HDL Cholesterol Efflux and Serum Cholesterol Loading Capacity Alterations Associate to Macrophage Cholesterol Accumulation in FH Patients with Achilles Tendon Xanthoma
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
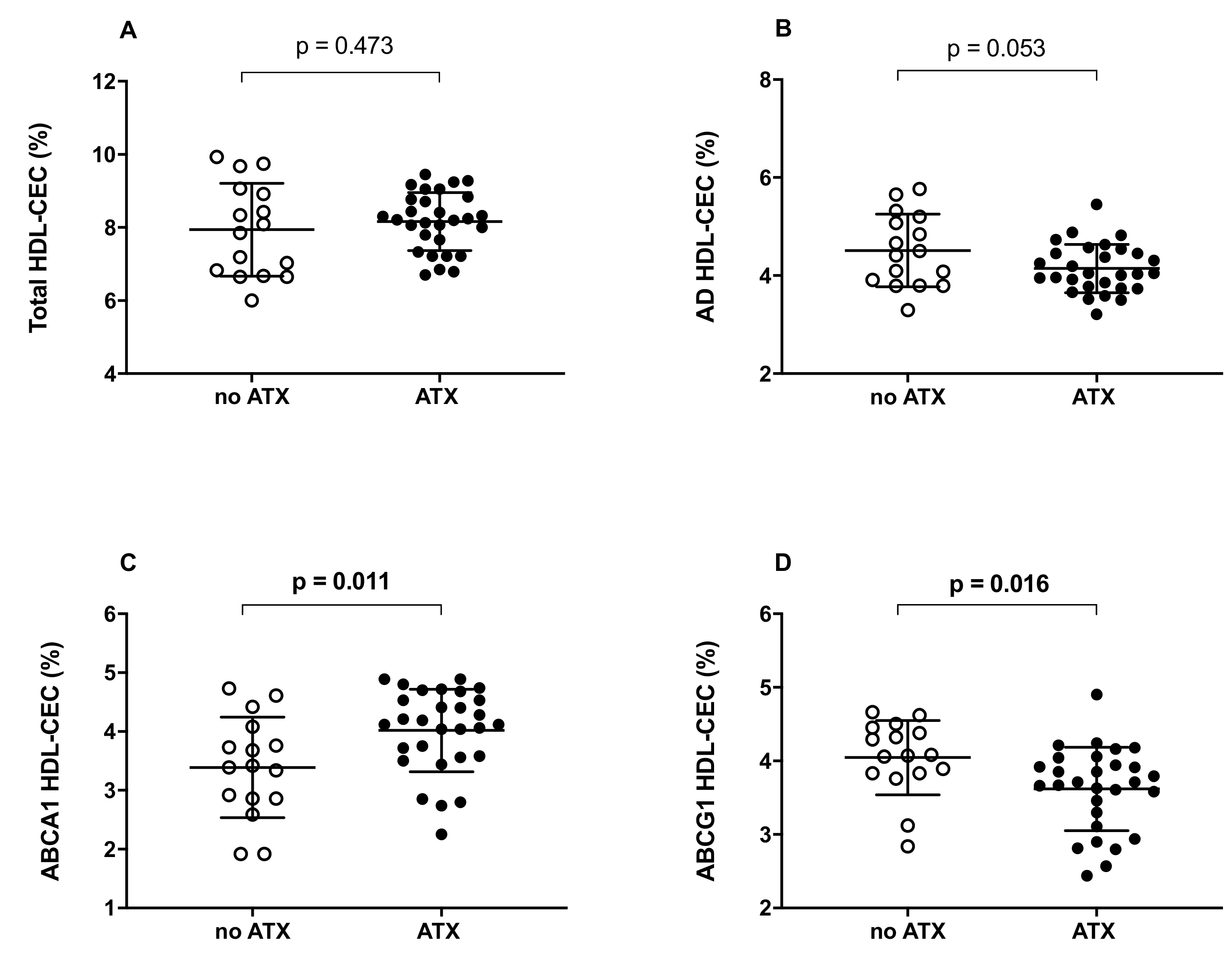
2.2. HDL Cholesterol Efflux Capacity (CEC)
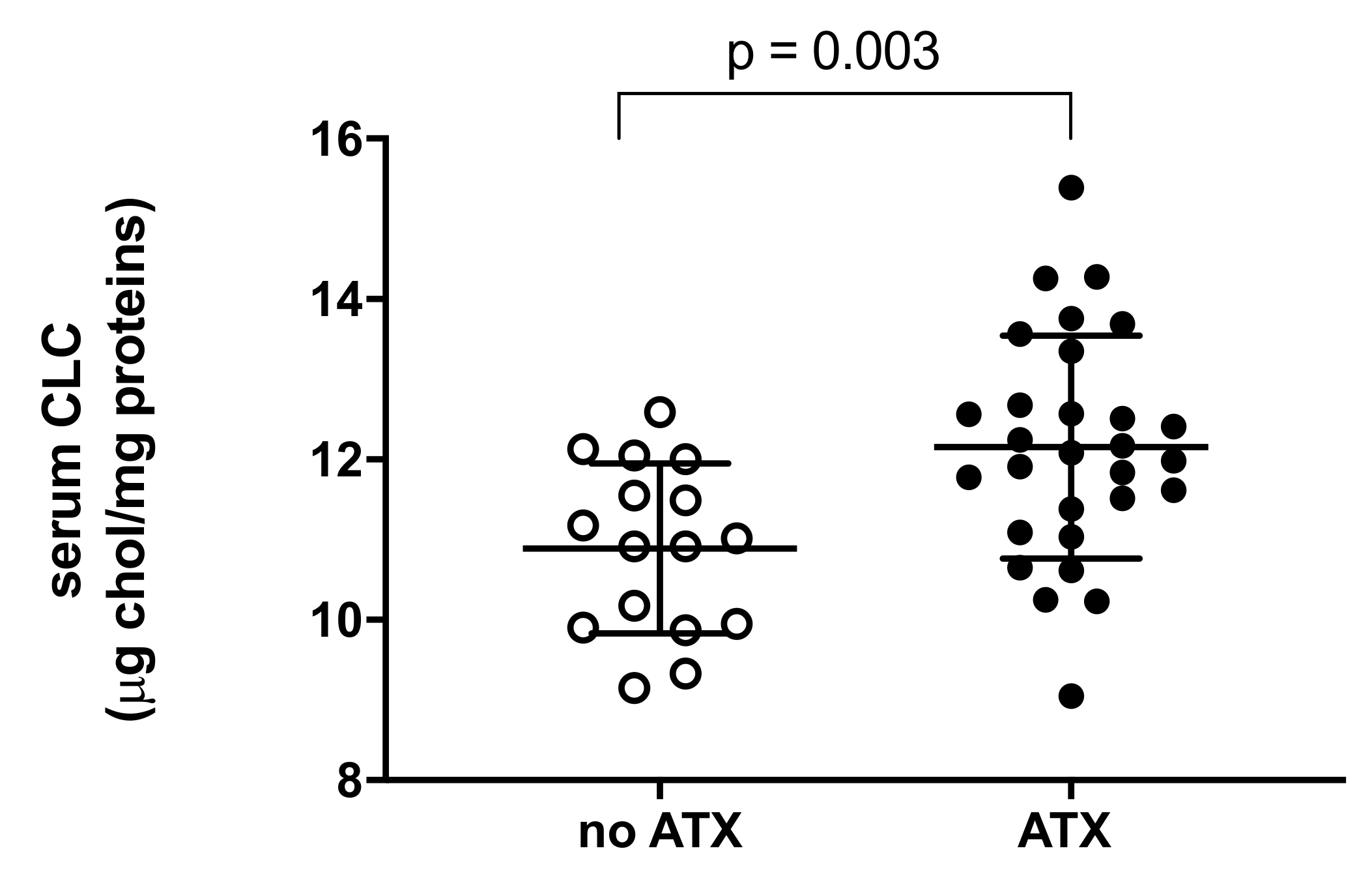
2.3. Serum Cholesterol Loading Capacity (CLC)
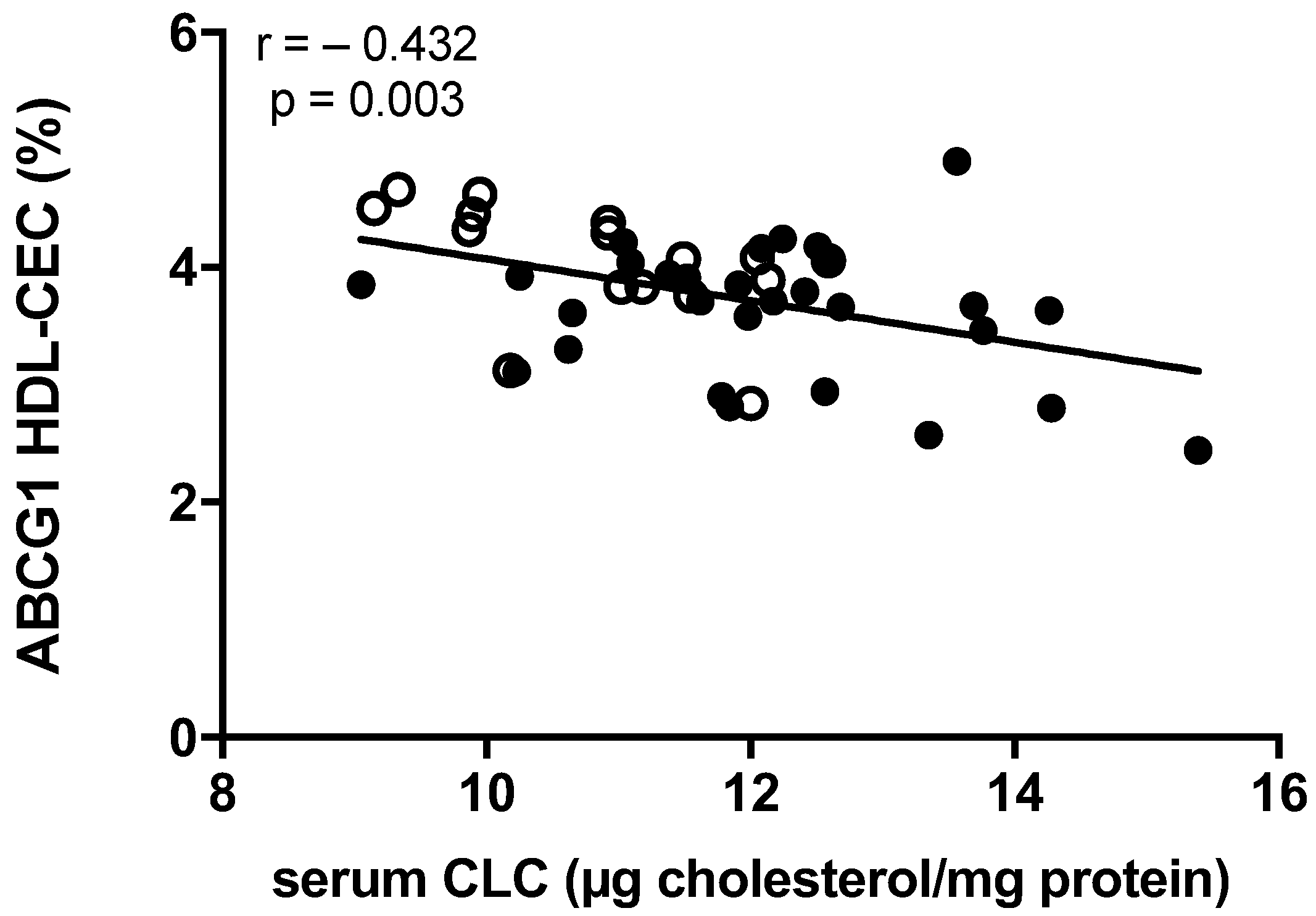
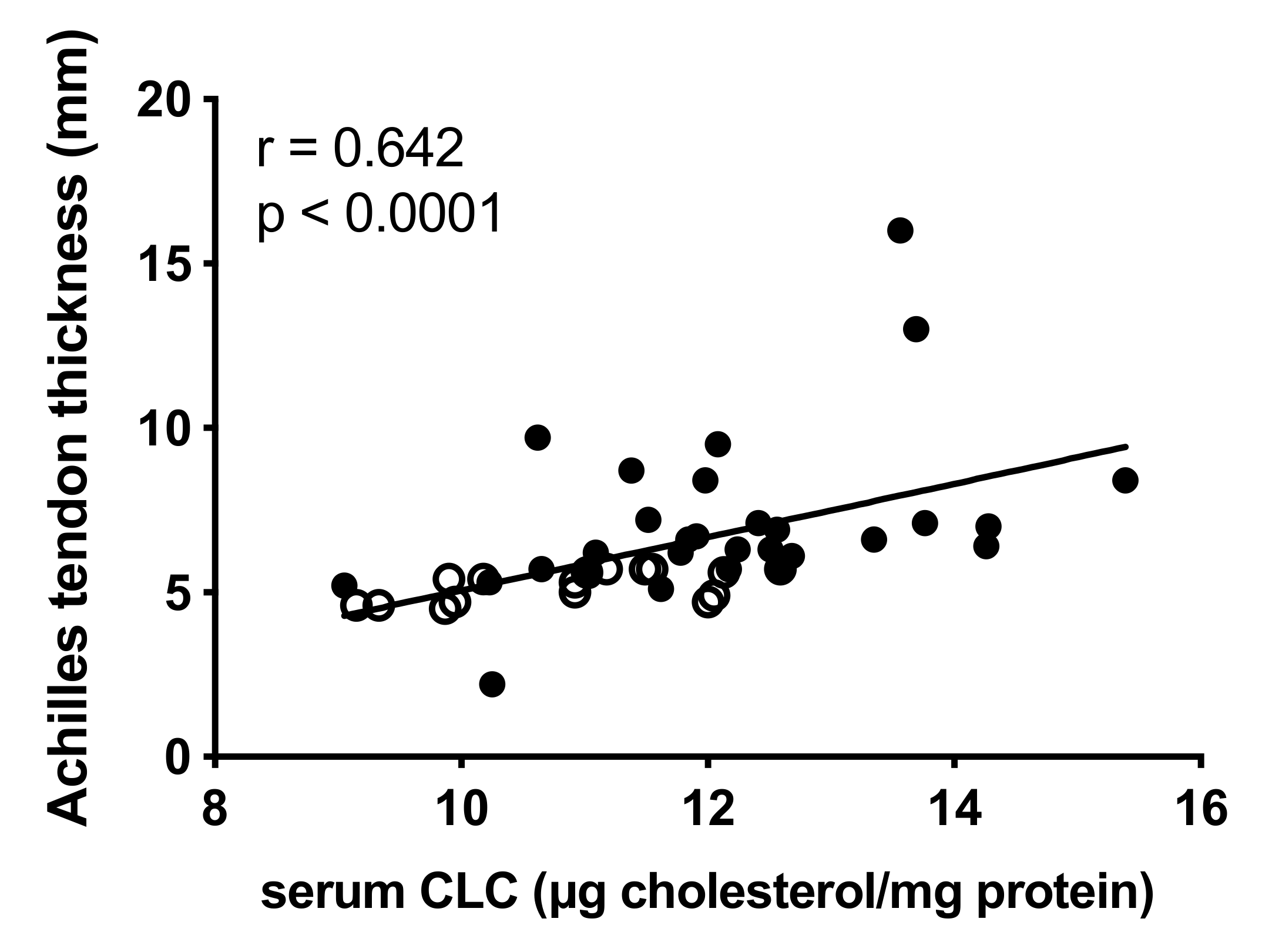
2.4. Correlation between Lipoprotein Functions and Achilles Tendon Thickness
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data Collection and Xanthoma Evaluation
4.3. Total Cholesterol, HDL Cholesterol, Triglycerides and hs-CRP Evaluation
4.4. HDL Cholesterol Efflux Capacity (CEC)
4.5. Total, Aqueous Diffusion and ABCA1 HDL-CEC
4.6. ABCG1-Mediated HDL-CEC
4.7. Serum Cholesterol Loading Capacity (CLC)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lui, D.T.W.; Lee, A.C.H.; Tan, K.C.B. Management of Familial Hypercholesterolemia: Current Status and Future Perspectives. J. Endocr. Soc. 2021, 5, bvaa122. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Familial Hypercholesterolemia and Coronary Heart Disease: A HuGE Association Review. Am. J. Epidemiol. 2004, 160, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial Hypercholesterolaemia Is Underdiagnosed and Undertreated in the General Population: Guidance for Clinicians to Prevent Coronary Heart Disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Scott, A.; Zahradnik, T.M.; Squier, K.; Beck, C.; Brunham, L.R. Diagnostic Accuracy of Ultrasound and MRI for Achilles Tendon Xanthoma in People with Familial Hypercholesterolemia: A Systematic Review. J. Clin. Lipidol. 2019, 13, 40–48. [Google Scholar] [CrossRef]
- Wiegman, A.; Gidding, S.S.; Watts, G.F.; Chapman, M.J.; Ginsberg, H.N.; Cuchel, M.; Ose, L.; Averna, M.; Boileau, C.; Borén, J.; et al. Familial Hypercholesterolaemia in Children and Adolescents: Gaining Decades of Life by Optimizing Detection and Treatment. Eur. Heart J. 2015, 36, 2425–2437. [Google Scholar] [CrossRef]
- Civeira, F.; Castillo, S.; Alonso, R.; Meriño-Ibarra, E.; Cenarro, A.; Artied, M.; Martín-Fuentes, P.; Ros, E.; Pocoví, M.; Mata, P. Tendon Xanthomas in Familial Hypercholesterolemia Are Associated with Cardiovascular Risk Independently of the Low-Density Lipoprotein Receptor Gene Mutation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1960–1965. [Google Scholar] [CrossRef]
- Mangili, L.C.; Miname, M.H.; Silva, P.R.S.; Bittencourt, M.S.; Rocha, V.Z.; Mangili, O.C.; Salgado Filho, W.; Chacra, A.P.; Jannes, C.E.; Pereira, A.C.; et al. Achilles Tendon Xanthomas Are Associated with the Presence and Burden of Subclinical Coronary Atherosclerosis in Heterozygous Familial Hypercholesterolemia: A Pilot Study. Atherosclerosis 2017, 263, 393–397. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.-A.; Nohara, A.; Inazu, A.; Mabuchi, H.; Yamagishi, M. Impact of Clinical Signs and Genetic Diagnosis of Familial Hypercholesterolaemia on the Prevalence of Coronary Artery Disease in Patients with Severe Hypercholesterolaemia. Eur. Heart J. 2017, 38, 1573–1579. [Google Scholar] [CrossRef]
- Kitahara, H.; Nakayama, T.; Fujimoto, Y.; Kobayashi, Y. Association between Achilles Tendon Xanthoma and Severity of Coronary Artery Disease in Patients Undergoing Percutaneous Coronary Intervention. J. Cardiol. 2020, 75, 654–658. [Google Scholar] [CrossRef]
- Taylor, B.; Cheema, A.; Soslowsky, L. Tendon Pathology in Hypercholesterolemia and Familial Hypercholesterolemia. Curr. Rheumatol. Rep. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Tsouli, S.G.; Kiortsis, D.N.; Argyropoulou, M.I.; Mikhailidis, D.P.; Elisaf, M.S. Pathogenesis, Detection and Treatment of Achilles Tendon Xanthomas. Eur. J. Clin. Investig. 2005, 35, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.; Vincent, V.; Sen, A.; Singh, A.; Roy, A. Changing Perspectives on HDL: From Simple Quantity Measurements to Functional Quality Assessment. J. Lipids 2021, 2021, 5585521. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ronda, N.; Bernini, F.; Zimetti, F. High Density Lipoprotein Cholesterol Efflux Capacity and Atherosclerosis in Cardiovascular Disease: Pathophysiological Aspects and Pharmacological Perspectives. Cells 2021, 10, 574. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.-A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Soria-Florido, M.T.; Schröder, H.; Grau, M.; Fitó, M.; Lassale, C. High Density Lipoprotein Functionality and Cardiovascular Events and Mortality: A Systematic Review and Meta-Analysis. Atherosclerosis 2020, 302, 36–42. [Google Scholar] [CrossRef]
- Tall, A.R.; Rader, D.J. Trials and Tribulations of CETP Inhibitors. Circ. Res. 2018, 122, 106–112. [Google Scholar] [CrossRef]
- Cheng, W.; Rosolowski, M.; Boettner, J.; Desch, S.; Jobs, A.; Thiele, H.; Buettner, P. High-Density Lipoprotein Cholesterol Efflux Capacity and Incidence of Coronary Artery Disease and Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2022, 21, 47. [Google Scholar] [CrossRef]
- Adorni, M.P.; Zimetti, F.; Cangiano, B.; Vezzoli, V.; Bernini, F.; Caruso, D.; Corsini, A.; Sirtori, C.R.; Cariboni, A.; Bonomi, M.; et al. High-Density Lipoprotein Function Is Reduced in Patients Affected by Genetic or Idiopathic Hypogonadism. J. Clin. Endocrinol. Metab. 2019, 104, 3097–3107. [Google Scholar] [CrossRef]
- Adorni, M.P.; Zimetti, F.; Puntoni, M.; Bigazzi, F.; Sbrana, F.; Minichilli, F.; Bernini, F.; Ronda, N.; Favari, E.; Sampietro, T. Cellular Cholesterol Efflux and Cholesterol Loading Capacity of Serum: Effects of LDL-Apheresis. J. Lipid Res. 2012, 53, 984–989. [Google Scholar] [CrossRef]
- Soslowsky, L.J.; Fryhofer, G.W. Tendon Homeostasis in Hypercholesterolemia. Adv. Exp. Med. Biol. 2016, 920, 151–165. [Google Scholar] [CrossRef]
- De Sá, A.; Hart, D.A.; Khan, K.; Scott, A. Achilles Tendon Structure Is Negatively Correlated with Body Mass Index, but Not Influenced by Statin Use: A Cross-Sectional Study Using Ultrasound Tissue Characterization. PLoS ONE 2018, 13, e0199645. [Google Scholar] [CrossRef]
- Hirata, Y.; Okawa, K.; Ikeda, M.; Seike, M.; Matsumoto, M.; Kodama, H. Low Density Lipoprotein Oxidized in Xanthoma Tissue Induces the Formation and Infiltration of Foam Cells. J. Dermatol. Sci. 2002, 30, 248–255. [Google Scholar] [CrossRef]
- Oosterveer, D.M.; Versmissen, J.; Yazdanpanah, M.; Hamza, T.H.; Sijbrands, E.J.G. Differences in Characteristics and Risk of Cardiovascular Disease in Familial Hypercholesterolemia Patients with and without Tendon Xanthomas: A Systematic Review and Meta-Analysis. Atherosclerosis 2009, 207, 311–317. [Google Scholar] [CrossRef]
- Heiberg, A. The Lipoprotein and Lipid Pattern in Xanthomatosis. Acta Med. Scand. 1975, 198, 183–195. [Google Scholar] [CrossRef]
- Mancuso, G.; La Regina, G.; Bagnoli, M.; Bittolo Bon, G.; Cazzolato, G.; Preda, P.; Berdondini, R.M.; Sangiorgi, Z.; Gaddi, A. “Normolipidemic” Tendinous and Tuberous Xanthomatosis. Dermatology 1996, 193, 27–32. [Google Scholar] [CrossRef]
- Kitahara, H.; Mori, N.; Saito, Y.; Nakayama, T.; Fujimoto, Y.; Kobayashi, Y. Prevalence of Achilles Tendon Xanthoma and Familial Hypercholesterolemia in Patients with Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Heart Vessel. 2019, 34, 1595–1599. [Google Scholar] [CrossRef]
- Ferrières, J.; Lambert, J.; Lussier-Cacan, S.; Davignon, J. Coronary Artery Disease in Heterozygous Familial Hypercholesterolemia Patients with the Same LDL Receptor Gene Mutation. Circulation 1995, 92, 290–295. [Google Scholar] [CrossRef]
- Tani, M.; Kawakami, A.; Mizuno, Y.; Imase, R.; Ito, Y.; Kondo, K.; Ishii, H.; Yoshida, M. Small Dense LDL Enhances THP-1 Macrophage Foam Cell Formation. J. Atheroscler. Thromb. 2011, 18, 698–704. [Google Scholar] [CrossRef]
- Gerber, P.A.; Nikolic, D.; Rizzo, M. Small, Dense LDL: An Update. Curr. Opin. Cardiol. 2017, 32, 454–459. [Google Scholar] [CrossRef]
- Mason, J.C.; Libby, P. Cardiovascular Disease in Patients with Chronic Inflammation: Mechanisms Underlying Premature Cardiovascular Events in Rheumatologic Conditions. Eur. Heart J. 2015, 36, 482–489. [Google Scholar] [CrossRef]
- Groenen, A.G.; Halmos, B.; Tall, A.R.; Westerterp, M. Cholesterol Efflux Pathways, Inflammation, and Atherosclerosis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 426–439. [Google Scholar] [CrossRef]
- Artieda, M.; Cenarro, A.; Junquera, C.; Lasierra, P.; Martínez-Lorenzo, M.J.; Pocoví, M.; Civeira, F. Tendon Xanthomas in Familial Hypercholesterolemia Are Associated with a Differential Inflammatory Response of Macrophages to Oxidized LDL. FEBS Lett. 2005, 579, 4503–4512. [Google Scholar] [CrossRef]
- Hjuler Nielsen, M.; Irvine, H.; Vedel, S.; Raungaard, B.; Beck-Nielsen, H.; Handberg, A. Elevated Atherosclerosis-Related Gene Expression, Monocyte Activation and Microparticle-Release Are Related to Increased Lipoprotein-Associated Oxidative Stress in Familial Hypercholesterolemia. PLoS ONE 2015, 10, e0121516. [Google Scholar] [CrossRef]
- Ronda, N.; Favari, E.; Borghi, M.O.; Ingegnoli, F.; Gerosa, M.; Chighizola, C.; Zimetti, F.; Adorni, M.P.; Bernini, F.; Meroni, P.L. Impaired Serum Cholesterol Efflux Capacity in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2014, 73, 609–615. [Google Scholar] [CrossRef]
- Zimetti, F.; De Vuono, S.; Gomaraschi, M.; Adorni, M.P.; Favari, E.; Ronda, N.; Ricci, M.A.; Veglia, F.; Calabresi, L.; Lupattelli, G. Plasma Cholesterol Homeostasis, HDL Remodeling and Function during the Acute Phase Reaction. J. Lipid Res. 2017, 58, 2051–2060. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Oram, J.F.; Asztalos, B.F.; Vaughan, A.M.; Lund-Katz, S.; Adorni, M.P.; Phillips, M.C.; Rothblat, G.H. Effects of Acceptor Composition and Mechanism of ABCG1-Mediated Cellular Free Cholesterol Efflux. J. Lipid Res. 2009, 50, 275–284. [Google Scholar] [CrossRef]
- Tosheska Trajkovska, K.; Topuzovska, S. High-Density Lipoprotein Metabolism and Reverse Cholesterol Transport: Strategies for Raising HDL Cholesterol. Anatol. J. Cardiol. 2017, 18, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.-L.; Cochran, B.J.; Manandhar, B.; Thomas, S.; Rye, K.-A. HDL Maturation and Remodelling. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2022, 1867, 159119. [Google Scholar] [CrossRef]
- Escolà-Gil, J.C.; Rotllan, N.; Julve, J.; Blanco-Vaca, F. Reverse Cholesterol Transport Dysfunction Is a Feature of Familial Hypercholesterolemia. Curr. Atheroscler. Rep. 2021, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Momtazi-Borojeni, A.A.; Banach, M.; Kovanen, P.T.; Gotto, A.M.J.; Sahebkar, A. HDL Functionality in Familial Hypercholesterolemia: Effects of Treatment Modalities and Pharmacological Interventions. Drug Discov. Today 2018, 23, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Familial Hypercholesterolemia: Do HDL Play a Role? Biomedicines 2021, 9, 810. [Google Scholar] [CrossRef]
- Ogura, M.; Hori, M.; Harada-Shiba, M. Association Between Cholesterol Efflux Capacity and Atherosclerotic Cardiovascular Disease in Patients with Familial Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 181–188. [Google Scholar] [CrossRef]
- Prosser, H.C.; Ng, M.K.C.; Bursill, C.A. The Role of Cholesterol Efflux in Mechanisms of Endothelial Protection by HDL. Curr. Opin. Lipidol. 2012, 23, 182–189. [Google Scholar] [CrossRef]
- Gomaraschi, M.; Ossoli, A.; Favari, E.; Adorni, M.P.; Sinagra, G.; Cattin, L.; Veglia, F.; Bernini, F.; Franceschini, G.; Calabresi, L. Inflammation Impairs ENOS Activation by HDL in Patients with Acute Coronary Syndrome. Cardiovasc. Res. 2013, 100, 36–43. [Google Scholar] [CrossRef][Green Version]
- Holven, K.B.; Myhre, A.M.; Aukrust, P.; Hagve, T.A.; Ose, L.; Nenseter, M.S. Patients with Familial Hypercholesterolaemia Show Enhanced Spontaneous Chemokine Release from Peripheral Blood Mononuclear Cells Ex Vivo. Dependency of Xanthomas/Xanthelasms, Smoking and Gender. Eur. Heart J. 2003, 24, 1756–1762. [Google Scholar] [CrossRef][Green Version]
- Gazzotti, M.; Casula, M.; Olmastroni, E.; Averna, M.; Arca, M.; Catapano, A.L. How Registers Could Enhance Knowledge and Characterization of Genetic Dyslipidaemias: The Experience of the LIPIGEN in Italy and of Other Networks for Familial Hypercholesterolemia. Atheroscler. Suppl. 2020, 42, e35–e40. [Google Scholar] [CrossRef]
- Olmastroni, E.; Gazzotti, M.; Arca, M.; Averna, M.; Pirillo, A.; Catapano, A.L.; Casula, M. Twelve Variants Polygenic Score for Low-Density Lipoprotein Cholesterol Distribution in a Large Cohort of Patients with Clinically Diagnosed Familial Hypercholesterolemia with or without Causative Mutations. J. Am. Heart Assoc. 2022, 11, e023668. [Google Scholar] [CrossRef]
- Wang, J.; Dron, J.S.; Ban, M.R.; Robinson, J.F.; McIntyre, A.D.; Alazzam, M.; Zhao, P.J.; Dilliott, A.A.; Cao, H.; Huff, M.W.; et al. Polygenic Versus Monogenic Causes of Hypercholesterolemia Ascertained Clinically. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2439–2445. [Google Scholar] [CrossRef]
- Besseling, J.; Kindt, I.; Hof, M.; Kastelein, J.J.P.; Hutten, B.A.; Hovingh, G.K. Severe Heterozygous Familial Hypercholesterolemia and Risk for Cardiovascular Disease: A Study of a Cohort of 14,000 Mutation Carriers. Atherosclerosis 2014, 233, 219–223. [Google Scholar] [CrossRef]
- Zenti, M.G.; Altomari, A.; Lupo, M.G.; Botta, M.; Bonora, E.; Corsini, A.; Ruscica, M.; Ferri, N. From Lipoprotein Apheresis to Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors: Impact on Low-Density Lipoprotein Cholesterol and C-Reactive Protein Levels in Cardiovascular Disease Patients. Eur. J. Prev. Cardiol. 2018, 25, 1843–1851. [Google Scholar] [CrossRef]
- Van Velzen, D.M.; Adorni, M.P.; Zimetti, F.; Strazzella, A.; Simsek, S.; Sirtori, C.R.; den Heijer, M.; Ruscica, M. The Effect of Transgender Hormonal Treatment on High Density Lipoprotein Cholesterol Efflux Capacity. Atherosclerosis 2021, 323, 44–53. [Google Scholar] [CrossRef]
- Asztalos, B.F.; de la Llera-Moya, M.; Dallal, G.E.; Horvath, K.V.; Schaefer, E.J.; Rothblat, G.H. Differential Effects of HDL Subpopulations on Cellular ABCA1- and SR-BI-Mediated Cholesterol Efflux. J. Lipid Res. 2005, 46, 2246–2253. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Ohkawa, R.; Lai, S.-J.; Shimano, S.; Hagihara, M.; Tohda, S.; Kameda, T.; Tozuka, M. Usefulness of Apolipoprotein B-Depleted Serum in Cholesterol Efflux Capacity Assays Using Immobilized Liposome-Bound Gel Beads. Biosci. Rep. 2019, 39, BSR20190213. [Google Scholar] [CrossRef]
- Favari, E.; Zimetti, F.; Bortnick, A.E.; Adorni, M.P.; Zanotti, I.; Canavesi, M.; Bernini, F. Impaired ATP-Binding Cassette Transporter A1-Mediated Sterol Efflux from Oxidized LDL-Loaded Macrophages. FEBS Lett. 2005, 579, 6537–6542. [Google Scholar] [CrossRef]
- Zimetti, F.; Weibel, G.K.; Duong, M.; Rothblat, G.H. Measurement of Cholesterol Bidirectional Flux between Cells and Lipoproteins. J. Lipid Res. 2006, 47, 605–613. [Google Scholar] [CrossRef]
| Characteristics | Xanthoma | p Value | ||
|---|---|---|---|---|
| None (n = 16) | Present (n = 29) | |||
| Age—years | 42.1 ± 18.6 | 46.3 ± 13.9 | n.s. | |
| Male—n. (%) | 5 (31.2%) | 11 (37.9%) | n.s. | |
| BMI—Kg/m2 | 24.2 ± 5.3 | 25.9 ± 5.8 | n.s. | |
| hs-CRP—µg/mL | 0.54 (1.7) | 0.25 (0.94) | n.s. | |
| DLCN score | 6.4 ± 3.1 | 11.0 ± 5.0 | <0.001 | |
| Cardiovascular risk factors—n. (%) | ||||
| Smoking | 8 (50.0%) | 6 (20.7%) | 0.04 | |
| Arterial Hypertension | 1 (6.3%) | 3 (10.3%) | n.s. | |
| Diabetes mellitus | 0 | 0 | n.s. | |
| ASCVD familiarity | 3 (18.8%) | 15 (51.7%) | 0.03 | |
| Early cardiovascular events—n. (%) | 3 (18.8%) | 3 (10.3%) | n.s. | |
| Lipid profile—mg/dL | ||||
| Total Cholesterol | 223.8 ± 66.8 | 260.4 ± 80.7 | n.s. | |
| HDL Cholesterol | 61.2 ± 16.6 | 55.9 ± 14.0 | n.s. | |
| LDL Cholesterol | 141.3 ± 57.2 | 180.1 ± 77.0 | n.s. | |
| Triglyceride | 106.9 ± 49.0 | 124.5 ± 77.1 | n.s. | |
| Oxidized LDL | 56.3 ± 13.7 | 74.4 ± 34.3 | n.s | |
| apoB | 109.7 ± 26.3 | 131.5 ± 44.2 | n.s. | |
| Hypolipemic therapy in progress—n. (%) | ||||
| Statin | 11 (68.6%) | 16 (55.2%) | n.s. | |
| Ezetimibe | 4 (25.0%) | 13 (44.8%) | n.s. | |
| None | 5 (31.2%) | 13 (44.8%) | n.s. | |
| HDL-CEC Pathways | r | p Value |
|---|---|---|
| Total HDL-CEC | −0.104 | 0.497 |
| AD HDL-CEC | −0.342 | 0.021 |
| ABCA1 HDL-CEC | 0.186 | 0.221 |
| ABCG1 HDL-CEC | −0.367 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adorni, M.P.; Biolo, M.; Zimetti, F.; Palumbo, M.; Ronda, N.; Scarinzi, P.; Simioni, P.; Lupo, M.G.; Ferri, N.; Previato, L.; et al. HDL Cholesterol Efflux and Serum Cholesterol Loading Capacity Alterations Associate to Macrophage Cholesterol Accumulation in FH Patients with Achilles Tendon Xanthoma. Int. J. Mol. Sci. 2022, 23, 8255. https://doi.org/10.3390/ijms23158255
Adorni MP, Biolo M, Zimetti F, Palumbo M, Ronda N, Scarinzi P, Simioni P, Lupo MG, Ferri N, Previato L, et al. HDL Cholesterol Efflux and Serum Cholesterol Loading Capacity Alterations Associate to Macrophage Cholesterol Accumulation in FH Patients with Achilles Tendon Xanthoma. International Journal of Molecular Sciences. 2022; 23(15):8255. https://doi.org/10.3390/ijms23158255
Chicago/Turabian StyleAdorni, Maria Pia, Marta Biolo, Francesca Zimetti, Marcella Palumbo, Nicoletta Ronda, Paolo Scarinzi, Paolo Simioni, Maria Giovanna Lupo, Nicola Ferri, Lorenzo Previato, and et al. 2022. "HDL Cholesterol Efflux and Serum Cholesterol Loading Capacity Alterations Associate to Macrophage Cholesterol Accumulation in FH Patients with Achilles Tendon Xanthoma" International Journal of Molecular Sciences 23, no. 15: 8255. https://doi.org/10.3390/ijms23158255
APA StyleAdorni, M. P., Biolo, M., Zimetti, F., Palumbo, M., Ronda, N., Scarinzi, P., Simioni, P., Lupo, M. G., Ferri, N., Previato, L., Bernini, F., & Zambon, A. (2022). HDL Cholesterol Efflux and Serum Cholesterol Loading Capacity Alterations Associate to Macrophage Cholesterol Accumulation in FH Patients with Achilles Tendon Xanthoma. International Journal of Molecular Sciences, 23(15), 8255. https://doi.org/10.3390/ijms23158255







