyceride |
References
- Francque, S.; Szabo, G.; Abdelmalek, M.F.; Byrne, C.D.; Cusi, K.; Dufour, J.-F.; Roden, M.; Sacks, F.; Tacke, F. Nonalcoholic steatohepatitis: The role of peroxisome proliferator-activated receptors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F. Nonalcoholic fatty liver disease: Another leap forward. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 85–86. [Google Scholar] [CrossRef]
- Tanwar, S.; Rhodes, F.; Srivastava, A.; Trembling, P.M.; Rosenberg, W. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J. Gastroenterol. 2020, 26, 109–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Luo, X.; Li, Z.-H.; Wu, J.-C.; Luo, S.-Z.; Xu, M.-Y. Zinc-α2-glycoprotein 1 attenuates non-alcoholic fatty liver disease by negatively regulating tumour necrosis factor-α. World J. Gastroenterol. 2019, 25, 5451–5468. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [Green Version]
- Wagner, K.; Inceoglu, B.; Hammock, B.D. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins Other Lipid Mediat. 2011, 96, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Duan, J.; Yang, H.; Zhang, C.; Sun, C.; Guan, X.; Xiong, J.; Zu, C.; Tao, J.; Zhou, Y.; et al. Epoxyeicosatrienoic acids inhibit the activation of NLRP3 inflammasome in murine macrophages. J. Cell. Physiol. 2020, 235, 9910–9921. [Google Scholar] [CrossRef] [PubMed]
- Esamokhvalov, V.; Evriend, J.; Jamieson, K.L.; Akhnokh, M.K.; Emanne, R.; Falck, J.R.; Seubert, J.M. PPARγ signaling is required for mediating EETs protective effects in neonatal cardiomyocytes exposed to LPS. Front. Pharmacol. 2014, 5, 242. [Google Scholar] [CrossRef]
- Sun, C.-P.; Zhang, X.-Y.; Zhou, J.-J.; Huo, X.-K.; Yu, Z.-L.; Morisseau, C.; Hammock, B.D.; Ma, X.-C. Inhibition of sEH via stabilizing the level of EETs alleviated Alzheimer’s disease through GSK3β signaling pathway. Food Chem. Toxicol. 2021, 156, 112516. [Google Scholar] [CrossRef]
- Guan, X.-X.; Rao, D.-N.; Liu, Y.-Z.; Zhou, Y.; Yang, H.-H. Epoxyeicosatrienoic Acids and Fibrosis: Recent Insights for the Novel Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 10714. [Google Scholar] [CrossRef]
- Harris, T.R.; Hammock, B.D. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene 2013, 526, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Narumiya, S. Physiology and pathophysiology of prostanoid receptors. Proc. Jpn. Acad. Ser. B 2007, 83, 296–319. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-H.; Duan, J.-X.; Liu, S.-K.; Xiong, J.-B.; Guan, X.-X.; Zhong, W.-J.; Sun, C.-C.; Zhang, C.-Y.; Luo, X.-Q.; Zhang, Y.-F.; et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics 2020, 10, 4749–4761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Duan, J.; Yang, H.; Sun, C.; Zhong, W.; Tao, J.; Guan, X.; Jiang, H.; Hammock, B.D.; Hwang, S.H.; et al. COX-2/sEH dual inhibitor PTUPB alleviates bleomycin-induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J. 2020, 287, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Sun, C.-C.; Duan, J.-X.; Yang, H.-H.; Zhang, C.-Y.; Xiong, J.-B.; Zhong, W.-J.; Zu, C.; Guan, X.-X.; Jiang, H.-L.; et al. A COX-2/sEH dual inhibitor PTUPB ameliorates cecal ligation and puncture-induced sepsis in mice via anti-inflammation and anti-oxidative stress. Biomed. Pharmacother. 2020, 126, 109907. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.; Zhang, C.-Y.; Duan, J.-X.; Guan, X.-X.; Yang, H.-H.; Jiang, H.-L.; Hammock, B.D.; Hwang, S.H.; Zhou, Y.; Guan, C.-X.; et al. PTUPB ameliorates high-fat diet-induced non-alcoholic fatty liver disease via inhibiting NLRP3 inflammasome activation in mice. Biochem. Biophys. Res. Commun. 2020, 523, 1020–1026. [Google Scholar] [CrossRef]
- Moustakas, I.; Katsarou, A.; Legaki, A.-I.; Pyrina, I.; Ntostoglou, K.; Papatheodoridi, A.-M.; Gercken, B.; Pateras, I.; Gorgoulis, V.; Koutsilieris, M.; et al. Hepatic Senescence Accompanies the Development of NAFLD in Non-Aged Mice Independently of Obesity. Int. J. Mol. Sci. 2021, 22, 3446. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Papatheodoridi, A.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The Role of Senescence in the Development of Nonalcoholic Fatty Liver Disease and Progression to Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhou, D.; Strakovsky, R.; Zhang, Y.; Pan, Y.-X. Hepatic cellular senescence pathway genes are induced through histone modifications in a diet-induced obese rat model. Am. J. Physiol. Liver Physiol. 2012, 302, G558–G564. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jiang, X.; Dong, F.; Zhang, F.; Ji, X.; Xue, M.; Yang, F.; Chen, J.; Hu, X.; Bao, Z. Lipid accumulation-induced hepatocyte senescence regulates the activation of hepatic stellate cells through the Nrf2-antioxidant response element pathway. Exp. Cell Res. 2021, 405, 112689. [Google Scholar] [CrossRef]
- Sun, C.; Simon, S.I.; Foster, G.A.; Radecke, C.E.; Hwang, H.V.; Zhang, X.; Hammock, B.D.; Chiamvimonvat, N.; Knowlton, A.A. 11,12-Epoxyecosatrienoic acids mitigate endothelial dysfunction associated with estrogen loss and aging: Role of membrane depolarization. J. Mol. Cell. Cardiol. 2016, 94, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhao, Q.; Li, M.; Duan, Q.; Zhao, Y.; Zhang, H. The effects of endothelium-specific CYP2J2 overexpression on the attenuation of retinal ganglion cell apoptosis in a glaucoma rat model. FASEB J. 2019, 33, 11194–11209. [Google Scholar] [CrossRef] [Green Version]
- Wils, J.; Djerada, Z.; Roca, F.; Duflot, T.; Iacob, M.; Remy-Jouet, I.; Joannides, R.; Bellien, J. Alteration in the availability of epoxyeicosatrienoic acids contributes with NO to the development of endothelial dysfunction in conduit arteries during aging. Atherosclerosis 2018, 275, 239–245. [Google Scholar] [CrossRef]
- Bellaver, B.; Souza, D.G.; Souza, D.O.; Quincozes-Santos, A. Hippocampal Astrocyte Cultures from Adult and Aged Rats Reproduce Changes in Glial Functionality Observed in the Aging Brain. Mol. Neurobiol. 2016, 54, 2969–2985. [Google Scholar] [CrossRef]
- Domínguez-González, M.; Puigpinós, M.; Jové, M.; Naudi, A.; Portero-Otín, M.; Pamplona, R.; Ferrer, I. Regional vulnerability to lipoxidative damage and inflammation in normal human brain aging. Exp. Gerontol. 2018, 111, 218–228. [Google Scholar] [CrossRef]
- Kourtis, N.; Tavernarakis, N. Autophagy and cell death in model organisms. Cell Death Differ. 2009, 16, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the hallmarks of aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef]
- Madeo, F.; Tavernarakis, N.; Kroemer, G. Can autophagy promote longevity? Nat. Cell Biol. 2010, 12, 842–846. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, D.; Tang, L.; Li, H.; Liu, Z.; Gao, J.; Edin, M.L.; Zhang, H.; Zhang, K.; Chen, J.; et al. Soluble epoxide hydrolase deficiency attenuates lipotoxic cardiomyopathy via upregulation of AMPK-mTORC mediated autophagy. J. Mol. Cell. Cardiol. 2021, 154, 80–91. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.; Li, Q.; Zhan, C.; Xu, X.; Zhang, X.; Ai, D.; Zhu, Y.; Wen, Z.; Wang, D.W. CYP2J2-derived EETs attenuated ethanol-induced myocardial dysfunction through inducing autophagy and reducing apoptosis. Free Radic. Biol. Med. 2018, 117, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Mishra, K.P.; Thakur, A.K. Inhibition of Cyclooxygenase-2 (COX-2) Initiates Autophagy and Potentiates MPTP-Induced Autophagic Cell Death of Human Neuroblastoma Cells, SH-SY5Y: An Inside in the Pathology of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 8038–8050. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gonzalez, S.; Lu, W.-Y.; Raven, A.; Dwyer, B.; Man, T.Y.; O’Duibhir, E.; Lewis, P.J.S.; Campana, L.; Kendall, T.J.; Bird, T.; et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat. Commun. 2018, 9, 1020. [Google Scholar] [CrossRef]
- Engelmann, C.; Tacke, F. The Potential Role of Cellular Senescence in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 652. [Google Scholar] [CrossRef]
- Zhang, Y.; Whaley-Connell, A.T.; Sowers, J.R.; Ren, J. Autophagy as an emerging target in cardiorenal metabolic disease: From pathophysiology to management. Pharmacol. Ther. 2018, 191, 1–22. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, Á.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lo Iacono, O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-H.; Park, S.-H.; Ahn, J.; Hong, S.P.; Lee, E.; Jang, Y.-J.; Ha, T.-Y.; Huh, Y.H.; Ha, S.-Y.; Jeon, T.-I.; et al. Mir214-3p and Hnf4a/Hnf4α reciprocally regulate Ulk1 expression and autophagy in nonalcoholic hepatic steatosis. Autophagy 2021, 17, 2415–2431. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Yu, C.; Pan, Z.; Liu, Y.; Zhao, J.; Wang, X.; Yun, F.; Zhao, H.; Yan, S.; et al. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am. J. Physiol. Metab. 2015, 309, E925–E935. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhong, P.; Wang, Y.; Zhang, Q.; Li, J.; Liu, Z.; Ji, A.; Li, Y. Hydrogen Sulfide Attenuates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease by Inhibiting Apoptosis and Promoting Autophagy via Reactive Oxygen Species/Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Signaling Pathway. Front. Pharmacol. 2020, 11, 585860. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Singh, R. Autophagy and aging. Adv. Exp. Med. Biol. 2015, 847, 73–87. [Google Scholar]
- Singh, B.K.; Tripathi, M.; Sandireddy, R.; Tikno, K.; Zhou, J.; Yen, P.M. Decreased autophagy and fuel switching occur in a senescent hepatic cell model system. Aging 2020, 12, 13958–13978. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Yang, X.; Dong, W.; Yang, J.; Hu, Q.; Zhang, C.; Fang, H.; Liu, A. Growth differentiation factor 11 accelerates liver senescence through the inhibition of autophagy. Aging Cell 2022, 21, e13532. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Li, Y.-M.; Yin, S.; Xu, M.-J.; Feng, D.; Zhou, Z.; Zang, M.; Mukhopadhyay, P.; Varga, Z.V.; Pacher, P.; et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J. Hepatol. 2017, 66, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J.; et al. Deacetylation of Nuclear LC3 Drives Autophagy Initiation under Starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Li, X.; Zhang, P.; Chen, W.-D.; Zhang, H.-L.; Li, D.-D.; Deng, R.; Qian, X.-J.; Jiao, L.; Ji, J.; et al. Acetylation of Beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat. Commun. 2015, 6, 7215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Jia, C.; Wu, D.; Jin, H.; Lin, Z.; Pan, J.; Li, X.; Wang, W. Fibroblast growth factor 21 (FGF21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway. Cell Death Dis. 2021, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Wagner, K.M.; Morisseau, C.; Liu, J.-Y.; Dong, H.; Wecksler, A.T.; Hammock, B.D. Synthesis and Structure−Activity Relationship Studies of Urea-Containing Pyrazoles as Dual Inhibitors of Cyclooxygenase-2 and Soluble Epoxide Hydrolase. J. Med. Chem. 2011, 54, 3037–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.-Y.; Guan, X.-X.; Song, Z.-H.; Jiang, H.-L.; Liu, Y.-B.; Chen, P.; Duan, J.-X.; Zhou, Y. COX-2/sEH Dual Inhibitor PTUPB Attenuates Epithelial-Mesenchymal Transformation of Alveolar Epithelial Cells via Nrf2-Mediated Inhibition of TGF-β1/Smad Signaling. Oxidative Med. Cell. Longev. 2022, 2022, 5759626. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.-J.; Duan, J.-X.; Liu, T.; Yang, H.-H.; Guan, X.-X.; Zhang, C.-Y.; Yang, J.-T.; Xiong, J.-B.; Zhou, Y.; Guan, C.-X.; et al. Activation of NLRP3 inflammasome up-regulates TREM-1 expression in murine macrophages via HMGB1 and IL-18. Int. Immunopharmacol. 2020, 89, 107045. [Google Scholar] [CrossRef] [PubMed]

| Antibodies | Source | Catalog |
|---|---|---|
| Anti-collagen III polyclonal antibody | Proteintech | 22734-1-AP |
| Anti- collagen I monoclonal antibody | CST | #84336 |
| Anti- αSMA polyclonal antibody | SAB | 41550 |
| Anti-p53 polyclonal antibody | Proteintech | 10442-1-AP |
| Anti-p21 polyclonal antibody | Servicebio | GB11153 |
| Anti-p16 monoclonal antibody | Abcam | ab211542 |
| Anti-COX-2 polyclonal antibody | Proteintech | 12375-1-AP |
| Anti-sEH monoclonal antibody | Abcam | ab155280 |
| Anti-γH2AX monoclonal antibody | Boster | BM4841 |
| Anti-LC3 monoclonal antibody | CST | 12741 |
| Anti-Fundc1 monoclonal antibody | CST | 49240 |
| Anti-p62 polyclonal antibody | Immunoway | YT7058 |
| Anti-ki67 polyclonal antibody | Abways | CY5542 |
| Anti-PCNA polyclonal antibody | Proteintech | 10205-2-AP |
| Anti-Sirt1 monoclonal antibody | Proteintech | 60303-1-Ig |
| Anti-mTOR (phospho ser2448) antibody | CST | 2972S |
| Anti-mTOR polyclonal antibody | Proteintech | 20657-1-AP |
| Anti-PI3K p85α (phospho Tyr607) antibody | Affinity | AF3241 |
| Anti-PI3K p85α monoclonal antibody | Immunoway | YM3503 |
| Anti- AKT (phospho ser473) polyclonal antibody | Immunoway | YP0846 |
| Anti-AKT polyclonal antibody | Immunoway | YT0185 |
| Anti-α-Tubulin polyclonal antibody | Servicebio | GB11200 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| m-p16 | CTCTGCTCTTGGGATTGGC | GTGCGATATTTGCGTTCCG |
| m-p19 | GAGGCCGGCAAATGATCATAGA | GTGGATACCGGTGGACTGTG |
| m-β-actin | TTCCAGCCTTCCTTCTTG | GGAGCCAGAGCAGTAATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-Y.; Tan, X.-H.; Yang, H.-H.; Jin, L.; Hong, J.-R.; Zhou, Y.; Huang, X.-T. COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR. Int. J. Mol. Sci. 2022, 23, 8267. https://doi.org/10.3390/ijms23158267
Zhang C-Y, Tan X-H, Yang H-H, Jin L, Hong J-R, Zhou Y, Huang X-T. COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR. International Journal of Molecular Sciences. 2022; 23(15):8267. https://doi.org/10.3390/ijms23158267
Chicago/Turabian StyleZhang, Chen-Yu, Xiao-Hua Tan, Hui-Hui Yang, Ling Jin, Jie-Ru Hong, Yong Zhou, and Xiao-Ting Huang. 2022. "COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR" International Journal of Molecular Sciences 23, no. 15: 8267. https://doi.org/10.3390/ijms23158267
APA StyleZhang, C.-Y., Tan, X.-H., Yang, H.-H., Jin, L., Hong, J.-R., Zhou, Y., & Huang, X.-T. (2022). COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR. International Journal of Molecular Sciences, 23(15), 8267. https://doi.org/10.3390/ijms23158267






